Abstract
In arid regions, starchy agricultural products such as wheat and rice provide essential carbohydrates, minerals, fibers and vitamins. However, drought, desiccation, high salinity, potentially toxic metals and hydrocarbon accumulation are among the most notable stresses affecting soil quality and cereal production in arid environments. Certain soil bacteria, referred to as Plant Growth-Promoting Rhizobacteria (PGPR), colonize the plant root environment, providing beneficial advantages for both soil and plants. Beyond their ability to improve plant growth under non-stressed conditions, PGPR can establish symbiotic and non-symbiotic interactions with plants growing under stress conditions, participating in soil bioremediation, stress alleviation and plant growth restoration. Moreover, the PGPR ability to fix nitrogen, to solubilize insoluble forms of nutrients and to produce other metabolites such as siderophores, phytohormones, antibiotics and hydrolytic enzymes makes them ecofriendly alternatives to the excessive use of unsuitable and cost-effective chemicals in agriculture. The most remarkable PGPR belong to the genera Arthrobacter, Azospirillum, Azotobacter, Bacillus, Enterobacter, Klebsiella, Pseudomonas, etc. Therefore, high cereal production in arid environments can be ensured using PGPR. Herein, the potential role of such bacteria in promoting wheat and rice production under both normal and derelict soils is reviewed and highlighted.
1. Introduction
In 2019, the United Nations Organization signaled that demographic growth had reached eight billion inhabitants on the planet, where most of the population live in arid and semi-arid environments (Asia: 60% and Africa: 16%). In addition, the global population is expected to reach 8.5 billion by 2030, 9.7 billion by 2050 and 10.9 billion by 2100 [1]. This rapidly growing population requires increasing food production, essentially coming from agriculture. Thus, a doubling of food and feed production is needed in the next forty years to respond to these new requirements [2].
Arid environments are extremely diverse in terms of landforms, soils, fauna, flora, water balances and human activities. Thus, no practical definition of arid environments can be derived, whereas the one binding element to all arid regions is “aridity”. It is important to underline the fact that arid agriculture receives rising attention because of its related problems in mostly undeveloped countries; where a third of all human beings live in 41% of the globe’s surface, getting the larger part of their food from cereals and vegetables [3].
The increase in aridity decreases water availability, crop yield and agricultural productivity [4]. Several studies have indicated that increased carbon dioxide concentrations in the atmosphere lead to global warming [5,6,7]. Consequently, an increase in aridity is predicted in some model scenarios where drought would persist in some areas of the globe [8,9,10]. According to Le Houérou [11], there has been an increase of 0.5 °C in global temperature over the past 100 years, which is partially due to excessive urbanization and industrialization. Otherwise, abiotic stresses such as drought, salinity, extreme temperatures, chemical toxicity and oxidative stress are serious threats to agriculture and the natural status of arid environments [12].
In addition, several human activities related to industry, such as the excessive use of petroleum, but also to other agricultural practices such as the hysterical application of fertilizers, pesticides and herbicides, have improved human life quality. However, such activities have also led to the accumulation of alarming amounts of salts and other toxic chemicals, leading to environmental degradation and soil deprivation [13]. These phenomena do not only affect plant growth, but also the soil’s microbial community, including beneficial soil bacteria. In this context, the results obtained by Maestre et al. [14] suggest that changes in aridity, following the climate-change models, may reduce microbial abundance and diversity, a response that will likely impact soil fertility and climate regulation. Therefore, several practices have been adopted over time to remediate soils and to enhance plant growth under stress conditions. Among these solutions, certain soil bacteria, referred to as Plant Growth-Promoting Rhizobacteria (PGPR), can colonize the surfaces or inner tissues of plant roots, providing beneficial advantages for both soils and plants [15]. Beyond their ability to improve plant growth under non-stressed conditions, some PGPR are able to establish symbiotic and non-symbiotic interactions with plants growing under stress conditions, participating in soil bioremediation, stress alleviation and plant growth restoration [16]. The ability of PGPR to fix nitrogen, to solubilize nutrients and to produce metabolites such as siderophores, phytohormones, antibiotics and hydrolytic enzymes makes them one of the most ecofriendly alternatives to avoid the excessive use of unsuitable and cost-effective chemicals in agriculture [17]. In the last few years, PGPR have retained both scientists’ and farmers’ attention as interesting substitutes for chemicals for their sustainable and healthy effect on the environment, but also their promising roles in bioremediation [18,19].
Recently, some reviews have highlighted the role of PGPR as abiotic stress alleviators in soil and the prospects of their application to mitigate soil metal contamination [20,21,22]. However, none was directed to describe their impact on soil bioremediation and cereal growth enhancement in arid environments. In this paper, we highlight the importance of using beneficial soil bacteria for both soil quality restoration and plant growth enhancement in arid environments. We also summarize scientific works revealing the place of such soil bacteria in improving wheat and rice production under stress circumstances. It is important to mention that cereals, particularly wheat and rice, are known to be the most important crops in the world. Together with maize, they constitute more than 50% of all the calories consumed by human beings over the world [23]. In addition, human diets, especially in developing countries where aridity is dominant, are essentially based on cereals. For example, global cereal demand is expected to increase from 585 million to 828 million tons by 2025, corresponding to an increase of 42% [2]. Furthermore, developing countries’ rice and wheat production is supposed to jump from 4.2 and 3.1 to 4.7 and 3.5 tons/ha, between the year 2015 and 2030, respectively. Such rising cereal production would seem to be unsatisfactory to meet the accelerated growth of the human population that is predicted to reach 8.5 billion by the end of 2030 [1,24].
Accordingly, this review aims to understand the prospective functions of Plant Growth-Promoting Rhizobacteria (PGPR), which can participate in soil bioremediation, stress alleviation and plant growth restoration. In fact, the positive effects of PGPR could be exploited to promote agricultural practices in stressed environments and help specialists to manage decisions for more ecofriendly practices in agriculture.
2. Main Aspects of Low Soil Fertility
With industrialization, anthropogenic activities such as crude oil exploitation, mining, urban development and the excessive use of chemical fertilizers, pesticides and herbicides have strongly affected soil’s physical, physiological and biochemical properties, but also its intrinsic heterogeneous microbial diversity [13,25]. Compounds resulting from such activities are known to be hardly biodegradable and contain high amounts of potentially toxic elements (hereafter: PTE) and other pollutants, hence persisting in nature and affecting vegetal development [19]. In addition, the microbial communities in these contaminated soils are disturbed, which affects their important roles in organic matter recycling, plant disease control, vegetal growth enhancement and the detoxification of the deleterious chemicals in the soil [26,27]. Among PTEs, lead (Pb), chromium (Cr), arsenic (As), zinc (Zn), cadmium (Cd), copper (Cu), mercury (Hg) and nickel (Ni) are the most encountered. Unlike other pollutants, soil is the main tank of such PTE. At high concentrations, these compounds are highly toxic for both plants and microorganisms [28]. Moreover, global warming, together with water scarcity, has led to excessive irrigation and chemical fertilizer application to meet global food requirements, which has resulted in salt accumulation in soil. High concentrations of salt ions in soil, mainly Na+ and Cl−, but also other ions such as (K+, Ca2+, (CO3)2−, etc.), reduce water acquisition by plant roots, disturb soil microflora and accentuate phytopathogen virulence [29,30,31]. Recently, using certain soil bacteria for soil health maintaining and plant growth restauration under abiotic stresses attracts both farmers and scientists as a potential alternative to chemicals. Table 1 summarizes the most recent scientific advances in using beneficial soil bacteria for bioremediation and crop growth restoration.

Table 1.
Recent advances in soil remediation and plant growth restoration using beneficial soil bacteria.
3. Plant Growth-Promoting Rhizobacteria, A Potential Approach for Bioremediation
One of the safer tools to alleviate environmental deterioration is the application of ecofriendly living agents such as bacteria, fungi, algae and higher plants to eliminate the toxic chemicals, oil spills and toxic metals present in the altered sites, which is known as “bioremediation” [57]. To discriminate between the use of these biological agents for polluted sites’ decontamination and their use in biorecycling processes designed to reduce the emission of toxins at source, bioremediation is defined as “a biological response to environmental abuse” [58,59].
Apart from their implications as plant growth enhancers, PGPR are also known to have a primordial role in practically all bioremediation aspects of contaminated soils (PTE, fungicides, organic pollutants, etc.) [60,61,62]. For example, some PGPR can interact with the soil’s toxic metals by binding them to their cell surfaces or incorporating them in some metabolic functions after captivation. Some microorganisms can reduce metal ions such as Hg2+ and Ag+ to Hg0 and Ag0 and provide a perfect model for total metal removal from the soil [63]. It has been experimentally demonstrated that some rhizobacteria produce extracellular enzymes such as peroxidases, reductases, Cytochome P450, lacases and glutathione-S-transferase, having important roles in polycyclic aromatic hydrocarbon degradation, molecules present in crude oil and known for their toxicity and carcinogenicity [64]. Meliani [65] produced a detailed review about PGPR application in soil decontamination, focusing on the genus Pseudomonas, and some of the most important strategies used by PGPR in bioremediation such as the production of biosurfactants, biofilms, toxic metal solubilization and siderophore production.
Plant Growth-Promoting Rhizobacteria utilize a wide range of mechanisms to improve plant growth and soil quality under stress conditions. Under ionic stress, mostly related to high salinity, drought and desiccation, certain bacteria can provide compatible solutes (proline, glycine betaine, sugars and derivatives, etc.) for root cells to avoid ion accumulation in the cytoplasm and, thus, water deficiency. Others can synthesize exopolysaccharides (EPS) in the rhizosphere. Bacterial EPS enhance water and ion (K+, Ca+, Na+) uptake. They also play a major role in soil structure stabilization and aggregation under high ion concentrations. Certain PGPR express ion antiporters in their membranes (Na+ (K+)/H+) to maintain their water balance in the cytoplasm under ionic stress. They also produce stress mitigation enzymes such as 1-aminocyclopropane-1-carboxylate (ACC) deaminase (see Section 5.2) and antioxidant enzymes such as peroxidase (POD), catalase (CAT) and superoxide dismutase (SOD) to eliminate the high amounts of reactive oxygen species (ROS) produced under abiotic stresses. In addition, PGPR phytohormones such as auxins, gibberellins, abscisic acid and cytokines (see Section 5.4) are inevitable for the stabilization of plants’ physiology under water stresses [66]. In PTE bioremediation, several mechanisms are used by beneficial soil bacteria to adsorb, transform and uptake toxic elements in the soil. In this context, the toxic species is either passively adsorbed (biosorption) to the cell surface or internalized (bioaccumulation) to its interior through “metabolic independent mechanisms”. Inside the cytoplasm, toxic metal behavior follows more complex and, often, “metabolic dependent mechanisms” that may include: (1) metal compartmentation within specific organelles, (2) enzymatic detoxification (methylation, oxidation, dealkylation, reduction, etc.), and (3) efflux pumps that transport the modified and harmless toxic metal forms outside the cytoplasm (Figure 1) [67].
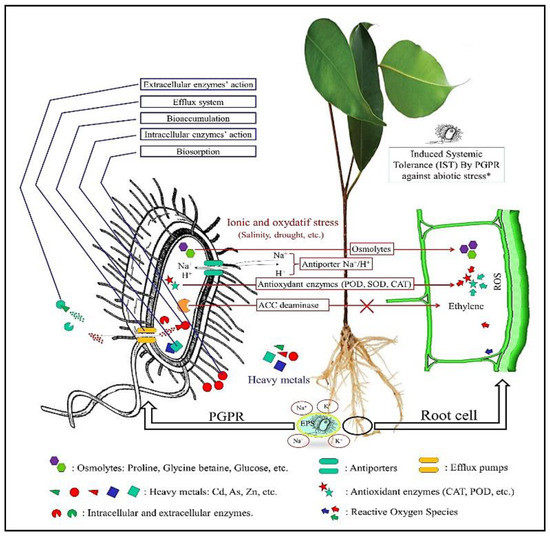
Figure 1.
Principal mechanisms of bacterial bioremediation and plant growth regulation under abiotic stresses. * (IST): see Section 3.
At a cellular level, microbial populations permanently communicate between themselves, but at the same time with their surrounding root systems. In addition, some positive PGPR effects are controlled by small diffusible signaling molecules, mainly N-acyl homoserine lactone molecules (AHLs), that regulate gene expression in response to bacterial population density and interactions with plants, particularly under stress conditions [68,69]. For example, the N-3-oxo-hexanoyl homoserine lactone (3OC6-HSL) was found to be highly effective in restoring wheat root length, shoot length and fresh weight under salt stress conditions. In fact, 3OC6-HSL upregulated salt-responsive gene expressions such as (1) Abscisic Acid (ABA)-dependent osmotic stress genes: COR15a, RD22, ADH and P5CS1, (2) ABA-independent gene: ERD1, and (3) ion-homeostasis regulation genes: SOS1, SOS2 and SOS3 in Arabidopsis under salt stress conditions [70]. Moreover, Sheng et al. [71] carried out impressive work on the role of quorum sensing systems during microbial biofilm formation while degrading the pollutant 1,2,4-trichlorobenzene (1,2,4-TCB). For them, there was no doubt about the positive correlation between the 1,2,4-TCB mineralization, microbial biofilm abundance and AHL (3-oxo-C12:1-HSL; 3-oxo-C10:1-HSL, 3-oxo-C14:1-HSL; OH-C14:1-HSL, etc.) production.
Among other PGPR, a Pseudomonas aeruginosa PS1 significantly promoted root and shoot nitrogen, root and shoot phosphorus and the seed yield of greengram (Vigna radiata) plants at all tested concentrations of tebuconazole, a fungicide belonging to the triazole group and largely used in agriculture. At high concentrations, tebuconazole may be accumulated in soils and plants, becoming toxic, damaging plant tissues and affecting crop yield [60]. Bensidhoum et al. [72] studied the effect of a Pseudomonas protegens S5LiBe on barley growth restoration under toxic metal contamination. The results showed an increase in germination rate, shoot and root fresh weight, shoot and root dry weight and shoot length. Moreover, the two siderophore producers, Alcaligenes feacalis RZS2 and Pseudomonas aeruginosa RZS3, showed a high ability to promote wheat growth when seeds were sown in PTE-contaminated soil; their bioremediation potential was higher than other metal chelators such as EDTA or citric acid [73]. Muratova et al. [74] realized a pot experiment in a growth chamber, proving the ability of Azospirillum lipoferum strain 5 to enhance the development of wheat root systems growing in crude oil-contaminated soil. In addition, a greenhouse experiment was conducted by Gomaa et al. [75] on the wheat growth stimulation potential of the two bacteria Azospirillum lipoferum and Rhizobium leguminosarum under different concentrations of Zn and Cd. The treatments: Azospirillum; Azospirillum + Rhizobium; Rhizobium + 200 ppm Zn; Azospirillum + Rhizobium + 300-ppm Cd; Rhizobium + 300 ppm Zn and Azospirillum + 300 ppm Zn resulted in an increase in wheat growth parameters compared to controls without bacterial inoculation. Similarly, a Pseudomonas sp. strain, isolated from hydrocarbon-contaminated soil, showed an important effect in stimulating rice’s root and shoot elongation and enhanced its final yield in hydrocarbon and toxic metal-contaminated soils [76].
4. Plant Growth-Promoting Rhizobacteria Implication in Induced Systemic Tolerance and Induced Systemic Resistance
Currently, modern agriculture is facing extremely dangerous biotic and abiotic problems. Some of these constraints are the result of the human populations’ growth explosion in some regions of the planet. Others are the direct consequence of environmental degradation due to drought, salinity, oxidative stress, toxic metals, nutrient deficiency and pathogens, loss of biodiversity and global climate changes [77,78,79,80,81,82]. In addition, the biggest part of the agricultural loss is due to abiotic factors. For example, the average yield of wheat is about 1500 kg/ha, while crop damage was estimated at 2000 and 14,500 kg/ha due to biotic and abiotic stresses, respectively [83].
Several works have highlighted the role of some PGPR as inducers of plant tolerance to abiotic stress by provoking physiological and biochemical changes in their tissues, which result in enhancing their tolerance to environmental stresses such as drought, salinity and toxic metals. Such complex interactions between plants and bacteria are known under the term “Induced Systemic Tolerance” (IST) [80,84,85,86,87]. Among other bacteria, Arthrobacter, Azospirillum, Azotobacter, Klebsiella and Pseudomonas are known for their ability to promote wheat and rice growth under high salinity conditions. [84,88,89,90,91,92,93,94,95,96,97,98,99,100,101].
Other works have described PGPR’s effect on drought stress mitigation in wheat (Burkholderia, Bacillus, Paenibacillus, Azospirillum and Azotobacter) and rice (Pseudomonas, Bacillus, Arthrobacter, Azospirillum) [102,103,104,105,106,107,108,109,110]. Recently, PGPR conferring plant tolerance to toxic metals (Cd, Zn, etc.) have increasingly attracted researchers’ attention. Thus, a Pseudomonas sp. SNA5 was efficiently used by Verma et al. [111] to promote wheat growth under high concentrations of cadmium, which is associated with high phosphate fertilization. Islam et al. [112] found that a P. aeruginosa was an ideal candidate for wheat growth enhancement against Zn-induced oxidative stress by improving the necessary nutrients’ availability, as well as by lowering Zn metal uptake. In addition, ref [113] used PGPR to stimulate wheat growth under high Cr concentrations. Moreover, Gontia-Mishra et al. [114] found that Enterobacter ludwigii (HG 2) and Klebsiella pneumoniae (HG 3) could significantly promote wheat seedling growth under mercury stress. Furthermore, the two bacteria Ochrobactrum sp. and Bacillus spp. revealed a high potential for rice growth promotion under toxic metal-contaminated soil [115].
It is largely recognized that many PGPR are implicated in plant defense stimulation against phytopathogens (viruses, bacteria, fungi and insects), which is designated “Induced Systemic Resistance” (ISR) [80,84,85,86,87]. Bacteria such as Acinetobacter, Alcaligenes, Bacillus, Burkholderia, Enterobacter, Pantoea, Pseudomonas and Staphylococcus have exhibited high antagonistic activities against Alternaria alternata, Botrytis cinerea, Fusarium culmorum, F. oxysporum, F. solani, Gaeumanomyces graminis var. tritici, Phytophthora cryptogea, Pythium and promoted wheat growth and defenses against these same phytopathogens [87,116,117,118]. Also, Rice inoculation with a combination of three PGP-Pseudomonas fluorescens (Pf1, TDK1 and PY15) increased chitinase accumulation and enhanced disease resistance in rice plants against sheath rot disease provoked by Sarocladium oryzae [119]. Elsewhere, PGPR such as Bacillus, Pseudomonas, Rhizobium and Serratia were used as biocontrol agents against rice pathogens (Burkholderia glumae, Cnaphalocrocis medinalis, Cochliobolus myiabeanus, Hirschmanniella oryzae, Magnaporthe oryzae, Meloidogyne graminicola, Meloidogyne javanica, Pyricularia grisea, Rhizoctonia solani and Xanthomonas oryzae pv. oryzae) [80,116,120,121].
5. Plant Growth-Promoting Metabolites for Soil Remediation and Crop Improvement
The ability of PGPR to fix nitrogen, solubilize nutrients and to produce other metabolites such as siderophores, phytohormones, antibiotics and hydrolytic enzymes makes them ecofriendly alternatives for avoiding the excessive use of unsuitable and cost-effective chemicals in agriculture (Figure 2).
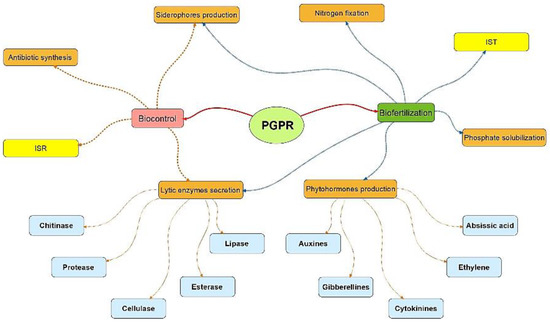
Figure 2.
PGPR metabolites for soil remediation and crop improvement. ISR: Induced Systemic Resistance. IST: Induced Systemic Tolerance (realized using the Visual Understanding Environment software, VUE 3.3.0-developped by Tufts university, Medford, MA, USA).
5.1. Bacterial Siderophores in Soil
Iron is a key compound in almost all the electron transfer enzymes. In soil, Fe3+ is the most found form (clays, oxides, hydroxides, etc.). The extremely insoluble nature of such forms constitutes a limiting factor for both plant and microorganism development [86]. Most soil bacteria and fungi, but also some plants, can produce chelators with a high affinity to iron. When iron is limited in soil, these chelators, namely siderophores, are secreted to collect it from various sources and deliver it to the producer, but also to some of the other cohabitating organisms [122]. Some siderophores producing PGPR can competitively participate in phytopathogen inhibition, especially when iron is limited in soil [123]. In addition, siderophores are also implicated in vegetal growth enhancement by providing accessible iron to plants [124]. Otherwise, some microorganisms produce siderophores with a high affinity to toxic metals, providing them with the ability to adsorb the metal in their biomass on metal-induced outer membrane protein or by bioprecipitation [125,126,127].
Two siderophore-producing and toxic metal-resistant bacteria, namely, Enterobacter ludwigii (HG 2) and Klebsiella pneumoniae (HG 3), were efficiently used by Gontia-Mishra et al. [114] to alleviate Hg toxicity in wheat (Triticum durum), suggesting their utility as potential candidates for Hg stress alleviation and wheat growth improvement. [115] studied the role of toxic metal-resistant and siderophore-producing Ochrobactrum sp. (CdSP9) and Bacillus spp. (AsSP9) strains in stimulating rice growth and remediating contaminated soils. The two strains reduced metal toxicity and enhanced overall rice biomass and root/shoot ratio.
In a work realized by Islam et al. [112], Zn-stressed wheat plants showed more green color intensity on their leaves and more iron availability when inoculated with a PGP-Pseudomonas, which was attributed to siderophore production by the bacteria. The bacterium also promoted wheat growth under greenhouse conditions, where shoot and root dry biomass was enhanced by 23% and 45%, respectively; they attributed this, in part, to its ability to produce high amounts of siderophores. Moreover, Rana et al. [128] attributed the significant role of a Providencia strain in the enhancement of wheat biomass, grain yield and macro- (NPK) and micronutrient contents to its ability to exhibit siderophore production, antifungal activity and synergistic interactions with other bacteria in wheat rhizosphere. A Pseudomonas fluorescens WCS374r was found to be implicated in the ISR of rice against Magnaporthe oryzae, which is based on pseudobactin (siderophore)-mediated priming for a salicylic acid-repressible multifaceted defense response [129]. Another P. fluorescens, together with its siderophore iron complex, was found to enhance phenol content and phenol-oxidizing enzyme content in rice plants, hence inducing systemic resistance in rice against the phytopathogen Pyricularia oryzae [130]. In addition, a complex of siderophore-producing PGPR consortia was used by Naureen et al. [131] to enhance rice growth and induce its systemic resistance to the phytopathogen fungus Rhizoctonia solani.
5.2. Plant Ethylene Balancing via Bacterial ACC Deaminase
Salinity affects approximately 6% of the land surface worldwide and about 20% of irrigated areas, posing a major threat to agriculture [15]. It affects plant growth and yield, but also provokes an imbalance of microorganism distribution in the rhizosphere and increases, in some cases, pathogen virulence [31]. Salinity also affects nutrient availability for crops. For example, phosphate (P) tends to precipitate with calcium in saline soils, which makes it difficult to be captured by plants [132], although some PGPR can be responsible for physical and chemical changes in plant tissues, participating in stress mitigation and plant growth restoration. The bacterial enzyme “1-amino cyclopropane-1-carboxylate (AAC) deaminase” plays an important role in regulating ethylene levels in plant tissues by degrading its precursor (ACC). Ethylene is a plant hormone that regulates several aspects of plant growth. However, its synthesis is accelerated under high salinity, and then it acts as a negative plant growth regulator as its concentration exceeds the required level [133].
The use of ACC deaminase-producing bacteria has been widely adopted in agriculture, especially for crop enhancement under different stresses [134]. Thus, Govindasamy et al. [135] reported the effect of ACC-deaminase-producing rhizobacteria on wheat growth promotion under cadmium stress. The tested isolates significantly enhanced root elongation and minimized ethylene synthesis in wheat seedlings under such stressed conditions. Indeed, the most efficient strains, among others, in promoting wheat growth (Pseudomonas sp. PS 2–3 and Pseudomonas fluorescens PS 7–12) showed the presence of acdS gene coding for ACC deaminase. Abbas et al. [136] obtained better yield and chlorophyll content with rice inoculated with ACC deaminase-producing bacteria under salt stress. The ACC deaminase-producing Pseudomonas putida CEN7 and Pseudomonas fluorescens CEN8 showed a significant increase in the root length and root colonization of rice plants [137]. In addition, the bacterium Rhizobium leguminosarum (SN10) promoted the biomass, root branching and N content of four different rice varieties. Not only this, but the bacterium also displayed a strong chemotaxis response towards the rice seed and its root exudates [138]. Many other works have reported the role of ACC deaminase-producing PGPR in the enhancement of rice growth under different stresses such as salinity, flooding and toxic metals [137,139,140]. Moreover, similar results were found with wheat inoculated by ACC deaminase-producing bacteria such as Pseudomonas, Serratia, Burkholderia under salt and drought stress conditions [92,141,142,143,144,145].
5.3. Phosphate Solubilization
Phosphorus is one of the most important elements for plant nutrition. In agriculture, it is generally compensated through the addition of chemical fertilizers to soil. However, phosphorus coming from such fertilizers is rapidly immobilized, becoming useless for plants [146]. In addition, the high release of contaminants into the main product, gas steam and by-products, but also toxic metal accumulation in both the soil and crop due to the repetitive use of phosphoric fertilizers, has obliged producers to look for better tools to reduce the use of such chemical fertilizers [147,148]. Among these alternatives, the use of phosphate-solubilizing bacteria (PSB) is one of the most ecofriendly options to avoid or to minimize the exaggerated use of chemicals [149].
Ahemad [150] reviewed the role of metal phytoremediation in association with PSB and reported that such associations considerably overcome the practical drawbacks imposed by metal stress on plants. Furthermore, Paul and Sinha [151] isolated and selected a group of PSB with a high ability to tolerate toxic metal stress. They suggested that using toxic metal PSB in metal-contaminated areas might be exploited for bioremediation studies. Otherwise, Kaur and Reddy [152] found that inoculation with the two phosphate-solubilizing bacteria Pantoea cypripedii (PSB-3) and Pseudomonas plecoglossicida (PSB-5), together with rock phosphate fertilization, increased shoot height, shoot and root dry biomass, grain yield and total phosphorus uptake in both maize and wheat as compared to the control. The application of phosphate-solubilizing Azotobacter strains, alone or together with chemical fertilizers, improved the yield and root biomass of three wheat varieties under greenhouse conditions [153].
Several works have shown the efficiency of PSB such as Pantoea, Azotobacter, Rhizobium, Pseudomonas and Serratia in nitrogen uptake and wheat growth enhancement under different stress conditions [152,154,155,156,157]. The in vitro experiment, conducted by Panhwar et al. [158], to study the influence of two phosphate-solubilizing Bacillus spp. (PSB9 and PSB16), together with triple supper phosphate on aerobic rice growth, showed that the coupled “bacteria-triple supper phosphate” increased phosphate uptake, available soil phosphate and rice growth. The two phosphate-solubilizing bacteria PSB 12 identified as Gluconacetobacter sp. (MTCC 8368) and PSB 73 identified as Burkholderia sp. (MTCC 8369) were examined for their potential ability to enhance rice growth. They revealed high growth promotion ability under pot culture assays and were presumed to be of potential to develop as biofertilizers [159]. Vahed et al. [160] and Panhwar et al. [158] have also discussed the role of some PSB in improving phosphate uptake, soluble soil phosphate and rice growth.
5.4. Bacterial Phytohormones
Phytohormones are natural organic substances that influence plant development and regulate, at low concentrations, their physiology. The name auxin was given by Charles Darwin to the first discovered phytohormone, referring to “αυξειν”, a Greek word that means grow or increase. Later, gibberellins, ethylene, cytokinin and abscisic acid joined auxins to be regarded as “the classical five phytohormones” [161,162]. Phytohormones affect several aspects of plant growth, nutrition, biotic and abiotic stress response and physiology. Their actions are in complex and continued interaction with each other, with plants, but also with the surrounding environment. Recently, phytohormones were classified, based on their physiological functions and structure, into different classes: abscisic acids (ABAs), auxins, cytokinins (CTKs), gibberellins (GAs), strigolactones, brassinosteroids, Jasmonic acid (JA), salicylic acid (SA) and ethylene [163]. While the hormonal classes are often associated with various characteristics and biological effects, increasing evidence suggests that multiple phytohormones often mediate the same biological processes by additive, synergistic or antagonistic actions, forming intricate signaling networks [164].
- a.
- Auxins
Indole 3-Acetic Acid (IAA) is the most studied auxin. Its importance in promoting plant growth makes it an important line to select efficient PGPR [165,166,167]. Thus, the bacterial strains (Pseudomonas putida, P. fluorescens and Azospirillum lipoferum) were used by Sharma et al. [168] to enhance rice growth. The three bacteria showed a high ability to produce IAA and helped inoculated plants to express higher photosynthetic capacity and chlorophyll content, but also increased their root/shoot dry mass. Another Pseudomonas putida (BHUJY23) was found to produce high amounts of IAA and to be helpful for rice production and as an antagonistic agent against phytopathogens [169]. Hasan et al. [170] confirmed the beneficial effects of two IAA-producing bacteria, belonging to the genera Rhizobium and Azospirillum, on rice growth and yield. Bacteria belonging to the genera Bacillus and Citrobacter showed a significant improvement of root/shoot growth in inoculated rice plants. The results were attributed to IAA production by the studied isolates [171,172]. Torres-Rubio et al. [173], Tsavkelova et al. [174], Jha and Kumar [175], Soltani et al. [176] and Kumar et al. [177] reported that bacteria such as Flavobacterium, Pseudomonas, Achromobacter and Azotobacter can produce large quantities of IAA and promote wheat plant growth.
- b.
- Gibberellins
The first gibberellin (gibberellic acid GA) was discovered in 1962 with the fungus Fusarium moniliforme (Gibberella fujikuroi in its sexual form), while the first report of bacterial gibberellins was in 1988 with the species Rhizobium meliloti [178,179]. Bacterial gibberellin synthesis starts with geranylgeranyl-PP conversion into ent-kaurene, which is then converted to GA12-aldehyde. After that, GA12-aldehyde is oxidized to GA12 and metabolized to another GA [180]. Hasan et al. [170] studied the capacity of some PGPR strains (Enterobacter spp., Azospirillum spp.) to enhance rice growth under controlled conditions, alone and in combination with Azospirillum or rhizobium. Their results revealed a significant increase in gibberellic acid content in both the shoots and roots of the inoculated plants. Similar results have been reported by Caba et al. [181]. Moreover, Inoculation by the gibberellin-producing Azospirillum sp. and bacillus sp. resulted in increasing nitrogen uptake by wheat roots [182]. Furthermore, the water stress alleviation in wheat by PGPR was partially attributed to bacterial gibberellin production [183,184]. Many other reports have mentioned the beneficial effect of gibberellins on wheat and/or rice growth [185,186,187,188,189,190].
- c.
- Cytokinins
Cytokinins are an important trait to search for in PGPR selection. They play a crucial role in the control of plant cell division, cell cycle, leaf senescence and nutrient mobilization, shoot apical meristem formation, seed dormancy and germination, floral development, etc. Chemically, cytokinins are N6-substituted aminopurines or adenine compounds with an isoprene, modified isoprene, aromatic side chain attached to the N6-amino group or zeatin and trans-zeatin [86,179,191].
Bacteria such as Azospirillum [192], Agrobacterium [193], Azotobacter [194], Pseudomonas [193], Paenibacillus [195], Achromobacter [196], Enterobacter [197], Bacillus [198] and Klebsiella [192] are known for their implication in plant growth regulation via cytokinin production. Zahir et al. [199] conducted an interesting experiment to study the effect of kinetin (a synthetic cytokinin) and its physiological precursors (adenine + isopentyl alcohol) on rice growth. The results showed that the precursor was more effective than kinetin on yield improvement. It significantly enhanced plant height, tiller and panicle number, paddy yield and (NPK) content, hence suggesting that the precursor of kinetin was converted to cytokinin by rhizospheric microorganisms before absorption or transformed into cytokinins inside plant tissues. Otherwise, Kudoyarova et al. [200] studied the separate effect of the synthetic cytokinin trans-zeatin, Bacillus subtilis IB-22 (able to produce zeatin-type cytokinin) or Bacillus subtilis IB-21 (unable to produce cytokinins) on amino acid release from wheat roots in a split-root system. This system allowed the spatial separation of the zeatin or rhizobacterial application to one compartment and analyses of amino acid rhizodeposition into the other compartment. The application of B. subtilis IB-22 or trans-zeatin greatly enhanced amino acid liberation in the soil solution compared to B. subtilis IB-21 or the untreated plants, suggesting that the ability of cytokinin-producing B. subtilis IB-22 to enhance rhizodeposition may constitute an important process in improving the rhizobacterial colonization of the wheat rhizosphere.
- d.
- Abscisic acid
Abscisic acid (ABA) is the most important hormone produced by plants in response to abiotic stresses. However, ABA is also synthesized by bacteria, fungi, algae and animals. The prokaryotic pathway for synthesizing this fifteen-carbon sesquiterpene originates from isoprene, known as isopentenyl pyrophosphate, which is synthesized from the mevalonate pathway [201,202].
Travaglia et al. [203] investigated the influence of abscisic acid on wheat physiology and yield under field conditions with limited amounts of water during anthesis and postanthesis. Abscisic acid application significantly enhanced leaf area, chlorophyll and carotenoid content in flag leaf and soluble carbohydrates in shoots at anthesis. Yang et al. [204] found that the application of exogenous abscisic acid significantly promoted proline accumulation (as a response to water stress and/or dark-induced senescence) in the detached rice leaf when investigated in both dark and light conditions. Moreover, Azuma et al. [205] studied the promotive effect of abscisic acid on floating rice growing at a low relative humidity and its interactive role together with ethylene and gibberellin in internodal elongation. Their results revealed that the separate application of the abscisic acid had no promoting effect on the internodal elongation of the stem sections. However, the simultaneous application of abscisic acid, gibberellin and ethylene efficiently enhanced the internodal elongation of the stem sections, suggesting that abscisic acid could be a good enhancer of the ethylene- and gibberellin-induced internodal elongation at low humidity, hence preventing the reduction of water potential via apoplast transpiration.
5.5. Bacterial Nitogen Recycling for Soil Maintenance and Crop Improvement
Nitrogen makes up 78% of the atmospheric volume and constitutes a major limiting factor for many physiological processes in soil. Molecular nitrogen cannot be assimilated by photosynthesizing plants or the majority of microorganisms [206]. The inter-conversion between nitrogen forms (nitrogen fixation, mineralization, nitrification and denitrification) represents the biogeochemical cycle of nitrogen, mainly made of biological processes in which microorganisms play a predominant role. Metabolically, microorganisms preferentially uptake ammonium while plants and some microorganisms assimilate nitrate [207].
Nitrogen fixation is the first step in the process of making nitrogen usable by plants, where nitrogen-fixing bacteria play a crucial role in changing dinitrogen into ammonium via nitrogenase activity. After that, the produced ammonium needs to be converted into nitrates before assimilation by plants. This nitrification process, in which bacteria play an important role, allows nitrogen assimilation by plant roots to be used in amino acids, nucleic acids and chlorophyll composition. When a plant or animal dies, decomposers such as fungi and bacteria turn nitrogen back into ammonium so it can reenter the nitrogen cycle through denitrification [208].
Unfortunately, the excessive use of fertilizers to supply nitrogen in soil and nitrous oxide emission in the atmosphere due to other human activities results in the unbalancing of the nitrogen cycle in both the atmosphere and soil [209]. To avoid such exaggerated application of chemical nitrogen to soil, N-fixing bacteria such as Azospirillum [210,211,212,213,214,215], Pseudomonas [210,211,216,217], Bacillus [218], Herbaspirillum [215,219], Azotobacter [210,220], Rhizobium and Enterobacter [221], Klebsiella [222] and Burkholderia [223,224] have proved their efficiency in reducing or replacing chemical fertilizers for wheat and rice crop enhancement.
In addition to nitrogen fixation, the presence of a positive correlation between bacterial denitrification and the rhizosphere colonization potential has permitted researchers to consider denitrification as an important trait in isolating and selecting efficient PGPR. In this context, a fluorescent PGP-pseudomonas associated with rice was isolated and selected by Kumar et al. [177], considering the denitrification potential as an important trait in selecting competitive PGPR. Moreover, Muriel et al. [225], considered denitrification in the PGP-Pseudomonas fluorescens F113 as an important character. The NO produced by the PGP-Azospirillum brasilense Sp245 via denitrification could be a major signal implicated in wheat root branching stimulation when the dissimilatory nitrite reductase gene (nirK) is upregulated [226].
6. Conclusions
In the last few decades, soil salinization and contamination with petroleum, hydrocarbons and toxic metals have seemed to be directly linked to some environmentally uncontrolled anthropogenic activities and population expansion. In arid environments, such alarming problems have become a major threat to global cereal production. In addition, applying physicochemical techniques to maintain soil health and ensure enough food production is often disruptive, labor intensive and relatively expensive. Recently, PGPR application for soil bioremediation and cereal growth improvement has received considerable attention for its ecofriendly, efficient and cost-effective advantages. Thus, bacteria such as Arthrobacter, Azotobacter, Bacillus, Enterobacter, Pseudomonas, etc., have proved their efficiency as plant growth promoters and soil quality remediators in arid environments. However, most of the reported experiments have been realized in lab- or greenhouse-controlled conditions, and we still lack information about their interactions with plants and other microorganisms once in the field, certainly a more complex environment. Progressively, and with increasing knowledge about the interactive aspects between plants, bacteria and soil, but also the understanding of the key signal molecules implicated in such interactions, PGPR’s place in modern agriculture is now undeniable as biocontrol, biofertilization and bioremediation agents. However, more investigations are needed for a better understanding of some problems related to bacterial long-term stability and efficiency in the field, their large-scale production once selected, their storage, transportation and delivery conditions, but also their long-term effects on the innoculated environment. Moreover, incorporating more data about the impact of aridity expansion on soil composition, and both microbial and plant diversity, is crucial to direct the already obtained results for better PGPR applications.
Author Contributions
All authors have contributed substantially to the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This work is a literature review that does not require ethical approval. The submitted work is a review. It does not contain any human/animal experiments. The manuscript is rriginal; it is not submitted to another journal for simultaneous consideration and is not published elsewhere in any form or language.
Informed Consent Statement
This work is a literature review that does not require any consent statement.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects: Highlights; ST/ESA/SER.A/423; United Nations Secretariat: New York, NY, USA, 2019. [Google Scholar]
- Waughray, D. Water Security: The Water-Food-Energy-Climate Nexus: The World Economic Forum Water Initiative; Island Press: Washington, DC, USA, 2011. [Google Scholar]
- Golla, B. Agricultural production system in arid and semi-arid regions. J. Agric. Sci. Food Technol. 2021, 7, 234–244. [Google Scholar]
- Tabari, H.; Aghajanloo, M.B. Temporal pattern of aridity index in Iran with considering precipitation and evapotranspiration trends. Int. J. Climatol. 2013, 33, 396–409. [Google Scholar] [CrossRef]
- Zhang, X.; Aguilar, E.; Sensoy, S.; Melkonyan, H.; Tagiyeva, U.; Ahmed, N.; Kutaladze, N.; Rahimzadeh, F.; Taghipour, A.; Hantosh, T.H.; et al. Trends in Middle East climate extreme indices from 1950 to 2003. J. Geophys. Res. Atmos. 2005, 110, 1–12. [Google Scholar] [CrossRef]
- Andrighetti, M.; Zardi, D.; Franceschi, M. History and analysis of the temperature series of Verona (1769–2006). Meteorol. Atmos. Phys. 2009, 103, 267–277. [Google Scholar] [CrossRef]
- Tabari, H.; Talaee, P.H. Recent trends of mean maximum and minimum air temperatures in the western half of Iran. Meteorol. Atmos. Phys. 2011, 111, 121–131. [Google Scholar] [CrossRef]
- Paltineanu, C.; Mihailescu, I.F.; Seceleanu, I.; Dragota, C.; Vasenciuc, F. Using aridity indices to describe some climate and soil features in Eastern Europe: A Romanian case study. Theor. Appl. Climatol. 2007, 90, 263–274. [Google Scholar] [CrossRef]
- Seager, R.; Ting, M.; Held, I.; Kushnir, Y.; Lu, J.; Vecchi, G.; Huang, H.P.; Harnik, N.; Leetmaa, A.; Lau, N.C.; et al. Model projections of an imminent transition to a more arid climate in southwestern North America. Science 2007, 316, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Giorgi, F. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Glob. Planet. Chang. 2008, 62, 195–209. [Google Scholar] [CrossRef]
- Le Houérou, H.N. Climate change, drought and desertification. J. Arid Environ. 1996, 34, 133–185. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Instinctive plant tolerance towards abiotic stresses in arid regions. In Artificial Photosynthesis; Najafpour, M., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 219–238. [Google Scholar]
- Tak, H.I.; Ahmad, F.; Babalola, O.O. Advances in the application of Plant Growth-Promoting Rhizobacteria in phytoremediation of heavy metals. In Reviews of Environmental Contamination and Toxicology; Whitacre, D.M., Ed.; Springer: New York, NY, USA, 2013; pp. 33–52. [Google Scholar]
- Maestre, F.T.; Delgado-Baquerizo, M.; Jeffries, T.C.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Quero, J.L.; García-Gómez, M.; Gallardo, A.; Ulrich, W.; et al. Increasing aridity reduces soil microbial diversity and abundance in global drylands. Proc. Natl. Acad. Sci. USA 2015, 112, 15684–15689. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Nabti, E. Plant Growth-Promoting Bacteria: Importance in Vegetable Production. In Microbial Strategies for Vegetable Production; Zaidi, A., Khan, M.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 23–48. [Google Scholar]
- Rai, A.; Cherif, A.; Cruz, C.; Nabti, E. Extracts from seaweeds and Opuntia ficus-indica Cladodes enhance diazotrophic-PGPR halotolerance, their enzymatic potential, and their impact on wheat germination under salt stress. Pedosphere 2018, 2, 241–254. [Google Scholar] [CrossRef]
- Tabli, N.; Rai, A.; Bensidhoum, L.; Palmieri, G.; Gogliettino, M.; Cocca, E.; Consiglio, C.; Cillo, F.; Bubici, G.; Nabti, E. Plant growth promoting and inducible antifungal activities of irrigation well water-bacteria. Biol. Cont. 2018, 117, 78–86. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Roy, A.S.; Baruah, R.; Borah, M.; Singh, A.K.; Boruah, H.P.D.; Saikia, N.; Deka, M.; Dutta, N.; Bora, T.C. Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int. Biodeterior Biodegrad. 2014, 94, 79–89. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Ilyas, N.; Tabassum, B.; Hashem, A.; Abd_Allah, E.F.; Jadhav, H.P. Plausible role of plant growth-promoting rhizobSingh acteria in future climatic scenario. In Environmental Biotechnology: For Sustainable Future; Sobti, R., Arora, N., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 175–197. [Google Scholar]
- Goswami, M.; Malakar, C.; Deka, S. Rhizosphere microbes for sustainable maintenance of plant health and soil fertility. In Rhizosphere Microbes Vol. 23; Sharma, S.K., Singh, U.B., Sahu, P.K., Singh, H.V., Sharma, P.K., Eds.; Springer: Singapore, 2020; pp. 35–72. [Google Scholar]
- Guo, J.; Muhammad, H.; Lv, X.; Wei, T.; Ren, X.; Jia, H.; Atif, S.; Hua, L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: A review. Chemosphere 2020, 246, 125823. [Google Scholar] [CrossRef]
- Awika, J.M. Major cereal grains production and use around the world. In Advances in Cereal Science: Implications to Food Processing and Health Promotion ACS Symposium Series; Awika, J.M., Piironen, V., Bean, S., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 1–13. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World agriculture towards 2030/2050: The 2012 revision. In ESA Working Paper; No. 12-03; FAO: Rome, Italy, 2012. [Google Scholar]
- Nonnoi, F.; Chinnaswamy, A.; de la Torre, V.S.G.; de la Peña, T.C.; Lucas, M.M.; Pueyo, J.J. Metal tolerance of rhizobial strains isolated from nodules of herbaceous legumes (Medicago spp. and Trifolium spp.) growing in mercury-contaminated soils. Appl. Soil Ecol. 2012, 61, 49–59. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Pečiulytė, D.; Dirginčiutė-Volodkienė, V. Effect of long-term industrial pollution on microorganisms in soil of deciduous forests situated along a pollution gradient next to a fertilizer factory: 2. Abundance and diversity of soil fungi. Ekologija 2009, 55, 133–141. [Google Scholar] [CrossRef][Green Version]
- Wuana, R.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Poustini, K.; Siosemardeh, A. Ion distribution in wheat cultivars in response to salinity stress. Field Crops Res. 2004, 85, 125–133. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Prášil, I.T.; Renaut, J. Plant proteome changes under abiotic stress—Contribution of proteomics studies to understanding plant stress response. J. Proteom. 2011, 74, 1301–1322. [Google Scholar] [CrossRef]
- Dikilitas, M.; Karakas, S. Behavior of plant pathogens for crops under stress during the determination of physiological, biochemical, and molecular approaches for salt stress tolerance. In Crop Protection for Agricultural Improvement; Ashraf, A., Öztürk, M., Ahmad, M.A., Aksoy, A., Eds.; Springer: Dordrecht, The Netherland, 2012; pp. 417–441. [Google Scholar]
- Mazhar, R.; Ilyas, N.; Arshad, M.; Khalid, A.; Hussain, M. Isolation of Heavy Metal-Tolerant PGPR strains and amelioration of chromium effect in Wheat in combination with Biochar. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 1–12. [Google Scholar] [CrossRef]
- Xiao, A.W.; Li, Z.; Li, W.C.; Ye, Z.H. The effect of plant growth-promoting rhizobacteria (PGPR) on arsenic accumulation and the growth of rice plants (Oryza sativa L.). Chemosphere 2020, 242, 125136. [Google Scholar]
- Ajmal, A.W.; Yasmin, H.; Hassan, M.N.; Khan, N.; Jan, B.L.; Mumtaz, S. Heavy Metal–Resistant Plant Growth–Promoting Citrobacter werkmanii Strain WWN1 and Enterobacter cloacae Strain JWM6 Enhance Wheat (Triticum aestivum L.) Growth by Modulating Physiological Attributes and Some Key Antioxidants Under Multi-Metal Stress. Front. Microbiol. 2022, 13, 500. [Google Scholar] [CrossRef] [PubMed]
- Shilev, S.; Babrikova, I.; Babrikov, T. Consortium of plant growth-promoting bacteria improves spinach (Spinacea oleracea L.) growth under heavy metal stress conditions. J. Chem. Technol. Biotechnol. 2020, 95, 932–939. [Google Scholar] [CrossRef]
- Sumranwanich, T.; Leartsiwawinyu, W.; Meeinkuirt, W.; Chayapan, P. Application of plant growth-promoting rhizobacteria (PGPR) associated with energy plant, Pennisetum purpurenum, in cadmium and zinc contaminated soil. Res. Sq. 2022, 1–15. [Google Scholar] [CrossRef]
- Jian, L.; Bai, X.; Zhang, H.; Song, X.; Li, Z. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. Peer J. 2019, 7, e6875. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Sidhu, G.K.; Datta, S.; Dhanjal, D.S.; Koul, B.; Janeja, H.S.; Singh, J. Plant growth promoting rhizobacteria from heavy metal contaminated soil promote growth attributes of Pisum sativum L. Biocatal. Agric. Biotechnol. 2019, 17, 665–671. [Google Scholar] [CrossRef]
- Sohaib, M.; Zahir, Z.A.; Khan, M.Y.; Ans, M.; Asghar, H.N.; Yasin, S.; Al-Barakah, F.N. Comparative evaluation of different carrier-based multi-strain bacterial formulations to mitigate the salt stress in wheat. Saudi J. Biol. Sci. 2020, 27, 777–787. [Google Scholar] [CrossRef]
- Afridi, M.S.; Amna; Sumaira; Mahmood, T.; Salam, A.; Mukhtar, T.; Mehmood, S.; Ali, J.; Khatoon, Z.; Bibi, M.; et al. Induction of tolerance to salinity in wheat genotypes by plant growth promoting endophytes: Involvement of ACC deaminase and antioxidant enzymes. Plant Physiol. Biochem. 2019, 139, 569–577. [Google Scholar] [CrossRef]
- Albdaiwi, R.N.; Khyami-Horani, H.; Ayad, J.Y.; Alananbeh, K.M.; Al-Sayaydeh, R. Isolation and characterization of Halotolerant Plant Growth Promoting Rhizobacteria from Durum Wheat (Triticum turgidum subsp. durum) Cultivated in Saline Areas of the Dead Sea Region. Front. Microbiol. 2019, 10, 1639. [Google Scholar] [PubMed]
- Boumaaza, B. Effect of Salinity-NaCl and Pseudomonas fluorescens on the germination of Wheat Genotypes (Triticum durum L.) cultivated in arid regions of Algeria. Singap. J. Sci. Res. 2020, 10, 182–189. [Google Scholar] [CrossRef]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M.; El-Shehawi, A.M. Bacillus tequilensis strain ‘UPMRB9′improves biochemical attributes and nutrient accumulation in different rice varieties under salinity stress. PLoS ONE 2021, 16, e0260869. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional alterations reveal Bacillus amyloliquefaciens-rice cooperation under salt stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.; Guan, C. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant 2020, 42, 42. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, V.; Gupta, V.K.; Shukla, R.; Prabha, R.; Sarma, B.; Patel, J.S. Microbial inoculation in rice regulates antioxidative reactions and defense related genes to mitigate drought stress. Sci. Rep. 2020, 10, 4818. [Google Scholar] [CrossRef]
- Danish, S.; Kiran, S.; Fahad, S.; Ahmad, N.; Ali, M.A.; Tahir, F.A.; Rasheed, M.K.; Shahzad, K.; Li, X.; Wang, D.; et al. Alleviation of chromium toxicity in maize by Fe fortification and chromium tolerant ACC deaminase producing plant growth promoting rhizobacteria. Ecotoxicol. Environ. Saf. 2019, 185, 109706. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-Ul-Hye, M.; Mohsin, F.; Hussain, M. ACC-deaminase producing plant growth promoting rhizobacteria and biochar mitigate adverse effects of drought stress on maize growth. PLoS ONE 2020, 15, e0230615. [Google Scholar] [CrossRef]
- Jochum, M.; McWilliams, K.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.-K. Bioprospecting Plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microb. 2019, 10, 2106. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Z.; Zhang, X.; Lang, D.; Zhang, X. Growth-promoting bacteria alleviates drought stress of G. uralensis through improving photosynthesis characteristics and water status. J. Plant Interact. 2019, 14, 580–589. [Google Scholar] [CrossRef]
- Danish, S.; Zafar-ul-Hye, M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 2019, 9, 5999. [Google Scholar] [CrossRef] [PubMed]
- Zafar-Ul-Hye, M.; Danish, S.; Abbas, M.; Ahmad, M.; Munir, T.M. ACC deaminase producing PGPR Bacillus amyloliquefaciens and Agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy 2019, 9, 343. [Google Scholar] [CrossRef]
- Ashraf, A.; Bano, A.; Ali, S.A. Characterisation of plant growth-promoting rhizobacteria from rhizosphere soil of heat-stressed and unstressed wheat and their use as bio-inoculant. Plant Biol. 2019, 21, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.-T.; Yen, J.-H.; Liao, C.-S.; Chen, W.-C.; Chao, Y.-T. Screening of rice endophytic biofertilizers with fungicide tolerance and plant growth-promoting characteristics. Sustainability 2019, 11, 1133. [Google Scholar] [CrossRef]
- Ahmad, A.G.M.; Attia, A.Z.G.; Mohamed, M.S.; Elsayed, H.E. Fermentation, formulation and evaluation of PGPR Bacillus subtilis isolate as a bioagent for reducing occurrence of peanut soil-borne diseases. J. Integr. Agric. 2019, 18, 2080–2092. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, J.; Zhang, D.; Li, Y.; Li, H.; He, H. Effect of heterocystous nitrogen-fixing cyanobacteria against rice sheath blight and the underlying mechanism. Appl. Soil Ecol. 2020, 153, 103580. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kumari, R. Bioremediation: A sustainable tool for environmental management–A review. Ann. Rev. Res. Biol. 2013, 3, 974–993. [Google Scholar]
- Colleran, E. Use of bacteria in bioremediation. In Methods in Biotechnology, Vol. 2. Bioremediation Protocols; Sheehan, D., Ed.; Humana Press Inc.: Totowa, NJ, USA, 1997; pp. 3–22. [Google Scholar]
- Juwarkar, A.A.; Misra, R.R.; Sharma, J.K. Recent trends in bioremediation. In Geomicrobiology and Biogeochemistry, Soil Biology 39; Parmar, N., Singh., A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 81–100. [Google Scholar]
- Ahemad, M.; Khan, M.S. Alleviation of fungicide-induced phytotoxicity in greengram [Vigna radiata (L.) Wilczek] using fungicide-tolerant and plant growth promoting Pseudomonas strain. Saudi J. Biol. Sci. 2012, 19, 451–459. [Google Scholar] [CrossRef]
- Phieler, R.; Voit, A.; Kothe, E. Microbially supported phytoremediation of heavy metal contaminated soils: Strategies and applications. Adv. Biochem. Eng. Biotechnol. 2014, 141, 211–235. [Google Scholar]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae–bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef]
- Tekaya, S.B.; Tipayno, S.; Kim, K.; Subramanian, P.; Sa, T. Rhizobacteria: Restoration of heavy metal- contaminated soils. In Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment: Vol. 2; Ahmad, P., Wani, M.R., Eds.; Springer Science and Business Media: New York, NY, USA, 2014; pp. 297–323. [Google Scholar]
- Sarma, H.; Prasad, M.N.V. Plant-Microbe association-assisted removal of heavy metals and degradation of polycyclic aromatic hydrocarbons. In Petroleum Geosciences: Indian Contexts; Mukherjee, S., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 219–236. [Google Scholar]
- Meliani, A. Bioremediation strategies employed by Pseudomonas species. In Bacterial Metabolites in Sustainable Agroecosystem, Sustainable Development and Biodiversity 12; Maheshwari, D.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 351–383. [Google Scholar]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, D.S.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- de Alencer, F.L.S.; Navoni, J.A.; do Amaral, V.S. The use of bacterial bioremediation of metals in aquatic environments in the twenty-first century: A systematic review. Environ. Sci. Pollut. Res. 2017, 24, 16545–16559. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Khan, A.R.; Hong, S.-J.; Park, G.-S.; Park, Y.-J.; Kim, H.-J.; Jeon, H.-J.; Khan, M.A.; Waqas, M.; Lee, I.-J.; et al. Quorum sensing activity of the plant growth-promoting rhizobacterium Serratia glossinae GS2 isolated from the sesame (Sesamum indicum L.) rhizosphere. Ann. Microbiol. 2017, 67, 623–632. [Google Scholar] [CrossRef]
- Fiorela, L.N.; Pablo, C.B.; Walter, G. Quorum sensing signaling molecules and their inhibitors in legume-associated bacteria. In Abiotic Stress and Legumes; Vijay, P.S., Samiksha, S., Durgesh, K.T., Sheo, M.P., Renu, B., Devendra, K.C., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 277–289. [Google Scholar]
- Zhao, Q.; Yang, X.Y.; Li, Y.; Liu, F.; Cao, X.Y.; Jia, Z.H.; Song, S.S. N-3-oxo-hexanoyl-homoserine lactone, a bacterial quorum sensing signal, enhances salt tolerance in Arabidopsis and wheat. Bot. Stud. 2020, 61, 8. [Google Scholar] [CrossRef]
- Sheng, H.; Harir, M.; Boughner, L.A.; Jiang, X.; Schmitt-Kopplin, P.; Schroll, R.; Wang, F. N-acyl-homoserine lactone dynamics during biofilm formation of a 1,2,4-trichlorobenzene mineralizing community on clay. Sci. Total Environ. 2017, 605–606, 1031–1038. [Google Scholar] [CrossRef]
- Bensidhoum, L.; Nabti, E.; Tabli, N.; Kupferschmied, P.; Weiss, A.; Rothballer, M.; Schmid, M.; Keel, C.; Hartmann, A. Heavy metal tolerant Pseudomonas protegens isolates from agricultural well water in northeastern Algeria with plant growth promoting, insecticidal and antifungal activities. Eur. J. Soil Biol. 2016, 75, 38–46. [Google Scholar] [CrossRef]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar]
- Muratova, A.Y.; Turkovskaya, O.V.; Antonyuk, L.P.; Makarov, O.E.; Pozdnyakova, L.I.; Ignatov, V.V. Oil-oxidizing potential of associative rhizobacteria of the genus Azospirillum. Microbiology 2005, 74, 210–215. [Google Scholar] [CrossRef]
- Gomaa, A.M.; Al-Fassi, F.A.; Al-Kenawy, Z.; Al-Gharbawi, H.T. Role of Azospirillum and Rhizobium in bio-remediating Cd and Zn polluted soil cultivated with wheat plant. Aust. J. Basic Appl. Sci. 2012, 6, 550–556. [Google Scholar]
- Deepthi, M.S.; Reena, T.; Deepu, M.S. In vitro study on the effect of heavy metals on PGPR microbes from two different soils and their growth efficiency on Oryza sativa (L.). J. Biopest. 2014, 7, 64–72. [Google Scholar]
- Vivian, A.; Murillo, J.; Jackson, R.W. The roles of plasmids in phytopathogenic bacteria: Mobile arsenals? Microbiology 2001, 147, 763–780. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, R.; Prats, E.; Jorrín-Novo, J.V. Proteomics of plant pathogenic fungi. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Ye, W.; Tredway, L.; Martin, S.; Martin, M. Taxonomy and morphology of plant-parasitic nematodes associated with turfgrasses in North and South Carolina, USA. Zootaxa 2012, 3452, 1–46. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Varma, A. Microbial-Mediated Induced Systemic Resistance in Plants; Springer Science and Business Media: Singapore, 2016. [Google Scholar]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique KH, M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–9. [Google Scholar]
- Krishnamurthy, A.; Rathinasabapathi, B. Oxidative stress tolerance in plants. Plant Signal. Behav. 2013, 8, e2576. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Ahmad, M.S.A.; Öztürk, M.; Aksoy, A. Crop Improvement through different means: Challenges and prospects. In Crop Production for Agricultural Improvement; Ashraf, A., Öztürk, M., Ahmad, M.A., Aksoy, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–15. [Google Scholar]
- Chaudhary, D.; Narula, N.; Sindhu, S.S.; Behl, R.K. Plant growth stimulation of wheat (Triticum aestivum L.) by inoculation of salinity tolerant Azotobacter strains. Physiol. Mol. Biol. Plants 2013, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Jha, C.K.; Saraf, M. Hormonal signaling by PGPR improves plant health under stress conditions. In Bacteria in Agrobiology: Stress Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 119–140. [Google Scholar]
- Niranjana, S.R.; Hariprasad, P. Understanding the mechanism involved in PGPR-Mediated growth promotion and suppression of biotic and abiotic stress in plants. In Future Challenges in Crop Protection against Fungal Pathogens, Fungal Biology; Goyal, A., Manoharachary, C., Eds.; Springer: New York, NY, USA, 2014; pp. 59–108. [Google Scholar]
- Nadeem, S.M.; Naveed, M.; Ahmad, M.; Zahir, Z.A. Rhizosphere Bacteria for crop production and improvement of stress tolerance: Mechanisms of action, applications, and future prospects. In Plant Microbes Symbiosis: Applied Facets; Arora, N.K., Ed.; Springer: New Delhi, India, 2015; pp. 1–36. [Google Scholar]
- Bacilio, M.; Rodríguez, H.; Moreno, M.; Hernandez, J.-P.; Bashan, Y. Mitigation of salt stress in wheat seedlings by a gfp-tagged Azospirillum lipoferum. Biol. Fertil. Soils 2004, 40, 188–193. [Google Scholar] [CrossRef]
- Nabti, E.H.; Sahnoune, M.; Adjrad, S.; Van Dommelen, A.; Ghoul, M.; Schmid, M.; Hartmann, A. A halophilic and osmotolerant Azospirillum brasilense strain from Algerian soil restores wheat growth under saline conditions. Eng. Life Sci. 2007, 7, 354–360. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Spaepen, S.; Versées, W.; Gocke, D.; Pohl, M.; Steyaert, J.; Vanderleyden, J. Characterization of Phenylpyruvate Decarboxylase, involved in Auxin Production of Azospirillum brasilense. J. Bacteriol. 2007, 189, 7626–7633. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Ghani, U.; Naveed, M.; Nadeem, S.M.; Asghar, H.N. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch. Microbiol. 2009, 191, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR inoculation on growth and antioxidant status of wheat under saline conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, M.; Kim, S.-R.; Ryu, H.; Cho, Y.-G. Insights into genomics of salt stress response in rice. Rice 2013, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springer Plus 2013, 2, 68. [Google Scholar] [CrossRef] [PubMed]
- Saghafi, K.; Ahmadi, J.; Asgharzadeh, A.; Bakhtiari, S. The effect of microbial inoculants on physiological responses of two wheat cultivars under salt stress. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 421–431. [Google Scholar]
- Sahoo, R.K.; Ansari, M.W.; Pradhan, M.; Dangar, T.K.; Mohanty, S.; Tuteja, N. A novel Azotobacter vinellandii (SRIAz3) functions in salinity stress tolerance in rice. Plant Signal. Behav. 2014, 9, e29377. [Google Scholar] [CrossRef]
- Sen, S.; Chandrasekhar, C.N. Effect of PGPR on growth promotion of rice (Oryza sativa L.) under salt stress. Asian J. Plant Sci. Res. 2014, 4, 62–67. [Google Scholar]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.; Cherif-Silini, H.; Yahiaoui, B. Growing varieties durum wheat (Triticum durum) in response to the effect of osmolytes and inoculation by Azotobacter chroococcum under salt stress. Afr. J. Microbiol. Res. 2016, 10, 387–399. [Google Scholar]
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; de Salamone, I.E.G.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- El-Afry, M.M.; El-Nady, M.F.; Abdelmonteleb, E.B.; Metwaly, M.M.S. Anatomical studies on drought-stressed wheat plants (Triticum aestivum L.) treated with some bacterial strains. Acta Biol. Szeged. 2012, 56, 165–174. [Google Scholar]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; El-Daim, A.; Islam, A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using Plant-Growth-Promoting Bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar] [CrossRef]
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, U. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. Soil Biol. Biochem. 2014, 9, e96086. [Google Scholar] [CrossRef] [PubMed]
- Gusain, S.Y.; Singh, U.S.; Sharma, A.K. Enzymatic amelioration of drought stress in rice through the application of Plant Growth Promoting Rhizobacteria (PGPR). Int. J. Curr. Res. 2014, 6, 4487–4491. [Google Scholar]
- Gusain, Y.S.; Singh, U.S.; Sharma, A.K. Bacterial mediated amelioration of drought stress in drought tolerant and susceptible cultivars of rice (Oryzae sativa L.). Afr. J. Biotechnol. 2015, 14, 764–773. [Google Scholar] [CrossRef]
- Verma, C.; Singh, P.; Kumar, R. Isolation and characterization of heavy metal resistant PGPR and their role in enhancement of growth of wheat plant under metal (Cadmium) stress condition. Arch. Appl. Sci. Res. 2015, 7, 37–43. [Google Scholar]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef]
- Khan, M.Y.; Asghar, H.N.; Jamshaid, M.U.; Akhtar, M.J.; Zahir, Z.A. Effect of microbial inoculation on wheat growth and phytostabilization of chromium contaminated soil. Pak. J. Bot. 2013, 45, 27–34. [Google Scholar]
- Gontia-Mishra, I.; Sapre, S.; Sharma, A.; Tiwari, S. Alleviation of mercury toxicity in wheat by the interaction of mercury-tolerant plant growth-promoting rhizobacteria. J. Plant Growth Regul. 2016, 35, 1000–1012. [Google Scholar] [CrossRef]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef]
- Annapurna, K.; Kumar, A.; Kumar, L.V.; Govindasamy, V.; Bose, P.; Ramadoss, D. PGPR-Induced systemic resistance (ISR) in plant disease management. In Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 405–425. [Google Scholar]
- Reddy, P.P. Plant Growth-Promoting Rhizobacteria (PGPR). In Recent Advances in Crop Protection; Reddy, P.P., Ed.; Springer: New Delhi, India, 2013; pp. 131–158. [Google Scholar]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and future prospects for development of sustainable agriculture. J. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar]
- Saravanakumar, D.; Lavanya, N.; Muthumeena, K.; Raguchander, T.; Samiyappan, R. Fluorescent pseudomonad mixtures mediate disease resistance in rice plants against sheath rot (Sarocladium oryzae) disease. Biocontrol 2009, 54, 273–286. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Höfte, M.; Bakker, P.A.H.M. Competition for iron and induced systemic resistance by siderophores of Plant Growth Promoting Rhizobacteria. In Microbial Siderophores; Varma, A., Chincholkar, S.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 12, pp. 121–133. [Google Scholar]
- Petrik, M.; Zhai, C.; Novy, Z.; Urbanek, L.; Haas, H.; Decristoforo, C. In Vitro and In Vivo comparison of selected Ga-68 and Zr-89 labelled siderophores. Mol. Imaging Biol. 2016, 18, 344–352. [Google Scholar] [CrossRef]
- Panpatte, D.G.; Jhala, Y.K.; Shelat, H.N.; Vyas, R.V. Pseudomonas fluorescens: A promising biocontrol agent and PGPR for sustainable agriculture. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2016; Volume 2, pp. 257–270. [Google Scholar]
- Zandi, P.; Basu, S.B. Role of Plant Growth-Promoting Rhizobacteria (PGPR) as biofertilizers in stabilizing agricultural ecosystems. In Organic Farming for Sustainable Agriculture, Sustainable Development and Biodiversity; Nandwani, D., Ed.; Springer International Publishing: Cham, Switzerland, 2016; Volume 9, pp. 71–87. [Google Scholar]
- Diels, L.; De Smet, M.; Hooyberghs, L.; Corbisier, P. Heavy metals bioremediation of soil. Mol. Biotechnol. 1999, 12, 149–158. [Google Scholar] [CrossRef]
- Kumar, V.V. Plant Growth-Promoting Microorganisms: Interaction with plants and soil. In Plant, Soil and Microbes; Hakeem, K.R., Akhtar, M.S., Abdullah, S.N.A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–16. [Google Scholar]
- Saha, M.; Sarkar, S.; Sarkar, B.; Sharma, B.K.; Bhattacharjee, S.; Tribedi, P. Microbial siderophores and their potential applications: A review. Environ. Sci. Pollut Res. 2016, 23, 3984–3999. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Djavaheri, M.; Bakker, P.A.; Hofte, M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008, 148, 1996–2012. [Google Scholar] [CrossRef] [PubMed]
- Amutharaj, P.; Sekar, C.; Natheer, S.E. Development and use of different formulations of Pseudomonas fluorescens siderophore for the enhancement of plant growth and induction of systemic resistance against Pyricularia oryzae in lowland rice. Int. J. Pharm. Bio Sci. 2013, 4, 831–838. [Google Scholar]
- Naureen, Z.; Hafeez, F.Y.; Hussain, J.; Al Harrasi, A.; Bouqellah, N.; Roberts, M.R. Suppression of incidence of Rhizoctonia solani in rice by siderophore producing rhizobacterial strains based on competition for iron. Eur. Sci. J. 2015, 11, 186–207. [Google Scholar]
- Singh, J.S.; Singh, D.P. Plant Growth Promoting Rhizobacteria (PGPR): Microbes in sustainable agriculture. In Management of Microbial Resources in the Environment; Malik, A., Grohmann, E., Alves, M., Eds.; Springer Science and Business Media: Dordrecht, The Netherlands, 2013; pp. 361–385. [Google Scholar]
- Kiani, M.Z.; Sultan, T.; Ali, A.; Rizvi, Z.F. Application of ACC-deaminase containing PGPR improves sunflower yield under natural salinity stress. Pak. J. Bot. 2016, 48, 53–56. [Google Scholar]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Govindasamy, V.; Senthilkumar, M.; Annapurna, K. Effect of mustard rhizobacteria on wheat growth promotion under Cadmium stress: Characterization of acdS gene coding ACC deaminase. Ann. Microbiol. 2015, 65, 1679–1687. [Google Scholar] [CrossRef]
- Abbas, T.; Pervez, M.A.; Ayyub, C.M.; Ahmad, R. Assessment of morphological, antioxidant, biochemical and ionic responses of salt tolerant and salt-sensitive okra (Abelmoschus esculentus) under saline regime. Pak. J. Life Soc. Sci. 2013, 11, 147–153. [Google Scholar]
- Etesami, H.; Hosseini, H.M.; Alikhani, H.A.; Mohammadi, L. Bacterial biosynthesis of 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase and Indole-3-Acetic Acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J. Plant Growth Regul. 2014, 33, 654–670. [Google Scholar] [CrossRef]
- Bhattacharjee, R.B.; Jourand, P.; Chaintreuil, C.; Dreyfus, B.; Singh, A.; Mukhopadhyay, S.N. Indole acetic acid and ACC Deaminase-producing Rhizobium leguminosarum bv. trifolii SN10 promote rice growth, and in the process undergo colonization and chemotaxis. Biol. Fertil. Soils 2012, 48, 173–182. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Han, Y.; Wang, R.; Yang, Z.; Zhan, Y.; Ma, Y.; Ping, S.; Zhang, L.; Lin, M.; Yan, Y. 1-Aminocyclopropane-1-Carboxylate Deaminase from Pseudomonas stutzeri A1501 facilitates the growth of Rice in the presence of salt or heavy metals. J. Microbiol. Biotechnol. 2015, 25, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Shaharoona, B.; Jamro, G.M.; Zahir, Z.A.; Arshad, M.; Memon, K.S. Effectiveness of various Pseudomonas spp. and Burkholderia caryophylli containing ACC-Deaminase for improving growth and yield of Wheat (Triticum aestivum L.). J. Microbiol. Biotechnol. 2007, 17, 1300–1307. [Google Scholar] [PubMed]
- Shakir, M.A.; Bano, A.; Arshad, M. Rhizosphere bacteria containing ACC-deaminase conferred drought tolerance in wheat grown under semi-arid climate. Soil Environ. 2012, 31, 108–112. [Google Scholar]
- Hassan, W.; Hussain, M.M.; Bashir, S.; Shah, A.; Bano, R.; David, J.C. ACC-deaminase and/or nitrogen fixing rhizobacteria and growth of wheat (Triticum aestivum L.). J. Soil Sci. Plant Nutr. 2015, 15, 232–248. [Google Scholar] [CrossRef]
- Pande, A.M.; Kulkarni, N.S.; Bodhankar, M.G. Effect of PGPR with ACC- Deaminase activity on growth performance of wheat cultivated under stress conditions. Int. J. Appl. Res. 2016, 2, 723–726. [Google Scholar]
- Singh, R.P.; Jha, P.N. Mitigation of salt stress in wheat plant (Triticum aestivum) by ACC deaminase bacterium Enterobacter sp. SBP-6 isolated from Sorghum bicolor. Acta Physiol. Plant 2016, 38, 110. [Google Scholar] [CrossRef]
- Sundaram, V.M.; Kathiresan, D.; Eswaran, S.; Sankaralingam, S.; Balakan, B.; Harinathan, B. Phosphate solubilization and phytohormones production by rhizosphere microorganisms. Adv. Agric. Biol. 2016, 5, 5–13. [Google Scholar]
- Song, O.R.; Lee, S.J.; Lee, Y.S.; Lee, S.C.; Kim, K.K.; Choi, Y.L. Solubilization of insoluble inorganic phosphate by Burkholderia Cepacia DA23 isolated from cultivated soil. Braz. J. Microbiol. 2008, 39, 151–156. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Kairunnisa, K.; Natarajan, S. Phosphate solubilization by rhizosphere Bacteria isolated from Rose Garden soils of Satkhol, India. J. Acad. Ind. Res. 2016, 4, 243–245. [Google Scholar]
- Ahemad, M. Phosphate-solubilizing bacteria-assisted phytoremediation of metalliferous soils: A review. 3Biotech 2015, 5, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Sinha, S.N. Isolation and characterization of a phosphate solubilizing heavy metal tolerant bacterium from River Ganga, West Bengal, India. Songklanakarin J. Sci. Technol. 2015, 37, 651–657. [Google Scholar]
- Kaur, G.; Reddy, M.S. Effects of Phosphate-solubilizing Bacteria, rock phosphate and chemical fertilizers on Maize-Wheat cropping cycle and economics. Pedosphere 2015, 25, 428–437. [Google Scholar] [CrossRef]
- Kumar, V.; Behl, R.K.; Narula, N. Establishment of phosphate-solubilizing strains of Azotobacter chroococcum in the rhizosphere and their effect on wheat cultivars under greenhouse conditions. Microbiol. Res. 2001, 156, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Narula, N. Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum mutants. Biol. Fertil. Soils 1999, 28, 301–305. [Google Scholar] [CrossRef]
- Afzal, A.; Bano, A. Rhizobium and phosphate solubilizing bacteria improve the yield and phosphorus uptake in wheat (Triticum aestivum L.). Int. J. Agric. Biol. 2008, 10, 85–88. [Google Scholar]
- Schoebitz, M.; Ceballos, C.; Ciampi, L. Effect of immobilized phosphate-solubilizing bacteria on wheat growth and phosphate uptake. J. Soil Sci. Plant Nutr. 2013, 13, 1–10. [Google Scholar] [CrossRef]
- Sarker, A.; Talukder, N.M.; Islam, M.T. Phosphate solubilizing bacteria promote growth and enhance nutrient uptake by wheat. Plant Sci. Today 2014, 1, 86–93. [Google Scholar] [CrossRef]
- Panhwar, Q.A.; Radziah, O.; Zaharah, A.R.; Sariah, M.; Razi, I.M. Role of phosphate solubilizing bacteria on rock phosphate solubility and growth of aerobic rice. J. Environ. Biol. 2011, 32, 607–612. [Google Scholar]
- Stephen, J.; Shabanamol, S.; Rishad, K.S.; Jisha, M.S. Growth enhancement of rice (Oryza sativa) by phosphate solubilizing Gluconacetobacter sp. (MTCC 8368) and Burkholderia sp. (MTCC 8369) under greenhouse conditions. 3Biotech 2015, 5, 831–837. [Google Scholar] [CrossRef]
- Vahed, H.S.; Shahinrokhsar, P.; Heydarnezhad, F. Performance of phosphate solubilizing bacteria for improving growth and yield of rice (Oryza sativa L.) in the presence of phosphorus fertilizer. Int. J. Agri. Crop Sci. 2012, 4, 1228–1232. [Google Scholar]
- Went, F.W.; Thimann, K.V. Phytohormones; The Macmillan Company: New York, NY, USA, 1937. [Google Scholar]
- Kende, H.; Zeevaart, J.A.D. The five “Classical” plant hormones. Plant Cell 1997, 9, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176. [Google Scholar] [CrossRef]
- Cao, Z.Y.; Sun, L.H.; Mou, R.X.; Zhang, L.P.; Lin, X.Y.; Zhu, Z.W.; Chen, M.X. Profiling of phytohormones and their major metabolites in rice using binary solid-phase extraction and liquid chromatography-triple quadrupole mass spectrometry. J. Chromatogr. A 2016, 1451, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Azeem, S.A.M.; Mehana, T.A.; Shabayek, A.A. Some plant growth promoting traits of rhizobacteria isolated from Suez Canal region, Egypt. In African Crop Science Conference Proceeding; African Crop Science Society: El-Minia, Egypt, 2007; Volume 8, pp. 1517–1525. [Google Scholar]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth promoting rhizobacteria for their growth promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Alikhani, H.A.; Hosseini, H.M. Indole-3-acetic acid (IAA) production trait, a useful screening to select endophytic and rhizosphere competent bacteria for rice growth promoting agents. MethodsX 2015, 2, 72–78. [Google Scholar] [CrossRef]
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S. Growth promotion of the rice genotypes by PGPRs isolated from rice rhizosphere. J. Soil Sci. Plant Nutr. 2014, 14, 505–517. [Google Scholar] [CrossRef]
- Lavakush, Y.J.; Verma, J.P. Isolation and characterization of effective Plant Growth Promoting Rhizobacteria from rice rhizosphere of Indian soil. Asian J. Biol. Sci. 2012, 5, 294–303. [Google Scholar]
- Murtaza, H.; Asghari, B.; Hassan, S.G.; Javed, I.; Umer, A.; Khan, K.A. Enhancement of rice growth and production of Growth-Promoting phytohormones by inoculation with Rhizobium and other Rhizobacteria. World Appl. Sci. J. 2014, 31, 1734–1743. [Google Scholar]
- Yadav, J.; Verma, J.P.; Tiwari, K.N. Plant growth promoting activities of Fungi and their effect on Chickpea plant growth. Asian J. Biol. Sci. 2011, 4, 291–299. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Saud, H.M.; Ismail, M.R.; Othman, R. Molecular characterization of stress tolerant plant growth promoting Rhizobacteria (PGPR) for growth enhancement of rice. Int. J. Agric. Biol. 2015, 18, 184–191. [Google Scholar] [CrossRef]
- Torres-Rubio, M.G.; Valencia-Plata, S.A.; Bernal-Castillo, J.; Martínez-Nieto, P. Isolation of Enterobacteria, Azotobacter sp. and Pseudomonas sp., producers of indole-3-acetic acid and siderophores, from Colombian rice rhizosphere. Rev. Latinoam. Microbiol. 2000, 42, 171–176. [Google Scholar]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A. Microbial producers of plant growth stimulators and their practical use: A Review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, A. Characterization of novel plant growth promoting endophytic Bacterium Achromobacter xylosoxidans from wheat plant. Microb. Ecol. 2009, 58, 179–188. [Google Scholar] [CrossRef]
- Soltani, A.-A.; Khavazi, K.; Asadi-Rahmani, H.; Omidvari, M.; Dahaji, P.A.; Mirhoseyni, H. Plant Growth Promoting characteristics in some Flavobacterium spp. isolated from soils of Iran. J. Agric. Sci. 2010, 2, 106–115. [Google Scholar] [CrossRef]
- RameshKumar, N.; Krishnan, M.; Kandeepan, C.; Kayalvizhi, N. Molecular and functional diversity of PGPR fluorescent Pseudomonas isolated from rhizosphere of rice (Oryza sativa L.). Int. J. Adv. Biotechnol. Res. 2014, 5, 490–505. [Google Scholar]
- Takahashi, N.; Phinney, B.O.; MacMillan, J. Gibberellins, with 176 Illustrations; Springer: New York, NY, USA, 1991. [Google Scholar]
- Maheshwari, D.K.; Dheeman, S.; Agarwal, M. Phytohormone-Producing PGPR for sustainable agriculture. In Bacterial Metabolites in Sustainable Agroecosystem, Sustainable Development and Biodiversity; Maheshwari, D.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 12, pp. 159–182. [Google Scholar]
- Kang, S.-M.; Waqas, M.; Khan, A.L.; Lee, I.-J. Plant-Growth-Promoting Rhizobacteria: Potential candidates for gibberellins production and crop growth promotion. In Use of Microbes for the Alleviation of Soil Stresses, Vol. 1; Miransari, M., Ed.; Springer Science+Business Media: New York, NY, USA, 2014; pp. 1–19. [Google Scholar]
- Caba, J.M.; Centeno, M.L.; Fernández, B.; Gresshoff, P.M.; Ligero, F. Inoculation and nitrate alter phytohormone levels in soya bean roots differences between a super nodulating mutant and the wild type. Planta 2000, 211, 98–104. [Google Scholar] [CrossRef]
- Kucey, R.M.N. Plant growth-altering effects of Azospirillum brasilense and Bacillus C-11-25 on two wheat cultivars. J. Appl. Bacteriol. 1988, 64, 187–196. [Google Scholar] [CrossRef]
- Creus, C.; Sueldo, R.; Barassi, C. Shoot growth and water status in Azospirillum-inoculated wheat seedlings grown under osmotic and salt stresses. Plant Physiol. Biochem. 1997, 35, 939–944. [Google Scholar]
- Bottini, R.; Cassán, F.; Piccoli, P. Gibberellin production by bacteria and its involvement in plant growth promotion and yield increase. Appl. Microbiol. Biotechnol. 2004, 65, 497–503. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P.; Saikia, R. Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr. Microbiol. 2009, 59, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Lenin, G.; Jayanthi, M. Indole Acetic Acid, Gibberellic Acid and Siderophore production by PGPR isolates from Rhizospheric soils of Catharanthus roseus. Int. J. Pharm. Biol. Arch. 2012, 3, 933–938. [Google Scholar]
- Sethi, S.K.; Adhikary, S.P. Azotobacter: A Plant-Growth Promoting Rhizobacteria used as biofertilizer. Dyn. Biochem. Process Biotechnol. Mol. Biol. 2012, 6, 68–74. [Google Scholar]
- Jha, Y.; Subramanian, R.B. Characterization of root-associated bacteria from paddy and its growth-promotion efficacy. 3Biotech 2014, 4, 325–330. [Google Scholar] [CrossRef][Green Version]
- Kumar, S.; Agarwal, M.; Dheeman, S.; Maheshwari, D.K. Exploitation of phytohormone-producing PGPR in development of multispecies bioinoculant formulation. In Bacterial Metabolites in Sustainable Agroecosystem, Sustainable Development and Biodiversity; Maheshwari, D.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 12, pp. 297–317. [Google Scholar]
- Lakzadeh, B.; Mir-Mahmoodi, T.; Jalilnezhad, N. Effects of Azospirillum Bacteria and Gibberellin hormone on morpho-physiological properties, yield and yield components of Corn (Zea mays L.). Biol. Forum 2015, 7, 986–993. [Google Scholar]
- Wong, W.S.; Tan, S.N.; Ge, L.; Chen, X.; Yong, J.W.H. The importance of phytohormones and microbes in biofertilizers. In Bacterial Metabolites in Sustainable Agroecosystem, Sustainable Development and Biodiversity; Maheshwari, D.K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; Volume 12, pp. 105–158. [Google Scholar]
- Conrad, K.; Bettin, D.; Neumann, S. The cytokinin production of Azospirillum and Klebsiella and its possible ecological effects. In Physiology and Biochemistry of Cytokinins in Plants: Proceeding of the International Symposium on Physiology and Biochemistry of Cytokinins in Plants; Kamínek, M., Mok, D.W., Zažímalová, E., Eds.; SPB Academic Publishing: The Hague, The Netherlands, 1992; pp. 401–405. [Google Scholar]
- Akiyoshi, D.E.; Regier, D.A.; Gordon, M.P. Cytokinin production by Agrobacterium and Pseudomonas spp. J. Bacteriol. 1987, 169, 4242–4248. [Google Scholar] [CrossRef]
- Taller, B.J.; Wong, T.Y. Cytokinins in Azotobacter vinelandii Culture Medium. Appl. Environ. Microbiol. 1989, 55, 266–267. [Google Scholar] [CrossRef]
- Timmusk, S.; Nicander, B.; Granhall, U.; Tillberg, E. Cytokinin production by Paenibacillus polymyxa. Soil Biol. Biochem. 1999, 31, 1847–1852. [Google Scholar] [CrossRef]
- Donderski, W.; Głuchowska, M. Production of Cytokinin-like substances by planktonic bacteria isolated from lake Jeziorak. Pol. J. Environ. Stud. 2000, 9, 369–376. [Google Scholar]
- Kämpfer, P.; Ruppel, S.; Remus, R. Enterobacter radicincitans sp. nov., a plant growth promoting species of the family Enterobacteriaceae. Syst. Appl. Microbiol. 2005, 28, 213–221. [Google Scholar] [CrossRef]
- Ortiz-Castro, R.; Valencia-Cantero, E.; Lopez-Bucio, J. Plant growth promotion by Bacillus megaterium involves cytokinin signaling. Plant Signal. Behav. 2008, 3, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Asghar, H.N.; Arshad, M. Cytokinin and its precursors for improving growth and yield of rice. Soil Biol. Biochem. 2001, 33, 405–408. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Timergalina, L.N.; Arkhipova, T.N.; Shendel, G.V.; Kuz’Mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Okamoto, M.; Koshiba, T. ABA biosynthetic and catabolic pathways. In Abscisic Acid: Metabolism, Transport and Signaling; Zhang, D.P., Ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 2014; pp. 21–46. [Google Scholar]
- Gomez-Cadenas, A.; Vives, V.; Zandalinas, S.; Manzi, M.; Sanchez-Perez, A.; Perez-Clemente, R.; Arbona, V. Abscisic Acid: A versatile phytohormone in plant signaling and beyond. Curr. Protein Pept. Sci. 2015, 16, 413–434. [Google Scholar] [CrossRef]
- Travaglia, C.; Cohen, A.C.; Reinoso, H.; Castillo, C.; Bottini, R. Exogenous abscisic acid increases carbohydrate accumulation and redistribution to the grains in wheat grown under field conditions of soil water restriction. J. Plant Growth Regul. 2007, 26, 285–289. [Google Scholar] [CrossRef]
- Yang, C.W.; Wang, J.W.; Kao, C.H. The relation between accumulation of Abscisic Acid and proline in detached rice leaves. Biol. Plant 2000, 43, 301–304. [Google Scholar] [CrossRef]
- Azuma, T.; Hatanaka, T.; Uchida, N.; Yasuda, T. Interactions between abscisic acid, ethylene and gibberellin in internodal elongation in floating rice: The promotive effect of abscisic acid at low humidity. Plant Growth Regul. 2003, 41, 105–109. [Google Scholar] [CrossRef]
- Golubyatnikov, L.L.; Mokhov, I.I.; Eliseev, A.V. Nitrogen cycle in the earth climatic system and its modeling. Izv. Atmos. Ocean Phys. 2013, 49, 229–243. [Google Scholar] [CrossRef]
- Bertrand, J.C.; Bonin, P.; Caumette, P.; Gattuso, J.P.; Grégori, G.; Guyoneaud, R.; Roux, X.L.; Matheron, R.; Poly, F. Biogeochemical cycles. In Environmental Microbiology: Fundamentals and Applications: Microbial Ecology; Bertrand, J.C., Caumette, P., Lebaron, P., Matheron, R., Normand, P., Ngando, T.S., Eds.; Springer Science and Business Media: Dordrecht, The Netherlands, 2015; pp. 511–617. [Google Scholar]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present, and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Garnier, J.; Leach, A.; Galloway, J.N. Food and feed trade as a driver in the global nitrogen cycle: 50-year trends. Biogeochemistry 2014, 118, 225–241. [Google Scholar] [CrossRef]
- Rao, V.R.; Jena, P.K.; Adhya, T.K. Inoculation of rice with nitrogen-fixing bacteria-problems and perspectives. Biol. Fertil. Soils 1987, 4, 21–26. [Google Scholar] [CrossRef]
- Hartmann, A. Biotechnological aspects of diazotrophic bacteria associated with rice. In Biological Nitrogen Fixation Associated with Rice Production; Rahman, M., Ed.; Springer Science and Business Media: Dordrecht, The Natherlands, 1996; pp. 211–224. [Google Scholar]
- Malik, K.; Bilal, R.; Mehnaz, S.; Rasul, G.; Mirza, M.; Ali, S. Association of nitrogen-fixing, plant-growth-promoting rhizobacteria (PGPR) with kallar grass and rice. Plant Soil 1997, 194, 37–44. [Google Scholar] [CrossRef]
- Santa, O.R.D.; Hernández, R.F.; Alvarez, G.L.M.; Junior, P.R.; Soccol, C.R. Azospirillum sp. Inoculation in Wheat, Barley and Oats Seeds Greenhouse Experiments. Braz. Arch. Biol. Technol. 2004, 47, 843–850. [Google Scholar] [CrossRef]
- Nabti, E.; Sahnoune, M.; Ghoul, M.; Fischer, D.; Hofmann, A.; Rothballer, M.; Schmid, M.; Hartmann, A. Restoration of growth of durum wheat (Triticum durum var. Waha) under saline conditions due to inoculation with the Rhizosphere Bacterium Azospirillum brasilense NH and extracts of the marine Alga Ulva lactuca. J. Plant Growth Regul. 2010, 29, 6–22. [Google Scholar] [CrossRef]
- Volpiano, C.G.; Estevam, A.; Saatkamp, K.; Furlan, F.; Vendruscolo, E.C.G.; Dos Santos, M.F. Physiological responses of the co-cultivation of PGPR with two wheat cultivars in vitro under stress conditions. BMC Proc. 2014, 8, P108. [Google Scholar] [CrossRef]
- Mirza, M.S.; Mehnaz, S.; Normand, P.; Prigent-Combaret, C.; Moënne-Loccoz, Y.; Bally, R.; Malik, K.A. Molecular characterization and PCR detection of a nitrogen-fixing Pseudomonas strain promoting rice growth. Biol. Fertil. Soils 2006, 43, 163–170. [Google Scholar] [CrossRef]
- Shabaev, V.P.; Voronina, L.P. Grain yield and quality of winter wheat inoculated with a mixed culture of Pseudomonas Bacteria against the background of increasing Nitrogen fertilizer Rates. Russ. Agric. Sci. 2007, 33, 311–313. [Google Scholar] [CrossRef]
- Park, M.; Kim, C.; Yang, J.; Lee, H.; Shin, W.; Kim, S.; Sa, T. Isolation and characterization of diazotrophic growth promoting bacteria from rhizosphere of agricultural crops of Korea. Microbiol. Res. 2005, 160, 127–133. [Google Scholar] [CrossRef]
- Brusamarello-Santos, L.C.C.; Pacheco, F.; Aljanabi, S.M.M.; Monteiro, R.A.; Cruz, L.; Baura, V.A.; Pedrosa, F.O.; Souza, E.; Wassem, R. Differential gene expression of rice roots inoculated with the diazotroph Herbaspirillum seropedicae. Plant Soil 2012, 356, 113–125. [Google Scholar] [CrossRef]
- Milošević, N.; Tintor, B.; Protić, R.; Cvijanović, G.; Dimitrijević, T. Effect of inoculation with Azotobacter chroococcum on wheat yield and seed quality. Rom. Biotechnol. Lett. 2012, 17, 7352–7357. [Google Scholar]
- Habibi, S.; Djedidi, S.; Prongjunthuek, K.; Mortuza, F.; Ohkama-Ohtsu, N.; Sekimoto, H.; Yokoyoma, T. Physiological and genetic characterization of rice nitrogen fixer PGPR isolated from rhizosphere soils of different crops. Plant Soil 2014, 379, 51–66. [Google Scholar] [CrossRef]
- Iniguez, A.L.; Dong, Y.; Triplett, E.W. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol. Plant Microbe Interact. 2004, 17, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Trân Van, V.; Berge, O.; Ngô Kê, S.; Balandreau, J.; Heulin, T. Repeated beneficial effects of rice inoculation with a strain of Burkholderia vietnamiensis on early and late yield components in low fertility sulphate acid soils of Vietnam. Plant Soil 2000, 218, 273–284. [Google Scholar] [CrossRef]
- Govindarajan, M.; Balandreau, J.; Kwon, S.-W.; Weon, H.-Y.; Lakshminarasimhan, C. Effects of the inoculation of Burkholderia vietnamensis and related endophytic diazotrophic bacteria on grain yield of Rice. Microb. Ecol. 2008, 55, 21–37. [Google Scholar] [CrossRef]
- Muriel, C.; Jalvo, B.; Redondo-Nieto, M.; Rivilla, R.; Martín, M. Chemotactic motility of Pseudomonas fluorescens F113 under aerobic and denitrification conditions. PLoS ONE 2015, 10, e0132242. [Google Scholar] [CrossRef]
- Pothier, J.; Prigent-Combaret, C.; Haurat, J.; Moënne-Loccoz, Y.; Wisniewski-Dyé, F. Duplication of Plasmid-Borne Nitrite Reductase Gene nirK in the Wheat-Associated Plant Growth–Promoting Rhizobacterium Azospirillum brasilense Sp245. Mol. Plant Microbe Interact. 2008, 21, 831–842. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).