Featured Application
Potential applications of the concepts and data presented in the article relate to inference systems and computational predictive models of job burnout, effective as second opinion systems.
Abstract
Occupational burnout, manifested by emotional exhaustion, lack of a sense of personal achievement, and depersonalization, is not a new phenomenon, but thusfar, there is no clear definition or diagnostic guidelines. The aim of this article wasto summarize all empirical studies to date that have used medical neuroimaging techniques to provide evidence or links regarding changes in brain function in occupational burnout syndrome from a neuroscientific perspective, and then use these to propose a fuzzy-based computational model of burnout.A comprehensive literature search was conducted in two major databases (PubMed and Medline Complete). The search period was 2006–2021, and searches were limited to the English language. Each article was carefully reviewed and appropriately selected on the basis of raw data, validity of methods used, clarity of results, and scales for measuring burnout. The results showed that the brain structures of patients with job burnout that are associated with emotion, motivation, and empathy weresignificantly different from healthy controls. These altered brain regions included the thalamus, hippocampus, amygdala, caudate, striatum, dorso-lateral prefrontal cortex, anterior cingulate cortex, posterior cingulate cortex, anterior insula, inferior frontal cingulate cortex, middle frontal cingulate cortex, temporoparietal junction, and grey matter. Deepening our understanding of how these brain structures are related to burnout will pave the way for better approaches fordiagnosis and intervention. As an alternative to the neuroimaging approach, the paper presents a late proposal of the PLUS (personal living usual satisfaction) parameter. It is based on a fuzzy model, wherein the data source is psychological factors—the same or similar to the neuroimaging approach. As the novel approach to searching for neural burnout mechanisms, we have shown that computational models, including those based on fuzzy logic and artificial neural networks, can play an important role in inferring and predicting burnout. Effective computational models of burnout are possible but need further development to ensure accuracy across different populations. There is also a need to identify mechanisms and clinical indicators of chronic fatigue syndrome, stress, burnout, and natural cognitive changes associated with, for example, ageing, in order to introduce more effective differential diagnosis and screening.
1. Introduction
Professional burnout syndrome, manifested by emotional exhaustion, lack of a sense of personal achievement, and depersonalization, is the result of chronic occupational stress. It is not a new phenomenon; it was first mentioned as early as the 1970s [1]. There has been a large amountof research on occupational burnout syndrome over the past few decades, but there is still no clear definition of burnout or diagnostic guidelines [2]. Although it has been included in the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10 CM) [3,4] under the heading “Factor affecting health and health care contact (Z00-Z99)” as “A state of life exhaustion”, and defined in ICD-11 [5] as “feelings of energy depletion or exhaustion”, occupational burnout is still not recognized as a disorder in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM 5) [6]. The main reason for this is the lack of evidence for an objective positive neurobiological marker of occupational burnout syndrome, which makes the final stages of the diagnosis process difficult [1]. The main aim of medical research is to provide up-to-date data for use by clinicians to improve the effectiveness of diagnosis, therapy, and care. This is sometimes difficult due to the lack of a comprehensive picture of the mechanisms of physiology and pathology of the observed phenomenon, as well as unclear links between research findings and theories that attempt to explain it comprehensively.
Burnout is a relatively common health problem, but its prevalence varies according to a number of factors, both professional issues and various demographic factors, e.g., worldwide, the prevalence of burnout varies from country to country; it ranges from 0% to 80.5% [7,8,9]. The aforementioned differences in results are also caused by differences in burnout definitions, assessment methods, and research quality.
The aim of this article is to summarize all empirical studies to date that have used medical neuroimaging techniques to provide evidence or links regarding changes in brain function in occupational burnout syndrome from a neuroscientific perspective, and then use these to propose fuzzy-based computational model of burnout.Burnout has a cumulative effect on the human body over time. Chronic stress can have multidirectional effects causing clinical implications at different levels: microscopic, macroscopic, and affective, with varying degrees of severity. Neuroimaging offers standardized and objective evidence on a macroscopic scale in addition to the subjective information that self-report studies can give us. Computational modeling (at various levels) involves not only reflecting the phenomena occurring in the structures being modeled, but also, through a data-driven approach, extracting new, perhaps not yet known mechanisms and relationships. As a result, we may be able to discover and understand the brain structures and neural networks that are important in burnout syndrome, leading to the development of more effective prevention and treatment methods.
To date, computational models have been widely regarded as an intermediate level of research, linking theoretical concepts to experimental results. However, nowadays, thanks to the development of artificial intelligence methods and tools, their functionalities have been extended to include inference analysis and prediction from data. This brings with it the opportunity to extend the diagnosis and treatment of burnout to an earlier stage: prevention within the framework of healthy people medicine. With the aforementioned approach, disorders can be detected at an earlier stage, and early diagnosis and prevention strategies will allow treatment to be limited to the most severe cases. Not insignificant are the issues that work requires increasingly more specialized knowledge, education of specialists takes longer and costs more, and a satisfied employee works better. It pays for employers to invest in prevention, gaining efficiency at work.
The proposed fuzzy approach is not completely new but was used for the first time in the presented applications. The novelty of the article is the integration of the approaches: neuroimaging and fuzzy-based computational modeling of burnout. Former studies concerning integration neuroimaging with fuzzy-based computational modeling, for example in Alzheimer’s disease, cannot be applied here due to their different neurological (not even neurodegenerative) nature.Models of this type can be more accurate and faster, and in some cases, personalized. This fits into the global trend of personalized medicine and targeted therapies, as well as technical support for clinical practice—in this case, not only the use of artificial intelligence for drug development, but also for advanced diagnostics of the human body.
1.1. Types of Neuroimaging Used to Study Brain Structure and Activity
1.1.1. Electroencephalogram
An electroencephalograph (EEG) records the spontaneous electrical activity generated in the cerebral cortex. This activity reflects the electric currents flowing in the extracellular spaces of the brain, which are the combined effect of the myriad excitatory and inhibitory potentials of synaptic between cortical neurons. This spontaneous activity of cortical neurons is strongly conditioned and synchronized by the subcortical structures, especially the thalamus and the reticular formation of the brainstem. Afferent impulses from these deep structures are likely responsible for stimulating cortical neurons to produce characteristic rhythmic patterns of the brain waves, such as the alpha rhythm and sleep spindles [10]. Event-related potentials (ERP) associated with stimulus and analyses of the EEG power in a resting-state condition can be used to assess burnout.
1.1.2. Positron Emission Tomography
Positron emission tomography (PET) images reflect the regional concentrations of systemically administered radioactive compounds. The concentration of the above-mentioned markers in different parts of the brain are determined by a system of radiation detectors, and tomographic (3D) images are constructed using techniques similar to those used in computed tomography. With PET, local patterns of brain blood flow, oxygen uptake, and glucose utilization can be measured. This study has been shown to be useful in classifying many pathological changes (e.g., distinguishing neoplastic tissue from radiation necrosis, or differentiating types of degenerative diseases). Despite its usefulness, this technology is expensive and does not always increase diagnostic confidence.
1.1.3. Magnetic Resonance Imaging and Functional Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) involves placing the patient in a strong magnetic field, which causes endogenous isotopes (atoms) in the tissues and the cerebrospinal fluid to align with the longitudinal orientation of the magnetic field. Applying to the above-mentioned field of a short radio frequency pulse causes the rearrangement of the atoms. When this pulse ceases, the energy that has been absorbed is emitted by the isotopes, producing a magnetic signal that is detected by the receiver coils. The signals are measured repeatedly after each impulse. The signals are stored in a data matrix from which 2D images are reconstructed after computer analysis. The image is a map of the hydrogen content in the tissue, and thusit largely reflects the water concentration. The difference between MRI and functional MRI (fMRI) is as follows: MRI scans the anatomical structure (allowing the study of organs, tissues, etc.), while fMRI allows for the hemodynamic functioning of the brain to be studied (inferring brain activity by measuring changes in blood flow) [10]. fMRI indirectly measures neural activity using a blood-oxygenation-level-dependent (BOLD) signal and can be used to assess burnout. T2/T2-weighted MRI images reflecting change in deoxyhemoglobin concentration at activated brain regions can be used to assess burnout.
1.2. Types of Computational Systems and Models Used to Study Brain Structures and Activity
In this subsection, we showed the rationale for the use of computational models in the analysis of central nervous system processes, including the physiopathology of burnout. Computational models begin the predominant way of linking current theoretical concepts to results of experimental studies, even though they are not obvious or hidden.Many researchers think that there is no further progress in, e.g., medicine or psychology, without bioinformatics, biocybernetics, and healthcare informatics [11,12]. Fuzzy medical knowledge can be used to model uncertainty and ambiguity in medical concepts and their sets. Furthermore, inference mechanisms based on fuzzy logic ensure continuity and completeness of inference despite uncertainty in the data. [13,14]. Early recognition of the possible occupations can create novel chances for early diagnosis, therapy, and care, even as a part of preventive medicine. Fuzzy hierarchical analysis [15,16] can be a key solution to achieve this. The rationale for the use of computational models in the analysis of central nervous system processes, including the physiopathology of burnout, stems from the difficulty in isolating the neural mechanisms of burnout. On the one hand, computational models make it possible to link existing theoretical concepts to experimental findings by extracting the mechanisms linking them, and on the other hand, they make it possible to test postulated/hypothesized mechanisms for the need for their existence and for gaps in their explanation of observed phenomena. Current areas of application of artificial intelligence (AI) in clinical practice include:
- Data analysis, including biomedical signals (from simple sequences of laboratory data to EEG analysis, still and moving images, gait, or speech analysis as a biomedical signal);
- Pattern recognition and classification, and machine learning (ML), including traditional and deep artificial neural networks (ANN), support vector machine (SVM), naïveBayesian classifier, kNN, regressions, decision trees, and random forests, etc.;
- Swarm intelligence, including ant colony optimization (ACO) and particle swarm optimization (PSO);
- Fuzzy systems, including ordered fuzzy numbers and trend analysis;
- Multidimensional scaling (MDS) and similar tools for attractor analysis;
- Multifractal analysis, including the analysis of the trend change, e.g., based on changes in the Hurst index;
- Artificial intelligence optimization of the production processes of medical devices (prostheses, orthoses, exoskeletons, implants, drugs, etc.) and therapeutic processes (e.g., optimization of rehabilitation robot algorithms);
- Second opinion systems;
- Predictive systems;
- Modeling of central nervous system processes on biologically faithful neural networks (Emergent, Genesis, NEURON);
- Modeling the processes of the peripheral nervous system based on the patterns of the signal theory;
- Dedicated data science systems in Matlab, R (Python), or other data analysis environments for predictive medicine purposes.
The number of various possible clinical applications of the aforementioned AI methods and technologies in the area of early identification, diagnosis, therapy, and care of burnout it is very large, and there are more of them almost every day. For the purposes of this article, some of them (fuzzy systems, multifractal analysis, biologically faithful neural networks in Emergent, dedicated data science systems in Matlab) have been applied to the models presented later in this article.
2. Materials and Methods
2.1. Literature Review on Neuroimaging in Burnout
An extensive literature search was performed on two major databases (PubMed and Medline Complete). The following search terms were entered: “burnout”, “neuroimaging”, “work stress”, and the like. After careful check definitions and entry terms in bibliometric databases including MeSH dictionary (PubMed), we clarified that for requirements of this review,
- “work stress”= “occupational stress”;
- “professional burnout syndrome”= “professional burnout”;
- “mental stress” = “stress” = “stress exhaustion syndrome.
The search period was2006–2021, and the search was limited to the English language. Eligibility criteria for the review were formulated as follows: peer-reviewed articles and scientific chapters in English, appropriate study design for the research question, medical neuroimaging studies used, study group(s) with specified conditions. Each article was carefully reviewed and properly selected on the basis of the input of raw data, the validity of the methods used, the clarity of the results, and the scales for measuring burnout.
2.2. Concept of Computational Model of Burnout
On the basis of the results of the literature review, a hierarchical fuzzy model was used to build a proposal for a computational burnout model.
3. Results of the Literature Review
A total of eleven studies meeting the selection criteria were selected. Of these, four studies used EEG, six studies used fMRI, and one study used PET. The number of fMRI tests hasgradually increased compared to previous years. The PRISMA flow diagram is presented in Figure 1.
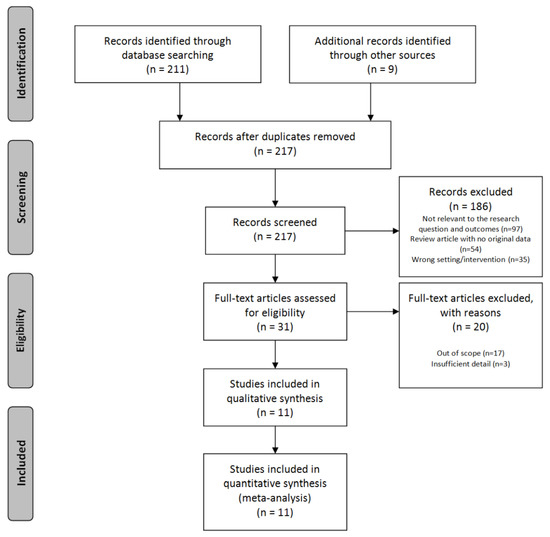
Figure 1.
PRISMA flow diagram [17].
An overview of all included studies and their results is presented in Table 1.

Table 1.
Included studies concerning burnout neuroimaging.
3.1. EEG Studies
Luijtelaar et al. [1] investigated the brain function of burnout patients by analyzing their EEG and neuropsychological results. Thirteen patients diagnosed with burnout syndrome and thirteen controls were compared using four different measures:
- Frontal asymmetry;
- P300 amplitude;
- Alpha peak frequency;
- Alpha and beta power comparison with eyes open and closed.
There were no observed differences in asymmetry between the burnout group and the control group, but the former showed significantly reduced P300 amplitude and a significantly lower alpha peak frequency than the control group. Comparing the power of alpha and beta with eyes open and closed,
- Beta potency was significantly reduced in burnout patients compared to the control group;
- There was no difference in alpha potency compared to the control group.
This study showed clear differences in the parameters obtained from the EEG in burnout patients compared to a healthy control group.
In a study by Tement et al. [18], 117 participants (42 of whom were male, 35.90%) were included from a large pool of individuals who completed questionnaires on burnout and depression. The aim of the study was to test whether individual alpha frequency (IAF) and IAF frequency power and coherence (IAF ± 1 Hz) differentiated between burnout and depression syndromes, i.e., to serve as potential biomarkers. Patients were seated in a chair and told to rest with their eyes closed while their EEG was recorded for three minutes. The results showed that gender was a significant predictor. Seven out of twelve interactions between EEG indicators and gender were significant. Connection patterns were also significant for depression, showing gender-related differences. A significant positive association was found between IAF and job burnout when observed at the posterior location. Statistical regression analysis showed that IAF was significantly associated with depressive symptomatology, while power was mainly associated with occupational burnout, suggesting the different value of IAF and power in predicting occupational burnout and depression. The results of this study provided a strong rationale for considering occupational burnout as a distinct clinical syndrome from depression.
In the study by Golonka et al., a significantly lower alpha power under open-eye conditions was observed in the burnout group compared to the control group. Possible development of compensatory mechanisms were also found in the burnout group. Gender appeared to be a differentiating factor in the association between EEG spectral characteristics and burnout symptoms [19].
For comparison, central fatigue was studied in 50 patients with chronic fatigue syndrome (CFS) and 50 healthy controls using resting EEG taken from 19 scalp sites for 3 min with eyes closed. Significant differences were found in the frequency bands: -delta (1–3 Hz): sources mainly in the frontal lobe, and -beta-2 (19–21 Hz): sources in the medial and superior parietal lobes. Left leading delta sources were correlated with clinically reduced motivation [20].
Abnormal cortical sources werealso seen in patients with CFS. This ledto the conclusion that early processes related to chronic stress and fatigue can be monitored by means of EEG. An increase in dysfunction of the brain’s regulatory systems was observed at different stages of burnout, correlating with an increase in the severity of clinical symptoms [21].
3.2. MRI and fMRI Studies
In this study, researchers investigated how burnout modulates brain activity during clinical reasoning in doctors. Using functional magnetic resonance imaging (fMRI), the brain activity ofinternal medicine residents was assessed:
- In the study group: N = 17 (15 men and 2 women);
- In the control group: N = 17 (10 men and 7 women) [21];
who answered multiple-choice questions while pondering the answers. All subjects completed the Maslach Burnout Inventory (MBI) [28] prior to fMRI. Whole-brain analysis of covariance was used to examine the blood-oxygen-dependent signal (BOLD) during response and reflection on clinical problems in relation to burnout scores. Higher scores in the area of depersonalization in the test group were reflected in lower BOLD signals in the right dorsolateral prefrontal cortex and middle frontal cortex during responses to clinical problems and, in the control group, lower BOLD signals in the bilateral prefrontal cortex during responses to clinical problems. In contrast, higher scores in the area of emotional exhaustion were associated in the control group with stronger BOLD signals in the right posterior cingulate cortex and middle frontal cortex. The study group (described above [21]: 40 burnout subjects (38 ± 6 years, range 19–46 years, 15 males, 25 females)) did not show any significant effect of burnout on brain activity. They were more susceptible to the effects of burnout on clinical reasoning, which may indicate that, as less experienced, they may need both cognitive and emotional support to improve their quality of life and optimize their performance and learning. In the samples studied, an imbalance in gender wasobserved. These findings organize the understanding of stress, cognitive control, and the theory of cognitive load [21].
The aim of the study by Savic et al. was to investigate structural changes in the brain related to occupational stress. The study compared the thickness of the cortex and the subcortical volume in 40 patients with burnout and 40 in the control group. Stress severity was measured using MBI. Patients with occupational burnout showed significant thinning of the medial frontal cortex. By examining the correlation between age and cortex thickness, we found that the effect of thinning of the frontal cortex was more pronounced in burnout patients. The results also showed that the volume of their amygdala increased bilaterally, while the volume of the caudate cortex decreased, accompanied by impaired motor function. Patients with burnout positively correlated with amygdala volume. This study found that burnout was associated with thinning of the cortex as well as selective changes in the subcortical volume with behavioral correlates. The results of this study support the hypothesis that stress-related excitotoxicity may be the underlying mechanism, and that the described condition is a stress-related disease [22].
Blix et al. conducted a comparative MRI study of brain volumes in the grey (GM) and white matter (WM) between patients with chronic occupational stress and healthy controls. The study also included volume analysis of structures known to be susceptible to neurotoxic changes of the hippocampus, caudate, and striatum (skin), but not the amygdala. The study group consisted of 30 right-handed, non-smoking patients (23 women and seven men) who were diagnosed with “coping with stress response and adjustment disorder” according to the International Classification of Diseases (ICD-10). Subjects were also required to have a mean stress burnout score >3.0 on the Maslach StressBurnout Inventory–General Survey (MBI-GS) [29]. The control group consisted of 68 healthy, right-handed, non-smoking volunteers (53 women and 15 men) without a history of chronic stress or hereditary neuropsychiatric disorders. The results of the study showed that the GM volume of the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex was significantly reduced in the stressed subjects. In addition, the volume of their caudate nucleus and shell cortex was reduced, correlating inversely with the degree of stress experienced. The results indicate a morphological involvement of the frontal striatal circuits in individuals exposed to stress. The finding of morphological changes, particularly GM atrophy in these areas, supports the previous conclusion that patients reporting classic symptoms of occupational stress have a medical condition that requires careful and precise diagnosis and treatment [23].
Tei et al. examined the relationship between self-reported burnout severity and psychological measures of empathic dissonance, emotional dissonance, and alexithymia in physicians to test two conflicting hypotheses to explain burnout:
- compassion fatigue—that is, individuals become overly emotionally involved;
- emotional dissonance—that is, a discrepancy between felt and expressed emotions along with decreased emotional regulation.
They then investigated whether increased or decreased brain activity related to empathy as measured by fMRI was associated with burnout severity scores and psychological measures. Twenty-five active nurses (twentywomen and five men) with work experience ranging from 1 to 10 years participated in the study. During the fMRI study, participants were presented with video clips showing the hand with and without pain. After the fMRI study, participants completed self-report measures:
- severity of burnout was assessed using the MBI;
- empathic disposition was assessed using the Interpersonal Reactivity Index (IRI) [24,30].
The study by Tei et al. was limited to a very specific population: nurses recruited via advertising from hospitals in Kyoto (Japan).
The results showed higher activation in the anterior cerebral hemisphere/inferior frontal gyrus (AI/IFG) and temporoparietal junction (TPJ) under pain conditions compared to the no-pain situation. Hence, the severity of burnout in medical professionals is explained by reduced brain activity related to empathy, and this reduced brain activity is correlated with stronger emotional dissonance and alexithymia and greater empathy. The results support the findings of previous studies that burnout is associated with emotional dysregulation. Predicting future burnout among doctors using neuroimaging may be an important addition to psychological research. A more complete understanding of burnout is possible by identifying specific aspects of trait and empathy status and neural processes that may be associated with burnout severity.
A study by Savic et al. in a group of patients exposed to chronic occupational stress found reduced thickness of the right prefrontal cortex (PFC) and left superior temporal gyrus (STG), enlarged amygdala volume, and reduced caudate volume, with these abnormalities more prominent in women, except for changes in caudate nucleus volume. These were all related to perceived stress, which was similar for both sexes. PFC thickness correlated with impaired ability to modulate negative emotions downwards. After 1–2 years, amygdala enlargement and left STG thinning persisted. In the control group, the above changes did not occur. Chronic occupational stress was associated with partially reversible structural abnormalities in key stress processing regions, correlated with the degree of stress experienced. The above changes weremore pronounced in women as a possible cause of increased brain vulnerability to stress-induced psychiatric disorders [25].
In Gavelin et al.’s study, burnout levels were positively correlated with neuronal activation in the rostral prefrontal cortex, posterior parietal cortex, and striatum, as observed by fMRI, and striatal activity decreased after therapy as a result of improved burnout levels [26]. The Shirom–Melamed Burnout Questionnaire (SMBQ) [31] containing 22 items in four subscales was used to evaluate burnout level.Cognitive training may bring improvements, but this requires further research on larger patient samples [32,33,34].
3.3. PET Studies
Jovanovic et al. [27] conducted a PET study on 16 chronically stressed individuals (mean stress burnout score >3.0 according to MBI-GS) and 16 non-stressed individuals. The function of their limbic system was examined by measuring cerebral blood flow at rest and during an odor activation paradigm. 5-HT1A receptor (BP) binding potential was studied by PET imaging and bolus injection. Radioactivity in the brain was measured in a series of 15 consecutive frames with a duration of 63 min, of which the first 9frames were acquired within 15 min. Stressed subjects showed a functional disconnect between the amygdala and anterior cingulate cortex (ACC)/m posterior prefrontal cortex (mPFC) and impaired olfactory activation of the ACC. They also showed reduced BP of 5-HT1A receptors in the ACC, hemispheric cortex, and hippocampus. Their performance in attention, odor discrimination, and semantic memory tasks was impaired and correlated with BP values in each region. The degree of perceived stress was inversely correlated with ACC and BP activation of 5-HT1A receptors in the amygdala and hippocampus. Thus, prolonged daily psychosocial stress appears to be associated with a limbic decline in 5-HT1A receptor binding and functional ACC/mPFC dissociation. These changes support the hypothesis of impaired top-down regulation of stressful stimuli and indicate potential targets for early treatment. PET has the potential to detect the detailed mechanisms of increased brain activity associated with burnout.
4. Computational Models of Burnout
There is no doubt that research into computational models of the central nervous system, including its diseases, is an important part of the current challenges in both computer science and biomedical engineering in medical science and clinical practice. Despite the validity of the problem of burnout and the availability of computational tools, computational approaches to the analysis and classification of burnout have started to be developed relatively recently [35,36]. The results of research to date are not satisfactory, and it is difficult to fully explain all the physiological and pathological mechanisms underlying burnout. This paper is one of the first to combine a review of neuroimaging studies that address burnout syndrome, with the proposal of fuzzy-based modeling of the selected aforementioned processes. Currently, there are no computational burnout models of satisfactory efficiency and accuracy. Hence, any new concept in this area, even a rather general one, is worth giving a chance, as attempts to construct such computational models to date have not ultimately been successful.
4.1. Fuzziness of Burnout
It seems that the fuzzy nature of well-being and occupational burnout was finally confirmed in the publications by Maija et al. and Dong et al. [37,38].
With the present state of knowledge, experience and clinical technique, a large part of clinical decisions can be based on the traditional deterministic approach (usuallydifferential diagnosis).In the case of well-being and burnout, the required approach is more complex. In these cases, it is also necessary to take into account the linguistic character of the description and numerous sources of uncertainty, including the interview with the patient and his self-esteem. Traditional factors of well-being and occupational stress overlap with numerous stressors, not only occupational but also related to pandemic, political, and economic crises. Such a complex approach to occupational stress analysis is unique and makes our computational approach unique.
4.2. Current Fuzzy Models of Burnout
Describing the brain through cognitive structures is one of the approaches/parts of neuroimaging [39]. It is based on a structure divided into individual functions. In this capacity, fuzzy logic opens up new possibilities.A fuzzy approach to the computational classification of occupational burnout was shown in the work of Prokopowicz et al. [40,41]. ANN-based predictive analysis of burnout, resilience, and COVID-19 risk among teachers was proposed by Martínez-Ramón et al. [42]. Blain et al. described the neuro-computational mechanism playing a key role in overtraining syndrome as a form of burnout, defined in endurance athletes [43]. Effective computational models of burnout are possible but require further development to ensure accuracy across different populations. Potential applications of the work concern inference systems and computational predictive models of job burnout, effective as second opinion systems. Fuzzy logic allows us to define models on the basis of incomplete and inaccurate knowledge, where a typical mathematical model has not been recognized. This has interesting potential of using them in the computational brain models in the area of theoretical and experimental computational neuroscience. This also applies to clinical applications.Details are shown in Figure 2a–d. Figure 2a presents a conceptual view of the model based on a previous review of literature. Figure 2b shows a traditional own model based on layers of point neurons (Emergent software) or compartmental neurons (Neuron or Genesis software). Our own approach based on fuzzy logic is presented in Figure 2c (single fuzzy inference block—FIB) and cascade of FIBs (Figure 2d). The fuzzy approach is original, partly based on our earlier concept published in [44].
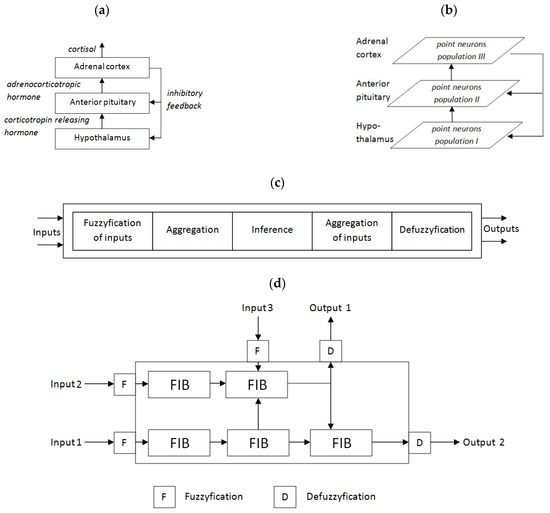
Figure 2.
An example of the possible use of fuzzy systems in the computational model of the HPAaxis (own concept): (a) hypothalamic–pituitary–adrenal (HPA) axis, (b) traditional model based on point or compartmental neurons, (c) single fuzzy inference block (FIB), (d) complex fuzzy cascade model (CFCM)—own approach based partly on our previous own concept [44].
The model presented in Figure 3 tries to reflect the structures that are responsible for the burnout phenomena shown in Figure 2a. A hierarchical cascade of fuzzy logic systems can provide descriptive insight into the behavior of a particular patient. It is connected with the linguistic nature of rules interpretation in the fuzzy system. Mechanisms built into the model allow for easier configuration and the use of complex sets of descriptive features. The complex structures of the model are based on simple elements called fuzzy inference blocks (FIBs) that provide intrinsic fuzzification and defuzzification (Figure 2b). More complicated structures and dependencies are modeled by cascade of FIBs called the complex fuzzy cascade model (CFCM) [41]. The most complex processes and structures can be modeled using cascades of CFCMs (Figure 3). Despite the relatively high complexity of the models, we can model these phenomena in a fuzzy manner.
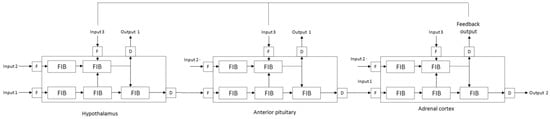
Figure 3.
Computational fuzzy-based model of the HPA axis (own concept).
The aforementioned models, despite fuzziness, can be more accurate in modeling complex phenomenon such as burnout, especially at thesystem level, where a single element of the circuit may not be clearly visible, but its internal structure should be ready to explore or adjust. This way, complex behavior emerges from synergy of the many simple elements (agents).
4.3. Further Computational Studies
Being innovative and more effective, a fuzzy approach can provide a better, clearer, and more understandable explanation processes underlying the emergence of stress and occupational burnout, giving birth to normal brain functioning as a result of stressors of varying intensity. The proposed fuzzy approach, once refined, may not only provide the basis for the whole family of innovative methods that effectively and reliably reflect the processes taking place in the median nervous system during stress and occupational burnout but also make a significant progress in knowledge affecting a number of basic and applied sciences. The multi-agent system as a model of the central nervous system is in some ways consistent with the above-mentioned concept of our related mini fuzzy systems. In such a structure, we can assume that each FIB represents one agent with a multi-agent architecture.
5. Discussion
The mechanisms of burnout are still poorly understood, especially in neuroimaging studies. The relationship between its neuronal substrate components and cognitive performance may be more complex than previously thought [45]. Results from neuroimaging studies indicate that the brain structures of patients with burnout that are related to emotion, motivation, and empathy differ significantly from the same structures in healthy control subjects. These brain structures include the thalamus, hippocampus, amygdala, caudate, striatum, dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), anterior cerebral hemisphere (AI), inferior frontal gyrus (IFG), middle frontal gyrus (MFG), temporoparietal junction (TPJ), and grey matter (GM). The ACC is also known to show alterations on PET imaging in burnout patients [10], as are the insular cortex and hippocampus. According to two recent cross-sectional meta-analyses, both AI and ACC are involved in empathy [43,44]. Several behavioral studies indicate a strong association between job burnout and empathy [46,47,48,49,50,51,52]. Interestingly, there are two conflicting theories regarding job burnout and empathy:
- the conventional compassion fatigue theory which maintains that occupational burnout is associated with excessive empathy [53];
- which maintains that occupational burnout is associated with reduced emotional regulation, which causes a disconnect between felt and expressed emotions [54].
Preliminary studies have shown that a model of empathy is emerging in which connections between mirror neurons and other brain structures may facilitate the observed components of empathy [55,56]. Thus, on the basis of the results of the aforementioned studies, it can be postulated that there is a direct link between empathy and mirror neurons, as lack of empathy is one of the sub-elements of burnout syndrome. Further research should be conducted to verify this hypothesis, with the ultimate goal being the development of a biomarker to diagnose occupational burnout.
Brain structures such as the amygdala, hippocampus, caudate, and putamen are different in burnout individuals due to the nature of the neurophysiological functions of these structures. The amygdala and hippocampus are part of the limbic system, which plays an important role in memory, emotion, emotional learning and behavior, motivation, and reward. A theory can be formulated about burnout syndrome in relation to the limbic system and its structures: it has a direct impact on all three main dimensions of burnout syndrome: emotional exhaustion, depersonalization, and lack of personal achievement. The striatum functions as an input nucleus to the basal ganglia—its main function is to receive excitatory input from cortical and subcortical structures. Input to the striatum allows the basal ganglia to integrate information from different cortical and subcortical areas and allows the basal ganglia to initiate our body movements and, also importantly, the motor expression of our emotions [57]. This provides a basis for further research in this area in the search for information on the correlation between the caudate ganglion, putamen, and occupational burnout.
EEG studies in patients with burnout have found reduced P300 amplitude, lower peak alpha frequency, and reduced beta power [1], with power being mainly associated with burnout, but with gender being a significant predictor [17]. Neuronal generators of P300 are mainly cortical (medial temporal area, superior temporal area, and inferior parietal area), but can be located in the hippocampus and amygdala [58]. As the hippocampus is part of the limbic system, it plays a role in visceral and endocrine functioning, as well as in the expression of emotions and emotional behavior [57]. We know that occupational burnout only develops after prolonged stress, which again is associated with hippocampal dysfunction, and reduced P300 amplitude may be a result of changes in the hippocampus, in which chronic elevated HPA axis activity may be involved [59]. P300 is commonly associated with memory updating and attentional allocation [49,60]. Reduced P300 amplitude may also be seen as a contributing factor to or as objective physiological confirmation of attention and memory problems in patients with burnout [51]. It should be noted here that alpha power can be quantified in two ways: peak alpha frequency (PAF) and individual alpha frequency (IAF). The IAF is considered a more accurate measure of the spectral distribution than the PAF. IAF is assumed to be related to features of white matter structure, such as fiber density, axon diameter, and myelination, and reflects different neuronal processes other than alpha power [52]. PAF correlates negatively with subjective ratings of “fatigue today” and “total fatigue”. PAF has been shown to be consistently associated with reduced cerebral blood flow and reduced cerebral oxygenation [61]. PAF has also been proposed as an indicator of “cognitive readiness” [62], as reduced PAF is associated with poorer performance on memory tasks [63]. Interestingly, the reported daytime fatigue and mild cognitive changes in patients with CSF may be explained by poor basal sleep drive [64]. Thus, classifying alpha power as a biomarker of burnout may be true as it is non-invasive, reliable, has trait-like characteristics, and is heritable. However, the specificity of alpha power, which is necessary to distinguish burnout from other disorders, is lacking. The P300 seems reasonable as a second indicator that can be added to diagnostic specificity and can help discriminate between psychopathologies.
The goal of discovering and understanding the brain structures and neuronal networks relevant to burnout is related to the work construct. The discovery and understanding of the above mechanisms bring the prevention and treatment of burnout closer. Further research is needed to confirm the findings described above and help develop interventions and strategies to help those suffering from job burnout. This is of great scientific, clinical, social, and economic importance, as job burnout can significantly reduce the ability to perform work tasks [65]. Work-related fatigue is common among workers, such as those in car factories [66]. Serious illnesses (stroke, neuromuscular diseases) and injuries can affect job burnout and the ability to be employed and work, but knowledge of individual factors is limited [67,68]. Further diagnostic methods need to be linked to being an employee. The phenomenon of job burnout in remote working, which is becoming increasingly common during the COVID-19 pandemic period, also requires research.
Previous research has focused on the relationships between stress, burnout, and biomarkers, while the review by Ciobanu et al. [69] summarized the immune and endocrine changes found in patients with burnout. The main interest in the literature seems to be the hypothalamic–pituitary–adrenal (HPA) axis, which can be disturbed by chronic stress and can be tested by measuring hormonal reactivity:
- corticotropin-releasing hormone (CRH);
- adrenocorticotropic hormone (ACTH);
- cortisol;
- prolactin;
- thyroid hormones;
in the conditions of their pulsatile and diurnal fluctuation and heterogeneity of burnout measures [69,70]. BDNF, the plasma level of which is higher in people with chronic stress, may play a key role in the mechanisms of stress-related disorders, and increasing its peripheral levels may contribute to the protection of neurons under stress [71]. Results of demand modeling and labor resources using structural equations with least squares estimation weighted with mean and variance showed that such a model was well suited to the data and that occupational burnout had a statistically significant negative structural relationship with work efficiency, but work efficiency did not explain statistically significant variance of neither psychological stress nor intention of rotation apart from occupational burnout. Thus, defining work efficiency as a result of occupational burnout in the process of employee handicap in the model of requirements and labor resources may not be useful [72].
The problem lies also in the early diagnosis of burnout. It has been observed that more complex tests may be more sensitive in detecting cognitive dysfunctions in non-clinical burnout. Moreover, a relationship was found between the performance of two tasks and the performance of work, and insomnia was associated with subjective cognitive functioning, but not with the performance of work [73].
In conclusion, thusfar, six biological areas for the basis of individual differences in stress resistance (i.e., the ability to adapt successfully to stressful work events) were identified:
- The influence of the immune system on stress sensitivity [74];
- The role of norepinephrine and serotonin [3];
- The role of selected neuropeptides in the inhibitory mechanism of the impact of stress [75];
- The role of the hypothalamic–pituitary–adrenal axis in influencing stress susceptibility and resistance to stress [76];
- The role of epigenetic modulation of gene expression in influencing stress susceptibility [77];
- Neurobiological mechanisms by which factors such as diet, exercise, and peer support influence stress resistance [78].
They all fit into the directions of our further research described below.
5.1. Directions for Future Research
As part of research on the above factors and mechanisms for clinical practice, it is crucial to identify modifiable factors and help to develop/modify effective strategies to prevent stress and burnout—including increasing resilience both among individuals and, for example, professional groups [78]. Possible interventions include dietary changes, lifestyle modifications, work environment modifications, psychosocial interventions, pharmacological therapies, and others [78,79].
Computational models, including those based on fuzzy numbers, extend this perspective with new possibilities, including the simultaneous and multi-level interaction of many factors within hierarchical models. It becomes possible to take into account non-contingencies that reflect not only gaps in knowledge or hypothetical mechanisms, but also the individual variability of the nervous system, both healthy and pathologically functioning. Further selection of model parameters aimed at increasing their reliability is an important direction of further research on computational models of severe stress and occupational burnout. Reflecting the continuity of disease processes in the computational model will show the successive phases of pathology growth in an apparently healthy organism.
This discussion also addresses fuzzy variants of analysis, reasoning, and prediction from data. As an alternative to the neuroimaging approach, the paper presents a late proposal of the PLUS (personal living usual satisfaction) parameter. It is based on a fuzzy model, wherein the data source is psychological factors—the same or similar to the neuroimaging approach [40,51]. It is believed that there is a common denominator because both neuroimaging and our PLUS are based on MBI, and thusboth in the layer of neural changes and the test results that reflect them, there is room for connection and consideration. There must be consistency in the results achieved with the different methods, techniques, and tools at the different levels of clinical analysis. Computational solutions are becoming the same clinician’s tool as neuroimaging studies or patient functioning tests.
The directions for further research also include novel methods, in particular, randomized controlled clinical trials (RCTs) in larger samples, using concurrent EEG/fMRI or similar studies as well as data-driven analysis to assess the correlation of burnout. The findings thusfar still require additional research to verify their clinical significance. Introducing a more objective assessment in the form of standardized criteria for interpreting and reporting the above data can help predict burnout risk;significantly reduce unnecessary surgeries;allow planning of preventive strategies;and, if necessary, provide the timely delivery of individualized treatment to at-risk patients. Research can be accelerated thanks to the application of new solutions of computational models of cognitive deficits [80,81]. Cognitive deficits, depression, and anxiety appear to be common in burnout, and family support is essential for both mental health and cognitive functioning [47,69].
5.2. Limitations of Own Studies
Most of the work in the review was producedbefore the COVID-19 pandemic and the war in Ukraine. Thus, it should be noted that the expected number of stressors may increase. We have had the opportunity to observe this recently: the COVID-19 pandemic, the war in Ukraine, and the energy and economic crisis are groups of stressors that overlap with each other and with work-related stress, especially in groups of medical specialists. Computational models, thanks to prediction, will allow not only for the prediction but also the assessment of the effectiveness of various strategies for the prevention of stressors of particular types. This will support and complement the existing tools at the disposal of medical specialists, providingthem with new opportunities to help patients.
Resolution of neuroimaging techniques constitute a limitation on the reported results. It must be taken into account that the neuroimaging methods, despite their high spatial resolution (e.g., one voxel in fMRI has a typical resolution 3.4 × 3.4 × 4 mm) vs. low spatial resolution of EEG usually have a low temporal resolution (fMRI 1–4 s, while EEG 1–10 ms). Hence, simultaneous fMRI + EEG studies would be better.Moreover, in the samples studied, an imbalance in gender wasobserved.
To sum up, the limitations of the current studies concern both the number of relevant studies that meet the requirements of the evidence-based medicine (EBM) paradigmand methodological issues (small sample size, sample recruitment—convenience sample vs. randomized, no standardization of data analysis, the need for interval studies, etc.). Such a small amount of data hardly meet the requirements of computational models, although for several years, machine learning methods for small datasets (20 < N < 100) have appeared. One should also bear in mind the limitations of the applied neuroimaging techniques: limited spatial resolution of EEG (despite attempts to compute localization of diffuse EEG sources) and limited temporal resolution of fMRI. Therefore, innovative suggestions such as simultaneous EEG/fMRI should be used. Connectomics (comprehensive map(s) of connections within the nervous system) may offer more advanced tools in the future [82,83,84,85,86], but thusfar, scarce publications have been observed on connectionism in occupational burnout [24,81]. For big data approaches dealing with a specific classification (e.g., healthy traits vs. disease symptoms), a convolutional neural network can create new solutions [87,88].
6. Conclusions
More research is still needed to understand the mechanisms observed in the neural correlates of burnout syndrome, including through artificial intelligence. In our review, only eleven studies met the inclusion criteria. For the aforementioned reasons, more studies should be conducted to replicate and confirm previous findings. The data summarized and analyzed suggest that burnout syndrome is strongly associated with changes in brain structures, particularly in regions responsible for emotion, motivation, and stress such as the HPA axis.
Neuroimaging techniques give us an advantage over traditional subjective methods (self-report, interview) by providing standardized, objective measurements of brain structures. With this approach, specific brain structures involved in the pathogenesis of burnout and providingthe earliest symptoms can be isolated. This will increase the overall understanding and knowledge of burnout, allowing for more effective approaches in prevention, diagnosis, and therapy.
The computational models of job burnout analyzed in this article will help to develop the foundations of computational psychiatry and computational psychology, supporting professionals from the fields of psychiatry and psychology, but also in occupational medicine, in their daily efforts to reduce the symptoms of job burnout.
As the novel approach to searching for neural burnout mechanisms, we showed that both our PLUS models as far as computational models, including those based on fuzzy logic and artificial neural networks, can play an important role in inferring and predicting burnout. There is also a need to identify mechanisms and clinical indicators of chronic fatigue syndrome, work-related stress, job burnout, and natural cognitive changes associated with, for example, ageing, in order to introduce more effective differential diagnosis and screening.
Author Contributions
Conceptualization, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; methodology, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; software, P.P. and D.M.; validation, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; formal analysis, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; investigation, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; data curation, P.P. and D.M.; writing—original draft preparation, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; writing—review and editing, E.M., P.P., Y.C., J.M., D.M., G.M.W., B.W., A.R.E. and M.O.; supervision, E.M., P.P., J.M. and G.M.W.; project administration, E.M., P.P., J.M. and G.M.W.; funding acquisition, E.M. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luijtelaar, G.V.; Verbraak, M.; Bunt, M.V.; Keijsers, G.; Arns, M. EEG findings in burnout patients. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Plieger, T.; Melchers, M.; Montag, C.; Meermann, R.; Reuter, M. Life stress as potential risk factor for depression and burnout. Burn. Res. 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Shanafelt, T. Physician distress and burnout: The neurobiological perspective. Mayo Clin. Proc. 2021, 96, 763–769. [Google Scholar] [CrossRef]
- American Medical Association. International Classification of Diseases. In Clinical Modification(ICD-10-CM) 2022: The Complete Official Codebook with Guidelines, 10th ed.; American Medical Association: Chicago, IL, USA, 2021. [Google Scholar]
- World Health Organization. International Classification of Diseases, 11th ed.; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; DSM-5; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Al-Haddad, A.; Al-Omar, F.; Al-Khaleel, A.; Al-Khalaf, A. Prevalence of burnout syndrome and its related risk factors among physicians working in primary health care centers of the Ministry of Health, Al Ahsa Region, Saudi Arabia, 2018–2019. J. Fam. Med. Prim. Care 2020, 9, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Messias, E.; Gathright, M.M.; Freeman, E.S.; Flynn, V.; Atkinson, T.; Thrush, C.R.; Clardy, J.A.; Thapa, P. Differences in burnout prevalence between clinical professionals and biomedical scientists in an academic medical centre: A cross-sectional survey. BMJ Open 2019, 9, e023506. [Google Scholar] [CrossRef] [PubMed]
- Rotenstein, L.S.; Torre, M.; Ramos, M.A.; Rosales, R.C.; Guille, C.; Sen, S.; Mata, D.A. Prevalence of burnout among physicians: A systematic review. JAMA 2018, 320, 1131–1150. [Google Scholar] [CrossRef]
- Roberts, B.L.; Karatsoreos, I.N. Brain-body responses to chronic stress: A brief review. Fac. Rev. 2021, 10, 83. [Google Scholar] [CrossRef]
- Markram, H. Seven challenges for neuroscience. Funct. Neurol. 2013, 28, 145–151. [Google Scholar] [CrossRef]
- Frégnac, Y. How blue is the sky? eNeuro 2021, 8, ENEURO.0130-21.2021. [Google Scholar] [CrossRef]
- Boegl, K.; Adlassnig, K.-P.; Hayashi, Y.; Rothenfluh, T.E.; Leitich, H. Knowledge acquisition in the fuzzy knowledge representation framework of a medical consultation system. Artif. Intell. Med. 2004, 30, 1–26. [Google Scholar] [CrossRef]
- Torres, A.; Nineto, J.J. Fuzzy logic in medicine and bioinformatics. BioMed Res. Int. 2006, 2006, 091908. [Google Scholar] [CrossRef]
- Buckley, J.J.; Feuring, T.; Hayashi, Y. Fuzzy hierarchical analysis revisited. Eur. J. Oper. Res. 2001, 121, 48–64. [Google Scholar] [CrossRef]
- Staszecka, E. Combining uncertainty and imprecision in models of medical diagnosis. Inf. Sci. 2006, 176, 3026–3059. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Tement, S.; Pahor, A.; Jaušovec, N. EEG alpha frequency correlates of burnout and depression: The role of gender. Biol. Psychol. 2016, 114, 1–12. [Google Scholar] [CrossRef]
- Golonka, K.; Gawlowska, M.; Mojsa-Kaja, J.; Marek, T. Psychophysiological characteristics of burnout syndrome: Resting-state EEG analysis. Biomed. Res. Int. 2019, 2019, 3764354. [Google Scholar] [CrossRef] [PubMed]
- Yakovenko, E.A.; Rem, A.V.; Surushkina, S.Y.; Chutko, L.S. Electroencephalographic signs of emotional burnout syndrome. Neurosci.Behav. Phys. 2021, 51, 155–157. [Google Scholar] [CrossRef]
- Durning, S.J.; Costanzo, M.; Artino, A.R.; Dyrbye, L.N.; Beckman, T.J.; Schuwirth, L.; Holmboe, E.; Roy, M.J.; Wittich, C.M.; Lipner, R.S.; et al. Functional neuroimaging correlates of burnout among internal medicine residents and faculty members. Front. Psychiatry 2013, 4, 131. [Google Scholar] [CrossRef] [PubMed]
- Savic, I. Structural changes of the brain in relation to occupational stress. Cereb Cortex 2013, 25, 1554–1564. [Google Scholar] [CrossRef] [PubMed]
- Blix, E.; Perski, A.; Berglund, H.; Savic, I. Long-term occupational stress is associated with regional reductions in brain tissue volumes. PLoS ONE 2013, 8, e64065. [Google Scholar] [CrossRef]
- Tei, S.; Becker, C.; Kawada, R.; Fujino, J.; Jankowski, K.F.; Sugihara, G.; Murai, T.; Takahashi, H. Can we predict burnout severity from empathy-related brain activity? Transl. Psychiatry 2014, 4, e393. [Google Scholar] [CrossRef]
- Savic, I.; Perski, A.; Osika, W. MRI shows that exhaustion syndrome due to chronic occupational stress is associated with partially reversible cerebral changes. Cereb Cortex 2018, 28, 894–906. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Neely, A.S.; Andersson, M.; Eskilsson, T.; Järvholm, L.S.; Boraxbekk, C.J. Neural activation in stress-related exhaustion: Cross-sectional observations and interventional effects. Psychiatry Res. Neuroimaging 2017, 269, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, H.; Perski, A.; Berglund, H.; Savic, I. Chronic stress is linked to 5-HT1A receptor changes and functional disintegration of the limbic networks. NeuroImage 2011, 55, 1178–1188. [Google Scholar] [CrossRef] [PubMed]
- Maslach, C.; Jackson, S.E. The measurement of experienced burnout. J. Occup. Behav. 1981, 2, 99–113. [Google Scholar] [CrossRef]
- Maslach, C.; Leiter, M.P.; Jackson, S.E. Making a significant difference with burnout interventions: Researcher and practitioner collaboration. J. Organ. Behav. 2012, 33, 296–300. [Google Scholar] [CrossRef]
- Davis, M.H. A multidimensional approach to individual differences in empathy. JSAS Cat. Sel. Doc. Psychol. 1980, 10, 85. [Google Scholar]
- Lundgren-Nilsson, Å.; Jonsdottir, I.H.; Pallant, J. Internal construct validity of the Shirom-Melamed Burnout Questionnaire (SMBQ). BMC Public Health 2012, 12, 1. [Google Scholar] [CrossRef]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef]
- Bherer, L. Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Ann. N. Y. Acad. Sci. 2015, 1337, 1–6. [Google Scholar] [CrossRef]
- Li, B.Y.; Tang, H.D.; Qiao, Y.; Chen, S.D. Mental training for cognitive improvement in elderly people: What have we learned from clinical and neurophysiologic studies? Curr. Alzheimer Res. 2015, 12, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Dutra, H.S.; Guirardello, E.B.; Li, Y.; Cimiotti, J.P. Nurse burnout revisited: A comparison of computational methods. J. Nurs. Meas. 2019, 27, E17–E33. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.S.; Liu, H.; Warner, B.C.; Harford, D.; Lu, C.; Kannampallil, T. Predicting physician burnout using clinical activity logs: Model performance and lessons learned. J. Biomed. Inform. 2022, 127, 104015. [Google Scholar] [CrossRef] [PubMed]
- Maija, K.; Katri, K. The moral orders of work and health: A case of sick leave due to burnout. Sociol. Health Illn. 2019, 41, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yan, S. A multicriteria approach for measuring employee well-being. Front. Psychol. 2022, 13, 795960. [Google Scholar] [CrossRef] [PubMed]
- MacCormack, J.K.; Stein, A.G.; Kang, J.; Giovanello, K.S.; Satpute, A.B.; Lindquist, K.A. Affect in the aging brain: A neuroimaging meta-analysis of older vs. younger adult affective experience and perception. Affect. Sci. 2020, 1, 128–154. [Google Scholar] [CrossRef]
- Prokopowicz, P.; Mikołajewski, D. Fuzzy approach to computational classification of burnout—Preliminary findings. Appl. Sci. 2022, 12, 3767. [Google Scholar] [CrossRef]
- Mikołajewski, D.; Prokopowicz, P. Effect of COVID-19 on selected characteristics of life satisfaction reflected in a fuzzy model. Appl. Sci. 2022, 12, 7376. [Google Scholar] [CrossRef]
- Martínez-Ramón, J.P.; Morales-Rodríguez, F.M.; Pérez-López, S. Burnout, resilience, and COVID-19 among teachers: Predictive capacity of an artificial neural network. Appl. Sci. 2021, 11, 8206. [Google Scholar] [CrossRef]
- Gavelin, H.M.; Neely, A.S.; Dunås, T.; Eskilsson, T.; Järvholm, L.S.; Boraxbekk, C.J. Mental fatigue in stress-related exhaustion disorder: Structural brain correlates, clinical characteristics and relations with cognitive functioning. Neuroimage Clin. 2020, 27, 102337. [Google Scholar] [CrossRef]
- Prokopowicz, P.; Mikołajewski, D. Fuzzy-based computational simulations of brain functions—Preliminary concept. Bio-Algorithms Med. Syst. 2016, 12, 99–104. [Google Scholar] [CrossRef]
- Bellucci, G.; Camilleri, J.A.; Eickhoff, S.B.; Krueger, F. Neural signatures of prosocial behaviors. Neurosci. Biobehav. Rev. 2020, 118, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Mercer Lindsay, N.; Chen, C.; Gilam, G.; Mackey, S.; Scherrer, G. Brain circuits for pain and its treatment. Sci. Transl. Med. 2021, 13, eabj7360. [Google Scholar] [CrossRef] [PubMed]
- De Hert, S. Burnout in healthcare workers: Prevalence, impact and preventative strategies. Local Reg. Anesthesia 2020, 13, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Weisbaum, E.; Chadi, N. Applied mindfulness for physician wellbeing: A prospective qualitative study protocol. Front. Public Health 2022, 10, 807792. [Google Scholar] [CrossRef]
- Laughey, W.F.; Atkinson, J.; Craig, A.M.; Douglas, L.; Brown, M.E.; Scott, J.L.; Alberti, H.; Finn, G.M. Empathy in medical education: Its nature and nurture—A qualitative study of the views of students and tutors. Med. Sci. Educ. 2021, 31, 1941–1950. [Google Scholar] [CrossRef]
- Paiva-Salisbury, M.L.; Schwanz, K.A. Building compassion fatigue resilience: Awareness, prevention, and intervention for pre-professionals and current practitioners. J. Health Serv. Psychol. 2022, 3, 39–46. [Google Scholar] [CrossRef]
- Lu, L.; Ko, Y.M.; Chen, H.Y.; Chueh, J.W.; Chen, P.Y.; Cooper, C.L. Patient safety and staff well-being: Organizational culture as a resource. Int. J. Environ. Res. Public Health 2022, 19, 3722. [Google Scholar] [CrossRef]
- Krebs, C.; Akesson, E.J.; Weinberg, J. Lippincott’s Illustrated Review of Neuroscience; Wolters Kluwer/Lippincott Williams & Wilkins Health: Amsterdam, The Netherlands, 2012. [Google Scholar]
- De Koninck, B.P.; Guay, S.; Blais, H.; De Beaumont, L. Parametric study of transcranial alternating current stimulation for brain alpha power modulation. Brain Commun. 2021, 3, fcab010. [Google Scholar] [CrossRef]
- Rosenzopf, H.; Wiesen, D.; Basilakos, A.; Yourganov, G.; Bonilha, L.; Rorden, C.; Fridriksson, J.; Karnath, H.O.; Sperber, C. Mapping the human praxis network: An investigation of white matter disconnection in limb apraxia of gesture production. Brain Commun. 2022, 4, fcac004. [Google Scholar] [CrossRef]
- Kelly, R.J.; Hearld, L.R. Burnout and leadership style in behavioral health care: A literature review. J. Behav. Health Serv. Res. 2020, 47, 581–600. [Google Scholar] [CrossRef] [PubMed]
- Lamm, C.; Majdandžić, J. The role of shared neural activations, mirror neurons, and morality in empathy—A critical comment. Neurosci. Res. 2015, 90, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, N.; Nagamine, T.; Fujiwara, N.; Yazawa, S.; Shibasaki, H. Cortical-hippocampal auditory processing identified by magnetoencephalography. J. Cogn. Neurosci. 1998, 10, 231–247. [Google Scholar] [CrossRef]
- Montoro, C.I.; Winterholler, C.; Terrasa, J.L.; Montoya, P. Somatosensory gating is modulated by anodal transcranial direct current stimulation. Front. Neurosci. 2021, 15, 651253. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, Y.; Shiner, T.; Bregman, N.; Fahoum, F.; Giladi, N.; Maidan, I.; Mirelman, A. Event-related oscillations differentiate between cognitive, motor and visual impairments. J. Neurol. 2022, 269, 3529–3540. [Google Scholar] [CrossRef] [PubMed]
- Duma, G.M.; Di Bono, M.G.; Mento, G. Grounding adaptive cognitive control in the intrinsic, functional brain organization: An HD-EEG resting state investigation. Brain Sci. 2021, 11, 1513. [Google Scholar] [CrossRef]
- Cannard, C.; Wahbeh, H.; Delorme, A. Electroencephalography correlates of well-being using a low-cost wearable system. Front. Hum. Neurosci. 2021, 15, 745135. [Google Scholar] [CrossRef]
- Millard, S.K.; Bokelmann, K.; Schalbroeck, R.; van der Wee, N.J.A.; van Loey, N.E.E.; van Laarhoven, A.I.M. No indications for altered EEG oscillatory activity in patients with chronic post-burn itch compared to healthy controls. Sci. Rep. 2022, 12, 5184. [Google Scholar] [CrossRef]
- Maksoud, R.; Eaton-Fitch, N.; Matula, M.; Cabanas, H.; Staines, D.; Marshall-Gradisnik, S. Systematic review of sleep characteristics in myalgic encephalomyelitis/chronic fatigue syndrome. Healthcare 2021, 9, 568. [Google Scholar] [CrossRef]
- Hong, Q.N.; Coutu, M.F.; Berbiche, D. Evaluating the validity of the Work Role Functioning Questionnaire (Canadian French version) using classical test theory and item response theory. Work 2017, 57, 501–515. [Google Scholar] [CrossRef]
- Guo, F.; Wang, T.; Ning, Z. Subjective measures of work-related fatigue in automobile factory employees. Work 2017, 58, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Norstedt, M. The (im)possibilities of returning to work after a stroke. Work 2017, 56, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Lexell, E.M.; Langdell, I.; Lexell, J. Vocational situation and experiences from the work environment among individuals with neuromuscular diseases. Work 2017, 56, 519–530. [Google Scholar] [CrossRef]
- Golkar, A.; Johansson, E.; Kasahara, M.; Osika, W.; Perski, A.; Savic, I. The influence of work-related chronic stress on the regulation of emotion and on functional connectivity in the brain. PLoS ONE 2014, 9, e104550. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.M.; Damian, A.C.; Neagu, C. Association between burnout and immunological and endocrine alterations. ROM J. Morphol. Embryol. 2021, 62, 13–18. [Google Scholar] [CrossRef]
- Buselli, R.; Veltri, A.; Baldanzi, S.; Marino, R.; Bonotti, A.; Chiumiento, M.; Girardi, M.; Pellegrini, L.; Guglielmi, G.; Dell’Osso, L.; et al. Plasma Brain-Derived Neurotrophic Factor (BDNF) and serum cortisol levels in a sample of workers exposed to occupational stress and suffering from adjustment disorders. Brain Behav. 2019, 9, e01298. [Google Scholar] [CrossRef]
- de Beer, L.T. Is there utility in specifying professional efficacy as an outcome of burnout in the employee health impairment process. Int. J. Environ. Res. Public Health 2021, 18, 6255. [Google Scholar] [CrossRef]
- Hoogduin, C.A.L.; Schaap, C.P.D.R.; Methorst, G.J. Burnout: Klinisch Beeld en Diagnostiek. In Behandelingsstrategieën in Bij Burnout, 2nd ed.; Hoogduin, C.A.L., Schaap, C.P.D.R., Kladler, A.J., Eds.; Houten/Diegem, Bohn Stafleu van Loghum: Houten, The Netherlands, 2001. [Google Scholar]
- Rajkumar, R.P. Harnessing the neurobiology of resilience to protect the mental well-being of healthcare workers during the COVID-19 pandemic. Front. Psychol. 2021, 12, 621853. [Google Scholar] [CrossRef]
- Verhaeghe, J.; Van Den Eede, F.; Van Den Ameele, H.; Sabbe, B.G. Neuro-endocrine correlates of burnout. Tijdschr. Psychiatry 2012, 54, 517–526. [Google Scholar]
- Lehmann, M.J.; Lormes, W.; Opitz-Gress, A.; Steinacker, J.M.; Netzer, N.; Foster, C.; Gastmann, U. Training and overtraining: An overview and experimental results in endurance sports. J. Sports Med. Phys. Fit. 1997, 37, 7–17. [Google Scholar]
- Leistner, C.; Menke, A. Hypothalamic-pituitary-adrenal axis and stress. Handb. Clin. Neurol. 2020, 175, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Weisel, F.; Shlomchik, M. Memory B cells of mice and humans. Annu. Rev. Immunol. 2017, 35, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Romito, B.T.; Okoro, E.N.; Ringqvist, J.R.B.; Goff, K.L. Burnout and wellness: The anesthesiologist’s perspective. Am. J. Lifestyle Med. 2020, 15, 118–125. [Google Scholar] [CrossRef]
- Wojcik, G.M.; Masiak, J.; Kawiak, A.; Kwasniewicz, L.; Schneider, P.; Postepski, F.; Gajos-Balinska, A. Analysis of decision-making process using methods of quantitative electroencephalography and machine learning tools. Front. Neuroinform. 2019, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, G.M.; Masiak, J.; Kawiak, A.; Kwasniewicz, L.; Schneider, P.; Polak, N.; Gajos-Balinska, A. Mapping the human brain in frequency band analysis of brain cortex electroencephalographic activity for selected psychiatric disorders. Front. Neuroinform. 2018, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.; Masiak, J.; Mikołajewska, E.; Mikołajewski, D.; Wójcik, G.M.; Wallace, B.; Eugene, A.; Olajossy, M. Limbic brain structures and burnout—A systematic review. Adv. Med. Sci. 2018, 63, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Koutsimani, P.; Montgomery, A.; Masoura, E.; Panagopoulou, E. Burnout and cognitive performance. Int. J. Environ. Res. Public Health 2021, 18, 2145. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, D.M.; van Rhenen, W.; Murre, J.M.J.; Verwijk, E. Cognitive functioning, sleep quality, and work performance in non-clinical burnout: The role of working memory. PLoS ONE 2020, 15, e0231906. [Google Scholar] [CrossRef]
- Guggisberg, A.G.; Koch, P.J.; Hummel, F.C.; Buetefisch, C.M. Brain networks and their relevance for stroke rehabilitation. Clin. Neurophysiol. 2019, 130, 1098–1124. [Google Scholar] [CrossRef]
- Miyawaki, A. Brain clearing for connectomics. Microscopy 2015, 64, 5–8. [Google Scholar] [CrossRef]
- Middlebrooks, E.H.; Grewal, S.S. Brain connectomics. Neuroimaging Clin. N. Am. 2022, 32, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.S.; Ahmad, I.; Bin, L.; Khan, S.; Rodrigues, J.J.P.C. Deep learning recognition of diseased and normal cell representation. Trans. Emerg. Telecommun. Technol. 2020, 42, e4017. [Google Scholar] [CrossRef]
- Iqbal, M.S.; El-Ashram, S.; Hussain, S.; Khan, T.; Huang, S.; Mehmood, R.; Luo, B. Efficient cell classification of mitochondrial images by using deep learning. J. Opt. 2019, 48, 113–122. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).