A Low-Temperature and Low-Pressure Distillation Plant for Dairy Wastewater
Abstract
Featured Application
Abstract
1. Introduction
- The degree of purification achievable on the polluting load;
- The possible recovery of valuable products (as whey proteins have excellent functional properties, they are widely utilized in the food industry);
- The overall energetic cost of the treatment per unit of raw, processed product.
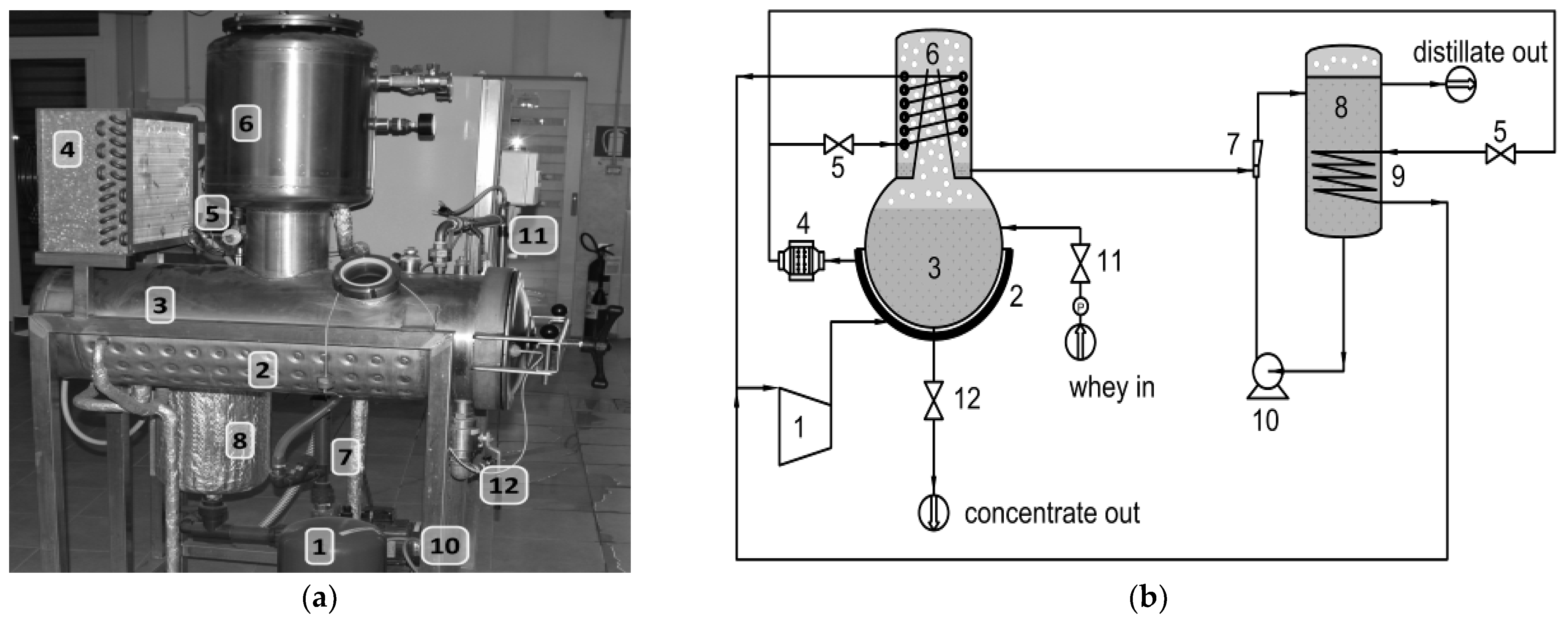
2. Materials and Methods
- At the tank of influent flow, where the raw whey resided;
- At the outlet of the distillate tank, where the effluent distillate resided;
- At the vacuum chamber, where the concentrate resided (at the end of the run).
3. Results and Discussion
3.1. Concentrate
3.2. Distillate (Evaporate)
- The amount of pollutant in the starting raw whey;
- The volume of the treated raw whey;
- The pollutant in the distilled outflow and the volume of the distillate.
3.3. Evaporation Capacity and Energy Costs
- Viscosity: the viscosity of the concentrate increased over the time, bringing a reduction in the overall heat transfer coefficient;
- Ebullioscopic raising: since the vapor pressure of an aqueous solution is lower than that of pure water at the same temperature, at a given pressure, the solution boiled at a temperature higher than that of pure water, bringing an overall reduction in the amount of evaporated water from the concentrate.
4. Conclusions
- When the plant is required to reduce the polluting load in the distillate, while the concentrate quality is neglected, a quick solution might be operating at a high treatment temperature with high operating capability, low energy costs, and large amounts of processed whey;
- Alternatively, when a high quality of the recovered product is required, then a low treatment temperature must be adopted, however, consequences will be a worse processing rate and a decreased cost saving.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| BOD | Biochemical oxygen demand |
| BOD5 | Biochemical oxygen demand after five days |
| COD | Chemical oxygen demand |
| COP | Coefficient of performance of the equipment |
| LTPD | Low-temperature and low-pressure distillation |
| RC | Retaining capability |
| SC | Solid content |
| TDS | Total dissolved solids |
| TN | Total nitrogen |
| TP | Total phosphorus |
| TSS | Total suspended solids |
References
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Fenton, O. Dairy Processing Sludge and Co-Products: A Review of Present and Future Re-Use Pathways in Agriculture. J. Clean. Prod. 2021, 314, 128035. [Google Scholar] [CrossRef]
- Guerreiro, R.C.S.; Jerónimo, E.; Luz, S.; Pinheiro, H.M.; Prazeres, A.R. Cheese Manufacturing Wastewater Treatment by Combined Physicochemical Processes for Reuse and Fertilizer Production. J. Environ. Manag. 2020, 264, 110470. [Google Scholar] [CrossRef] [PubMed]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological Utilization with a Focus on Anaerobic Treatment of Cheese Whey: Current Status and Prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef]
- Das, B.; Sarkar, S.; Sarkar, A.; Bhattacharjee, S.; Bhattacharjee, C. Recovery of Whey Proteins and Lactose from Dairy Waste: A Step towards Green Waste Management. Process Saf. Environ. Prot. 2016, 101, 27–33. [Google Scholar] [CrossRef]
- Zandona, E.; Blažić, M.; Režek Jambrak, A. Whey Utilization: Sustainable Uses and Environmental Approach. Food Technol. Biotechnol. 2021, 59, 147. [Google Scholar] [CrossRef]
- Janet Joshiba, G.; Senthil Kumar, P.; Femina, C.C.; Jayashree, E.; Racchana, R.; Sivanesan, S. Critical Review on Biological Treatment Strategies of Dairy Wastewater. Desalin. Water Treat. 2019, 160, 94–109. [Google Scholar] [CrossRef]
- Bickers, P.O.; Bhamidimarri, R. Aerobic Treatment of Reverse Osmosis Permeate in the Dairy Industry for Reuse. Water Sci. Technol. 1998, 38, 61–67. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Altieri, G. Whey Protein Concentrations Production Using a Membrane Ultrafiltration Pilot Plant. Production Optimisation with Different Pre-Treatment Systems. Riv. Di Ing. Agrar. (Italy) 2006, 37, 19–28. [Google Scholar]
- Dhineshkumar, V.; Ramaswamy, D. Review on Membrane Technology Applications in Food and Dairy Processing. J. Appl. Biotechnol. Bioeng. 2017, 3, 399–407. [Google Scholar] [CrossRef]
- Reig, M.; Vecino, X.; Cortina, J.L. Use of Membrane Technologies in Dairy Industry: An Overview. Foods 2021, 10, 2768. [Google Scholar] [CrossRef]
- Morr, C.V.; Ha, E.Y.W. Whey Protein Concentrates and Isolates: Processing and Functional Properties. Crit. Rev. Food Sci. Nutr. 1993, 33, 431–476. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharabsheh, S.; Goswami, D.Y. Experimental Study of an Innovative Solar Water Desalination System Utilizing a Passive Vacuum Technique. Sol. Energy 2003, 75, 395–401. [Google Scholar] [CrossRef]
- Scheffler, T.B.; Leao, A.J. Fabrication of Polymer Film Heat Transfer Elements for Energy Efficient Multi-Effect Distillation. Desalination 2008, 222, 696–710. [Google Scholar] [CrossRef]
- McCabe, D.L.; Vivona, M.A. Treating Process Wastewater Employing Vacuum Distillation Using Mechanical Vapor Recompression. Environ. Prog. 1999, 18, 30–33. [Google Scholar] [CrossRef]
- Wang, Y.; Shu, F.; Xiang, X.; Zhang, Y.; Luo, G.; Huang, M. Research on Treatment of Abandoned Water-Based Drilling Fluid by Low Pressure Distillation Process. In Proceedings of the Society of Petroleum Engineers—IADC/SPE Asia Pacific Drilling Technology Conference 2012—Catching the Unconventional Tide: Winning the Future Through Innovation, Tianjin, China, 9–11 July 2012; Volume 1, pp. 435–442. [Google Scholar] [CrossRef]
- Criscuoli, A.; Bafaro, P.; Drioli, E. Vacuum Membrane Distillation for Purifying Waters Containing Arsenic. Desalination 2013, 323, 17–21. [Google Scholar] [CrossRef]
- Hsu, C.J.; Xiao, Y.Z.; Chung, A.; Hsi, H.C. Novel Applications of Vacuum Distillation for Heavy Metals Removal from Wastewater, Copper Nitrate Hydroxide Recovery, and Copper Sulfide Impregnated Activated Carbon Synthesis for Gaseous Mercury Adsorption. Sci. Total Environ. 2022, 855, 158870. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, M.C.; Gnisci, E.; Hilal, J.; Criscuoli, A. Direct Contact and Vacuum Membrane Distillation Application for the Olive Mill Wastewater Treatment. Sep. Purif. Technol. 2016, 169, 121–127. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. On the Recent Use of Membrane Technology for Olive Mill Wastewater Purification. Membranes 2015, 5, 513. [Google Scholar] [CrossRef]
- Frascari, D.; Bacca, A.E.M.; Zama, F.; Bertin, L.; Fava, F.; Pinelli, D. Olive Mill Wastewater Valorisation through Phenolic Compounds Adsorption in a Continuous Flow Column. Chem. Eng. J. 2016, 283, 293–303. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of Olive Mill Wastewater Phenols through Membrane Filtration and Resin Adsorption/Desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Nabbou, N.; Benyagoub, E.; Mellouk, A.; Benmoussa, Y. Risk Assessment for Chemical Pollution of Dairy Effluents from a Milk Processing Plant Located in Bechar (Southwest of Algeria). Appl. Water Sci. 2020, 10, 229. [Google Scholar] [CrossRef]
- Kilara, A. Whey and Whey Products. In Dairy Processing and Quality Assurance; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 349–366. [Google Scholar] [CrossRef]
- Koroleff, F. Simultaneous Oxidation of Nitrogen and Phosphorus Compounds by Persulfate, 2nd ed.; Grasshoff, K., Ehrhardt, M., Kremling, K., Eds.; Verlag Chemie Weinhein: New York, NY, USA, 1983; Volume 2. [Google Scholar]
- Qualls, R.G. The Biogeochemical Properties of Dissolved Organic Matter in a Hardwood Forest Ecosystem: Their Influence on the Retention of Nitrogen. Phosphorus and Carbon. Ph.D. Dissertation, University of Georgia, Athens, GA, USA, 1989. [Google Scholar]
| Tests | Trial Coding | Temperature (°C) | Intended SC% |
|---|---|---|---|
| 1-2-3 | P1 | 30 | |
| 4-5-6 | P2 | 60 | 50 |
| 7-8-9 | P3 | 70 | |
| 10-11-12 | P4 | 30 | |
| 13-14-15 | P5 | 53 | 50 |
| 16-17-18 | P6 | 70 | |
| 19-20-21 | P7 | 30 | |
| 22-23-24 | P8 | 46 | 50 |
| 25-26-27 | P9 | 70 | |
| 28-29-30 | P10 | 30 | |
| 31-32-33 | P11 | 39 | 50 |
| 34-35-36 | P12 | 70 |
| Characteristic | Range | Mean | Standard Deviation |
|---|---|---|---|
| pH | 4.22 ÷ 4.88 | 4.49 | 0.22 |
| Conductivity (mS/cm) | 5.13 ÷ 6.36 | 5.66 | 0.38 |
| COD (mg/L) | 47,741 ÷ 143,224 | 97,756 | 29,130 |
| BOD5 (mg/L) | 15,257 ÷ 42,124 | 30,159 | 8624 |
| TN (g/L) | 1.65 ÷ 2.97 | 2.17 | 0.35 |
| TP (g/L) | 0.58 ÷ 1.25 | 0.80 | 0.21 |
| Protein content (g/L) | 12.3 ÷ 22.7 | 13.9 | 2.22 |
| Fat content (g/L) | 0.60 ÷ 1.32 | 0.96 | 0.25 |
| Lactose (g/L) | 3.73 ÷ 4.41 | 4.19 | 0.21 |
| Solid content (%) | 4.55 ÷ 7.09 | 6.05 | 0.79 |
| Density (g/L) | 1022 ÷ 1026 | 1024.1 | 1.1 |
| Trial | Density g/L | SC % | pH | Conductivity mS/cm | BOD5 mg/L | COD mg/L | TN g/L | TP g/L | Fat Content g/L | Lactose g/L | Protein g/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 1024 ± 1.0 | 6.55 ± 1.27 | 4.62 ± 0.26 | 5.39 ± 0.14 | 25,015 ± 1241 | 61,381 ± 1692 | 2.42 ± 0.44 | 1.02 ± 0.13 | 0.93 ± 0.14 | 4.07 ± 0.13 | 13.3 ± 0.86 |
| P2 | 1026 ± 0.6 | 7.09 ± 0.62 | 4.88 ± 0.26 | 5.50 ± 0.19 | 41,071 ± 1813 | 109,123 ± 1590 | 2.09 ± 0.51 | 1.04 ± 0.27 | 0.73 ±0.23 | 4.32 ± 0.08 | 14.4 ± 1.04 |
| P3 | 1024 ± 1.0 | 5.91 ± 0.47 | 4.36 ± 0.14 | 5.23 ± 0.22 | 32,415 ± 1691 | 64,791 ± 1711 | 2.97 ± 0.51 | 1.25 ± 0.35 | 0.67 ± 0.30 | 4.21 ± 0.08 | 12.7 ± 0.36 |
| P4 | 1025 ± 1.5 | 6.82 ± 0.45 | 4.59 ± 0.16 | 5.13 ± 0.25 | 35,125 ± 1457 | 95,482 ± 1621 | 2.09 ± 0.25 | 0.71 ± 0.07 | 1.09 ± 0.11 | 4.37 ± 0.06 | 12.5 ± 0.35 |
| P5 | 1023 ± 2.0 | 6.54 ± 0.42 | 4.32 ± 0.15 | 5.55 ± 0.46 | 15,257 ± 1328 | 119,353 ± 1336 | 2.09 ± 0.29 | 0.61 ± 0.07 | 1.25 ± 0.22 | 4.26 ± 0.08 | 13.2 ± 1.42 |
| P6 | 1024 ± 1.0 | 5.94 ± 0.55 | 4.31 ± 0.15 | 5.56 ± 0.40 | 20,420 ± 1241 | 143,224 ± 1669 | 1.65 ± 0.40 | 0.58 ± 0.07 | 1.29 ± 0.24 | 4.36 ± 0.10 | 12.9 ± 1.23 |
| P7 | 1022 ± 1.5 | 6.75 ± 0.58 | 4.58 ±0.19 | 6.36 ± 0.20 | 25,518 ± 1621 | 122,763± 1419 | 2.20 ± 0.28 | 0.71 ± 0.04 | 0.89 ± 0.27 | 4.20 ± 0.22 | 10.6 ± 1.04 |
| P8 | 1025 ± 1.5 | 5.70 ± 0.57 | 4.33 ± 0.15 | 5.98 ± 0.15 | 35,765 ± 1822 | 92,072 ± 1520 | 2.42 ± 0.32 | 0.63 ± 0.12 | 1.32 ± 0.26 | 4.38 ± 0.26 | 12.5 ± 0.47 |
| P9 | 1024 ± 2.3 | 5.79 ± 0.94 | 4.22 ± 0.16 | 6.04 ± 0.16 | 30,120 ± 1846 | 98,892 ± 1107 | 1.87 ± 0.32 | 0.68 ± 0.14 | 0.81 ± 0.28 | 3.95 ± 0.35 | 12.3 ± 0.52 |
| P10 | 1025 ± 0.6 | 6.73 ± 0.97 | 4.52 ± 0.29 | 5.76 ± 0.34 | 37,573 ± 1631 | 129,583 ± 1106 | 1.87 ± 0.29 | 0.86 ± 0.16 | 1.16 ± 0.29 | 4.41 ± 0.34 | 13.2 ± 0.55 |
| P11 | 1023 ± 1.5 | 5.84 ± 0.78 | 4.28 ± 0.40 | 6.05 ± 0.48 | 42,124 ± 2126 | 47,741 ± 1752 | 2.42 ± 0.31 | 0.59 ± 0.21 | 0.60 ± 0.12 | 3.73 ± 0.24 | 12.3 ± 0.07 |
| P12 | 1024 ± 2.0 | 5.94 ± 0.79 | 4.85 ± 0.22 | 5.37 ± 0.38 | 21,507 ± 2468 | 88,662 ± 1757 | 1.98 ± 0.35 | 0.89 ± 0.22 | 0.77 ± 0.25 | 4.07 ± 0.21 | 12.2 ± 0.90 |
| Coding | Temperature (°C) | Duration (Minutes) | SC (%) | pH | Conductivity (mS/cm) | BOD5 (mg/L) | COD (mg/L) | TN (g/L) | TP (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 140 ± 18 | 19.07 ± 4.28 | 4.27 ± 0.19 | 17.97 ± 2.36 | 83,333 ± 1324 | 204,603 ± 1839 | 8.07 ± 1.19 | 3.30 ± 1.41 | |
| P2 | 60 | 240 ± 21 | 47.82 ± 3.34 | 4.21 ± 0.18 | 36.00 ± 2.27 | 220,540 ± 1430 | 545,615 ± 2073 | 21.00 ± 4.08 | 4.69 ± 1.54 |
| P3 | 315 ± 31 | 65.92 ± 4.16 | 4.09 ± 0.11 | 56.90 ± 2.91 | 246,914 ± 4455 | 701,052 ± 2794 | 24.44 ± 3.35 | 6.11 ± 1.74 | |
| P4 | 269 ± 22 | 22.24 ± 2.17 | 4.22 ± 0.11 | 20.52 ± 2.79 | 140,513 ± 2165 | 381,928 ± 1903 | 8.36 ± 1.81 | 3.04 ± 1.73 | |
| P5 | 53 | 322 ± 25 | 43.61 ± 2.23 | 4.05 ± 0.11 | 37.00 ± 2.48 | 166,121 ± 2877 | 795,687 ± 1814 | 13.93 ± 1.55 | 3.17 ± 1.69 |
| P6 | 434 ± 37 | 69.92 ± 5.18 | 3.99 ± 0.11 | 65.41 ± 3.77 | 235,294 ± 4672 | 1130,245 ± 2920 | 19.41 ± 3.73 | 4.36 ± 1.60 | |
| P7 | 370 ± 19 | 23.43 ± 2.62 | 4.34 ± 0.14 | 29.58 ± 2.76 | 116,279 ± 1209 | 570,991 ± 1709 | 10.23 ± 1.03 | 3.17 ± 1.88 | |
| P8 | 46 | 475 ± 23 | 47.50 ± 3.81 | 4.11 ± 0.12 | 42.41 ± 2.84 | 248,227 ± 1408 | 652,993 ± 2340 | 17.16 ± 1.80 | 3.70 ± 1.77 |
| P9 | 579 ± 35 | 63.20 ± 8.24 | 4.07 ± 0.13 | 60.40 ± 2.32 | 276,300 ± 2490 | 988,920 ± 4909 | 18.70 ± 2.54 | 4.88 ± 1.74 | |
| P10 | 487 ± 28 | 20.03 ± 2.26 | 4.22 ± 0.21 | 19.20 ± 2.91 | 125,150 ± 1740 | 431,943 ± 2290 | 6.23 ± 1.78 | 3.43 ± 1.96 | |
| P11 | 39 | 584 ± 37 | 40.78 ± 5.24 | 4.12 ± 0.31 | 39.41 ± 2.51 | 199,080 ± 3384 | 530,128 ± 4037 | 15.77 ± 1.62 | 3.91 ± 1.97 |
| P12 | 764 ± 41 | 68.30 ± 7.24 | 4.05 ± 0.15 | 56.53 ± 3.18 | 226,316 ± 4521 | 933,284 ± 3899 | 20.84 ± 2.93 | 5.28 ± 1.98 |
| Coding | Temperature (°C) | SC | pH | Conductivity | BOD5 | COD | TN | TP |
|---|---|---|---|---|---|---|---|---|
| P1 | 2.91 ± 1.22 | 0.92 ± 0.09 | 3.33 ± 0.52 | 3.33 ± 0.22 | 3.33 ± 0.12 | 3.33 ± 1.10 | 3.24 ± 1.79 | |
| P2 | 60 | 6.74 ± 1.06 | 0.86 ± 0.08 | 6.55 ± 0.64 | 5.37 ± 0.27 | 5.00 ± 0.09 | 10.05 ± 4.40 | 4.51 ± 2.65 |
| P3 | 11.15 ± 1.59 | 0.94 ± 0.06 | 10.88 ± 1.01 | 7.62 ± 0.53 | 10.82 ± 0.33 | 8.23 ± 2.54 | 4.89 ± 2.76 | |
| P4 | 3.26 ± 0.53 | 0.92 ± 0.06 | 4.00 ± 0.74 | 4.00 ± 0.23 | 4.00 ± 0.09 | 4.00 ± 1.34 | 4.28 ± 2.86 | |
| P5 | 53 | 6.67 ± 0.77 | 0.94 ± 0.06 | 6.67 ± 1.00 | 10.89 ± 1.14 | 6.67 ± 0.09 | 6.67 ± 1.67 | 5.20 ± 3.37 |
| P6 | 11.77 ± 1.96 | 0.93 ± 0.06 | 11.76 ± 1.52 | 11.52 ± 0.93 | 7.89 ± 0.11 | 11.76 ± 5.11 | 7.52 ± 3.67 | |
| P7 | 3.47 ± 0.69 | 0.95 ± 0.07 | 4.65 ± 0.58 | 4.56 ± 0.34 | 4.65 ± 0.07 | 4.65 ± 1.06 | 4.46 ± 2.90 | |
| P8 | 46 | 8.33 ± 1.50 | 0.95 ± 0.06 | 7.09 ± 0.65 | 6.94 ± 0.39 | 7.09 ± 0.14 | 7.09 ± 1.68 | 5.87 ± 3.93 |
| P9 | 10.92 ± 3.20 | 0.96 ± 0.07 | 10.00 ± 0.65 | 9.17 ± 0.64 | 10.00 ± 0.16 | 10.00 ± 3.07 | 7.18 ± 4.04 | |
| P10 | 2.98 ± 0.76 | 0.93 ± 0.11 | 3.33 ± 0.70 | 3.33 ± 0.19 | 3.33 ± 0.05 | 3.33 ± 1.47 | 3.99 ± 3.02 | |
| P11 | 39 | 6.98 ± 1.83 | 0.96 ± 0.16 | 6.51 ± 0.93 | 4.73 ± 0.32 | 11.10 ± 0.49 | 6.52 ± 1.50 | 6.63 ± 5.70 |
| P12 | 11.50 ± 2.75 | 0.84 ± 0.07 | 10.53 ± 1.34 | 10.52 ± 1.42 | 10.53 ± 0.25 | 10.53 ± 3.34 | 5.93 ± 3.69 |
| Parameter | Slope (RC) | Intercept | R2 |
|---|---|---|---|
| pH | 0.00 (−0.01,0.01) | 0.94 | 0.03 |
| Conductivity | 0.85 (0.75,0.95) | 0.97 | 0.97 |
| BOD5 | 0.72 (0.37,1.06) | 1.66 | 0.68 |
| COD | 0.72 (0.39,1.04) | 1.86 | 0.70 |
| TN | 0.76 (0.51,1.01) | 1.68 | 0.82 |
| TP | 0.30 (0.13,0.46) | 3.16 | 0.62 |
| Coding | Temperature (°C) | pH | Conductivity (uS/cm) | BOD5 (mg/L) | COD (mg/L) | TN (mg/L) |
|---|---|---|---|---|---|---|
| P1 | 5.9 ± 0.3 | 180 ± 17 | 598 ± 133 | 975 ± 232 | 31.6 ± 1.6 | |
| P2 | 60 | 6.5 ± 0.5 | 171 ± 23 | 280 ± 84 | 728 ± 217 | 30.3 ± 5.5 |
| P3 | 6.1 ± 0.5 | 236 ± 40 | 323 ± 77 | 682 ± 156 | 28.4 ± 4.3 | |
| P4 | 6.6 ± 0.4 | 324 ± 42 | 543 ± 112 | 816 ± 168 | 19.9 ± 3.3 | |
| P5 | 53 | 5.5 ± 0.7 | 284 ± 52 | 308 ± 98 | 767 ± 213 | 25.3 ± 3.6 |
| P6 | 6.3 ± 0.4 | 331 ± 61 | 355 ± 70 | 634 ± 131 | 26.0 ± 3.6 | |
| P7 | 6.8 ± 0.3 | 323 ± 44 | 433 ± 131 | 805 ± 120 | 31.9 ± 3.3 | |
| P8 | 46 | 5.9 ± 0.4 | 287 ± 44 | 362 ± 88 | 742 ± 153 | 25.3 ± 12.7 |
| P9 | 5.7 ± 0.3 | 161 ± 31 | 345 ± 70 | 709 ± 157 | 27.7 ± 11.6 | |
| P10 | 5.8 ± 0.4 | 317 ± 44 | 452 ± 88 | 833 ± 162 | 48.4 ± 10.3 | |
| P11 | 39 | 6.5 ± 0.3 | 307 ± 50 | 370 ± 77 | 753 ± 142 | 28.9 ± 2.8 |
| P12 | 6.8 ± 0.2 | 295 ± 50 | 325 ± 74 | 646 ± 173 | 32.9 ± 6.9 |
| Parameter | Min | Max | Median | Mean | Standard Deviation |
|---|---|---|---|---|---|
| Conductivity | 94.6% | 97.7% | 95.9% | 96.1% | 1.0% |
| BOD5 | 98.3% | 99.5% | 98.9% | 98.9% | 0.4% |
| COD | 98.7% | 99.6% | 99.4% | 99.3% | 0.3% |
| TN | 98.2% | 99.3% | 98.9% | 98.9% | 0.3% |
| Coding | Treatment Temperature (°C) | Processed Whey Measured Flow Rate (L/h) | Measured Electrical Energy Consumption Per Unit Volume of Processed Whey (kWh/L) | Coefficient of Performance of the Plant (COP) |
|---|---|---|---|---|
| P1-P2-P3 | 60 | 5.79 ± 1.43 | 0.35 ± 0.02 | 1.48 ± 0.15 |
| P4-P5-P6 | 53 | 3.65 ± 0.49 | 0.38 ± 0.03 | 1.47 ± 0.19 |
| P7-P8-P9 | 46 | 2.61 ± 0.34 | 0.76 ± 0.08 | 0.75 ± 0.11 |
| P10-P11-P12 | 39 | 2.03 ± 0.26 | 0.99 ± 0.04 | 0.55 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altieri, G.; De Luca, V.; Genovese, F.; Matera, A.; Scarano, L.; Di Renzo, G.C. A Low-Temperature and Low-Pressure Distillation Plant for Dairy Wastewater. Appl. Sci. 2022, 12, 11465. https://doi.org/10.3390/app122211465
Altieri G, De Luca V, Genovese F, Matera A, Scarano L, Di Renzo GC. A Low-Temperature and Low-Pressure Distillation Plant for Dairy Wastewater. Applied Sciences. 2022; 12(22):11465. https://doi.org/10.3390/app122211465
Chicago/Turabian StyleAltieri, Giuseppe, Vincenzo De Luca, Francesco Genovese, Attilio Matera, Luciano Scarano, and Giovanni Carlo Di Renzo. 2022. "A Low-Temperature and Low-Pressure Distillation Plant for Dairy Wastewater" Applied Sciences 12, no. 22: 11465. https://doi.org/10.3390/app122211465
APA StyleAltieri, G., De Luca, V., Genovese, F., Matera, A., Scarano, L., & Di Renzo, G. C. (2022). A Low-Temperature and Low-Pressure Distillation Plant for Dairy Wastewater. Applied Sciences, 12(22), 11465. https://doi.org/10.3390/app122211465







