Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy
Abstract
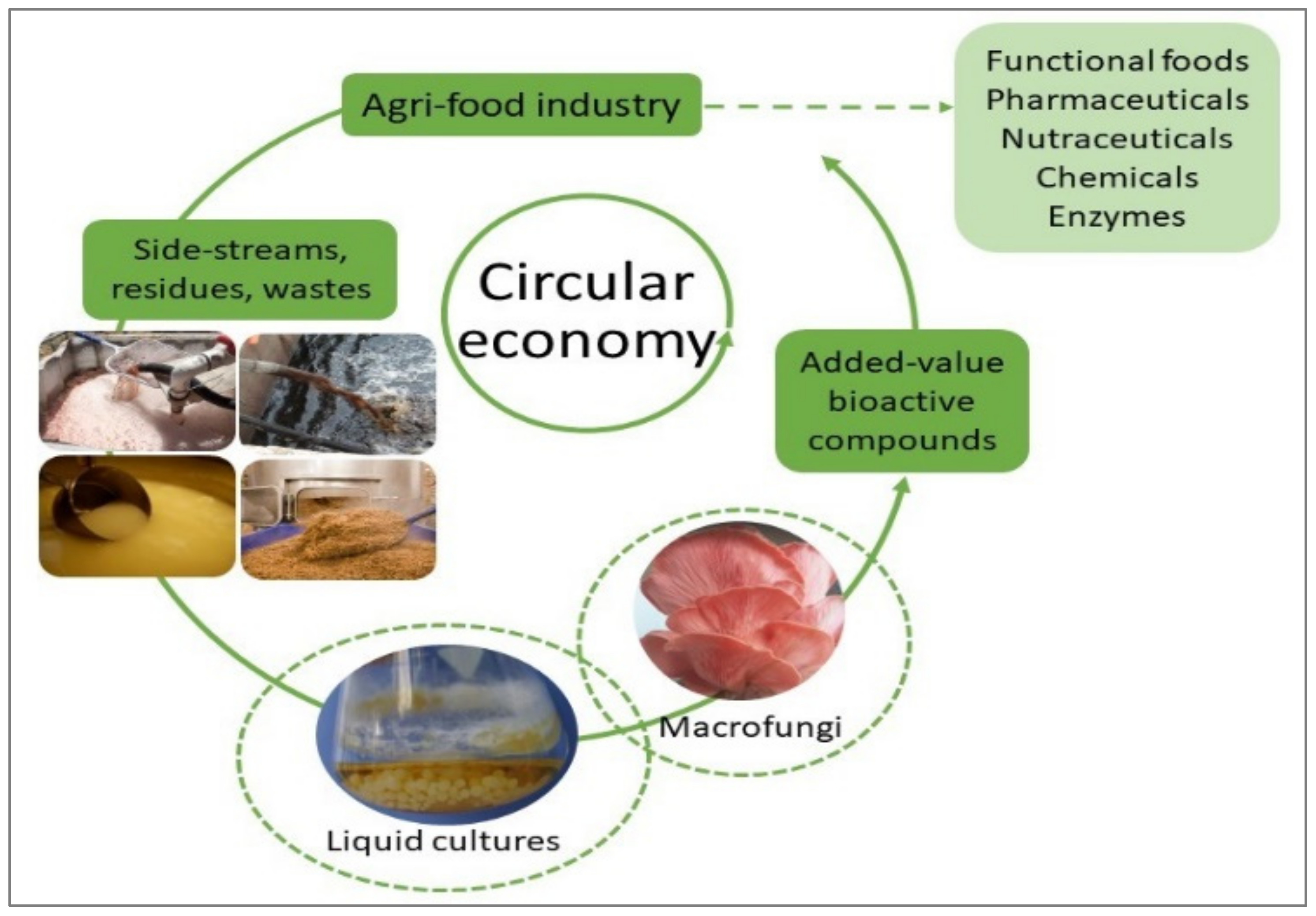
1. Introduction
2. Agri-Food Side Streams: Residues, By-Products, and Wastes
3. Macrofungi: Ascomycota and Basidiomycota
4. Bioactive Metabolites
4.1. Carbohydrates: Polysaccharides (EPS and IPS) and Oligosaccharides
4.2. Proteins and Peptides
4.3. Fatty Acids and Lipids
4.4. Phenols
4.5. Terpenes and Terpenoids
4.6. Nutrients
4.7. Other Compounds
5. Liquid Mycelial Cultivation of Macrofungi: Methods and Strategies
- Kinetic, heat, and mass transfer parameters can be estimated;
- Easy control and adjustment of the operating parameters;
- Very good reproducibility when the medium composition is characterized;
- Defined medium allows for easier purification of desired substances;
- High yield potential;
- Mixing and stirring allow for very good diffusion of nutrients;
- Temperature control is precise and easier because of the high-water content and stirring;
- Existing kinetic and transfer information of bioprocess can guide the design and operation of bioreactors;
- A Variety of online sensors are available, and more are being developed;
- Sensors allow for the automatic addition of the proper reagents to control the ongoing process;
- Easy manipulation of environmental conditions, growth factors, and nutritional requirements;
- Short overall time of cultivation compared to solid state cultivation of fruit bodies that takes several months, a large volume of substrate and space, and the quality is not always reliable.
- Crude medium ingredients may need processing and/or solubilization and characterization which increases the cost of the process.
- Downstream processing of the products requires removal of large water volumes and can be more expensive;
- Media dilution leads to lower volumetric productivity;
- High substrate concentrations may cause rheological problems;
- Gas transfer from the gas to liquid requires high air pressure and may be slow and limiting;
- Scaling up from flasks to bioreactors can create technical problems that need to be tackled, such as increased broth viscosity and sufficient oxygen supply;
- Submerged cultivation can be more demanding in energy, water, and labor compared to solid-state fermentation;
- Optimization is necessary.
6. Bioconversion of Wastes by Submerged Fermentation of Macrofungi
7. Challenges and Future Perspective
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.-P. Grape winery waste as feedstock for bioconversions: Applying the biorefinery concept. Waste Biomass Valorization 2017, 8, 1011–1025. [Google Scholar] [CrossRef]
- Kevin van Langen, S.; Vassillo, C.; Ghisellini, P.; Restaino, D.; Passaro, R.; Ulgiati, S. Promoting circular economy transition: A study about perceptions and awareness by different stakeholders groups. J. Clean. Prod. 2021, 316, 128166. [Google Scholar] [CrossRef]
- D’Amato, D. Sustainability Narratives as Transformative Solution Pathways: Zooming in on the Circular Economy. Circ. Econ. Sustain. 2021, 1, 231–242. [Google Scholar] [CrossRef]
- Carus, M.; Dammer, L. The circular bioeconomy—Concepts, opportunities, and limitations. Ind. Biotechnol. 2018, 14, 83–91. [Google Scholar] [CrossRef]
- Hamelin, L.; Borzęcka, M.; Kozak, M.; Pudełko, R. A spatial approach to bioeconomy: Quantifying the residual biomass potential in the EU-27. Renew. Sustain. Energy Rev. 2019, 100, 127–142. [Google Scholar] [CrossRef]
- Javourez, U.; O’Donohue, M.; Hamelin, L. Waste-to-nutrition: A review of current and emerging conversion pathways. Biotechnol. Adv. 2021, 53, 107857. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating biowaste into circular bioeconomy: A critical review of current trend and scaling up feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A comprehensive review on the framework to valorise lignocellulosic biomass as biorefinery feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A roadmap towards a circular and sustainable bioeconomy through waste valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Tsouko, E.; Maina, S.; Ladakis, D.; Kookos, I.K.; Koutinas, A. Integrated biorefinery development for the extraction of value-added components and bacterial cellulose production from orange peel waste streams. Renew. Energy 2020, 160, 944–954. [Google Scholar] [CrossRef]
- Di Piazza, S.; Benvenuti, M.; Damonte, G.; Cecchi, G.; Mariotti, M.G.; Zotti, M. Fungi and Circular Economy: Pleurotus ostreatus Grown on a Substrate with Agricultural Waste of Lavender, and Its Promising Biochemical Profile. Recycling 2021, 6, 40. [Google Scholar] [CrossRef]
- Challa, S.; Dutta, T.; Neelapu, N.R.R. Fungal White Biotechnology Applications for Food Security: Opportunities and Challenges. In Recent Advancement in White Biotechnology Through Fungi: Volume 2: Perspective for Value-Added Products and Environments; Yadav, A.N., Singh, S., Mishra, S., Gupta, A., Eds.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2019; pp. 119–148. [Google Scholar]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A Boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Jaros, D.; Köbsch, J.; Rohm, H. Exopolysaccharides from Basidiomycota: Formation, isolation and techno-functional properties. Eng. Life Sci. 2018, 18, 743–752. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.; Zervakis, G. Fungal bioconversions of various lignocellulosic by-products to edible biomass. New Biotechnol. 2014, 31, S212. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Seraphim, P.; Aggelis, G.; Philippoussis, A. Adaptation of Volvariella volvacea metabolism in high carbon to nitrogen ratio media. Food Chem. 2016, 196, 272–280. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Katsarou, E.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part II: Study of Volvariella volvacea. Appl. Biochem. Biotechnol. 2012, 167, 1890–1906. [Google Scholar] [CrossRef] [PubMed]
- Ahlborn, J.; Stephan, A.; Meckel, T.; Maheshwari, G.; Rühl, M.; Zorn, H. Upcycling of food industry side streams by basidiomycetes for production of a vegan protein source. Int. J. Recycl. Org. Waste Agric. 2019, 8, 447–455. [Google Scholar] [CrossRef]
- Sarris, D.; Philippoussis, A.; Mallouchos, A.; Diamantopoulou, P. Valorization of low-cost, carbon-rich substrates by edible ascomycetes and basidiomycetes grown on liquid cultures. FEMS Microbiol. Lett. 2020, 367, fnaa168. [Google Scholar] [CrossRef] [PubMed]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part II: Effect on productivity and quality of carposomes. Carbon Resour. Convers. 2022, 5, 52–60. [Google Scholar] [CrossRef]
- Diamantis, I.; Melanouri, E.-M.; Dedousi, M.; Panagopoulou, I.; Papanikolaou, S.; Stoforos, N.G.; Diamantopoulou, P. Sustainable and eco-friendly conversions of olive mill mastewater-based media by Pleurotus pulmonarius cultures. Fermentation 2022, 8, 129. [Google Scholar] [CrossRef]
- Dedousi, M.; Fourtaka, K.; Melanouri, E.-M.; Argyropoulos, D.; Psallida, C.; Diamantis, I.; Papanikolaou, S.; Diamantopoulou, P. Detoxification of molasses and production of mycelial mass and valuable metabolites by Morchella Species. Appl. Sci. 2021, 11, 9481. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Kassim, F.O.; Thomas, C.L.P.; Afolabi, O.O.D. Integrated conversion technologies for sustainable agri-food waste valorization: A critical review. Biomass Bioenergy 2022, 156, 106314. [Google Scholar] [CrossRef]
- Alexander, P.; Brown, C.; Arneth, A.; Finnigan, J.; Moran, D.; Rounsevell, M.D.A. Losses, inefficiencies and waste in the global food system. Agric. Syst. 2017, 153, 190–200. [Google Scholar] [CrossRef]
- Schramski, J.R.; Woodson, C.B.; Brown, J.H. Energy use and the sustainability of intensifying food production. Nat. Sustain. 2020, 3, 257–259. [Google Scholar] [CrossRef]
- Loboguerrero, A.M.; Campbell, B.M.; Cooper, P.J.M.; Hansen, J.W.; Rosenstock, T.; Wollenberg, E. Food and earth systems: Priorities for climate change adaptation and mitigation for agriculture and food systems. Sustainability 2019, 11, 1372. [Google Scholar] [CrossRef]
- Mbow, H.-O.P.; Reisinger, A.; Canadell, J.; O’Brien, P. Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (SR2); IPCC: Ginevra, Switzerland, 2017. [Google Scholar]
- Wilson, D.C.; Rodic, L.; Modak, P.; Soos, R.; Carpintero, A.; Velis, K.; Iyer, M.; Simonett, O. Global Waste Management Outlook; United Nations Environment Programme (UNEP): Nairobi, Kenya; International Solid Waste Association (ISWA): Vienna, Austria, 2015. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Otterdijk, R.V.; Meybeck, A. Globalfood losses and food waste: Extent, causes and prevention. 2011. Available online: https://www.fao.org/3/i2697e/i2697e.pdf (accessed on 1 December 2021).
- FAO; IFAD. The State of Food and Agriculture 2019. Moving forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; pp. 2–13. Available online: https://www.fao.org/3/i3991e/i3991e.pdf (accessed on 3 February 2022).
- FAO. Food Wastage Footprint Full-Cost Accounting: Final Report; FAO: Rome, Italy, 2014. [Google Scholar]
- Sanchez Lopez, J.; Patinha Caldeira, C.; De Laurentiis, V.; Sala, S.; Avraamides, M. Brief on food waste in the European Union. European Commission, JRC121196. 2020. Available online: https://ec.europa.eu/jrc/en/publication/brief-food-waste-european-union (accessed on 3 December 2021).
- Cherubin, M.R.; da Silva Oliveira, D.M.; Feigl, B.J.; Pimentel, L.G.; Lisboa, I.P.; Gmach, M.R.; Varanda, L.L.; Morais, M.C.; Satiro, L.S.; Popin, G.V.; et al. Crop residue harvest for bioenergy production and its implications on soil functioning and plant growth: A review. Sci. Agric. 2018, 75, 255–272. [Google Scholar] [CrossRef]
- Pimentel, D.; Marklein, A.; Toth, M.A.; Karpoff, M.; Paul, G.S.; McCormack, R.; Kyriazis, J.; Krueger, T. Biofuel Impacts on World Food Supply: Use of Fossil Fuel, Land and Water Resources. Energies 2008, 1, 41–78. [Google Scholar] [CrossRef]
- Georganas, A.; Giamouri, E.; Pappas, A.C.; Papadomichelakis, G.; Galliou, F.; Manios, T.; Tsiplakou, E.; Fegeros, K.; Zervas, G. Bioactive Compounds in Food Waste: A Review on the Transformation of Food Waste to Animal Feed. Foods 2020, 9, E291. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Jain, A.; Kumar Awasthi, S.; Duan, Y.; Kumar Awasthi, M.; Shi, J. Microbial dynamics for lignocellulosic waste bioconversion and its importance with modern circular economy, challenges and future perspectives. Bioresour. Technol. 2019, 291, 121905. [Google Scholar] [CrossRef]
- Parfitt, J.; Barthel, M.; Macnaughton, S. Food waste within food supply chains: Quantification and potential for change to 2050. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3065–3081. [Google Scholar] [CrossRef]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of global issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Sujarit, K.; Penkhrue, W.; Kakumyan, P.; Jatuwong, K.; Vadthanarat, S.; Lumyong, S. Cultivation of nushrooms and their lignocellulolytic enzymep roduction through the utilization of agro-industrial waste. Molecules 2020, 25, 2811. [Google Scholar] [CrossRef]
- Economou, C.; Diamantopoulou, P.; Philippoussis, A. Valorization of spent oyster mushroom substrate and laccase recovery through successive solid state cultivation of Pleurotus, Ganoderma, and Lentinula strains. Appl. Microbiol. Biotechnol. 2017, 101, 5213–5222. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii mushroom strains on agro-industrial residues in solid-state fermentation. Part I: Screening for growth, endoglucanase, laccase and biomass production in the colonization phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Philippoussis, A.N.; Diamantopoulou, P.A.; Zervakis, G.I. Correlation of the properties of several lignocellulosic substrates to the crop performance of the shiitake mushroom Lentinula edodes. World J. Microbiol. Biotechnol. 2003, 19, 551–557. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G. Solid-state fermentation of plant residues and agro-industrial wastes for the production of medicinal mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Agrawal, D.C., Tsay, H.-S., Shyur, L.-F., Wu, Y.-C., Wang, S.-Y., Eds.; Springer: Singapore, 2017; pp. 365–396. [Google Scholar]
- García, C.A.; Hodaifa, G. Real olive oil mill wastewater treatment by photo-Fenton system using artificial ultraviolet light lamps. J. Clean. Prod. 2017, 162, 743–753. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee Silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Meneses, N.; Martins, S.; Teixeira, J.; Mussatto, S. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Vlyssides, A.; Barampouti, E.; Mai, S. Wastewater characteristics from Greek wineries and distilleries. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2005, 51, 53–60. [Google Scholar] [CrossRef]
- Santana, N.A.; Jacques, R.J.S.; Antoniolli, Z.I.; Martínez-Cordeiro, H.; Domínguez, J. Changes in the chemical and biological characteristics of grape marc vermicompost during a two-year production period. Appl. Soil Ecol. 2020, 154, 103587. [Google Scholar] [CrossRef]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crops Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- De Iseppi, A.; Lomolino, G.; Marangon, M.; Curioni, A. Current and future strategies for wine yeast lees valorization. Food Res. Int. 2020, 137, 109352. [Google Scholar] [CrossRef]
- Chiaraluce, G.; Bentivoglio, D.; Finco, A. Circular Economy for a sustainable agri-food supply chain: A review for current trends and future pathways. Sustainability 2021, 13, 9294. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Petre, M.; Pătrulescu, F.; Teodorescu, R.I. Chapter 3—Controlled Cultivation of Mushrooms on Winery and Vineyard Wastes. In Mushroom Biotechnology; Petre, M., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 31–47. [Google Scholar]
- Philippoussis, A.N. Production of mushrooms using agro-industrial residues as substrates. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 163–196. [Google Scholar]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The amazing potential of fungi: 50 ways we can exploit fungi industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Miles, P.G.; Chang, S.-T. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Hawksworth, D.L.; Lücking, R. Fungal Diversity Revisited: 2.2 to 3.8 Million Species. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Meenu, M.; Xu, B. Application of vibrational spectroscopy for classification, authentication and quality analysis of mushroom: A concise review. Food Chem. 2019, 289, 545–557. [Google Scholar] [CrossRef]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Govorushko, S.; Rezaee, R.; Dumanov, J.; Tsatsakis, A. Poisoning associated with the use of mushrooms: A review of the global pattern and main characteristics. Food Chem. Toxicol. 2019, 128, 267–279. [Google Scholar] [CrossRef]
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged Culture Fermentation of “Higher Fungi”: The Macrofungi. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2008; Volume 63, pp. 33–103. [Google Scholar]
- Grimm, D.; Wösten, H.A.B. Mushroom cultivation in the circular economy. Appl. Microbiol. Biotechnol. 2018, 102, 7795–7803. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Barzee, T.J.; Cao, L.; Pan, Z.; Zhang, R. Fungi for future foods. J. Future Foods 2021, 1, 25–37. [Google Scholar] [CrossRef]
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 2015, e376387. [Google Scholar] [CrossRef] [PubMed]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Pat. 2018, 28, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef]
- Bogue, J.; Collins, O.; Troy, A.J. Market analysis and concept development of functional foods. In Developing New Functional Food and Nutraceutical Products; Elsevier: Amsterdam, The Netherlands, 2017; pp. 29–45. [Google Scholar]
- Rathore, H.; Prasad, S.; Kapri, M.; Tiwari, A.; Sharma, S. Medicinal importance of mushroom mycelium: Mechanisms and applications. J. Funct. Foods 2019, 56, 182–193. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C.F.R. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y. An Overview of Polysaccharides and the Influence Factors of Hypoglycemic Activity. In Structure and Health Effects of Natural Products on Diabetes Mellitus; Springer: Singapore, 2021; pp. 163–177. [Google Scholar]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Wen, C.; Liu, J.; Xu, X.; Liu, G.; Kan, J.; Qian, C.; Jin, C. New light on Grifola frondosa polysaccharides as biological response modifiers. Trends Food Sci. Technol. 2021, 119, 565–578. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, L.; Yan, F.; Wu, X. Chain Conformation of water-insoluble hyperbranched polysaccharide from fungus. Biomacromolecules 2007, 8, 2321–2328. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Y.; Xue, Z.; Li, N.; Liu, J.; Chen, H. Recent developments in Inonotus obliquus (Chaga mushroom) polysaccharides: Isolation, structural characteristics, biological activities and application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Pandya, U.; Dhuldhaj, U.; Sahay, N.S. Bioactive mushroom polysaccharides as antitumor: An overview. Nat. Prod. Res. 2019, 33, 2668–2680. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, MBI.S10957. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhu, L.-W.; Li, H.-M.; Li, D.-S. Submerged culture of mushrooms in bioreactors—challenges, current state-of-the-art, and future prospects. Food Technol. Biotechnol. 2007, 45, 221–229. [Google Scholar]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef]
- Kim, H.O.; Yun, J.W. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J. Appl. Microbiol. 2005, 99, 728–738. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotus ostreatus mushrooms and correlate well with cultivation performance parameters. World J. Microbiol. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Gardeli, C.; Papanikolaou, S. Impact of olive mill wastewaters on the physiological behavior of a wild-type new Ganoderma resinaceum isolate. Environ. Sci. Pollut. Res. 2021, 28, 20570–20585. [Google Scholar] [CrossRef]
- Osińska-Jaroszuk, M.; Jarosz-Wilkołazka, A.; Jaroszuk-Ściseł, J.; Szałapata, K.; Nowak, A.; Jaszek, M.; Ozimek, E.; Majewska, M. Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 2015, 31, 1823–1844. [Google Scholar] [CrossRef]
- Kho, C.-H.; Kan, S.-C.; Chang, C.-Y.; Cheng, H.-Y.; Lin, C.-C.; Chiou, P.-C.; Shieh, C.-J.; Liu, Y.-C. Analysis of exopolysaccharide production patterns of Cordyceps militaris under various light-emitting diodes. Biochem. Eng. J. 2016, 112, 226–232. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Kuang, Y.; Zheng, S. Sonoporation of Ganoderma lucidum mycelium for high biomass and exopolysaccharide productivity in submerged culture. Int. J. Agric. Biol. 2016, 18, 773–779. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Patterns of major metabolites biosynthesis by different mushroom fungi grown on glucose-based submerged cultures. Bioprocess Biosyst. Eng. 2014, 37, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwijk, H.P.; Bast, A.; de Boer, A. Immunomodulating effects of fungal beta-glucans: From traditional use to medicine. Nutrients 2021, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Kleftaki, S.-A.; Simati, S.; Amerikanou, C.; Gioxari, A.; Tzavara, C.; Zervakis, G.I.; Kalogeropoulos, N.; Kokkinos, A.; Kaliora, A.C. Pleurotus eryngii improves postprandial glycaemia, hunger and fullness perception, and enhances ghrelin suppression in people with metabolically unhealthy obesity. Pharmacol. Res. 2022, 175, 105979. [Google Scholar] [CrossRef]
- Vlassopoulou, M.; Yannakoulia, M.; Pletsa, V.; Zervakis, G.I.; Kyriacou, A. Effects of fungal beta-glucans on health—A systematic review of randomized controlled trials. Food Funct. 2021, 12, 3366–3380. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Pillai, T.G.; Uma Devi, P. Mushroom beta glucan: Potential candidate for post irradiation protection. Mutat. Res. Toxicol. Environ. Mutagen. 2013, 751, 109–115. [Google Scholar] [CrossRef]
- Ostroff, G.; Ross, G.; Ross, T. Methods of using Beta Glucan as a Radioprotective Agent. European Patent EP1545208A4, 29 June 2005. [Google Scholar]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus mushrooms content in glucans and ergosterol assessed by ATR-FTIR spectroscopy and multivariate analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of olive by-products as substrates for the Cultivation of Ganoderma lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.; Shan, W.; Shi, L.; Chang, X.; Zhu, Y.; Chen, F.; Han, X. Lentinan protects pancreatic β cells from STZ-induced damage. J. Cell. Mol. Med. 2016, 20, 1803–1812. [Google Scholar] [CrossRef]
- Xue, J.; Tong, S.; Wang, Z.; Liu, P. Chemical characterization and hypoglycaemic activities in vitro of two polysaccharides from Inonotus obliquus by submerged culture. Molecules 2018, 23, 3261. [Google Scholar] [CrossRef]
- Chen, C.-H.; Kang, L.; Lo, H.-C.; Hsu, T.-H.; Lin, F.-Y.; Lin, Y.-S.; Wang, Z.-J.; Chen, S.-T.; Shen, C.-L. Polysaccharides of Trametes versicolor improve bone properties in diabetic rats. J. Agric. Food Chem. 2015, 63, 9232–9238. [Google Scholar] [CrossRef]
- Jiang, X.; Meng, W.; Li, L.; Meng, Z.; Wang, D. Adjuvant Therapy with Mushroom polysaccharides for diabetic complications. Front. Pharmacol. 2020, 11, 168. [Google Scholar] [CrossRef]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Polysaccharides in Grifola frondosa mushroom and their health promoting properties: A review. Int. J. Biol. Macromol. 2017, 101, 910–921. [Google Scholar] [CrossRef]
- Jing, X.; Mao, D.; Geng, L.; Xu, C. Medium optimization, molecular characterization, and bioactivity of exopolysaccharides from Pleurotus eryngii. Arch. Microbiol. 2013, 195, 749–757. [Google Scholar] [CrossRef]
- Moradali, M.-F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.-A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Leathers, T.D.; Nunnally, M.S.; Stanley, A.M.; Rich, J.O. Utilization of corn fiber for production of schizophyllan. Biomass Bioenergy 2016, 95, 132–136. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Khosravi-Darani, K.; Hosseini, H.; Farshi, P.; Reihani, S.F.S. Mycoproteins as safe meat substitutes. J. Clean. Prod. 2020, 253, 119958. [Google Scholar] [CrossRef]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvador, M.; Dillon, A.J.P. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2008, 35, 1149. [Google Scholar] [CrossRef] [PubMed]
- Gang, J.; Liu, H.; Liu, Y. Optimization of Liquid Fermentation Conditions and protein nutrition evaluation of mycelium from the caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2016, 18, 745–752. [Google Scholar] [CrossRef]
- Argyropoulos, D.; Psallida, C.; Sitareniou, P.; Flemetakis, E.; Diamantopoulou, P. Biochemical Evaluation of Agaricus and Pleurotus strains in batch cultures for production optimization of valuable metabolites. Microorganisms 2022, 10, 964. [Google Scholar] [CrossRef]
- Rahgo, Z.; Samadlouie, H.R.; Mojerlou, S.; Jahanbin, K. Statistical optimization of culture conditions for protein production by a newly isolated Morchella fluvialis. BioMed Res. Int. 2019, 2019, e7326590. [Google Scholar] [CrossRef]
- Tagkouli, D.; Kaliora, A.; Bekiaris, G.; Koutrotsios, G.; Christea, M.; Zervakis, G.I.; Kalogeropoulos, N. Free amino acids in three Pleurotus species cultivated on agricultural and agro-Industrial by-products. Molecules 2020, 25, 4015. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Diling, C.; Chaoqun, Z.; Jian, Y.; Jian, L.; Jiyan, S.; Yizhen, X.; Guoxiao, L. Immunomodulatory activities of a fungal protein extracted from Hericium erinaceus through regulating the gut microbiota. Front. Immunol. 2017, 8, 666. [Google Scholar] [CrossRef]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A critical review on submerged production of mushroom and their bioactive metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef]
- Singh, S.S.; Wang, H.; Chan, Y.S.; Pan, W.; Dan, X.; Yin, C.M.; Akkouh, O.; Ng, T.B. Lectins from edible mushrooms. Molecules 2015, 20, 446–469. [Google Scholar] [CrossRef]
- Lin, C.-H.; Sheu, G.-T.; Lin, Y.-W.; Yeh, C.-S.; Huang, Y.-H.; Lai, Y.-C.; Chang, J.-G.; Ko, J.-L. A new immunomodulatory protein from Ganoderma microsporum inhibits epidermal growth factor mediated migration and invasion in A549 lung cancer cells. Process Biochem. 2010, 45, 1537–1542. [Google Scholar] [CrossRef]
- Puri, M.; Kaur, I.; Perugini, M.A.; Gupta, R.C. Ribosome-inactivating proteins: Current status and biomedical applications. Drug Discov. Today 2012, 17, 774–783. [Google Scholar] [CrossRef]
- Jose Alves, M.; Ferreira, I.C.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on antifungalactivity of mushroom (Basidiomycetes) extracts and isolated compounds. Curr. Top. Med. Chem. 2013, 13, 2648–2659. [Google Scholar] [CrossRef]
- Xiao, C.; Feng, S.; Wang, H.; Gong, Z.; Ng, T. Purification and characterization of a ribonuclease with antiproliferative activity from the mystical wild mushroom Tuber indicum. J. Basic Microbiol. 2014, 54 (Suppl. 1), S102–S108. [Google Scholar] [CrossRef]
- Dan, X.; Liu, W.; Wong, J.H.; Ng, T.B. A ribonuclease isolated from wild Ganoderma lucidum suppressed autophagy and triggered apoptosis in colorectal cancer cells. Front. Pharmacol. 2016, 7, 217. [Google Scholar] [CrossRef]
- Pahl, A.; Lutz, C.; Hechler, T. Amanitins and their development as a payload for antibody-drug conjugates. Drug Discov. Today Technol. 2018, 30, 85–89. [Google Scholar] [CrossRef]
- Di Pasquale, M.G. The essentials of essential fatty acids. J. Diet. Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef]
- Sande, D.; de Oliveira, G.P.; e Moura, M.A.F.; Martins, B.D.A.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Parikh, P.; McDaniel, M.C.; Ashen, M.D.; Miller, J.I.; Sorrentino, M.; Chan, V.; Blumenthal, R.S.; Sperling, L.S. Diets and cardiovascular disease: An evidence-based assessment. J. Am. Coll. Cardiol. 2005, 45, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part I: Screening various mushroom species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of mushrooms as a potential source of dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Tagkouli, D.; Bekiaris, G.; Kaliora, A.; Tsiaka, T.; Tsiantas, K.; Chatzipavlidis, I.; Zoumpoulakis, P.; Kalogeropoulos, N.; Zervakis, G.I. Enhancing the nutritional and functional properties of Pleurotus citrinopileatus mushrooms through the exploitation of winery and olive mill wastes. Food Chem. 2022, 370, 131022. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible mushrooms as functional ingredients for development of healthier and more sustainable muscle foods: A flexitarian approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Xin, G.; Sun, B.; Bao, X.; Wei, Y.; Zhao, X.; Xu, H. Advances in umami taste and aroma of edible mushrooms. Trends Food Sci. Technol. 2020, 96, 176–187. [Google Scholar] [CrossRef]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in wild and cultivated mushrooms: Nutrition and health. Phytochem. Rev. 2021, 21, 339–383. [Google Scholar] [CrossRef]
- Tešanović, K.; Pejin, B.; Šibul, F.; Matavulj, M.; Rašeta, M.; Janjušević, L.; Karaman, M. A comparative overview of antioxidative properties and phenolic profiles of different fungal origins: Fruiting bodies and submerged cultures of Coprinus comatus and Coprinellus truncorum. J. Food Sci. Technol. 2017, 54, 430–438. [Google Scholar] [CrossRef]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F.R.; Soler-Rivas, C. Mushrooms do not contain flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, J. Enhanced phenolic antioxidants production in submerged cultures of Inonotus obliquus in a ground corn stover medium. Biochem. Eng. J. 2011, 58–59, 103–109. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, X. Stimulatory Effect of Different Lignocellulosic Materials for phenolic compound production and antioxidant activity from Inonotus obliquus in submerged fermentation. Appl. Biochem. Biotechnol. 2013, 169, 2138–2152. [Google Scholar] [CrossRef]
- Wang, K.; Bao, L.; Xiong, W.; Ma, K.; Han, J.; Wang, W.; Yin, W.; Liu, H. Lanostane triterpenes from the tibetan medicinal mushroom Ganoderma leucocontextum and their inhibitory effects on HMG-CoA reductase and α-glucosidase. J. Nat. Prod. 2015, 78, 1977–1989. [Google Scholar] [CrossRef]
- Lakornwong, W.; Kanokmedhakul, K.; Kanokmedhakul, S.; Kongsaeree, P.; Prabpai, S.; Sibounnavong, P.; Soytong, K. triterpene lactones from ultures of Ganoderma sp. KM01. J. Nat. Prod. 2014, 77, 1545–1553. [Google Scholar] [CrossRef]
- Luo, Q.; Di, L.; Dai, W.-F.; Lu, Q.; Yan, Y.-M.; Yang, Z.-L.; Li, R.-T.; Cheng, Y.-X. Applanatumin A, a new dimeric meroterpenoid from Ganoderma applanatum that displays potent antifibrotic activity. Org. Lett. 2015, 17, 1110–1113. [Google Scholar] [CrossRef]
- Yin, R.-H.; Zhao, Z.-Z.; Chen, H.-P.; Yin, X.; Ji, X.; Dong, Z.-J.; Li, Z.-H.; Feng, T.; Liu, J.-K. Tremulane sesquiterpenes from cultures of the fungus Phellinus igniarius and their vascular-relaxing activities. Phytochem. Lett. 2014, 10, 300–303. [Google Scholar] [CrossRef]
- Isaka, M.; Sappan, M.; Supothina, S.; Srichomthong, K.; Komwijit, S.; Boonpratuang, T. Alliacane sesquiterpenoids from submerged cultures of the basidiomycete Inonotus sp. BCC 22670. Phytochemistry 2017, 136, 175–181. [Google Scholar] [CrossRef]
- Ishikawa, N.K.; Fukushi, Y.; Yamaji, K.; Tahara, S.; Takahashi, K. Antimicrobial cuparene-type sesquiterpenes, enokipodins C and D, from a mycelial culture of Flammulina velutipes. J. Nat. Prod. 2001, 64, 932–934. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Wittstein, K.; Rascher, M.; Rupcic, Z.; Löwen, E.; Winter, B.; Köster, R.W.; Stadler, M. Corallocins A–C, nerve growth and brain-derived neurotrophic factor inducing metabolites from the mushroom Hericium coralloides. J. Nat. Prod. 2016, 79, 2264–2269. [Google Scholar] [CrossRef]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium erinaceus, an amazing medicinal mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, R.-N.; Tang, Q.-J.; Zhang, J.-S.; Yang, Y.; Shang, X.-D. A new diterpene from the fungal mycelia of Hericium erinaceus. Phytochem. Lett. 2015, 11, 151–156. [Google Scholar] [CrossRef]
- Rupcic, Z.; Rascher, M.; Kanaki, S.; Köster, R.W.; Stadler, M.; Wittstein, K. Two new cyathane diterpenoids from mycelial cultures of the medicinal mushroom Hericium erinaceus and the rare species, Hericium flagellum. Int. J. Mol. Sci. 2018, 19, 740. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Z.; Wang, S.H.; Ai, X.R.; Yao, L.; Sun, Z.W.; Lei, C.; Wang, Y.; Wang, Q. Composition and characterization of cordyxanthins from Cordyceps militaris fruit bodies. J. Funct. Foods 2013, 5, 1450–1455. [Google Scholar] [CrossRef]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef]
- Feeney, M.J.; Miller, A.M.; Roupas, P. Mushrooms—Biologically distinct and nutritionally unique: Exploring a “Third Food Kingdom”. Nutr. Today 2014, 49, 301–307. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Bailey, A.M.; Alberti, F.; Kilaru, S.; Collins, C.M.; de Mattos-Shipley, K.; Hartley, A.J.; Hayes, P.; Griffin, A.; Lazarus, C.M.; Cox, R.J.; et al. Identification and manipulation of the pleuromutilin gene cluster from Clitopilus passeckerianus for increased rapid antibiotic production. Sci. Rep. 2016, 6, 25202. [Google Scholar] [CrossRef]
- Hetland, G.; Johnson, E.; Bernardshaw, S.V.; Grinde, B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID-19 and its pneumonic superinfection and complicating inflammation? Scand. J. Immunol. 2020, 93, e12937. [Google Scholar] [CrossRef]
- De Silva, D.D.; Rapior, S.; Sudarman, E.; Stadler, M.; Xu, J.; Aisyah Alias, S.; Hyde, K.D. Bioactive metabolites from macrofungi: Ethnopharmacology, biological activities and chemistry. Fungal Divers. 2013, 62, 1–40. [Google Scholar] [CrossRef]
- Schüffler, A. Secondary Metabolites of Basidiomycetes. In Physiology and Genetics: Selected Basic and Applied Aspects; Anke, T., Schüffler, A., Eds.; The Mycota; Springer International Publishing: Cham, Switzerland, 2018; pp. 231–275. [Google Scholar]
- Chen, H.-P.; Liu, J.-K. Secondary Metabolites from Higher Fungi. In Progress in the Chemistry of Organic Natural Products 106; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Progress in the Chemistry of Organic Natural Products; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–201. [Google Scholar]
- Ho, L.-H.; Zulkifli, N.A.; Tan, T.-C. Edible Mushroom: Nutritional Properties, Potential Nutraceutical Values, and Its Utilisation in Food Product Development; IntechOpen: London, UK, 2020. [Google Scholar]
- Zhou, X.-W.; Su, K.-Q.; Zhang, Y.-M. Applied modern biotechnology for cultivation of Ganoderma and development of their products. Appl. Microbiol. Biotechnol. 2012, 93, 941–963. [Google Scholar] [CrossRef]
- Wasser, S. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345. [Google Scholar] [CrossRef]
- Schügerl, K. Progress in monitoring, modeling and control of bioprocesses during the last 20 years. J. Biotechnol. 2001, 85, 149–173. [Google Scholar] [CrossRef]
- Formenti, L.R.; Nørregaard, A.; Bolic, A.; Hernandez, D.Q.; Hagemann, T.; Heins, A.-L.; Larsson, H.; Mears, L.; Mauricio-Iglesias, M.; Krühne, U.; et al. Challenges in industrial fermentation technology research. Biotechnol. J. 2014, 9, 727–738. [Google Scholar] [CrossRef]
- Gregory, F.J.; Healy, E.M.; Agersborg, H.P.K., Jr.; Warren, G.H. Studies on antitumor substances produced by Basidiomycetes. Mycologia 1966, 58, 80–90. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Olsen, L.M.; Ruthes, A.C.; Czelusniak, P.A.; Santana-Filho, A.P.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohydr. Polym. 2012, 87, 368–376. [Google Scholar] [CrossRef]
- Chan, J.S.L.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of caterpillar medicinal fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef]
- Papaspyridi, L.-M.; Aligiannis, N.; Topakas, E.; Christakopoulos, P.; Skaltsounis, A.-L.; Fokialakis, N. Submerged Fermentation of the edible mushroom Pleurotus ostreatus in a batch stirred tank bioreactor as a promising alternative for the effective production of bioactive metabolites. Molecules 2012, 17, 2714–2724. [Google Scholar] [CrossRef]
- Berovic, M. Cultivation of medicinal mushroom biomass by solid-state bioprocessing in bioreactors. In Solid State Fermentation: Research and Industrial Applications; Steudler, S., Werner, A., Cheng, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar]
- Sarris, D.; Economou, C.N.; Papanikolaou, S. Food waste management: The role of biotechnology. Prog. Food Biotechnol. 2018, 4, 383–430. [Google Scholar]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon 2020, 6, e04205. [Google Scholar] [CrossRef]
- Tiwari, A.; Khawas, R. Food waste and agro by-products: A step towards food sustainability. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food by-Products; IntechOpen: London, UK, 2021. [Google Scholar]
- Mao, X.-B.; Zhong, J.-J. Hyperproduction of cordycepin by two-stage dissolved oxygen control in submerged cultivation of medicinal mushroom Cordyceps militaris in Bioreactors. Biotechnol. Prog. 2004, 20, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Wei, T.; Lin, Y.; Ye, Z.-W.; Lin, J.-F.; Guo, L.-Q.; Yun, F.; Kang, L. Developing a novel two-stage Process for carotenoid production by Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2019, 21, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2004, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.A. Fungal morphology: A challenge in bioprocess engineering industries for product development. Curr. Opin. Chem. Eng. 2022, 35, 100729. [Google Scholar] [CrossRef]
- Petre, M.; Petre, V. Chapter 1—Biotechnology of mushroom growth through submerged cultivation. In Mushroom Biotechnology; Petre, M., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 1–18. [Google Scholar]
- Wu, C.-Y.; Liang, Z.-C.; Tseng, C.-Y.; Hu, S.-H. Effects of illumination pattern during cultivation of fruiting body and bioactive compound production by the caterpillar medicinal mushroom, Cordyceps militaris (Ascomycetes). Int. J. Med. Mushrooms 2016, 18, 589–597. [Google Scholar] [CrossRef]
- Ha, S.-Y.; Jung, J.-Y.; Park, J.-H.; Lee, D.-H.; Choi, J.-W.; Yang, J.-K. Effect of light-emitting diodes on cordycepin production in submerged Cordyceps militaris cultures. J. Mushroom 2020, 18, 10–19. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Cheung, P.C.K. Use of Stimulatory Agents To Enhance the Production of Bioactive Exopolysaccharide from Pleurotus tuber-regium by Submerged Fermentation. J. Agric. Food Chem. 2011, 59, 1210–1216. [Google Scholar] [CrossRef]
- Tang, J.; Qian, Z.; Wu, H. Enhancing cordycepin production in liquid static cultivation of Cordyceps militaris by adding vegetable oils as the secondary carbon source. Bioresour. Technol. 2018, 268, 60–67. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Wei, T.; Chen, Q. Stimulatory effects of oleci acid and fungal elicitor on betulinic acid production by submerged cultivation of medicinal mushroom Inonotus obliquus. J. Fungi 2021, 7, 266. [Google Scholar] [CrossRef]
- Guru, A.; Dwivedi, P.; Kaur, P.; Pandey, D.K. Exploring the role of elicitors in enhancing medicinal values of plants under in vitro condition. S. Afr. J. Bot. 2021, 149, 1029–1043. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, Y.; Lian, D.; Zhong, X.; Liu, X. Production of triterpenoids from Ganoderma lucidum: Elicitation strategy and signal transduction. Process Biochem. 2018, 69, 22–32. [Google Scholar] [CrossRef]
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Asatiani, M.; Kvesitadze, G. Use of Pleurotus dryinus for lignocellulolytic enzymes production in submerged fermentation of mandarin peels and tree leaves. Enzyme Microb. Technol. 2006, 38, 998–1004. [Google Scholar] [CrossRef]
- Elisashvili, V.; Penninckx, M.; Kachlishvili, E.; Tsiklauri, N.; Metreveli, E.; Kharziani, T.; Kvesitadze, G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour. Technol. 2008, 99, 457–462. [Google Scholar] [CrossRef]
- Metreveli, E.; Kachlishvili, E.; Singer, S.W.; Elisashvili, V. Alteration of white-rot basidiomycetes cellulase and xylanase activities in the submerged co-cultivation and optimization of enzyme production by Irpex lacteus and Schizophyllum commune. Bioresour. Technol. 2017, 241, 652–660. [Google Scholar] [CrossRef]
- Taskin, M.; Erdal, S.; GENISEL, M. Biomass and exopolysaccharide production by Morchella esculenta in submerged culture using the extract from waste loquat (Eriobotrya japonica L.) kernels. J. Food Process. Preserv. 2011, 35, 623–630. [Google Scholar] [CrossRef]
- Mokochinski, J.B.; Sovrani, V.; Dalla Santa, H.S.; Felsner, M.L.; Sawaya, A.C.H.F.; González-Borrero, P.P.; Bataglion, G.A.; Eberlin, M.N.; Torres, Y.R. Biomass and sterol production from vegetal substrate fermentation using Agaricus brasiliensis. J. Food Qual. 2015, 38, 221–229. [Google Scholar] [CrossRef]
- Ogidi, C.O.; Ubaru, A.M.; Ladi-Lawal, T.; Thonda, O.A.; Aladejana, O.M.; Malomo, O. Bioactivity assessment of exopolysaccharides produced by Pleurotus pulmonarius in submerged culture with different agro-waste residues. Heliyon 2020, 6, e05685. [Google Scholar] [CrossRef]
- Davitashvili, E.; Kapanadze, E.; Kachlishvili, E.; Elisashvili, V. Lectin activity of species of genus Cerrena S.F. Gray (Aphyllophoromycetideae) in submerged fermentation of lignocellulosic materials. Int. J. Med. Mushrooms 2011, 13, 159–166. [Google Scholar] [CrossRef]
- Kapri, M.; Singh, U.; Behera, S.M.; Srivastav, P.P.; Sharma, S. Nutraceutical augmentation of agro-industrial waste through submerged fermentation using Calocybe indica. LWT 2020, 134, 110156. [Google Scholar] [CrossRef]
- Beltrame, G.; Hemming, J.; Yang, H.; Han, Z.; Yang, B. Effects of supplementation of sea buckthorn press cake on mycelium growth and polysaccharides of Inonotus obliquus in submerged cultivation. J. Appl. Microbiol. 2021, 131, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Mshandete, A.M.; Mgonja, J.R. Submerged liquid fermentation of some tanzanian basidiomycetes for the production of mycelial biomass, exopolysaccharides and mycelium protein using wastes peels media. J. Agric. Biol. 2009, 4, 13. [Google Scholar]
- Do Rosário Freixo, M.; Karmali, A.; Arteiro, J.M. Production of polygalacturonase from Coriolus versicolor grown on tomato pomace and its chromatographic behaviour on immobilized metal chelates. J. Ind. Microbiol. Biotechnol. 2008, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Elisashvili, V.; Kachlishvili, E.; Asatiani, M.D. Efficient production of lignin-modifying enzymes and phenolics removal in submerged fermentation of olive mill by-products by white-rot basidiomycetes. Int. Biodeterior. Biodegrad. 2018, 134, 39–47. [Google Scholar] [CrossRef]
- Apohan, E.; Yesilada, O. Enhancement of laccase production of pre-grown fungal pellets in wastewater of olive oil mills. Fresenius Environ. Bull. 2011, 20, 1216–1224. [Google Scholar]
- Vaidya, V.; Carota, E.; Calonzi, D.; Petruccioli, M.; D’Annibale, A. Production of lignin-modifying enzymes by Trametes ochracea on high-molecular weight fraction of olive mill wastewater, a byproduct of olive oil biorefinery. New Biotechnol. 2019, 50, 44–51. [Google Scholar] [CrossRef]
- Zerva, A.; Papaspyridi, L.-M.; Christakopoulos, P.; Topakas, E. Valorization of Olive Mill Wastewater for the Production of β-glucans from Selected Basidiomycetes. Waste Biomass Valorization 2017, 8, 1721–1731. [Google Scholar] [CrossRef]
- Reverberi, M.; Di Mario, F.; Tomati, U. β-Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl. Microbiol. Biotechnol. 2004, 66, 217–225. [Google Scholar] [CrossRef]
- Papadaki, A.; Kachrimanidou, V.; Papanikolaou, S.; Philippoussis, A.; Diamantopoulou, P. Upgrading grape pomace through Pleurotus spp. Cultivation for the production of enzymes and fruiting bodies. Microorganisms 2019, 7, 207. [Google Scholar] [CrossRef]
- Hultberg, M.; Bodin, H. Fungi-based treatment of brewery wastewater—Biomass production and nutrient reduction. Appl. Microbiol. Biotechnol. 2017, 101, 4791–4798. [Google Scholar] [CrossRef]
- Wolters, N.; Schabronath, C.; Schembecker, G.; Merz, J. Efficient conversion of pretreated brewer’s spent grain and wheat bran by submerged cultivation of Hericium erinaceus. Bioresour. Technol. 2016, 222, 123–129. [Google Scholar] [CrossRef]
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of brewers’ spent grain for the production of laccase and polyphenols: Biovalorization of brewers’ spent grain. J. Inst. Brew. 2018, 124, 182–186. [Google Scholar] [CrossRef]
- Pinheiro, V.E.; Michelin, M.; Vici, A.C.; de Almeida, P.Z.; Teixeira de Moraes Polizeli, M.D.L. Trametes versicolor laccase production using agricultural wastes: A comparative study in Erlenmeyer flasks, bioreactor and tray. Bioprocess Biosyst. Eng. 2020, 43, 507–514. [Google Scholar] [CrossRef]
- Han, S.H.; Ahn, Y.; Lee, H.J.; Suh, H.J.; Jo, K. Antioxidant and immunostimulatory activities of a submerged culture of Cordyceps sinensis using spent coffee. Foods 2021, 10, 1697. [Google Scholar] [CrossRef]
- Oh, N.S.; Sung, N.Y.; Byun, E.H.; Kim, Y.E.; Cho, E.J.; Oh, G.H. Changes in mycelial growth and antioxidant activities of Basidiomycetous strains added with hydrolysates of spent coffee grounds as a substrate. J. Korean Soc. Food Sci. Nutr. 2018, 47, 90–95. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Chatterjee, S.; Chatterjee, B.P.; Guha, A.K. Enhancement of biomass production of edible mushroom Pleurotus sajor-caju grown in whey by plant growth hormones. Process Biochem. 2005, 40, 1241–1244. [Google Scholar] [CrossRef]
- Wu, X.; Hansen, C.L. Effects of whey permeate-based medium on the proximate composition of Lentinus edodes in the submerged culture. J. Food Sci. 2006, 71, M174–M179. [Google Scholar] [CrossRef]
- Asada, C.; Okumura, R.; Sasaki, C.; Nakamura, Y. Acceleration of Hericium erinaceum mycelial growth in submerged culture using yogurt whey as an alternative nitrogen source. Adv. Biosci. Biotechnol. 2012, 03, 828–832. [Google Scholar] [CrossRef]
- Velez, M.E.V.; da Luz, J.M.R.; da Silva, M.D.C.S.; Cardoso, W.S.; Lopes, L.D.S.; Vieira, N.A.; Kasuya, M.C.M. Production of bioactive compounds by the mycelial growth of Pleurotus djamor in whey powder enriched with selenium. LWT 2019, 114, 108376. [Google Scholar] [CrossRef]
- Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Gonou-Zagou, Z.; Kopsahelis, N. Trametes versicolor as a natural source of bioactive compounds for the production of whey protein films with functional properties: A holistic approach to valorize cheese whey. Waste Biomass Valorization 2022, 13, 3989–3998. [Google Scholar] [CrossRef]
- Kausar, S.; Zia, S.; Nadeem, Z.; Murtaza, M.A.; Hafiz, I.; Shaheen, B.A.; Hazrat, A.; Jan, T.; Mukhtiar, M.; Sher, F.; et al. Submerged cultivation of medicinal mushroom in hydrolysate of ligno- cellulosic material. Pak. J Pharm. Sci. 2019, 33, 2835–2841. [Google Scholar]
- Jones, M.P.; Lawrie, A.C.; Huynh, T.T.; Morrison, P.D.; Mautner, A.; Bismarck, A.; John, S. Agricultural by-product suitability for the production of chitinous composites and nanofibers utilising Trametes versicolor and Polyporus brumalis mycelial growth. Process Biochem. 2019, 80, 95–102. [Google Scholar] [CrossRef]
- André, A.; Diamantopoulou, P.; Philippoussis, A.; Sarris, D.; Komaitis, M.; Papanikolaou, S. Biotechnological conversions of bio-diesel derived waste glycerol into added-value compounds by higher fungi: Production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 2010, 31, 407–416. [Google Scholar] [CrossRef]
- Kim, S.P.; Park, S.O.; Lee, S.J.; Nam, S.H.; Friedman, M. A polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against a Salmonella lipopolysaccharide-induced endotoxemia. J. Agric. Food Chem. 2013, 61, 10987–10994. [Google Scholar] [CrossRef]
- Xie, C.; Wu, Z.; Guo, H.; Gu, Z. Release of feruloylated oligosaccharides from wheat bran through submerged fermentation by edible mushrooms. J. Basic Microbiol. 2014, 54 (Suppl. 1), S14–S20. [Google Scholar] [CrossRef]
- Meng, F.; Liu, X.; Jia, L.; Song, Z.; Deng, P.; Fan, K. Optimization for the production of exopolysaccharides from Morchella esculenta SO-02 in submerged culture and its antioxidant activities in vitro. Carbohydr. Polym. 2010, 79, 700–704. [Google Scholar] [CrossRef]
- Chiranjeevi, P.; Pandian, M.; Thadikamala, S. Enhancement of Laccase Production from Pleurotus ostreatus PVCRSP-7 by altering the Nutritional Conditions using Response Surface Methodology. BioResources 2014, 9, 4212–4225. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, P.; Zhu, Z.; Chen, C.; Xu, X. Production of phenolic compounds and antioxidant activity via bioconversion of wheat straw by Inonotus obliquus under submerged fermentation with the aid of a surfactant. J. Sci. Food Agric. 2021, 101, 1021–1029. [Google Scholar] [CrossRef]
- Lee, J.; Cho, K.; Shin, S.G.; Bae, H.; Koo, T.; Han, G.; Hwang, S. Nutrient recovery of starch processing waste to Cordyceps militaris: Solid state cultivation and submerged liquid cultivation. Appl. Biochem. Biotechnol. 2016, 180, 274–288. [Google Scholar] [CrossRef]
- Gang, J.; Fang, Y.; Wang, Z.; Liu, Y. Fermentation optimization and antioxidant activities of mycelia polysaccharides from Morchella esculenta using soybean residues. Afr. J. Biotechnol. 2013, 12, 1239–1249. [Google Scholar] [CrossRef]
- Shi, M.; Yang, Y.; Guan, D.; Zhang, Y.; Zhang, Z. Bioactivity of the crude polysaccharides from fermented soybean curd residue by Flammulina velutipes. Carbohydr. Polym. 2012, 89, 1268–1276. [Google Scholar] [CrossRef]
- Kurbanoglu, E.; Algur, O.; Zulkadir, A. Submerged production of edible mushroom Agaricus bisporus mycelium in ram horn hydrolysate. Ind. Crops Prod. 2004, 19, 225–230. [Google Scholar] [CrossRef]
- Mohamed, H.; Hassane, A.; Atta, O.; Song, Y. Deep learning strategies for active secondary metabolites biosynthesis from fungi: Harnessing artificial manipulation and application. Biocatal. Agric. Biotechnol. 2021, 38, 102195. [Google Scholar] [CrossRef]
- Turło, J. The biotechnology of higher fungi—Current state and perspectives. Acta Univ. Lodz. Folia Biol. Oecologica 2014, 10, 49–65. [Google Scholar] [CrossRef]
- Chai, K.F.; Ng, K.R.; Samarasiri, M.; Chen, W.N. Precision Fermentation to Advance Fungal Food Fermentations. Curr. Opin. Food Sci. 2022, 47, 100881. [Google Scholar] [CrossRef]
| Agri-Food Waste | Composition (% w/w) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Ash | Total Solids | Moisture | C/N Ratio | Reference | |
| Barley straw | 33.8 | 21.9 | 13.8 | 11 | - | - | - | [18] |
| Wheat straw | 32.9 | 24 | 8.9 | 6.7 | 95.6 | 7 | 50–80 | [18,26,48] |
| Wheat bran | 30 | 50 | 15 | - | - | - | 19 | [18,26] |
| Rice straw | 39.2 | 23.5 | 36.1 | 12.4 | 98.6 | 6.5 | 8–18 | [18,49] |
| Rice bran | 35 | 25 | 17 | - | - | 12–48 | [28] | |
| Oat straw | 39.4 | 27.1 | 17.5 | 8 | - | - | - | [18] |
| Sawdust | 45.1 | 28.1 | 24.2 | 1.2 | 98.5 | 1.12 | - | [18] |
| Sugar beet waste | 26.3 | 18.5 | 2.5 | 4.8 | 87.5 | 12.4 | - | [18] |
| Sugarcane bagasse | 30.2 | 56.7 | 13.4 | 1.9 | 91.6 | 4.8 | 70–120 | [18] |
| Corn stalks | 61.2 | 19.3 | 6.9 | 10.8 | 97.7 | 6.4 | - | [18,48] |
| Cotton stalks | 58.5 | 14.4 | 21.5 | 9.9 | - | 7.4 | 15 | [18,49] |
| Soya stalks | 34.5 | 24.8 | 19.8 | 10.3 | - | 11.8 | 20–40 | [18,45] |
| Sunflower stalks | 24.1 | 29.7 | 13.4 | 11.1 | - | - | 97 | [18,45] |
| Walnut shells | 3 | 628 | 43 | - | - | - | 175 | [28] |
| Almond shells | 38 | 29 | 30 | - | - | - | 61 | [28] |
| Chestnut shells | 21 | 16 | 36 | - | - | - | 8 | [28] |
| Pistachio shell | 43 | 25 | 16 | - | - | - | 43 | [28] |
| Hazelnut shell | 55 | 34 | 35 | - | - | - | 50–58 | [28] |
| Olive oil cake | 31 | 21 | 26 | - | - | - | 14–17 | [28] |
| Olive mill wastewater | - | - | - | 2.61 | 0.41 | - | 35–55 | [50] |
| Oil palm cake | 64 | 15 | 5 | - | - | - | - | [45] |
| Sunflower oil cake | 25 | 12 | 8 | - | - | - | - | [45] |
| Cotton seed hull | 31 | 20 | 18 | - | - | - | 59–67 | [45] |
| Potato peel waste | 2.2 | - | - | 7.7 | - | 9.89 | - | [45] |
| Orange peel | 9.2 | 10.5 | 0.84 | 3.5 | - | 11.86 | - | [45] |
| Lemon peel | 12 | 5 | 2 | [45] | ||||
| Pineapple peel | 18.11 | - | 1.37 | - | 93.6 | 91 | 41 | [45] |
| Apple pomace | 43 | 24 | 20 | - | - | - | 48 | [45] |
| Tomato pomace | 9 | 5 | 5 | - | - | - | - | [45] |
| Coffee skin | 23.7 | 16.6 | 28.5 | 5.3 | - | - | 14.4 | [51] |
| Spent coffee grounds | 12.4 | 39.1 | 23.9 | 1.3 | - | - | 16.9 | [26] |
| Cheese Whey | - | - | - | 0.5 | 0.1–22 | - | 57 | [52] |
| Brewer’s spent grain | 19 | 21 | 19 | 4.2 | 4.9 | - | - | [53] |
| Distillery wastes | - | - | - | - | 36 | - | 32 | [54] |
| Grape marc | 20.9 | 13.3 | 34.7 | - | 74 | 24 | [55,56] | |
| Wine lees | - | - | - | 10.5 | - | - | 6–13 | [57] |
| Mushroom Species | Bioactive Compound | Fruiting Body | Mycelium |
|---|---|---|---|
| Pleurotus ostreatus | Crude protein | 36% | 40.1% |
| Total lipids | 2% | 3% | |
| Non-polar lipids | 1.50% | 4% | |
| Fat | 1.90% | 2.6% | |
| Pleurotus ostreatoroseus | Free sugars | 26 mg/g | 58 mg/g |
| Total organic acids | 212 mg/g | 91 mg/g | |
| Pleurotus spp. | Proteins | 24.30% | 23.3% |
| Carbohydrates | 27.70% | 70.4% | |
| Fatty acids | 1.60% | 1.5% | |
| Pleurotus sajor-caju | Soluble carbohydrates | 19.55% | 4.1% |
| Proteins | 36.36% | 32.1% | |
| Total fat | 2.30% | 10.2% | |
| Agaricus campestris | Proteins | 46.30% | 44.4% |
| Fat | 2.80% | 2.8% | |
| Agaricus bisporus | Proteins | 45.90% | 47.1% |
| Fat | 2.70% | 5.8% | |
| Lentinus edodes | Proteins | 23–24% | 17% |
| L. edodes | Lipids | 4% | 20% |
| L. edodes | Proteins | 26.50% | 52.8% |
| Cordyceps militaris | Proteins | 18.47% | 21.1% |
| Total amino acids | 18.57% | 28.7% | |
| Tuber sinense | Total umami amino acids | 1.5 mg/g | 9.0 mg/g |
| Sterol content | 1883 μg/g | 5740.4 μg/g | |
| Tuber indigum | Total umami amino acids | 5.3 μg/g | 12.6 μg/g |
| Sterol content | 2204.2 μg/g | 6638.1 μg/g | |
| Tuber aestivum | Total umami amino acids | 7.1 mg/g | 11.6 mg/g |
| Sterol content | 3239.9 μg/g | 5792.5 μg/g |
| Sector | Substrate | Mushroom Species | Culture Conditions | Bioactive Compounds | Publication Year | Reference |
|---|---|---|---|---|---|---|
| Fruits and vegetables | Mandarin peels and tree leaves | Pleurotus dryinus | Shake flasks | Cellulase (47.7 U/mL), xylanase (71.7 U/mL), laccase (6493 U/mL), MnP (83 U/mL) | 2006 | [194] |
| Mandarin peels and tree leaves | P. dryinus, P. ostreatus, P. tuberregium | Shake flasks | Cellulase (62.3 U/mL), xylanase (84.1 U/mL), laccase (4103 U/mL) | 2008 | [195] | |
| Mandarin peels | Coculture of Irpex lacteus and Schizophyllum commune | Shake flasks | Cellulase (7 U/mL), endoglucanase (142 U/mL), xylanase (104 U/mL), β-glycosidase (5.2 U/mL) | 2017 | [196] | |
| Loquat kernel | Morchella esculenta | Shake flasks | EPS (5.4 g/L) | 2011 | [197] | |
| Apple pomace | P. sapidus | Shake flasks | Protein (25.4%) | 2019 | [24] | |
| Agrocybe aegerita | Protein (18.6%) | |||||
| Lentinula edodes | Protein (20.4%) | |||||
| P. sajor-caju | Protein (14.6%) | |||||
| P. salmoneostramineus | Protein (20.9%) | |||||
| Stropharia rugosoannulata | Protein (12.3 %) | |||||
| Apple pomace | Agaricus brasiliensis | Shake flasks | Sterols (1053 μg/g) Ergosterol (323 μg/g) | 2015 | [198] | |
| Plantain peels | P. pulmonarius | Shake flasks | EPS | 2020 | [199] | |
| Mango peels | EPS | |||||
| Pineapple peels | EPS | |||||
| Kiwi peels | Cerrena unicolor | Shake flasks | Lectins | 2011 | [200] | |
| Banana peels combined with groundnut cake | Calocybe indica | Shake flasks | Biomass (5.3 g/L), protein, glucans, minerals, flavonoids | 2018 | [201] | |
| Sea buckthorn press cake | Inonotus obliquus | Shake flasks | EPS (0.67 g/L) | 2021 | [202] | |
| Yam dextrose | Pleurotus spp. | Shake flasks | EPS (0.54 g/L), Protein (55%) | [203] | ||
| P. flabellatus | EPS (0.38 g/L), Protein (31%) | |||||
| Ganoderma lucidum | Protein (34%) | |||||
| Laetiporous sulphureus | Protein (39%) | |||||
| Tomato pomace | C. versicolor | Shake flasks | Polygalacturonase (1427 U/L) | 2008 | [204] | |
| Pectin | Polygalacturonase (3207 U/L) | |||||
| Olive oil | Olive mill wastewater | Cerrena unicolor | Shake flasks | Laccase (112.8 U/mL) | 2018 | [205] |
| P. οstreatus | Laccase (23.4 U/mL) | |||||
| G. lucidum | Laccase (18 U/mL) | |||||
| Pycnoporus coccineus | Laccase (18 U/mL) | |||||
| Trametella trogii | Laccase (29.7 U/mL) | |||||
| Trametes versicolor | Laccase (10.3 U/mL) | |||||
| Olive mill wastewater | T. versicolor | Shake flasks | Lacasse | 2011 | [206] | |
| Olive mill wastewater | Trametes ochracea | 3 L Bioreactors | Laccase (14,967 IU/L) MnP (16,856 IU/L) | 2009 | [207] | |
| Olive mill wastewater | G. resinaceum | Static flasks | EPS (0.79 g/L), IPS (5.2 g/L) | 2020 | [92] | |
| Olive mill wastewater | P. citrinopileatus | 2.5 L Lab scale bioreactor | Glucans (18.8 g/L) | 2016 | [208] | |
| G. lucidum | Glucans (19.7 g/L) | |||||
| P. ostreatus | Glucans (21.02 g/L) | |||||
| Olive mill wastewater | L. edodes | Shake flasks | β-1,3-Glucan synthase | 2005 | [60] | |
| P. ostreatus | β-1,3-Glucan synthase | |||||
| Olive mill wastewater and glycose | P. pulmonarius | Shake flasks | Biomass (32.76 g/L), IPS (4.38 g/L), lipids (2.85 g/L) | 2022 | [27] | |
| Winery, Brewery, Distillery | Grape marc | G. lucidum | 5 L Lab scale bioreactor | Protein (19.71%) | 2016 | [209] |
| Grape marc | L. edodes | Protein (19.96%) | ||||
| Grape marc | P. ostreatus | Protein (17.62%) | ||||
| Grape pomace hydrolysate | P. ostreatus | Shake flasks | Biomass (0.5 g/g of dry substrate), laccase, endoglucanase | 2019 | [210] | |
| P. pulmonarius | Biomass (0.54 g/g of dry substrate), laccase, endoglucanase | |||||
| Brewery wastewater | P. ostreatus | Shake flasks | Biomass (1.78 g/L) | 2017 | [211] | |
| T. versicolor | Biomass (1 g/L) | |||||
| Brewer’s spent grains | Hericium erinaceus | Shake flasks | Biomass, erinacine C (175 mg/g) | 2016 | [212] | |
| Brewer’s spent grains | T. versicolor | Shake flasks | Laccase (560 U/L), polyphenols | 2018 | [213] | |
| Vinasse and cotton gin waste | T. versicolor | Shake flasks, 3.3 bioreactor, static tray | Laccase (5005.55 U/L) | 2019 | [214] | |
| Malt | A. brasiliensis | Shake flasks | Sterols (2659 μg/g), Ergosterol (1267 μg/g) | 2015 | [198] | |
| Coffee | Spent coffee grounds | Cordyceps sinensis | Shake flasks | Glucosamine (140.3 μg/mL), Antioxidant activity (ABTS IC50, 0.93 mg/mL) | 2021 | [215] |
| Spent coffee grounds hydrolysate | Flammulina velutipes | Shake flasks | Biomass, improved antioxidant activity | 2018 | [216] | |
| P. ostreatus | Biomass, improved antioxidant activity | |||||
| C. militaris | Biomass, improved antioxidant activity | |||||
| P. linteus | Biomass, improved antioxidant activity | |||||
| Dairy | Deproteinized whey | P. sajor-caju | Shake flasks | Protein | 2005 | [217] |
| Whey permeate | L. edodes | Shake flasks | Biomass (2 g/L) | 2006 | [218] | |
| Yogurt whey | H. erinaceus | Shake flasks | β-glucans (1.7 g/L) | 2012 | [219] | |
| Whey powder | P. djamor | Shake flasks | β-glucans, ergosterol | 2019 | [220] | |
| Cheese whey | T. versicolor | Shake flasks | Biomass (26 g/L), protein 19.8% | 2022 | [221] | |
| Sugar industry | Beet molasses | M. rotunda | Static and shake flasks | EPS (3.94 g/L), IPS (3.34 g/L) | 2021 | [28] |
| M. vulgaris | EPS (3.93 g/L), IPS (4.24 g/L) | |||||
| M. conica | EPS (3.18 g/L), IPS (4.8 g/L) | |||||
| M. vulgaris | Static flasks | EPS (1.17 g/L), Lipids (2.32 g/L) | 2020 | [25] | ||
| M. elata | EPS (1.61 g/L), Lipids (1.93 g/L) | |||||
| Tuber aestivum | EPS (2.06 g/L), Lipids (5.91 g/L) | |||||
| Sugarcane bagasse hydrolysate | G. lucidum | Static flasks | Ganoderic acid (1.1 mg/L) | 2019 | [222] | |
| Blackstrap molasses | T. versicolor | Shake flasks | Biomass, ergosterol | 2019 | [223] | |
| Polyporus brumalis | Biomass, ergosterol | |||||
| Biodiesel | Bio-diesel derived glycerol | L. edodes | Shake flasks | Lipids (0.1 g/g of biomass) | 2010 | [224] |
| Bio-diesel derived glycerol | Τ. aestivum | Static flasks | EPS (0.54 g/L) | 2020 | [25] | |
| Cereals, legumes, nuts | Expired rice cereal hydrolysate | L. edodes | Static flasks | EPS (1.75 g/L), Lipids (0.28 g/L) | 2020 | [25] |
| M. vulgaris | EPS (1.67 g/L), Lipids (0.87 g/L) | |||||
| M. elata | EPS (2.17 g/L), Lipids (0.46 g/L) | |||||
| T. aestivum | EPS (1.80 g/L), Lipids (6.51 g/L) | |||||
| Black rice bran hydrolysate | L. edodes | Shake flasks | Bioprocessed polysaccharide | 2013 | [225] | |
| Expired wheat cereal hydrolysate | L. edodes | Shake flasks | EPS (0.98 g/L), Lipids (0.45 g/L) | 2020 | [25] | |
| M. vulgaris | EPS (0.59 g/L), Lipids (0.60 g/L) | |||||
| M. elata | EPS (1.34 g/L), Lipids (1.73 g/L) | |||||
| T. aestivum | EPS (1.25 g/L), Lipids (3.06 g/L) | |||||
| Wheat Bran | A. chaxingu | Shake flasks | Oligosaccharides (35.4 μM) | 2013 | [226] | |
| Wheat bran | M. esculenta | Shake flasks | EPS (2.91 g/L) | 2010 | [227] | |
| Wheat bran | P. ostreatus | Shake flasks | Laccase (12.124 U/L) | 2014 | [228] | |
| Wheat straw | I. obliquus | Shake flasks | Flavonoids (ECG 374.1 mg/g, EGCG 447.2 mg/g), Antioxidant activity (DPPH IC50 30.96 mg/L) | 2021 | [229] | |
| Wheat straw | G. lucidum | Shake flasks | Ganoderic acid (1.7 mg/L) | 2019 | [222] | |
| Starch processing waste | C. militaris | Shake flasks | Biomass (1.91 g/L/day) | 2016 | [230] | |
| Soybean residue | M. esculenta | Shake flasks | EPS (36.22 g/L) | 2013 | [231] | |
| Soybean curd residues | F. velutipes | Shake flasks | EPS (59.15 mg/g) | 2012 | [232] | |
| Groundnut shell | P. pulmonarius | Shake flasks | EPS (5.6 g/L) | 2020 | [199] | |
| Coconut coir | EPS (3.6 g/L) | |||||
| Walnut husk | EPS (2.1 g/L) | |||||
| Walnut leaves | C. unicolor | Shake flask | Protein (1.44 g/L), Lectins | 2011 | [200] | |
| Walnut pericarp | C. unicolor | Shake flasks | Protein (1.78 g/L), Lectins | |||
| Animal industry | Ram Horn Hydrolysate | A. bisporus | Shake flasks | Protein (47.1%), EPS | 2004 | [233] |
| Chicken feather hydrolysate | M. esculenta | Shake flasks | EPS (4.60 g/L) | 2011 | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilafidis, S.; Diamantopoulou, P.; Gkatzionis, K.; Sarris, D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Appl. Sci. 2022, 12, 11426. https://doi.org/10.3390/app122211426
Pilafidis S, Diamantopoulou P, Gkatzionis K, Sarris D. Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Applied Sciences. 2022; 12(22):11426. https://doi.org/10.3390/app122211426
Chicago/Turabian StylePilafidis, Sotirios, Panagiota Diamantopoulou, Konstantinos Gkatzionis, and Dimitris Sarris. 2022. "Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy" Applied Sciences 12, no. 22: 11426. https://doi.org/10.3390/app122211426
APA StylePilafidis, S., Diamantopoulou, P., Gkatzionis, K., & Sarris, D. (2022). Valorization of Agro-Industrial Wastes and Residues through the Production of Bioactive Compounds by Macrofungi in Liquid State Cultures: Growing Circular Economy. Applied Sciences, 12(22), 11426. https://doi.org/10.3390/app122211426







