Abstract
From the perspective of finding new, more environmental-friendly methods for the stabilization of sewage sludge to be used in agriculture, sludge treated with 15% bentonite, vermiculite or biochar was investigated as a soil amendment for white clover (Trifolium repens L.) growth, by means of a pot experiment. The sludge treatments, which were applied to two soils (an acid and an alkaline soil), in three replications, were the addition of 2% (≈80 Mg ha−1) treated sludge with the clay minerals or biochar, as well as limed or untreated (air-dried) sludge (for comparison reasons). Additional treatments with inorganic fertilization or neither organic nor inorganic fertilization (control) were also included. The application of 2% sludge to both soils significantly increased salinity compared to the control, which remained below harmful levels for sensitive crops, except for the case of untreated sludge. Furthermore, it significantly increased the soil-available macronutrients N, P and K, and micronutrients Cu, Zn (several times, especially in the case of untreated sludge) and B (up to three times) compared with the control. Moreover, the pH of the acid soil was improved, except for the case of limed sludge, where an undesirable pH increase close to 8.5 was observed. Addition of the treated sludge with the clay minerals or biochar and untreated sludge to the acid and alkaline soil significantly increased the aboveground biomass yield of white clover by 117–233% and 114–153%, respectively, compared to the control, whereas limed sludge had no effect. Plant nutrient uptake increased as well. In general, the effect of sludge on soil microbiological properties and arbuscular mycorrhizal fungal root colonization was ambiguous. It was concluded that 2% soil addition of sewage sludge treated with 15% bentonite, vermiculite or biochar could improve soil fertility and enhance plant growth; however, caution is needed with respect to potential risks of soil salinization or Zn and B phytotoxicities.
1. Introduction
Sewage sludge has long been utilized as a soil amendment to ameliorate soil properties and increase plant yield [1,2]. Sewage sludge contains considerable amounts of organic matter, nutrients essential for plant growth (mainly N, P), heavy metals, organic pollutants and pathogens, while its effect on soil environment is determined by treatment methods, soil properties and environmental factors [1]. Soil application of sewage sludge is regulated by relative legislations, e.g., the European Council Directive 86/278/EEC, which was adopted by Greek legislation, and the USEPA 625/R-95/001 [3,4], to protect human and environmental health by reducing pathogens and managing the heavy metal load. Although, sewage sludge’s fertilizing capacity is well renowned, the main methods of sludge treatment, i.e., aerobic and anaerobic digestion, composting, heating and alkaline treatment [5,6,7], do not focus on the preservation of its nutrient content. Reducing nutrient loss, especially N in the form of ammonia (NH3), during treatment, and thus enhancing its fertilizing efficiency and ameliorating capacity, could further increase sewage sludge’s merits for agriculture.
From the perspective of sludge’s agronomic use after its treatment with new, more environmental-friendly and easily-handled methods, the results of our previous research outline a reduction in the pathogen load, and also N loss after the treatment of sewage sludge with bentonite, vermiculite or biochar [8,9]. This was attributed to the considerable sorptive properties of these materials, which are well known from their ability to effectively adsorb heavy metals. Bentonite and vermiculite are 2:1 clay minerals with high cation exchange capacity (CEC), resulting in high cation and water-holding capacity. High CEC is due to both high negative charge and surface area [10]. Moreover, biochar has been investigated as an adsorbent of either organic or inorganic pollutants in the processes of soil and water decontamination [11]. Consequently, sewage sludge treated with bentonite, vermiculite or biochar could be used in agriculture as a soil amendment, although relevant studies are absent from the literature.
In sewage sludge-amended soils, the microbial respiration rate and biomass N (Nmic), metabolic quotient (qCO2) and arbuscular mycorrhizal fungal (AMF) root colonization could be investigated as indicators of soil health [12,13,14]. Initially, it is the labile C and N fractions of sewage sludge that promote microbial activity, whereas stress caused by potential increased heavy metal availability and salinity can be inhibitory [12]. AMF colonization can support nutrient and water uptake by plants and promote tolerance to soil abiotic stress (e.g., heavy metal toxicity, salinity and drought). Moreover, increased P uptake and reduced Zn uptake were evidenced in AMF-colonized white clover plants, grown in a Zn-contaminated soil [13]. However, AMF colonization on white clover’s roots has not been widely investigated after the soil application of sewage sludge.
Several researchers studied the effect of soil-applied sewage sludge on various plant species, including corn (Zea mays L.), wheat (Triticum aestivum L.), bean (Phaseolus Vulgaris L.), cannabis (Cannabis sativa L.), clovers (Trifolium repens L., Trifolium pratense L.) and ryegrass (Lolium perenne L.) [7,15,16]. White clover is the most important pasture legume, and is cultivated solely or along with other plants for its significant forage value [15]. Increasing cultivation and productivity of white clover through soil application of sewage sludge is in accordance with the European Green Deal since inorganic N fertilization in arable soils can be reduced by both sewage sludge’s N content and white clover’s atmospheric N2 fixation [17]. The response of white clover to liming and inorganic fertilization has also been investigated so far [18,19,20]. Clover has also been used as an indicator plant for investigating the effects of soil-applied sewage sludge [15]. However, white clover’s growth was usually studied in sludge-amended pastures or silvopastures where white clover was cultivated along with other plant species including grasses, legumes and trees. Thus, sole investigations of white clover’s growth in sludge-amended soils are rarely found in the literature [16].
Based on the above, and since the effects of sewage sludge treated with bentonite, vermiculite or biochar on soil properties and plant growth have not been investigated, sewage sludge stabilized with the aforementioned materials was evaluated as a soil amendment, in comparison to limed or untreated sludge, by means of a pot experiment with white clover (which was used as a test plant). Specifically, dewatered sewage sludge stabilized with 15% bentonite, vermiculite or biochar, or calcium hydroxide (Ca(OH)2) or air-dried sludge on its own were added to two soils (one acid and one alkaline and calcareous) at the rate of 2% (≈80 Mg ha−1), and the objectives of this study were to evaluate the effects of sludge on: (i) soil fertility, and chemical and microbiological properties and (ii) yield, composition and nutrient uptake by white clover plants, in comparison with inorganic fertilization, or no organic or inorganic fertilizer addition.
2. Materials and Methods
2.1. Materials
The clay minerals bentonite and vermiculite were provided from Milos island and northern Greece (n. Greece), respectively, whereas biochar was provided from Crete island. Certain properties of the materials are presented in Table 1. Briefly, all materials had alkaline to strongly alkaline reaction and low salinity. The CECs of the two minerals were considerable but close to the lowest range value reported in literature for smectites (i.e., bentonite) and vermiculite, 80–150 and 100–200 cmolc kg−1, respectively [21]. Among materials, only biochar contained N, P and B, from other plant nutrients compared with those presented in Table 1, at concentrations of 0.81 ± 0.09%, 0.11 ± 0.01% and 24.1 ± 0.9 mg kg−1, respectively [22]. As for the seven heavy metals, which are included in the European Council Directive 86/278/EEC [3] regarding the agronomic use of sewage sludge, Cu, Zn, Ni, Cr and Pb were detectable in all or some of the materials and Cd and Hg were not detectable in all cases [22].

Table 1.
Certain chemical properties and total composition (expressed on dry weight basis) of sewage sludge and the materials used for its stabilization. CEC: cation exchange capacity; EC: electrical conductivity; SAR: sodium adsorption ratio; ND: not detectable.
Moreover, dewatered sewage sludge was collected from the urban sewage sludge treatment plant of Thessaloniki (n. Greece) in July 2020 and some of its properties are presented in Table 1. In general, sludge had 86.2% moisture, almost neutral pH, low salinity and contained variable water-soluble and total amounts of macro- and micronutrients essential for plant growth (Table 1). Moreover, it contained 4.96 ± 0.52% N and 18.0 ± 1.6 mg kg−1 B, and the loss on ignition (LOI) was 63.0 ± 1.3% [22]. Cadmium and Hg were not detectable, whereas total concentrations of Cu, Zn, Ni, Cr and Pb had levels ranging far below the critical limits [22], according to the European Council Directive 86/278/EEC [3] and the 3rd draft of working document on sludge of European Commission (2000) for Cr [23].
The specific clay minerals, among others, as well as the biochar had been tested as materials for sewage sludge stabilization in a previous study of ours [9,22]. The results of that study showed that the aforementioned materials added to dewatered sewage sludge at 15% decreased its microbial load considerably; the observed reduction in fecal indicators was between one and two logarithmic units. Based on these results, quantity of treated sludge with 15% addition of bentonite (B), vermiculite (V) or biochar (Bc) was prepared. Additional sludge treatments were limed sludge (with 15% Ca(OH)2) (L) and untreated (addition of no material) (D) sludge. After air-drying of all sludge treatments, the treated and untreated sludge was ground to pass a 4-mm sieve and used for the preparation of the soil treatments of the current study.
2.2. Soil Treatments
Surface samples (0–20 cm) of two loamy soils were collected from n. Greece, one acid and one alkaline and calcareous. Soil samples were air-dried, ground to pass a 10-mm sieve and used for the pot experiment. Then, the treated and untreated (air-dried) sludge mixtures were added at a 2% rate (≈80 Mg ha−1) to the two soils. Additional treatments were inorganic fertilization, i.e., N-P-K (IF) and P-K (IFX) corresponding to 100 mg N kg−1 as NH4NO3, 60 mg P and 75 mg K kg−1 as KH2PO4, and no organic or inorganic fertilization (control). Specifically, eight treatments were added to each soil, in three replications: acid (Ac) soil with (i) bentonite-treated sludge (AcB), (ii) vermiculite-treated sludge (AcV), (iii) biochar-treated sludge (AcBc), (iv) limed sludge (AcL), (v) untreated (air-dried) sludge (AcD), (vi) N-P-K inorganic fertilization (AcIF), (vii) P-K inorganic fertilization (AcIFX) and (viii) control (AcC); alkaline (Al) soil with (i) bentonite-treated sludge (AlB), (ii) vermiculite-treated sludge (AlV), (iii) biochar-treated sludge (AlBc), (iv) limed sludge (AlL), (v) untreated (air-dried) sludge (AlD), (vi) N-P-K inorganic fertilization (AlIF), (vii) P-K inorganic fertilization (AlIFX) and (viii) control (AlC). After equilibration for ≈20 days, employing wetting with deionized water and mixing, a quantity of each air-dried soil treatment was passed through a 2-mm sieve for laboratory analysis and the rest material was used for the pot experiment.
Soil treatments were analyzed for the following properties: pH in a 1:2 (w/v) suspension with water, CaCO3 with a calcimeter, organic carbon (OC) by the wet oxidation method [24], Kjeldahl-N [25], cation exchange capacity (CEC) [26] and electrical conductivity of the saturation extract (ECse) along with water-soluble Na, Ca and Mg. In addition, soil-available NH4-N and NO3-N were extracted with 1 M KCl, P with 0.5 M NaHCO3, K, Ca and Mg with 1 M CH3COONH4, B with the hot water method and Cu, Zn, Fe and Mn with 0.005 M DTPA [27]. Moreover, total Cu, Zn, Ni, Cr, Cd, Pd and Hg were determined after digestion with aqua regia [28]. After the pot experiment, all soil treatments were air-dried, ground to pass through a 2-mm sieve, and all the aforementioned properties were determined again, except for CEC and total heavy metals. All analyses were conducted in triplicate.
As far as the methods used for the analytical determinations of the chemical species are concerned, NO3-N and NH4-N were determined by ultraviolet spectrometry and the sodium salicylate–sodium nitroprusside method [29], respectively, P by the molybdenum blue ascorbic acid [30], B by the azomethine-H method [31], K and Na by flame photometry and the other metals by atomic absorption spectrometry. Additionally, sodium adsorption ratio (SAR) was calculated from the water-soluble concentrations of Na, Ca and Mg.
2.3. Pot Experiment with White Clover
Pots were filled with one kg of soil treatment and sown with 0.5 g white clover seeds (≈830 seeds), at the beginning of May 2021. The experimental design was completely randomized (CRD) and randomization was repeated every 15 days. Pots received irrigation with deionized water regularly and as needed. The pot experiment lasted for ≈60 days and at the end of the experiment, aboveground biomass was harvested, oven-dried (70 °C) till constant weight and the aboveground biomass yield per pot was calculated. Then, aboveground biomass was analyzed in triplicate for Kjeldahl-N and, after dry ashing, for Ρ, Κ, Ca, Mg, B, Cu, Zn, Fe, Mn, Ni, Cr, Cd, Pb and Hg, employing the same methods of analytical determination reported in the previous subsection. Plant uptake of the elements per pot was calculated (dry aboveground biomass yield times concentration of the elements). After the pot experiment, roots were separated from soil treatments, washed, oven-dried (70 °C) till constant weight and the root biomass yield per pot was calculated. In addition, total dry biomass yield per pot was calculated by summation of the aboveground and root biomass.
2.4. Microbiological Properties
Microbial respiration rate and Nmic were determined in all treatments of both soils, before and after the pot experiment. Microbial respiration rate was determined by trapping CO2 in 1 M NaOH and titrating with 1 M HCl [32], while Nmic was determined with the chloroform fumigation extraction method [33], colorimetrically using ninhydrin. In addition, the qCO2 was calculated. After the experiment, a portion of roots were cleared in 10% KOH for 45 min at 80 °C, rinsed with tap water and immersed in 2.5% HCl for 30 min. Subsequently, roots were stained with Trypan blue [34] and AMF colonization was estimated on slides with a microscope [35].
2.5. Statistical Analysis
For each soil and plant property determined within the same soil, ANOVA was conducted using the statistical package SPSS, version 26 and the LSD test was used for mean comparisons, at p ≤ 0.05.
3. Results and Discussion
3.1. Effect of Treated Sludge on Soil Fertility, and Chemical and Microbiological Properties
In agreement with other researchers’ findings [2,7], soil application of treated or untreated sludge significantly increased the pH of the acid soil (Table 2). This could be beneficial for white clover’s growth since legume productivity is higher nearer to neutral pH values [36]. The highest pH value (8.4) was observed upon limed sludge addition because of the Ca(OH)2 content. The pH of alkaline soil treatments ranged at levels similar to the control because of the soil’s buffering capacity originating from its CaCO3 initial content (Table 2). The same trend has been reported upon the application of limed sludge to alkaline soils [37]. Although in all cases pH remained at levels below the critical value of 8.5 that stands for alkalinity problems [21], a pH value of 8.4 was rather undesirable since such high pH values represent a potential environmental hazard [38].

Table 2.
Certain properties of the soil treatments before the pot experiment with white clover. CEC: cation exchange capacity; ECse: electrical conductivity of the saturation extract; SAR: sodium adsorption ratio; ND: not detectable; NS: non-significant. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil and property, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
The CEC of alkaline soil, which was considerable, was not affected by treatment in any case, whereas it was significantly increased in all treatments with sludge on the acid soil. The latter was attributed to the pH increase, which resulted in an increase of the variable negative charge, due to deprotonation of structural and functional hydroxyls [39]. This agrees with the fact that the highest CEC increase was observed in the limed sludge treatment (AcL), which had a strongly alkaline reaction (Table 2). The ECse of both soils significantly increased in all treatments with the sludge compared to the control (Table 2), due to sludge’s water-soluble salt content. Although salinity is not inhibitory towards the growth of white clover [36], it is notable that the ECse of treatments with air-dried sludge (AcD, AlD) of both soils exceeded the critical value of 2 dS m−1 for a salinity hazard to sensitive crops [21], whereas it was lower in all other cases. The same treatments also had the significantly highest SAR values (Table 2), probably because of sludge’s considerable water-soluble Na. However, in all cases, no sodicity hazard was evidenced. The results concerning Kjeldahl-N were rather inconclusive, especially for the alkaline soil, whereas the treatment of the acid soil with the untreated sludge (AcD) had the highest N content. However, OC content significantly increased in most of the treatments with sludge on both soils compared to the control because of sludge’s organic matter content. Both soils with biochar-treated sludge (AcBc, AlBc) had the significantly highest OC content (Table 2) because of biochar’s contribution. As is reported in the literature, both the total N and OC contents of sewage sludge are not strongly affected by sludge’s treatment, whereas a major loss of water-soluble nutrients is denoted [1].
The beneficial effect of treated or untreated sludge on the pH and CEC of the acid soil also persisted after the pot experiment. Unfortunately, the treatment effect on the ECse of both soils also persisted. Specifically, not only did the treatments with air-dried sludge of both soils maintain ECse above the critical value of 2 dS m−1 [21], but the treatments with limed sludge of both soils and those with sludge treated with clay minerals or biochar of the alkaline soil did too. In all these cases, ECse was almost below 3 dS m−1. Since the biological experiment lasted for a short period of time and sewage sludge’s degradation is very slow, the Kjeldahl-N and OC contents of all soil treatments after the experiment remained at levels similar to the initial values (data not shown).
The initial heavy metal load of treated and untreated sludge [9,22] was either not detectable or below prohibitive levels for the soil application of sewage sludge according to the European Council Directive 86/278/EEC [3] and the third draft of the working document on sludge of the European Commission (2000) for Cr [23]. Consequently, upon soil application of treated or untreated sludge, although total concentrations of heavy metals slightly increased, they remained, in most cases, below the critical limits of legislation [3,23]. In acid soil treatments, the total concentrations of Cu, Zn, Ni, Cr and Pb ranged between 24 and 32, 51 and 85, 27 and 32, 10 and 13 and 24 and 28 mg kg−1, respectively, while in alkaline soil treatments, the corresponding concentrations ranged between 30 and 32, 44 and 55, 161 and 173, 107 and 112 and 9 and 11 mg kg−1. In all cases, Cd and Hg were not detectable. However, total Ni and Cr concentrations of the alkaline soil treatments were above the critical limits of the aforementioned legislation [3,23] due to the soil’s parent material.
Both treated and untreated sludge had considerable readily available nutrient contents [9,22]. Therefore, available N (NH4-N, NO3-N), P and K significantly increased in all sludge treatments of both soils, which agrees with the findings of other researchers [7,40], as well as in the inorganic fertilization treatments, compared to the control (Table 3). In most cases, N (NH4-N, NO3-N), P and K availability was higher in the inorganically fertilized treatments of both soils because N, P and K were applied in the forms of NH4NO3 and KH2PO4 solutions. Among the treatments with sludge of both soils, those with biochar-treated sludge (AcBc, AlBc) had the lowest available N content. For the acid soil, the highest NH4-N concentrations were observed in the treatments with air-dried sludge (AcD) and bentonite-treated sludge (AcB). Probably because of the presence of bentonite in the latter case, a considerable amount of NH4-N was retained as exchangeable, after its release from sludge during organic N mineralization. The treatment with the limed sludge had the highest concentration of NO3-N, probably because of the strongly alkaline pH of the particular treatment, which favors organic N mineralization–nitrification [41]. As far as the alkaline soil is concerned, the highest NH4-N concentration was obtained upon the addition of limed sludge, whereas the highest NO3-N concentration was observed in the AlD treatment (Table 3). The significant increase in available P in all sludge treatments of both soils was expected (Table 3) since the sewage sludge contained considerable amounts of P [9,22], and this is in agreement with other researchers’ findings [7]. Moreover, inorganic P accounts for approximately 70–90% of total P in sewage sludge [42]. Available concentrations of K of both soils were mainly indigenous, while a small portion was attributed to sludge’s K since its K content is usually very low [1]. As far as the available Ca and Mg are concerned, it is worth noting that for both soils, Ca concentration was the highest in the treatments with limed sludge (AcL, AlL) due to its Ca(OH)2 content, whereas Mg concentration was the highest in the treatments with vermiculite-treated sludge (AcV, AlV) due to the interlayer Mg of the mineral (Table 3). After the pot experiment, the available concentrations of NH4-N, NO3-N and P of all treatments on both soils remained increased compared to the control, whereas those of K ranged at levels similar to the control (data not shown).

Table 3.
Concentrations of available macronutrients of soil treatments before the pot experiment with white clover. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil and property, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
The available concentrations of Cu and Zn increased in all treatments with the sludge of both soils compared to the control (Table 4) due to the considerable content of sludge [22]. As far as Zn is concerned, its availability increased 6.5–9.5 and 5.0–7.5 times upon the addition of treated or untreated sludge to the acid and alkaline soil, respectively, in comparison to the control. The highest increases were observed in the air-dried sludge treatments of both soils (Table 4). Nickel and Pb are scarcely added to soils through sewage sludge [1]; in the current study the results concerning these heavy metals were rather inconclusive (Table 4). It is worth mentioning that the available concentrations of Fe and Mn decreased or remained unchanged upon the addition of sludge to both soils (Table 4). This was attributed to the pH increase in the acid soil and to the alkaline pH in all cases of the alkaline soil since pH is the main soil factor that regulates the availability of the two micronutrients [21]. The available B of both soils significantly increased upon the addition of treated or untreated sludge in comparison to the control (Table 4). Although in all cases B remained below or within the sufficiency range for most crops (0.5–2 mg kg−1) [34], the 1.5–3 times increase observed in certain treatments, and especially those with the air-dried sludge, is of concern since the range values of B deficiency–sufficiency–toxicity are too close to each other (0.5 mg kg−1; 0.5–2 mg kg−1; >2 mg kg−1) [43].

Table 4.
Concentrations of available micronutrients and heavy metals of soil treatments before the pot experiment with white clover. Available Cr, Cd and Hg were not detectable. NS: non-significant. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil and property, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
The same trends, observed for the soil-available metals and B of the initial treatments of both soils, were also observed, and after the pot experiment. It should be mentioned that the aforementioned increases in Zn and B persisted. More specifically, Zn was increased 2.7–7.4 and 5.1–11.6 times in treatments with treated or untreated sludge of the acid and alkaline soil, respectively, compared to the control. As far as B is concerned, its concentration in the acid soil treatments with sludge increased by up to 2.5 times relative to the control, whereas in the alkaline soil treatments B was at levels close to the control. Again, the highest increases for both elements and soils were observed in the air-dried sludge treatments (data not shown).
Before the establishment of the pot experiment, microbial respiration was similar among treatments compared to the control for both soils, and the overall mean values were 0.20 ± 0.19 and 0.26 ± 0.13 mg CO2 g−1 d−1 for the acid and alkaline soils, respectively. Similar results were obtained after the biological experiment and the respective overall mean values, much lower than the initial values, were 0.05 ± 0.02 and 0.08 ± 0.05 mg CO2 g−1 d−1, for the acid and alkaline soils. As far as Nmic is concerned, it significantly increased after the application of treated or untreated sludge to acid soil (19.9–30.8 mg g−1) compared to the control (1.2 mg g−1), indicating the conversion of added and indigenous soil N into microbial biomass. However, this increase was not apparent after the pot experiment, probably because a large part of the labile nutrient sources was consumed by plants and microorganisms [44]. For the alkaline soil, Nmic only significantly increased in the treatment with limed sludge (AlL) (12.5 mg g−1) before the pot experiment and in the treatment with vermiculite-treated sludge (AlV) (24.2 mg g−1) after the pot experiment, compared to the control of each case (7.4 mg g−1 and 12.2 mg g−1, respectively). That occurred probably because the particular treatments had the highest available N content for the respective periods. Only for the acid soil was qCO2 of the initial treatments with treated or untreated sludge significantly lower than the control. This could be attributed to the pH increase caused by sludge application and the consequent reduction in pH-induced stress [44]. However, no significant differences were obtained in respect to qCO2 among the treatments for both soils after the pot experiment.
3.2. Effect of Treated Sludge on Yield, Composition and Nutrient Uptake by White Clover
The aboveground and total (aboveground plus root) biomass yields of white clover were significantly affected by treatment, in both soils. In the acid soil treatments, the highest total and aboveground biomass yields were observed in air-dried sludge treatment (AcD), the latter also being similar to that of treatments AcB and AcV (Figure 1a). Moreover, acid soil treatments with bentonite-, vermiculite- and biochar-treated sludge (AcB, AcV, AcBc) had similar aboveground and total biomass yields, which were higher than the control but also higher than the AcL treatment and inorganically-fertilized treatments (AcIF, AcIFX). Specifically, aboveground biomass yields were significantly increased by 233, 150, 140 and 117% in AcD, AcV, AcB and AcBc treatments, respectively, compared to the control. The order of treatments regarding the root biomass yield was AcD > AcBc > AcB > AcIFX > AcV > AcC > AcIF > AcL. The beneficial effect of treated sludge with the clay minerals or biochar on the yield of white clover was attributed to the improvement in the soil’s physical and chemical properties due to the supply of soils with organic matter and nutrients, as was also reported in studies with crops other than white clover [45,46]. Moreover, the slight increase in the pH (>5.0) of acid soil upon the addition of the aforementioned treated sludge cannot be excluded as a beneficial factor, which resulted in the better growth and, consequently, the obtained (positive) response of white clover plants to the particular treatments. This can also justify the fact that no response of white clover was observed for the inorganically-fertilized treatments (AcIF, AcIFX) (pH < 5.0), and also for the limed sludge treatment (AcL) (pH = 8.4). Furthermore, similar increases in the aboveground biomass of white clover, to those obtained in the present study, have been reported for plants grown in an acid soil of pH 6.1 under different rates of P-K fertilization [20].
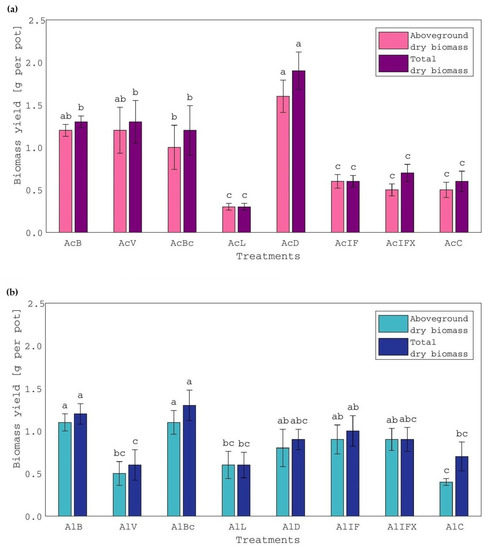
Figure 1.
Aboveground and total dry biomass yield of white clover plants, grown in (a) acid soil treatments and (b) alkaline soil treatments. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil and property, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
The concentrations of macronutrients in the aboveground biomass of the plants grown in acid soil treatments ranged between 19.9 and 43.8, 2.2 and 3.7, 5.6 and 42.9, 6.4 and 30.5 and 2.0 and 3.3 g kg−1 for N, P, K, Ca and Mg, respectively. Comparing these concentrations with those reported in the literature [19,47], it was evidenced that plant N, P, K and Mg contents were within the common range in all cases, whereas the Ca content was low in all cases, except for the limed sludge treatment (AcL).
In each acid soil treatment, the uptake of N, P and K by white clover plants followed the same pattern as the aboveground biomass yield. Specifically, the uptake of these macronutrients by plants grown in the treatments with bentonite- (AcB), vermiculite- (AcV) or biochar (AcBc)-treated or untreated (AcD) sludge was significantly higher than all other treatments and the control (Figure 2a). This was attributed again to the pH effect, meaning that the initial strongly acidic soil pH slightly increased, establishing better growing conditions for white clover, while sludge supplied soil with nutrients [1]. In the case of the limed sludge treatment (AcL), the pH increase to the strongly alkaline range probably depressed P uptake leading to insufficient development of the roots (the lowest root biomass among all treatments) and inevitably creating an inability for nutrient absorption (e.g., N, K). Therefore, the limed sludge treatment on the acid soil (AcL) delivered a lower aboveground dry biomass yield by 32% compared to the control (Figure 1a). The latter agrees with white clover’s sensitivity to P and K deficiency [12]. Although a higher uptake by plants grown in the inorganically-fertilized treatments was expected compared with that observed, since readily available forms of macronutrients were applied, the uptake was similar to the control in all cases of the macronutrients, probably because of the low soil pH (Figure 2a).
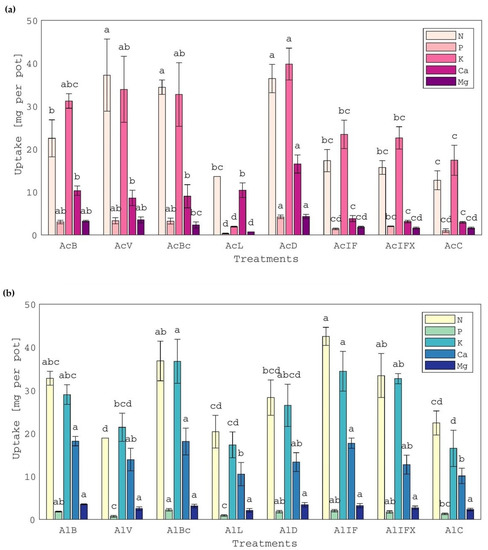
Figure 2.
Uptake of macronutrients by white clover plants, grown in (a) acid soil treatments and (b) alkaline soil treatments. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil and property, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
As far as the alkaline soil treatments are concerned, the highest total and aboveground biomass yields were observed in the bentonite- and biochar-treated sludge treatments (AlBc, AlB), which were also similar to the yields of the air-dried sludge (AlD) and the inorganic fertilization treatments (Figure 1b). Aboveground biomass yields significantly increased by 153, 147, 116, 114 and 107% in AlBc, AlB, AlIF, AlD and AlIFX treatments, respectively, compared to the control. According to root biomass yields, treatments followed the order AlB > AlBc > AlD > AlL > AlIF > AlC > AlV > AIFX. The nitrogen, P, K, Ca and Mg contents of white clover plants grown in alkaline soil treatments were within the common range [19,47] and the respective concentrations were 26.6–42.8, 1.7–2.2, 27.6–37.7, 14.3–42.1 and 3.2–5.2 g kg−1. Plant uptake of macronutrients followed the same pattern as biomass yield and the poor effect of treatments with limed and vermiculite-treated sludge (AlL, AlV) on white clover yield was accompanied by reduced N, P and K uptake (Figure 2b).
Soil pH increments promoted the availability and, consequently, the plant uptake of Cu, Zn and B in the acid soil treatments with bentonite- or vermiculite-treated or untreated sludge, compared to the control and also to the inorganic fertilization treatments (Figure 3a). Conversely, plant uptake of almost all micronutrients after the treatment with the limed sludge (AcL) was similar to or lower than the control, probably because of the strongly alkaline reaction of the specific treatment. It was observed that Zn uptake was depressed in the treatment with the biochar-treated sludge (AcBc) (Figure 3a); although no effect of the particular treatment on soil-available Zn was observed. Concentrations of Cu, Zn, Fe, Mn and B in white clover plants grown in acid soil treatments were either within usual ranges or excessive [19,47] and ranged between 11.3 and 42.4, 18.5 and 53.5, 341 and 1376, 179 and 1326 and 54.1 and 69.7 mg kg−1, respectively. Concentrations of Ni, Cd, Pb and Hg were not detectable in all of the treatments of both soils. In agreement with these findings, increased metal uptake has been reported for white clover plants grown in sewage sludge-amended soils with a pH of 5.1 [15]. In all alkaline soil treatments, micronutrient uptake occurred at levels several times lower than acid soil treatments, probably because the pH remained close to the initial strongly alkaline value in all cases (Figure 3b and compared with Figure 3a). However, Zn uptake increased in all treatments with sludge in comparison to the control and inorganically-fertilized treatments (Figure 3b), despite the strongly alkaline pH. Concentrations of micronutrients in white clover plants grown in the alkaline soil treatments were either within or below usual ranges [19,47] in all cases and ranged between 11.4 and 20.3, 9.5 and 27.4, 281 and 465, 71 and 85 and 29.5 and 39.4 mg kg−1, for Cu, Zn, Fe, Mn and B, respectively.
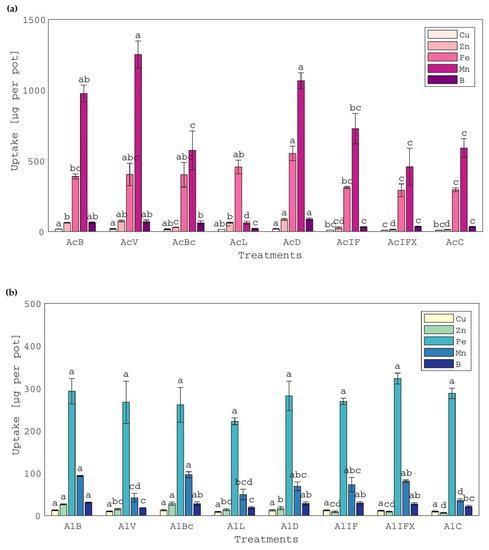
Figure 3.
Uptake of micronutrients by white clover plants, grown in (a) acid soil treatments and (b) alkaline soil treatments. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil and property, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
In agreement with the aforementioned findings of the current study concerning plant parameters, other researchers [7,48,49] also reported no clear effect of sewage sludge on plant composition. However, similar beneficial results have been reported for nutrient uptake by various crops, such as grasses, legumes, wheat and sugar beet, after their fertilization with sewage sludge in the form of raw, dewatered, composted, limed etc. [7,48,50].
Although AMF root colonization was the highest (51%) in inorganically-P-K-fertilized treatment of the acid soil (AcIFX) (Figure 4a), this had no effect on the yield and nutrient uptake of plants. As is shown in Figure 1a, a lower aboveground biomass was observed in that particular treatment compared with the treatments with sludge, except for the limed sludge (AcL) treatment, which was also similar to the control. It has been suggested that when P availability increases, the relationship between the clover host and the AMF changes from symbiosis to parasitism [51]. The AMF colonization ranged between 32 and 36% among all other treatments with no significant differences, including the control (Figure 4a). The alkaline soil offered better conditions for AMF colonization (41–59%), possibly due to lower P availability compared to acid soil, as this was deduced from P concentration ranges in the aboveground biomass of white clover (2.2–3.7 and 1.7–2.2 g kg−1, for acid and alkaline soil treatments, respectively). Nevertheless, no significant differences were observed among treatments as far as AMF root colonization is concerned (Figure 4b). According to the literature, the highest mycorrhizal fungal root colonization (60%) was reported when biochar was added along with AMF, and P fertilization was absent [15]. Although white clover is highly mycotrophic and AMF colonization is expected to promote plant growth and increase P absorption [13], the results of this study were rather poor or inconclusive, probably because the available P of all treatments of both soils (Table 3) was well above the lower limit of 10 mg kg−1 of the P sufficiency range [52]. Furthermore, no visible nodulation on the roots of white clover was achieved by indigenous rhizobia; thus, no determination or analysis were conducted. This was attributed to the absence of white clover-specific rhizobia strains (e.g., Rhizobium leguminosarum bv. trifolii) in both original soils that could nodulate plant roots, rather than potential sludge-induced stress [53].
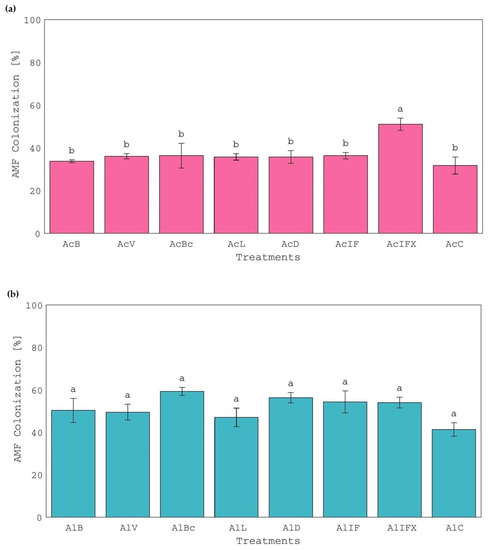
Figure 4.
Percentages of AMF root colonization of white clover plants, grown in (a) acid soil treatments and (b) alkaline soil treatments. Treatment of the acid and alkaline soil, respectively, with: AcB and AlB: bentonite-treated sludge; AcV and AlV: vermiculite-treated sludge; AcBc and AlBc: biochar-treated sludge; AcL and AlL: limed sludge; AcD and AlD: air-dried sludge; AcIF and AlIF: N-P-K inorganic fertilization; AcIFX and AlIFX: P-K inorganic fertilization; AcC and AlC: control. Different letters, within each soil, indicate significant differences among means, using the LSD test, at p ≤ 0.05.
4. Conclusions
In a previous study of ours, the clay minerals bentonite and vermiculite, as well as biochar, seemed to be promising materials for sewage sludge stabilization because of their sorptive and bactericidal properties. In the current study, sewage sludge, treated with 15% of the aforementioned materials, was evaluated as a soil amendment in comparison to limed or untreated sludge, using white clover as a test plant. It was concluded that soil application of 2% (≈80 Mg ha−1) sewage sludge treated with bentonite, vermiculite or biochar could improve the pH and cation exchange capacity of acid soils and enhance the fertility of acid and alkaline soils, with respect to both essential macro- and micronutrients for plant growth. As a result of the beneficial effect of treatment with minerals or biochar sludge on soil properties, plant aboveground and root biomass yields could increase considerably, as well as nutrient absorption and uptake by plants, whereas effects on soil microbiological properties and arbuscular mycorrhizal fungal root colonization were ambiguous. As far as undesirable effects are concerned, soil salinity along with Zn and B availability must be closely monitored in sludge-amended soils to prevent adverse effects on soils and crops. However, risks of either soil salinization or Zn and B phytotoxicity are more apparent in the case of using untreated sewage sludge. Needless to say that the microbial load of untreated sludge is expected to be considerable. As far as limed sludge is concerned, although its microbial load is expected to be undetectable, it can increase soil pH at the strongly alkaline range causing soil alkalinity problems, which can adversely affect plant growth.
Author Contributions
A.B.: writing—original draft preparation, and laboratory and statistical analysis; T.M.: conceptualization, writing—review and editing, supervision, project administration, experiment designation and funding acquisition; A.-G.K.: laboratory analysis; I.I.: supervision, experiment designation and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “First Call for H.F.R.I. Research Projects to support Faculty members and Researchers and the procurement of high-cost research equipment grant”, Project Number: HFRI-FM17-1907.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sterritt, R.M.; Lester, J.N. The value of sewage sludge to agriculture and effects of the agricultural use of sludges contaminated with toxic elements: A review. Sci. Total Environ. 1980, 16, 55–90. [Google Scholar] [CrossRef]
- Tsadilas, C.D.; Matsi, T.; Barbayiannis, N.; Dimoyiannis, D. Influence of sewage sludge application on soil properties and on the distribution and availability of heavy metal fractions. Commun. Soil Sci. Plant Anal. 1995, 26, 2603–2619. [Google Scholar] [CrossRef]
- European Council Directive. On the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture 86/278/EEC. 1986. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:31986L0278&from=EN (accessed on 8 November 2022).
- USEPA 625/R-95/001; Process Design Manual for Land Application of Sewage Sludge and Domestic Septage. United States Environmental Protection Agency, Office of Research and Development: Washington, DC, USA, 1995.
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Cieslik, B.M.; Namiesnik, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Samara, E.; Matsi, T.; Balidakis, A. Soil application of sewage sludge stabilized with steelmaking slag and its effect on soil properties and wheat growth. Waste Manag. 2017, 68, 378–387. [Google Scholar] [CrossRef]
- Samara, E.; Matsi, T.; Zdragas, A.; Barbayiannis, N. Use of clay minerals for sewage sludge stabilization and a preliminary assessment of the treated sludge’s fertilization capacity. Environ. Sci. Pollut. Res. 2019, 26, 35387–35398. [Google Scholar] [CrossRef]
- Balidakis, A.; Giannopoulos, G.; Kalderis, D.; Ipsilantis, I.; Matsi, T. Sewage sludge stabilization with clay minerals and biochar. In Proceedings of the 8th International Conference on Sustainable Solid Waste Management, Virtual, 23–25 June 2021. [Google Scholar]
- Yuan, G.; Theng, B.; Churchman, J.; Gates, W. Clays and clay minerals for pollution control. In Handbook of Clay Science. Part B. Techniques and Applications; Bergaya, F., Lagaly, G., Eds.; Developments in Clay Science 5; Elsevier: Amsterdam, The Netherlands, 2013; pp. 587–644. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, S.Y. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.C.; Lai, K.M.; Fang, M.; Ma, K.K. Effect of sewage sludge amendment on soil microbial activity and nutrient mineralization. Environ. Int. 1998, 24, 935–943. [Google Scholar] [CrossRef]
- Zhu, Y.; Christie, P.; Laidlaw, A.S. Uptake of Zn by arbuscular mycorrhizal white clover from Zn-contaminated soil. Chemosphere 2001, 42, 193–199. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, M.; Chen, L.; Ji, L.; Zhang, Z.; Wang, L.; Wei, L.; Zhang, Y. Growth and elemental uptake of Trifolium repens in response to biochar addition, arbuscular mycorrhizal fungi and phosphorus fertilizer applications in low-Cd-polluted soils. Environ. Pollut. 2020, 260, 113761. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Angle, J.S.; Chaney, R.L.; van Berkum, P. Sewage sludge and heavy metals effect on nodulation and nitrogen fixation of legumes. J. Environ. Qual. 1995, 24, 1199–1204. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Makela, P.S.A. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 104647. [Google Scholar] [CrossRef]
- Guyomard, H.; Bureau, J.-C.; Chatellier, V.; Detang-Dessendre, C.; Dupraz, P.; Jacquet, F.; Reboud, H.; Requillart, V.; Soler, L.-G.; Tysebaert, M. Research for AGRI Committee—The Green Deal and the CAP: Policy Implications to Adapt Farming Practices and to Preserve the EU’s Natural Resources; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2020. [Google Scholar]
- Rangeley, A.; Newbould, P. Growth response to lime and fertilizers and critical concentrations in herbage of white clover in Scottish hill soils. Grass Forage Sci. 1985, 40, 265–277. [Google Scholar] [CrossRef]
- Hart, A.L.; Collier, W.A. The effect of phosphorus and form of nitrogen supply on leaf cell size and nutrient content in Trifolium repens and Lotus uliginosus. Grass Forage Sci. 1994, 49, 96–104. [Google Scholar] [CrossRef]
- Bailey, J.S.; Laidlaw, A.S. Growth and development of white clover (Trifolium repens L.) as influenced by P and K nutrition. Ann. Bot. 1998, 81, 783–786. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Balidakis, A.; Matsi, T.; Karagianni, A.; Ipsilantis, I. Evaluation of certain clay minerals and biochar as materials for sewage sludge stabilization. Int. J. Environ. Sci. Technol. 2022. under review. [Google Scholar]
- European Commission. Working Document on Sludge; 3rd draft. ENV.E.3/LM; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen—Total. In Methods of Soil Analysis—Part 3—Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; SSSA; ASA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- ISO 23470; Soil Quality. Determination of Effective Cation Exchange Capacity (CEC) and Exchangeable Cations Using Hexamminecobalt Trichloride Solution. ISO: Geneva, Switzerland, 2007.
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci. Soc. Am. J. 1998, 42, 421–428. [Google Scholar] [CrossRef]
- ISO 11466; Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. ISO: Geneva, Switzerland, 1995.
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis—Part 3—Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; SSSA; ASA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Kuo, S. Phosphorus. In Methods of Soil Analysis—Part 3—Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; SSSA; ASA: Madison, WI, USA, 1996. [Google Scholar]
- Keren, R. Boron. In Methods of Soil Analysis—Part 3—Chemical Methods; SSSA Book Series 5; Sparks, D.L., Ed.; SSSA; ASA: Madison, WI, USA, 1996; pp. 603–626. [Google Scholar]
- Tinsley, J.; Taylor, T.G.; Moore, J.H. The determination of carbon dioxide derived from carbonates in agricultural and biological materials. Analyst 1951, 76, 300–310. [Google Scholar] [CrossRef]
- Horwath, W.R.; Paul, E.A. Microbial Biomass. In Methods of Soil Analysis-Part 2-Microbiological and Biochemical Properties; SSSA Book Series 5; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; SSSA: Madison, WI, USA, 1994; pp. 753–773. [Google Scholar] [CrossRef]
- Sylvia, D.M. Vesicular–arbuscular mycorrhizal (VAM) fungi. In Methods of Soil Analysis-Part 2-Microbiological and Biochemical Properties; SSSA Book Series 5; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; SSSA: Madison, WI, USA, 1994; pp. 351–378. [Google Scholar]
- McGonigle, T.; Miller, M.; Evans, D.; Fairchild, G.; Swan, J. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Moore, K.J.; Collins, M.; Nelson, C.J.; Redfearn, D.D. Forages: The Science of Grassland Agriculture, 7th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; Volume 2, pp. 263–275. [Google Scholar] [CrossRef]
- Akrivos, J.; Mamais, D.; Katsara, K.; Andreadakis, A. Agricultural utilization of sewage sludge. Water Sci. Technol. 2000, 42, 203–210. [Google Scholar] [CrossRef]
- Cerne, M.; Palcic, I.; Paskovic, I.; Major, N.; Romic, M.; Filipovic, V.; Igrc, M.D.; Percin, A.; Ban, S.G.; Zorko, B.; et al. The effect of stabilization on the utilization of municipal sewage sludge as a soil amendment. Waste Manag. 2019, 94, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Won, S.W.; Choi, S.B.; Yun, Y.-S. Binding sites and mechanisms of cadmium to the dried sewage sludge biomass. Chemosphere 2013, 93, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, J.C.; Adl, M.S.; Warman, P.R. A review of the use of composted municipal solid waste in agriculture. Agric. Ecosyst. Environ. 2008, 123, 1–14. [Google Scholar] [CrossRef]
- Rigby, H.; Clarke, B.O.; Pritchard, D.L.; Meehan, B.; Beshah, F.; Smith, S.R.; Porter, N.A. A critical review of nitrogen mineralization in biosolids-amended soil, the associated fertilizer value for crop production and potential for emissions to the environment. Sci. Total Environ. 2016, 541, 1310–1338. [Google Scholar] [CrossRef]
- Torri, S.I.; Correa, R.S.; Renella, G. Biosolids application to agricultural land-a contribution to global phosphorus cycle: A review. Pedosphere 2017, 27, 1–16. [Google Scholar] [CrossRef]
- Sims, J.T.; Johnson, G.V. Micronutrient soil tests. In Micronutrients in Agriculture, 2nd ed.; Mortvedt, J.J., Cox, F.R., Shuman, L.M., Welch, R.M., Eds.; SSSA: Madison, WI, USA, 1991; pp. 427–476. [Google Scholar]
- Hooda, P.S.; Alloway, B.J. The plant availability and DTPA extractability of trace metals in sludge-amended soils. Sci. Total Environ. 1993, 149, 39–51. [Google Scholar] [CrossRef]
- Gonçalves, P.W.B.; da Costa, C.A.; Cardoso, P.H.S.; Pegoraro, R.F.; Cardoso, G.R.; Versiani, L.C.F. Bioaccumulation of potentially toxic elements in lettuce and soil fertility treated with biosolid. J. Soil Sci. Plant Nutr. 2022, 22, 815–823. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Shams, M.S.; Ibrahim, S.M.; Elbehiry, F.A.; Antoniadis, V.; Hooda, P.S. Stabilization of sewage sludge by using various by-products: Effects on soil properties, biomass production, and bioavailability of copper and zinc. Water Air Soil Pollut. 2014, 225, 2014. [Google Scholar] [CrossRef]
- Frame, J.; Newbould, P. Agronomy of white clover. Adv. Agron. 1986, 40, 1–88. [Google Scholar] [CrossRef]
- Mantovi, P.; Baldoni, G.; Toderi, G. Reuse of liquid, dewatered, and composted sewage sludge on agricultural land: Effects of long-term application on soil and crop. Water Res. 2005, 39, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, M. Effect of different sewage sludge applications on growth and yield of Vigna radiata L. field crop: Metal uptake by plant. Ecol. Eng. 2010, 26, 969–972. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Poplawska, A.; Kolodziej, B.; Ciarkowska, K.; Gambus, F.; Bryk, M.; Babula, J. Application of ash and municipal sewage sludge as macronutrient sources in sustainable plant biomass production. J. Environ. Manag. 2020, 264, 110450. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, J.; Hart, A.L. Mineral nutrition. In White Clover; Baker, M.J., Williams, W.M., Eds.; CAB International: Wallingford, UK, 1987; pp. 153–183. [Google Scholar]
- Thomas, G.W.; Peaslee, D.E. Testing soils for phosphorus. In Soil Testing and Plant Analysis; Walsh, L.M., Beaton, J.D., Eds.; SSSA: Madison, WI, USA, 1973; pp. 115–132. [Google Scholar]
- Usman, A.; Kuzyakov, Y.; Stahr, K. Effect of clay minerals on immobilization of heavy metals and microbial activity in a sewage sludge-contaminated soil. J. Soils Sediments 2005, 5, 245–252. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).