The Effect of 6-Month Complex Exercise on Serum Bone Metabolism: Focused on the Elderly over 75 Years Old
Abstract
1. Introduction
2. Methods
2.1. Subjects of the Research
2.2. Measuring Items and Methods
2.2.1. Physical Examination
2.2.2. Blood Analysis
2.2.3. Exercise Program
2.2.4. Data Processing
2.3. Data Procesing
3. Result
3.1. Results of Osteocalcin
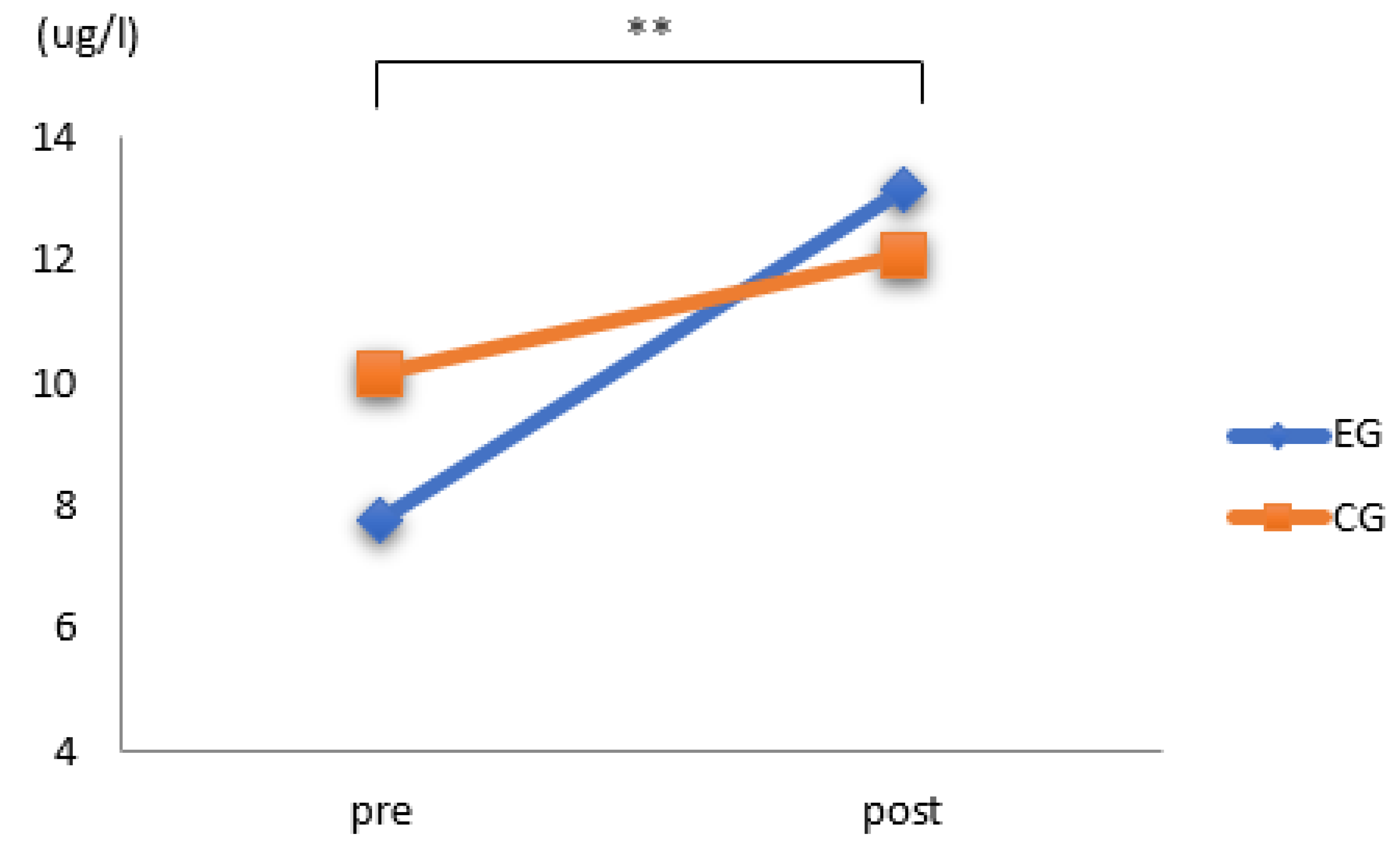
3.2. Results of ALP
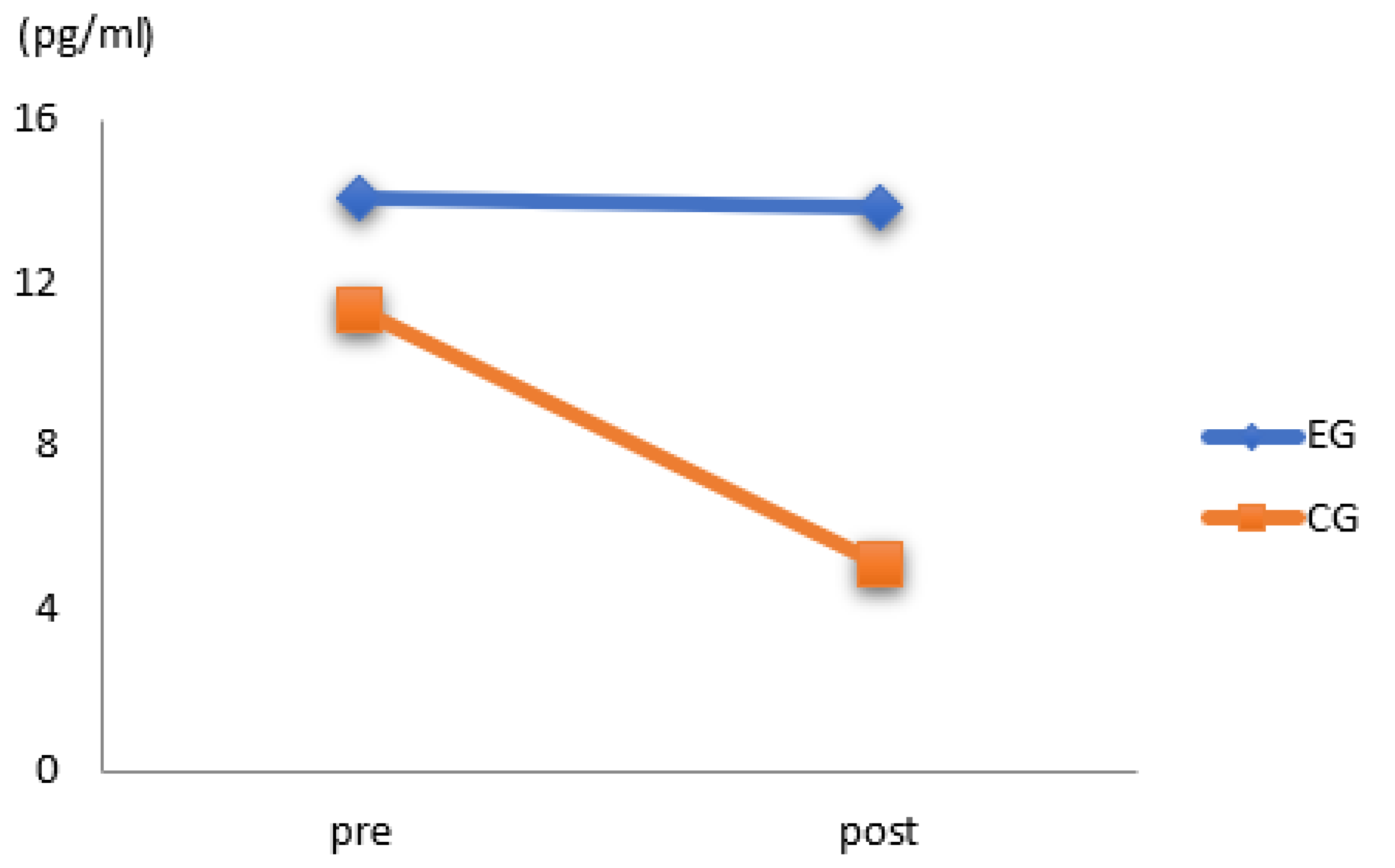
3.3. Results of Estradiol
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Statistics Korea. Social Indicators in Korea. 2021. Available online: https://kostat.go.kr/portal/korea/kor_nw/1/1/index.board?bmode=read&aSeq=403253 (accessed on 29 September 2021).
- Milanovic, Z.; Jorgić, B.; Trajković, N.; Sporis, P.S.; James, N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging 2013, 549. [Google Scholar] [CrossRef] [PubMed]
- Korea Institute for Health and Social Affairs. 2020 Survey of the Elderly; Korea Institute for Health and Social Affairs: Seoul, Korea, 2021. [Google Scholar]
- Nieto-Riveiro, L.; Groba, B.; Miranda, M.C.; Concheiro, P.; Pazos, A.; Pousada, T.; Pereira, J. Technologies for participatory medicine and health promotion in the elderly population. Medicine 2018, 97, e1079. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. The burden of osteoporosis. J. Endocrinol. Investig. 1999, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Bliuc, D.; Alarkawi, D.; Nguyen, T.V.; Eisman, J.A.; Center, J.R. Risk of subsequent fractures and mortality in elderly women and men with fragility fractures with and without osteoporotic bone density: The Dubbo Osteoporosis Epidemiology Study. J. Bone Miner. Res. 2015, 30, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029. [Google Scholar] [CrossRef]
- Chao, Y.P.; Kao, T.W.; Chen, W.L.; Peng, T.C.; Wu, L.W. Mid-arm muscle circumference as an indicator of osteoporosis in community-dwelling older men. Arch. Gerontol. Geriatr. 2020, 87, 103998. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Nevitt, M.C.; Brown Jr, B.W.; Kelsey, J.L. Increased falling as a risk factor for fracture among older women: The study of osteoporotic fractures. Am. J. Epidemiol. 2005, 161, 180–185. [Google Scholar] [CrossRef]
- Lee, S.R.; Ha, Y.C.; Kang, H.; Park, Y.G.; Nam, K.W.; Kim, S.R. Morbidity and mortality in Jeju residents over 50-years of age with hip fracture with mean 6-year follow-up: A prospective cohort study. J. Korean Med. Sci. 2013, 28, 1089–1094. [Google Scholar] [CrossRef][Green Version]
- Gallagher, C.M.; Kovach, J.S.; Meliker, J.R. Urinary cadmium and osteoporosis in US women ≥50 years of age: NHANES 1988–1994 and 1999–2004. Environ. Health Perspect. 2008, 116, 1338–1343. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. 2021. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=10478&pageIndex=1 (accessed on 22 November 2021).
- IOF. Broken Bones, Broken Lives UK. 2018. Available online: https://www.iofbonehealth.org/impact-osteoporosis (accessed on 10 February 2019).
- Lewiecki, E.M.; Ortendahl, J.D.; Vanderpuye-Orgle, J.; Grauer, A.; Arellano, J.; Lemay, J.; Singer, A.J. Healthcare policy changes in osteoporosis can improve outcomes and reduce costs in the United States. JBMR Plus 2019, 3, e10192. [Google Scholar] [CrossRef]
- Smith, S.M.; Zwart, S.R.; Heer, M.A.; Baecker, N.; Evans, H.J.; Feiveson, A.H.; Shackelford, L.C.; Leblanc, A.D. Effects of artificial gravity during bed rest on bone metabolism in humans. J. Appl. Physiol. 2009, 107, 47–53. [Google Scholar] [CrossRef]
- Babatunde, O.O.; Bourton, A.L.; Hind, K.; Paskins, Z.; Forsyth, J.J. Exercise interventions for preventing and treating low bone mass in the forearm: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2020, 101, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Marín-Cascales, E.; Alcaraz, P.E.; Ramos-Campo, D.J.; Rubio-Arias, J.A. Effects of multicomponent training on lean and bone mass in postmenopausal and older women: A systematic review. Menopause 2018, 25, 346–356. [Google Scholar] [CrossRef]
- Park, H.Y. The Effect of Physical Exercise Type on Bone Metabolism and Inflammatory Markers in Elderly Women; Hanyang University: Seoul, Korea, 2016. [Google Scholar]
- De Kam, D.; Smulders, E.; Weerdesteyn, V.; Smits-Engelsman, B.C.M. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: A systematic review of randomized controlled trials. Osteoporos. Int. 2009, 20, 2111–2125. [Google Scholar] [CrossRef] [PubMed]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Creed, G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef] [PubMed]
- Martyn-St James, M.; Carroll, S. A meta-analysis of impact exercise on postmenopausal bone loss: The case for mixed loading exercise programmes. Br. J. Sport. Med. 2009, 43, 898–908. [Google Scholar] [CrossRef]
- Ma, D.; Wu, L.; He, Z. Effects of walking on the preservation of bone mineral density in perimenopausal and postmenopausal women: A systematic review and meta-analysis. Menopause 2013, 20, 1216–1226. [Google Scholar] [CrossRef]
- Martyn-St James, M.; Carroll, S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone 2008, 43, 521–531. [Google Scholar] [CrossRef]
- Abrahin, O.; Rodrigues, R.P.; Marçal, A.C.; Alves, E.A.C.; Figueiredo, R.C.; Sousa, E.C.D. Swimming and cycling do not cause positive effects on bone mineral density: A systematic review. Rev. Bras. Reumatol. 2016, 56, 345–351. [Google Scholar] [CrossRef]
- Beger, T.; Yavuzer, H. Yaslilik ve yaslilik epidemiyolojisi. Klin. Gelisim 2012, 25, 1–3. [Google Scholar]
- Kang, H.Y.; Seo, N.S.; Kim, Y.H. Health pattern of elderly according to age group who living alone in an urban area. J. Korean Acad. Nurs. 2004, 34, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.J. The effects of integrated movement program on the health and quality of life for the elderly in rural areas. Korean J. Sports Sci. 2020, 29, 279–288. [Google Scholar] [CrossRef]
- Jang, S.H.; Hwang, B.D.; Yoon, H.J.; Lee, S.K. Effect of exercise program on grip strength, balance and bone mineral density of the elderly women in rural community. J. Korea Contents Assoc. 2009, 9, 214–223. [Google Scholar] [CrossRef][Green Version]
- Lee, S.G.; Park, S.K. The effects of a video strength exercise on grip strength, balance, TUG in the frail elderly women. J. Korean Soc. Phys. Med. 2013, 8, 91–98. [Google Scholar] [CrossRef]
- Jang, J.H.; Kim, J.C.; Kang, S.H.; Ko, J.U. Changes of physical fitness variables after 12-Week health promotion exercise program in elderly women: One-year follow-up. J. Korean Assoc. Phys. Educ. Sport Girls Women 2016, 30, 221–236. [Google Scholar] [CrossRef]
- Shin, J.H.; Hwang, Y.N.; Kim, W.W.; Kim, H.S.; Song, S.W. The relationship between the level of serum lipids and bone metabolism among pre and postmenopausal women. Korean J. Obes. 2007, 16, 162–169. [Google Scholar]
- Bonnick, S.L.; Shulman, L. Monitoring osteoporosis therapy: Bone mineral density, bone turnover markers, or both? Am. J. Med. 2006, 119, S25–S31. [Google Scholar] [CrossRef]
- Johansen, J.S.; Riss Delmas, P.D.; Chritianen, C. Clinical indication for bone mineral measurements. J. Bone Miner. Res. 1988, 4, 1–28. [Google Scholar]
- Woitge, H.W.; Friedmann, B.; Suttner, S.; Farahmand, I.; Müller, M.; Schmidt-Gayk, H.; Seibel, M.J. Changes in bone turnover induced by aerobic and anaerobic exercise in young males. J. Bone Miner. Res. 1998, 13, 1797–1804. [Google Scholar] [CrossRef]
- Ryan, A.S.; Treuth, M.S.; Hunter, G.R.; Elahi, D. Resistive training maintains bone mineral density in postmenopausal women. Calcif. Tissue Int. 1998, 62, 295–299. [Google Scholar] [CrossRef]
- Yasumura, S.; Aloia, J.F.; Gundberg, C.M.; Yeh, J.; Vaswani, A.N.; Yuen, K.; Cohn, S.H. Serum osteocalcin and total body calcium in normal pre-and postmenopausal women and postmenopausal osteoporotic patients. J. Clin. Endocrinol. Metab. 1987, 64, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, M.W.; Snead, B.D.; Slatopolsky, E.; Birge, S.J., Jr. Additive effects of weight-bearing exercise and estrogen on bone mineral density in older women. J. Bone Miner. Res. 1995, 10, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.Z.; Goettsch, B.M.; Van Ramshorst, R.D.; O’brien, J.A.; Jaque, S.V.; Sumida, K.D. Resistance training and bone mineral density during growth. Int. J. Sport. Med. 2008, 29, 316–321. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Banfi, G.; Lombardi, G.; Colombini, A.; Lippi, G. Bone metabolism markers in sports medicine. Sport. Med. 2010, 40, 697–714. [Google Scholar] [CrossRef]
- Ahn, N.Y.; Kim, K.J. Utility of Bone Metabolic Markers, Serum and Salivary Cortisol Concentration for Exercise Training Effect Analysis in Elderly with Osteoporosis. Exerc. Sci. 2016, 25, 1–9. [Google Scholar] [CrossRef]
- Leung, K.S.; Fung, K.P.; Sher, A.H.; Li, C.K.; Lee, K.M. Plasma bone-specific alkaline phosphatase as an indicator of osteoblastic activity. J. Bone Jt. Surg. Br. Vol. 1993, 75, 288–292. [Google Scholar] [CrossRef]
- Rudberg, A.; Magnusson, P.; Larsson, L.; Joborn, H. Serum isoforms of bone alkaline phosphatase increase during physical exercise in women. Calcif. Tissue Int. 2000, 66, 342–347. [Google Scholar] [CrossRef]
- Lee, C.J. The Effects of Resistance Training to Health-Related Fitness. Growth Hormone. Bone Metabolism & Bone Mineral Density by Grades of Male’s High School Students. Ph.D. Thesis, Graduate School of Pusan National University, Busan, Korea, 2005. [Google Scholar]
- Swissa-Sivan, A.; Azoury, R.; Statter, M.; Leichter, I.; Nyska, A.; Nyska, M.; Samueloff, S. The effect of swimming on bone modeling and composition in young adult rats. Calcif. Tissue Int. 1990, 47, 173–177. [Google Scholar] [CrossRef]
- Gundberg, C.M.; Looker, A.C.; Nieman, S.D.; Calvo, M.S. Patterns of osteocalcin and bone specific alkaline phosphatase by age, gender, and race or ethnicity. Bone 2002, 31, 703–708. [Google Scholar] [CrossRef]
- Delmas, P.D. Biochemical markers of bone turnover for the clinical investigation of osteoporosis. Osteoporos. Int. 1993, 3, 81–86. [Google Scholar] [CrossRef]
- Van Hoof, V.O.; De Broe, M.E. Interpretation and clinical significance of alkaline phosphatase isoenzyme patterns. Crit. Rev. Clin. Lab. Sci. 1994, 31, 197–293. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, A.; Raisz, L.G.; Brasel, J.A.; Cooper, D.M. Evidence for increased bone formation following a brief endurance-type training intervention in adolescent males. J. Bone Miner. Res. 1997, 12, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Humphries, B.; Newton, R.U.; Bronks, R.O.G.E.R.; Marshall, S.O.N.J.A.; McBRIDE, J.E.F.F.; Triplett-McBride, T.R.A.V.I.S.; Sly, N. Effect of exercise intensity on bone density, strength, and calcium turnover in older women. Med. Sci. Sport. Exerc. 2000, 32, 1043–1050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burger, H.G.; Hale, G.E.; Robertson, D.M.; Dennerstein, L. A review of hormonal changes during the menopausal transition: Focus on findings from the Melbourne Women’s Midlife Health Project. Hum. Reprod. Update 2007, 13, 559–565. [Google Scholar] [CrossRef]
- Lanfranco, F.; Gianotti, L.; Giordano, R.; Pellegrino, M.; Maccario, M.; Arvat, E. Ageing, growth hormone and physical performance. J. Endocrinol. Investig. 2003, 26, 861–872. [Google Scholar] [CrossRef]
- Ahn, K.J. 12-Week Combined Exercise Program on Bone Mineral Density and Sex Hormone in the Elderly; Chungbuk National University: Cheongju, Korea, 2008. [Google Scholar]
- Lee, J.H. Effects of Circuit Exercise on Physical Fitness, Blood Ipids and Estrogen in Elderly Women; Jeju National University: Jeju, Korea, 2010. [Google Scholar]
- Kim, Y.S.; Jeong, C.K.; Kang, H.S. The Effect of Elastic Resistance Exercise and Protein Intake on Functional Fitness and Aging Hormone in Elderly women. J. Korean Soc. Study Phys. Educ. 2016, 21, 111–124. [Google Scholar]
- Kim, C.H.; Lee, J.W.; Han, S.I. The Effect of Exercise Type Performance in Elderly Women on The Aging Hormone and De-pression Factor. J. Korea Soc. Wellness 2013, 8, 373–386. [Google Scholar]
- Zmuda, J.M.; Sheu, Y.T.; Moffett, S.P. Genetic epidemiology of osteoporosis: Past, present, and future. Curr. Osteoporos. Rep. 2005, 3, 111–115. [Google Scholar] [CrossRef]
- Mundy, G.R. Nutritional modulators of bone remodeling during aging. Am. J. Clin. Nutr. 2006, 83, 427S–430S. [Google Scholar] [CrossRef]
- Ferrari, S. Osteoporosis: A complex disorder of aging with multiple genetic and environmental determinants. World Rev. Nutr. Diet. 2005, 95, 35. [Google Scholar] [CrossRef]
- Ohshima, H. Bone loss and bone metabolism in astronauts during long-duration space flight. Clin. Calcium 2006, 16, 81–85. [Google Scholar] [PubMed]
- Iwamoto, J.; Takeda, T.; Sato, Y. Interventions to prevent bone loss in astronauts during space flight. Keio J. Med. 2005, 54, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Fontana, L.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Schechtman, K.B.; Holloszy, J.O. Bone mineral density response to caloric restriction–induced weight loss or exercise-induced weight loss: A randomized controlled trial. Arch. Intern. Med. 2006, 166, 2502–2510. [Google Scholar] [CrossRef]
- Nuti, R.; Martini, G.; Merlotti, D.; Valleggi, F.; De Paola, V.; Gennari, L. Professional sport activity and micronutrients: Effects on bone mass. J. Endocrinol. Investig. 2005, 28, 52–60. [Google Scholar]
- Kohrt, W.M. American College of Sports Medicine position stand on physical activity and bone health. Med. Sci. Sports Exer. 2004, 36, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Marcus, R. The muscle strength and bone density relationship in young women: Dependence on exercise status. J. Sport. Med. Phys. Fit. 2004, 44, 98–103. [Google Scholar]
- Bemben, D.A.; Buchanan, T.D.; Bemben, M.G.; Knehans, A.W. Influence of type of mechanical loading, menstrual status, and training season on bone density in young women athletes. J. Strength Cond. Res. 2004, 18, 220–226. [Google Scholar] [CrossRef]
- Kirchner, E.M.; Lewis, R.D.; O’Connor, P.J. Bone mineral density and dietary intake of female college gymnasts. Med. Sci. Sport. Exerc. 1995, 27, 543–549. [Google Scholar] [CrossRef]
- Marques, E.A.; Mota, J.; Viana, J.L.; Tuna, D.; Figueiredo, P.; Guimarães, J.T.; Carvalho, J. Response of bone mineral density, inflammatory cytokines, and biochemical bone markers to a 32-week combined loading exercise programme in older men and women. Arch. Gerontol. Geriatr. 2013, 57, 226–233. [Google Scholar] [CrossRef]
- Harridge, S.D.; Lazarus, N.R. Physical activity, aging, and physiological function. Physiology 2017, 32, 152–161. [Google Scholar] [CrossRef]
- Brach, J.S.; FitzGerald, S.; Newman, A.B.; Kelsey, S.; Kuller, L.; VanSwearingen, J.M.; Kriska, A.M. Physical activity and functional status in community-dwelling older women: A 14-year prospective study. Arch. Intern. Med. 2003, 163, 2565–2571. [Google Scholar] [CrossRef] [PubMed]

| Variable | Control Group (Mean ± SE) | Exercise Group (Mean ± SE) | p-Value |
|---|---|---|---|
| Age (year) | 80.80 ± 2.37 | 81.14 ± 3.98 | 0.429 |
| Height (cm) | 154.59 ± 2.14 | 155.14 ± 3.05 | 0.992 |
| Weight (kg) | 57.68 ± 3.15 | 59.26 ± 4.14 | 0.791 |
| Variable | Type | Time | Frequency | Intensity | |
|---|---|---|---|---|---|
| Warm-up exercise | Stretching | 5 min | Twice a week | RPE 2~3 | |
| Main exercise | Aerobic exercise | Walking in place 7 m shuttle walking | 20 min | HRmax 45~55% 11~14 RM 3 set | |
| Resistance exercise | Biceps curl, triceps curl, side band squat, kick back, knee up | 20 min | |||
| Balance exercise | One-leg standing | 10 min | |||
| Cool-down exercise | Stretching | 5 min | RPE 2~3 | ||
| Variable | Pre | Post | p | |
|---|---|---|---|---|
| Osteocalcin (ng/mL) | control group | 9.18 ± 3.09 | 8.47 ± 3.54 | 0.603 |
| exercise group | 8.04 ± 3.93 | 9.54 ± 5.48 | 0.683 | |
| ALP (ug/L) | control group | 10.15 ± 3.91 | 12.07 ± 3.71 | 0.181 |
| exercise group | 7.75 ± 4.47 | 13.16 ± 4.43 | 0.003 | |
| Estradiol (pg/mL) | control group | 11.29 ± 12.17 | 5.07 ± 0.25 | 0.946 |
| exercise group | 14.09 ± 12.76 | 13.83 ± 13.77 | 0.325 | |
| Variable | SS | df | MS | F | p Value | p eta-sq |
|---|---|---|---|---|---|---|
| Corrected model | 19.433 | 3 | 6.479 | 0.389 | 0.762 | 0.021 |
| Intercept | 4492.615 | 1 | 4492.615 | 269.520 | 0.000 | 0.833 |
| Group | 0.017 | 1 | 0.017 | 0.001 | 0.978 | |
| Time | 2.200 | 1 | 2.200 | 0.132 | 0.718 | |
| Group × Time | 17.623 | 1 | 17.623 | 1.057 | 0.308 | |
| Error | 900.124 | 54 | 16.669 | |||
| Total | 5418.120 | 58 | ||||
| Corrected total | 919.557 | 57 |
| Variable | SS | df | MS | F | p Value | p eta-sq |
|---|---|---|---|---|---|---|
| Corrected model | 238.357 | 3 | 79.452 | 4.651 | 0.006 | 0.205 |
| Intercept | 6734.303 | 1 | 6734.303 | 394.229 | 0.000 | 0.880 |
| Group | 6.241 | 1 | 6.241 | 0.365 | 0.548 | 0.007 |
| Time | 194.030 | 1 | 194.030 | 11.359 | 0.001 | 0.174 |
| Group × Time | 44.197 | 1 | 44.197 | 2.587 | 0.114 | 0.046 |
| Error | 922.440 | 54 | 17.082 | |||
| Total | 7917.280 | 58 | ||||
| Corrected total | 1160.797 | 57 |
| Variable | SS | df | MS | F | p Value | p eta-sq |
|---|---|---|---|---|---|---|
| Corrected model | 775.176 | 3 | 258.392 | 2.096 | 0.111 | 0.104 |
| Intercept | 7097.473 | 1 | 7097.473 | 57.580 | 0.000 | 0.516 |
| Group | 484.524 | 1 | 484.524 | 3.931 | 0.053 | 0.068 |
| Time | 152.235 | 1 | 152.235 | 1.235 | 0.271 | 0.022 |
| Group × Time | 128.428 | 1 | 128.428 | 1.042 | 0.312 | 0.019 |
| Error | 6656.249 | 54 | 123.264 | |||
| Total | 14409.880 | 58 | ||||
| Corrected total | 7431.424 | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, A.-R.; Lee, S.-E.; Shim, Y.-J.; Choi, S.-W. The Effect of 6-Month Complex Exercise on Serum Bone Metabolism: Focused on the Elderly over 75 Years Old. Appl. Sci. 2022, 12, 11373. https://doi.org/10.3390/app122211373
Kim A-R, Lee S-E, Shim Y-J, Choi S-W. The Effect of 6-Month Complex Exercise on Serum Bone Metabolism: Focused on the Elderly over 75 Years Old. Applied Sciences. 2022; 12(22):11373. https://doi.org/10.3390/app122211373
Chicago/Turabian StyleKim, A-Ram, So-Eun Lee, Yoo-Jin Shim, and Seung-Wook Choi. 2022. "The Effect of 6-Month Complex Exercise on Serum Bone Metabolism: Focused on the Elderly over 75 Years Old" Applied Sciences 12, no. 22: 11373. https://doi.org/10.3390/app122211373
APA StyleKim, A.-R., Lee, S.-E., Shim, Y.-J., & Choi, S.-W. (2022). The Effect of 6-Month Complex Exercise on Serum Bone Metabolism: Focused on the Elderly over 75 Years Old. Applied Sciences, 12(22), 11373. https://doi.org/10.3390/app122211373






