Abstract
Poultry farming plays a key role in agricultural air emissions. Particulate matter (PM) level tends to be high in broiler and cage-free layer houses, that may impair health and welfare of animals and their caretakers. To protect public health and welfare, the occupational exposure limit for PM10 and PM2.5 (i.e., PM diameters that are generally ≤10 and 2.5 μm, respectively) are suggested not to exceed 150 µg m−3 and 35 µg m−3, respectively, based on 24-h concentrations thresholds as suggested by US. EPA. However, the levels of PM10 and PM2.5 in poultry houses could be 100 times higher than that limit. For instance, PM10 and PM2.5 levels in cage-free henhouses are higher than 15,000 µg/m3 and 3500 µg/m3 in wintertime. Therefore, it is critical to identify the primary factors affecting PM generation in poultry houses and apply corresponding mitigation strategies. This review paper summarizes PM emission factors, mitigating strategies, and impacts on birds’ and caretakers’ health, and welfare. Generally, PM emissions are affected by various factors, including housing types, seasonal and diurnal variation, manure management, bedding materials, ventilation rates, and birds’ activities. High PM concentrations in poultry houses impair birds’ and caretakers’ liver, kidneys, and respiratory systems. Thus, different mitigating strategies are discussed in this study for addressing those issues. Effective mitigation strategies include frequent house cleaning, optimum light intensity, liquid spraying, bedding management, and air filtration systems. However, mitigation strategies can be cost-prohibitive and have side effects. Therefore, poultry farms should select mitigation strategies based on farm location, climate conditions, environmental policies, and available resources (government assistance programs).
1. Introduction
Animal feeding operations (AFOs) are important sources of air pollutant emissions into the environment [1,2,3,4,5]. The primary air emissions include particulate matter (PM) and other gases like greenhouse gases and ammonia (NH3), as these gases pose a high potential risk to air quality, public and animal health, and climate change [6,7,8,9,10,11,12]. Among these air pollutants, PM is considered one of the harmful air pollutants within and outside of animal houses because of its composition and emission rates at the animal and local levels [6]. According to the WHO (World Health Organization), the fine PM such as PM2.5 (inhalable particles with diameters 2.5 micrometers) causes 4.2 million premature deaths worldwide per year [11]. Moreover, the fine PM generated in the environment is the main source of haze in some parts of the United States [12,13,14]. In addition, depending on dust composition, settling down may cause lakes or streams to be acidic, reduce soil nutrients, and contribute to acid rain formation [12]. According to the European Environmental Agency, poultry and pig housings contributed approximately 50% and 30% of PM2.5 (PM with aerodynamic diameter ≤2.5 μm) and 57% and 32% of PM10 (PM with aerodynamic diameter ≤10 μm) emissions, respectively [15].
Particulate matters in confined animal housing are heterogeneous combinations of biologically generated materials and aerosolized pollutants such as feed additives, broken feather pieces, dried NH3, viable and nonviable bacteria, endotoxins, glucans, molds, and fungal spores [16,17,18]. In poultry houses, the primary sources of PM include feathers, feeds, urine mineral crystals, manure, and bedding materials. The PM generated from these sources shows harmful effects on the health of animals and caretakers [19,20]. For example, in birds, higher PM levels result in an increased risk of chronic bronchitis, cardiovascular illness, pneumonia lesions, asthma-like symptoms, and lung cancer [21,22], while in caretakers, it causes bronchitis, asthma, and organic dust toxic syndrome [20]. In addition, poultry farm workers are usually at high risk of occupationally being exposed to many respiratory problems leading to higher asthma rates or other respiratory symptoms at work [23].
This review study aims to discuss various factors that affect PM emissions and potential strategies to reduce its generation from poultry production systems. Therefore, the primary objectives of this study were to (1) summarize factors that affect PM or dust levels in poultry houses, (2) analyze potential strategies for mitigating PM generations, and (3) discuss the pros and cons of different methods and expectations on new strategies to improve air quality and health and welfare of animals and their caretakers.
2. Dust Composition and Mixture
Particulate matter composition varies according to animals and livestock housing [24]. In poultry housing, PM is entirely biological, organic, and inorganic in its origin (Figure 1), which typically consists of a complex mixture of solid and liquid materials such as bedding materials, feathers, feeds, skin, excreta, dander, and microorganism (Table 1). Particulate matter from poultry houses constitutes about 90% organic content [25]. Dander, excreta, feathers, feed, litter material, bacteria, and skin are all examples of organic PM [16,17,18], while inorganic PM is usually the consequence of secondary interactions between NH3 and acidic gases, which contribute to the fine PM fraction [26]. Based on particle sizes, feather-releasing airborne PM contributes about 4% to 43% fine PM and 6% to 35% coarse PM, while manure contributes ranges from 9–85% fine and 30–94% coarse PM [17]. Similarly, based on particle mass, feathers contribute about 17–68% in fine and 4–49% in coarse PM, while manure contributes 6–77% fine and 31–96% in coarse PM. In addition, in the case of poultry houses, PM could be rich in nitrogen content. According to Cambra-Lopez et al. (2010), elemental analysis of PM in poultry houses consists of N, O, C, S, P, Ca, Na, K, and Mg from feedstuffs, feces, and skin [21]. In addition, within PM, many pathogenic and nonpathogenic microorganisms are found attached to the surface [27].

Figure 1.
Poultry dust obtained from the exhaust fan of the cage-free laying hen house at the UGA research facility.
In broiler housing, the primary sources of PM are down feathers, mineral crystals from urine, and litter, whereas the most prominent sources of waste in layer barns are skin, feathers, excrement, urine, feed, and litter [25]. Based on particle size, PM is classified into PM1 (PM with aerodynamic diameter ≤1 μm), PM2.5 (PM with aerodynamic diameter ≤2.5 μm), PM4 (PM with aerodynamic diameter ≤4 μm), PM10 (PM with aerodynamic diameter ≤10 μm), and total suspended particle (TSP, PM with aerodynamic diameter ≤100 μm) [24,28,29]. The size of PM2.5 is 30 times smaller than the size of the average human hair [14]. The emission rate of PM10 was directly influenced by the activity of the hens, ambient temperature, and ventilation rate [30]. It has been found that a significant portion of the NH3 released contributes to the burden of PM2.5 [31,32].

Table 1.
Particulate matter composition varies with different housing systems.
Table 1.
Particulate matter composition varies with different housing systems.
| Sources | PM Type | PM Constitute | References |
|---|---|---|---|
| Broilers | TSP | Feathers, skin, bacteria, fungus, fecal matter, spilled feed, mold spores, and bedding fragments | [16] |
| Broilers | PM2.5 PM10 | 72.1% manure, 21.3% feathers, 5.8% wood shaving, and 0.7% ambient PM 95.6% manure and 4.4% feathers | |
| Layers | PM2.5 PM10 | 63.7% manure and 36.3% feathers 69.6% manure, 30.0% feathers, and 0.4% ambient PM | [17] |
| Layers | PM2.5 PM10 | 54.2% manure, 23.2% feed, 17.0% feathers, and 5.5% ambient PM 85.5% manure and 14.5% feathers | |
| Turkey | PM2.5 PM10 | 39.1% feathers, 34.8% manure, 26.1% wood shavings, and 0.1% ambient PM 51.9% manure, 25.1% feathers, and 22.9% wood shavings | |
| Broilers | TSP | 50% excreta, 30% litter, 15% feed, and 5% feathers | [18] |
| Poultry | TSP | 90% organic composition like a feather, feeds, urine mineral crystal, manure, and bedding materials | [25] |
| Poultry | TSP | Organic and inorganic particles: excreta, feathers, mites, dander, bacteria, fungi, fungal spores, and endotoxins | [33] |
| Poultry | TSP | Bedding materials and floor | [34] |
| Poultry | TSP | Feed, excreta, hair, and dander | [35] |
3. Factors Affecting Dust Generations
Dust emissions from poultry farms are affected by various factors and changes according to variable climatic conditions, applied management practices, the number of birds, and housing types. Various researchers have explained many factors that cause PM emission, as shown in Figure 2 [9,24,36,37,38,39].
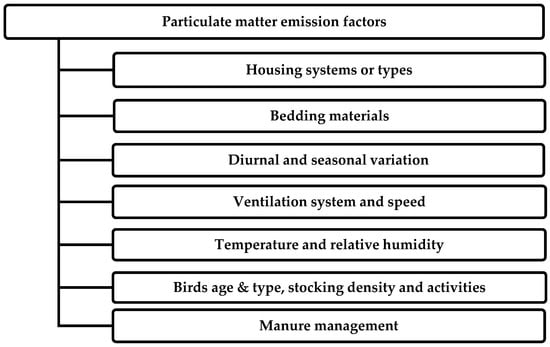
Figure 2.
Factors affecting PM emissions in poultry housing.
3.1. Effect of Housing Systems on PM
Poultry housing is the major source of PM emissions. Different housing systems (e.g., floor-raised, aviary, conventional caged, and enriched colony) show different PM emissions and concentrations. Among different housing types, the cage-free (CF) housing (aviary) system resulted in significantly higher PM concentrations and emissions [40]. The daily mean PM10 level in CF housing was about six to nine times higher than the conventional cage (CC) and the enriched colony housing (EC) systems [3]. Therefore, emission mitigation studies should consider CF housing systems as the priority. In addition, CF shows higher concentrations of airborne bacterial concentrations and emissions rates than CC and EC houses because PM is the primary carrier of airborne bacteria. In addition, house types can be divided into high-raised (HR) or manure belts (MB). According to Chai et al. (2012), the HR houses had higher NH3 concentrations but lower CO2, H2S, and PM10 concentrations than the MB house [9]. The detail of PM concentration and emissions in different houses is detailed in Table 2.

Table 2.
Particulate matter emissions and concentration of different PM sizes in various poultry housing systems.
3.2. Effect of Bedding Materials on PM Levels
Cage-free housing commonly uses bedding materials on the floor for producing hens with litter floor to perform natural behaviors of dust bathing and foraging [51,52,53,54,55,56,57,58]. In Europe, litter floor distribution should include bedding material covering at least 33% (one-third) and 100% of total space in laying hens and broiler houses, respectively [51]. This litter floor is the main source of PM emissions in CF houses. Particulate matter production from bedding material can be influenced by the type of bedding materials, moisture content, depth of bedding material, replacing or cleaning frequency [52]. Bedding materials can be organic (wood shaving or chips, straw, paper, rice hulls, maize silage, plant husk, or grass) or inorganic (stone, sand, and clay) in origin and must be nontoxic, highly absorbent, and comfortable for animals [36,53]. The management of bedding materials has been studied to control PM concentrations or emissions in animal houses [54,55], as summarized in Table 3. Different types of bedding materials, including peat, clay pellets, chopped straw, wood shaving, chopped paper, or gravel, peat, and clay pellets, were compared. Peat and clay pellets have shown higher efficiencies in PM reduction ranging from 19 to 64% [56].

Table 3.
Particulate matter emission due to different bedding materials used in the poultry housing system.
3.3. Effect of Lighting and Seasonal Variations on PM Levels
During the daytime, increased activities of birds lead to a higher PM concentration than the nighttime [60]. The concentration of PM2.5, PM10, and TSP were 151, 108, and 136% higher (p < 0.05) during the daytime (lights on) than at nighttime. During the daytime, birds were most active, ventilation rates were highest, and emissions rose. However, the ratio of PM2.5 and PM10 decreases at night because of low bird activities and the settling down of PM10 concentration [37].
The emission of PM is seasonally dependent and varies over time (Table 4) [37,38]. PM concentration increases in the winter compared to fall, spring, and summer [4,37,38,50,61]. According to Li et al. (2011), the concentration of PM10 was found to be lower during summer relative to winter due to higher air temperature and ventilation rates [50]. In addition, hens tend to move less if there are under heat stress in summer because the higher indoor air temperature may cause stagger, stupor, and reduced activities, and thus result in lower dust generation from the litter, while the cold season increases layers’ activities, thus generating higher PM from the poultry house litter floor [62]. Besides animal activities, house ventilation and litter moisture are critical for PM generations. Therefore, the total poultry house PM emissions could be higher in summer than in winter because of increased house ventilation and drier litter conditions [30].

Table 4.
Particulate matter concentration (mg/m3) as affected by seasons.
3.4. Effect of Ventilation System
PM emissions depend on the housing systems and ventilation types. Most of poultry housing systems are mechanically ventilation system that applies maximum ventilation in summer for removing extra heat and uses minimum ventilation in winter for moisture removing, which can improve air quality inside the house [66]. Poultry house ventilation rate affects the PM concentration [9]. Similarly, ventilation changes as affected by seasons that winter season has the highest concentration of PM among all four seasons [37]. Oppositely, increased ventilation during the summer dilutes the PM concentration [62]. Besides seasonal effect, housing style (e.g., natural ventilation vs. mechanical ventilation), ventilation types (e.g., negative vs. positive ventilation), and fan selection could also affect PM generations (Table 5). Measurements of PM in natural ventilation systems have higher variations as wind directions and speed are varying over time.

Table 5.
Emission of PM due to various ventilation systems used in the housing system.
3.5. Effect of Indoor Temperature and Relative Humidity
Temperature and relative humidity (RH) are inversely proportional to each other. Increased temperature decreases RH and is directly influenced by ventilation rates within poultry houses [70]. Temperature and RH change seasonally and depend on weather conditions and experimental house design. During the winter, ventilation rates are decreased, and heaters are turned on to make a room warm, reducing RH. A decrease in RH increases PM concentration. However, ventilation rates are increased during the summer season to bring cold air or moisture from outside (cooling pad). The moisture from outside makes RH higher inside the house and decreases PM concentration by making heavy PM settle down. According to Lin et al. (2017), PM2.5 and PM10 concentrations depend on RH due to ambient air [71]. Similarly, houses or rooms attached more to the outside environment possess higher RH due to individual room effects. Tang et al. (2020) tested the effect of different temperatures (21.1, 23.8, 26.4, and 29.2 °C) and RH (49.5, 74.7, 78.8, and 80.0%) on PM concentrations and found that PM1, PM2.5, PM10, and TSP were significantly lower in higher RH and lower temperature treatments because RH could affect litter moisture [64]. In addition, several experiments used liquid spray (oil or water) to reduce PM concentration by increasing LMC and RH [24,72,73,74,75,76]. In addition, Yang et al. (2022) found that RH directly affects LMC and varies between rooms and within rooms [77]. Higher LMC within rooms results in lower PM levels [24,78]. That is why temperature and RH have a direct influence on PM levels.
3.6. Other Factors
3.6.1. Manure Cleaning Methods
Poultry manure management plays an important role in dust emissions because manure contributes about 50% of total dust emissions in most housing systems with a raised floor [18]. Several studies show that floor-raised houses (broilers or layers) where manure gets deposited on the floor over time possess potentially higher PM concentrations than other poultry housing [17,40]. Similarly, PM10 and PM2.5 produced from deposited manure contribute up to 96% and 72% of total dust emissions, respectively, from poultry facilities [17]. In addition, poultry facilities having different kinds of manure removal or storage affects the PM concentration. For example, according to Chai et al. (2012), houses with MB usually have significantly higher dust concentrations than HR housing (manure deposited underneath the house) [9]. However, manures are removed continuously in MB housing to decrease PM levels. Similarly, manure removal frequency also affects PM production. An increase in manure removal frequency has shown the highest PM reduction compared to less frequent or stored manure facilities [45]. For example, furnished cages with manure belts with a manure removal frequency of two times per week resulted in lower PM concentration and bacteria counts than floor-raised with manure storage.
3.6.2. Bird Age, Stocking Density, and Behaviors
The chickens’ activity and dust emission depend on the birds’ age in poultry housing [78]. Recent research on pullets found that an increase in pullets’ age increases birds’ activities and significantly affects or increases dust production (p < 0.05). Similarly, Vucemilo et al. (2007) also found that increasing broiler age affects PM levels significantly [79]. Chicken activities during feeding mainly increase PM10 and TSP in the chicken house [80]. However, the perching behaviors and dust bathing in open spaces showed high PM production compared to the feeding and drinking behaviors [78]. Moreover, PM emission is also affected by housing stocking density and bird weight [9]. PM levels are higher with the increase of birds’ weight and stocking density.
4. Impacts of PM on the Health and Welfare of Chickens and Farm Workers
High levels of PM can negatively impact the health and welfare of animals and their caretakers. According to Zhao et al. (2016), PM acts as a major carrier for airborne bacteria and endotoxin, which, once inhaled, might cause harmful effects on the respiratory systems of animals and caretakers [81]. When toxins carried by PM10 (particle size less than 10 µm) reach the bloodstream after inhalation, they can harm the respiratory system, liver, kidneys, and nervous system [12,14,82]. On the other hand, PM is more harmful to humans and birds with pre-existing cardiac diseases like asthma, making breathing difficult [83]. A low level of ventilation rate within the animal house was linked to long-term lung function impairment in animals [20]. Higher PM10 levels can increase the risk of chronic bronchitis, cardiovascular illness, pneumonia lesions, asthma-like symptoms, and lung cancer in farmers and animals [21,22].
4.1. Impacts on Birds’ Health, Behaviors, and Welfare
High PM concentrations have been linked to higher avian mortality rates [84]. Particulate matters contain various airborne bacteria and endotoxin, which negatively impact health and welfare issues of birds. When birds inhale dust particles with dust-borne pathogens (especially Mycoplasma species) damage occurs to mucosal surface cilia present in the trachea [85]. Particulate matter of size PM2.5 was found to have lots of harmful microorganisms and endotoxins (Table 6) [86]. Long-term exposure to PM2.5 has been linked to impaired lung function, and fraction size up to PM10 has increased mortality risk [83]. According to Roque et al. (2015), the endotoxin of dust helps decrease the percentage of cell-mediated immunity B cells (CD3−la+ B cells) in layers [87]. A decrease in cell-mediated immunity B cells causes birds to have difficulty fighting against poultry pathogens and several health issues. In addition, birds raised on litter floors evinced a higher incidence of lung damage due to higher PM emissions [88].

Table 6.
Effects of various PM sizes or types on health, behavior, and welfare of birds.
4.2. Human Health, Behaviors, and Welfare
Particulate matter in poultry houses can pollute the air and affect caretaker health. Poultry caretakers are at high risk due to occupational exposure to PM, leading to more respiratory hazards at work than in other work environments. Similarly, male poultry workers who smoke showed a substantially higher prevalence of chronic cough, chronic phlegm, and chronic bronchitis than nonsmokers [91]. The most common symptoms caused by PM in poultry workers are characterized by cough, phlegm, eye irritation, dyspnea, chest tightness, weariness, nasal congestion, wheezing, sneezing, nasal discharge, headache, throat irritation, and fever [12,19,20,21,22,83].
Inhaled PM can penetrate deeper into the respiratory airways, impairing human respiratory health and leading to a rise in chronic bronchitis, allergic responses, chronic cough, phlegm, and asthma-like symptoms amongst caretakers [12,13,19,20]. It is found that long-term exposure to PM increases obstructive pulmonary disorder rates [23]. Moreover, high asthmatic (42.5%) and nasal (51.1%) symptoms are observed in poultry workers. In farmers, higher PM10 concentrations can cause chronic bronchitis, asthma-like symptoms, cardiovascular disease, pneumonia lesions, chronic obstructive pulmonary disease (COPD), and lung cancer [21,22,23]. The relationship between PM levels and COPD cases or human mortality has been investigated. A 10 g m−3 rise in PM2.5 was shown to be associated with a 24% increase in cardiovascular events and a 76% increase in mortality [92], while residents living near a high-volume highway would experience a 33% increase in COPD incidence for every 7 g m−3 increase in PM10 [93]. PM exposure affects children’s lung development and long-term lung function [94]. In addition, PM of different sizes is dangerous to humans with pre-existing cardiovascular diseases, such as asthma [12,83].
Among the different types of PM, PM2.5 and PM10 have adverse effects on human health (Table 7). The mass concentrations of PM10 and PM2.5 have commonly been used as an indicator for defining PM that significantly affects health [94]. Inhalable particles that are tiny enough to enter the thoracic area of the respiratory system are included in PM10 and PM2.5. In most places in Europe, PM2.5 accounts for 50–70% of PM10. Short-term and long-term exposure to PM10 negatively impacts the respiratory system and increases mortality rates, respectively, while long-term exposure to PM2.5 increases the risk of cardiopulmonary mortality. According to Dai et al. (2017), the distribution of PM2.5 in high-rise and manure-belt houses was found to damage human alveolar epithelial cells (A549 cell) [95]. PM2.5 collected reduced the viability of A549 cells in a time- and dose-dependent manner and produced an inflammatory response. Despite evidence confirming the adverse effects of poultry house exposures on employees’ respiratory health, the industry has mainly ignored the health of exposed workers [35]. The World Health Organization has stated the need to monitor PM10 and PM2.5 levels in many countries and to estimate population exposure, which will aid local authorities in developing strategies to improve air quality [94].

Table 7.
Effects of different PM sizes on health, behavior, and welfare of caretakers.
4.3. Poultry Production
Air quality is essential to increase production and plays an important role at an early stage of development. According to Willis et al. (1987), birds grow faster in a less dusty indoor environment than in a higher dust environment [99]. Birds’ body weight was recorded as 45 g and 165 g heavier at four and seven WOA in the less dusty environment than in the dusty environment. A dusty environment with high PM concentration can affect BW gain, reduce production performance, and cause specific humoral immune responsiveness in broilers [23,34,95]. According to Lai et al. (2009), a high level of dust-containing pathogens can cause a decrease in body weight gain, alter heart morphology, and increase immune reactivity [100]. Similarly, increased inhalation of PM causes lesions in the respiratory tract, which provides space to cause pathogenic effects of microorganisms [84,101]. With increased pathogenic effects of microorganisms, birds’ growth rate decreased and even increased the chances of mortality. Thus, poor air quality directly harms production and increases economic loss.
5. Mitigation Strategies Suppressing PM Levels in Poultry Houses
The high level of PM in poultry facilities is a major concern for the health and welfare of animals and their caretakers [102,103,104,105,106,107]. Among different PM sizes, PM10 and PM2.5 levels are considered measurement factors for most organizations and countries because of their harmful effects on the health and welfare of caretakers (Table 8). The World Health Organization (WHO) recently amended the ambient air quality standards in 2021 and proposed the maximum of PM10 to be 15 µg/m3 for the annual average and 45 µg/m3 for the 24-h mean, while for PM2.5 to be 5 µg/m3 for the annual average and 15 µg/m3 for the 24-h mean [11,106]. According to the EPA (2022), the National Ambient Air Quality Standard (NAAQS) has set an exposure limit of PM2.5 and PM10 as 35 µg/m3 and 150 µg/m3, respectively, for 24 h (98th percentile, averaged over 3 years) [103]. Therefore, everyone must follow OEL guidelines to improve the caretaker’s health.

Table 8.
Recommended guidelines for PM on occupational exposure limit of various countries or organizations.
6. Particulate Matter Emission Mitigating Strategies
The PM concentration in poultry housing is primarily affected by housing and feeding, animal species, stocking density, lighting duration, environment conditions (season), and existing mitigation practices [22,24,72,108]. It is important to possess a deep knowledge of PM morphology to evaluate their effects and propose the best mitigating technologies in animal housing. Particulate matter mitigating strategies can be classified into three different groups: dilution and effective room air distribution, source-control techniques to reduce PM from the source, and PM removal or cleaning techniques by using acid scrubbers, electrostatic precipitators, or ionizers [109]. Other techniques for improving air quality are oil spraying, manure handling, and electrolyzed water spray [24]. Controlling the living space environment, including temperature, humidity, air quality, and litter quality, is critical for poultry well-being [110]. Variations in indoor air quality have been linked to various factors, including barn architecture, manure management, animal densities, feed regimens, building ventilation, and farm management practices. Therefore, various biochemical, chemical, managerial, physical, and physiological practices must be implemented to decrease PM significantly lower than recommended guidelines (Figure 3).
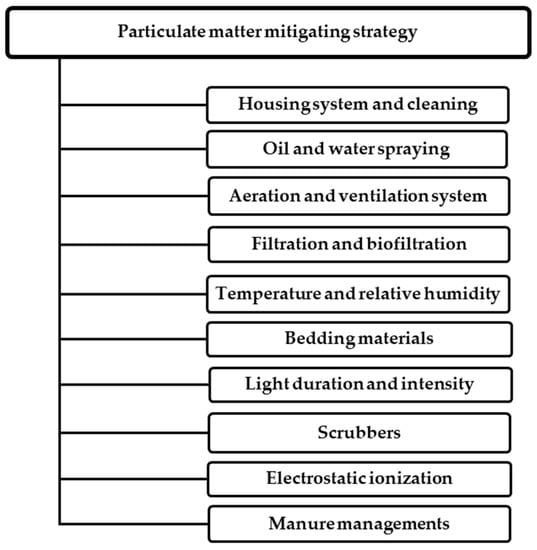
Figure 3.
Overview of the PM emission mitigating strategy used in poultry housing.
6.1. Housing Systems and Cleaning
Particulate matter emission differs according to housing types. Dust concentrations in caged buildings are influenced by cage design and rearing practices, but dust levels in floor housing are determined by litter management, hen age, temperature, and humidity control [41,78]. The air quality in CF houses is generally worse than in caged houses. Usually, PM concentration is higher in CF houses than in other housing types because of litter accumulation and hen activities on the floor [24,40]. In the floor housing system, an average concentration of respirable ambient dust of 0.37 mg/m3 was detected, which was more significant than average values in the caged system (0.13 mg/m3). Similarly, Le Bouquin et al. (2013) also found the highest concentration of dust (1.19 mg/m3) in AV housing [41]. The researchers evaluated PM levels in three-layer houses (AV, CC, and EC) and discovered that the daily mean PM10 level in AV was about six to nine times higher compared to CC and EC [40]. The emission rates for PM10 and PM2.5 were highest because of increased activities on the littered floor [40]. However, in CC and EC, PM emissions were similar, accounting for 16% of AV PM10 ER and 10–20% of AV PM2.5 ER, respectively. Thus, caged hen housing shows the lowest dust concentration, almost four to five times lower than floor-raised AV housing systems [111]. As a result, the type of housing and the amount of litter significantly impacted air quality. According to Guarino et al. (1999), PM concentration inside the farm were significantly higher during scraper cleaning and feed distribution [84]. As a result, reducing PM generation and emissions is critical for maintaining the health and well-being of laying hens and caregivers while also increasing the environmental stewardship production operation. Thus, housing types and cleaning procedures are vital in controlling PM emissions.
6.2. Oil and Water Spraying
Oil and water spraying in poultry housing helps to control indoor PM concentration. Spraying liquid agents, such as tap water, acidic water, electrolyzed water, or a combination of water and soybean or canola oil, over poultry buildings has been studied to lower PM levels and disinfect the houses [24,72,73,74,75,76]. According to Ogink et al. (2012), Spraying water on top of litter at an application rate of 150 to 600 mL m−2 reduced PM10 and PM2.5 emissions by 18 to 64% in an aviary hen house but increased NH3 emissions by 21 to 65% [76]. High liquid spray dosages decrease PM significantly while increasing NH3 levels due to the accumulation of litter moisture [112]. A spray dose of pH3 and 25-mL kg−1 dry litter d−1 showed a good combination for controlling PM levels in littered CF hen houses without creating unwanted increases in NH3 emissions [24]. Spraying a liquid agent like electrolyzed water over the litter of CF hen houses has been demonstrated to decrease PM successfully. Cautions should be paid as spraying water may lead to corrosion of metal equipment in poultry facilities [24].
Research on oil and water spraying shows significant PM and airborne bacteria reductions (Table 9). Total dust, airborne bacteria, and fungi were significantly reduced by soybean oil for 24 h after spraying [113]. Particulate matter removal efficiency of oil and water spray ranges from 18 to 89%. Increased spraying frequency can help reduce PM emissions even further, but it can also make surfaces oily and slippery, posing a safety risk to workers and animals [114].

Table 9.
Particulate matter reduction with liquid spray.
6.3. Filtration and Biofiltration
Filtration is one of the most well-known and extensively applied methods for removing particles from an air stream (Table 10). Filtration typically occurs through dry methods (without adding water), including impaction, interception, diffusion, and electrostatic and gravitational deposition [118]. Interception and impaction are major processes for larger particles, but diffusion is the primary mechanism for particles less than 0.5 µ [108]. Filters are usually used to remove dust particles from AFOs, while filters that utilize water as a scrubber media may catch NH3 gas from the air in poultry houses and clean it.
For many years, water filters, also known as trickling filters, have eliminated PM, NH3, sulfur compounds, and nitrous oxides in commercial operations [119]. Biofilters are generally designed to control NH3, with PM as a secondary concern [120], and help to biologically transform pollutants, such as NH3 gas, into inert forms [121,122]. Similarly, biofilters commonly use soil, compost, peat, activated carbon, municipal trash, bark, trimmings, and leaves as organic filter media [123].

Table 10.
Air filtration systems used to mitigate PM emissions.
Table 10.
Air filtration systems used to mitigate PM emissions.
| Filter/Biofilter | PM Size | PM Reduction (%) | References |
|---|---|---|---|
| Wood-chip Bio-filter 127 mm 254 mm | PM10 TSP | 62 and 89.7 62.9 and 96.3 | [9] |
| Stuffnix dry filter U-bend baffle filter | PM2.5 and PM10 | 41 and 64 19 and 22 | [44] |
| Dry filter | PM concentrations PM emissions | 55 72 | [62] |
| Dry filter | PM2.5 PM10 | 7.1 40.7 | [66] |
| Biotrickling filter and denitrification EBRT * = 3 s EBRT = 0.71 s EBRT = 3.6 s | PM10 | 38 60 69 | [124] |
| Stuffnix dry filter | Fine dust | 20–60 | [125] |
| Trickling biofilter using acidified water | PM10 | >80 | [126] |
| Bio-filter | TSP | 79–96 | [127] |
* EBRT = Empty bed air residence time.
6.4. Bedding Materials
Poultry litter/bedding material is well-known as the mixture of initial bedding material and the manure deposited by the birds on the floor [55]. Bedding material is the main contributor to PM emission from CF poultry facilities than caged. According to Van Harn et al. (2012), using different kinds of bedding material significantly reduces PM emissions; maize silage has shown a 19% PM2.5 reduction compared to wood shaving [55]. However, it does not show any significant differences in PM10 reduction. Similarly, when different kinds of bedding materials (chopped straw, gravel, peat, wood shaving, chopped paper, or clay pellets) were used in layer housing, peat or clay pellets resulted in lower PM production [54].
Several studies have shown that bedding increases dust concentrations, so changing bedding materials might be an alternative to reduce PM emissions [54,55] (Table 11). However, changing bedding materials in every flock (broilers) is impossible from an economic point of view, so there is a general trend in the US of reusing litter for several flocks [128]. Reusing bedding material might be a good idea to increase profitability, but it also inherits the challenges of higher PM emissions, so deep littering or topping of bedding materials can be an alternate way to reduce PM emissions from the farm. According to Bist et al. (unpublished), top application of different bedding materials (fine wood shaving, large wood shaving, and aspen wood chips) over reused litter significantly reduced PM concentration by up to 40%. Moreover, using deep bedding material has reported many beneficial aspects like increased growth rate, weight gain, and feed conversion ratio in poultry [129,130].

Table 11.
Particulate matter reduction by using different kinds of bedding materials.
6.5. Scrubbers
Scrubbers effectively remove air pollutants from poultry housing (Table 12). Scrubbers help remove airborne dust, bacteria, NH3, and even CO2 with the help of a multi-stage air scrubber design [132,133]. According to Zhao et al. (2011), three acid scrubbers (double-stage scrubber with filter, double-stage scrubber with a biofilter, and triple-stage scrubber) used in the experiment reduced PM10 by 61–93% and PM2.5 by 47–90% [133]. The double-stage acid scrubber reduced dust levels significantly higher than the triple-stage acid scrubber. Along with PM reduction, this multi-stage acid scrubber significantly reduced airborne total bacteria concentration from 46% to 85%. A scrubber can decrease the total dust emissions and airborne bacteria concentration by up to 88% and 85%, respectively.

Table 12.
Particulate matter and airborne bacteria reduction using scrubbers.
6.6. Electrostatic Ionization
For many years, the electrostatic ionization technique has been used to lower PM levels in AFOs. Recently, attempts have been made to employ the technology in animal housing conditions, and several studies have demonstrated the efficiency of this control technique in lowering airborne PM and bacteria [8,137,138,139,140,141,142]. For example, Mitchell and Waltman (2003) tested an electrostatic charging system (ESCS; −30 K Vdc and 0.2 mA) in the hatching cabinet and reduced dust from 77–79% [138]. Similarly, ESCS decreased Enterobacteriaceae and salmonella bacteria in the air from 93 to 96% and 33 to 83%, respectively. Furthermore, recent research used the prototype electrostatic precipitator (ESP) technique in different ventilation or weather condition (hot, warm, and cold weather) and found PM2.5 and PM10 reductions up to 97.8% and 99.0%, respectively [143]. Therefore, various research on electrostatic ionization has shown PM and airborne bacteria reduction up to 94 and 96%, respectively (Table 13).

Table 13.
Particulate matter and airborne bacteria reduction with electrostatic charging.
6.7. Other Management Practices
6.7.1. Aeration and Ventilation System
Aeration (Airflow speed) exhibited a significant and inverse relationship with PM and NH3 concentrations but a significant and direct relationship with temperature [80]. The contradiction between maintaining temperature and increased ventilation must be implemented to improve the air quality in the layer house. When there is insufficient ventilation, the concentration of air pollutants can build up to dangerous levels [147]. During the colder seasons, ventilation is used to remove moisture and manage humidity levels, whereas during the warmer seasons, ventilation is used to keep interior temperatures within the poultry barn’s thermal neutral zone. Furthermore, mechanical and natural ventilation systems help to dilute NH3 and PM concentrations by delivering fresh air into the indoor environment [148]. The mixing of air induced by the air inlets and exhaust fans might impact PM levels within the barn when mechanical ventilation is used. Decreased ventilation can cause dust concentrations to drop because of increased moisture content in air and litter compared to high ventilation [70]. However, houses with extremely high ventilation rates or natural ventilation resulted in decreased dust levels.
6.7.2. Lighting Management
Lighting programs are very important for causing variation in PM concentration [111] and strongly affect birds’ activities by changing circadian rhythms [149]. For example, particle formation rates in a layer house were significantly higher during light periods than during dark [25]. Similarly, the photoperiod duration shows increased dust emission from poultry housing as the light period increases birds’ activities. The mean respirable PM level is higher in light periods than in dark periods in broiler housing [150]. According to Calvet et al. (2009), average dust concentrations during the daytime are four times higher than during dark periods [149]. Therefore, adjusting light duration and intensity can help to reduce PM emissions from the farm.
6.7.3. Precision Control of Indoor Temperature and Relative Humidity
Temperature and RH are highly influenced by ventilation rates within poultry houses [70]. Particulate matter emissions depend on indoor RH and temperature. High RH and low temperature within the housing capture dust particles due to increased air moisture content. Similarly, higher RH due to low ventilation makes dust heavy and settle down, thus helping to lower PM emissions [70]. However, housing’s extremely high ventilation and natural ventilation rates can decrease PM emissions by pulling out PM from farms. Similarly, Tang et al. (2020) found that low temperature and high RH reduce PM emissions at higher levels, so controlling temperature and RH inside the house can help to decrease PM emissions from poultry houses [64].
7. Summary
Particulate matters (PM) found in poultry houses are biological, organic, and inorganic in composition, which originated from bedding materials, feathers, feeds, skin, excreta, bacteria, and feathers. Fine PM such as PM2.5 is crucial in affecting the health and well-being of birds and caretakers as that can enter animals’ respiratory system easier. According to the WHO, the occupational exposure limits of PM2.5 annual mean and 24-h mean should not exceed 5 µg/m3 and 15 µg/m3, respectively. The levels of PM in poultry houses could be 100 times of WHO limit or higher (e.g., PM2.5 levels in cage-free henhouse are higher than 1500 µg/m3 in most time of the year), and thus affect animals’ health and welfare, including eye irritation, throat irritation, cough, phlegm, chest tightness, sneezing, headache, fever, nasal congestion, and wheezing, especially in cold periods when the house will have limited ventilation. Furthermore, long-term exposure to PM increases obstructive pulmonary disorder, chronic bronchitis, chronic obstructive pulmonary disease, pneumonia lesions, cardiovascular disease, asthma-like symptoms, lung cancer, or even mortality in humans. Similarly, a higher level of PM with endotoxin in birds causes impaired lung function, chronic bronchitis, pneumonia lesions, cardiovascular illness, and cardiotoxicity in chicken embryos and hatchling chickens and might increase the risk of mortality rates. That is why it is very important to identify primary emissions factors and investigate PM mitigating strategies.
PM emissions depend on various factors and changes according to climatic conditions, housing type, applied manure management strategies, ventilation system, temperature and relative humidity, bird numbers, and bedding materials used. The factors that release significantly high PM levels must be managed and decreased to preserve and improve the environment, and human and animal health and welfare. Several studies have shown significant PM reduction by applying biochemical, chemical, managerial, physical, and physiological practices, which can be managing housing system and cleaning, light intensity, oil and water spraying, filtration and biofiltration, acid scrubber, bedding materials, and electrostatic ionization. Single or integrated mitigation has shown significant PM reduction in the past. Future research must be implemented by including integrated mitigating strategies to obtain much better results to improve air quality in poultry houses and enhance the health of both caretakers and birds. In addition, mitigation strategies could be cost prohibitive and have side effects. For instance, an acid scrubber has up to 95% efficiency in mitigating both dust and NH3, but the cost for installing the system is a primary barrier; the water spray has a lower cost in controlling PM generations in poultry houses, but the increased NH3 should be considered in quantifying the mitigation efficiency and costs. Additional strategies such as litter additives and new bedding will be needed for NH3 control if water spray results in higher NH3 generations. Therefore, poultry farms should select mitigation strategies based on a number of considerations, such as farm location, climate conditions, environmental policies, and available resources (assistance programs).
Author Contributions
L.C. and R.B.B. designed the concept; R.B.B. and L.C. collected data from published articles; R.B.B. and L.C. analyzed the data; R.B.B. and L.C. wrote the paper; L.C. provided resources. All authors have read and agreed to the published version of the manuscript.
Funding
Egg Industry Center at the Iowa State University; and USDA-Hatch projects: Future Challenges in Animal Production Systems: Seeking Solutions through Focused Facilitation (GEO00895; Accession Number: 1021519) and Enhancing Poultry Production Systems through Emerging Technologies and Husbandry Practices (GEO00894; Accession Number: 1021518); Georgia Research Alliance Venture Fund.
Acknowledgments
This project was supported by Egg Industry Center at the Iowa State University; and USDA-Hatch projects: Future Challenges in Animal Production Systems: Seeking Solutions through Focused Facilitation (GEO00895; Accession Number: 1021519) and Enhancing Poultry Production Systems through Emerging Technologies and Husbandry Practices (GEO00894; Accession Number: 1021518); Georgia Research Alliance Venture Fund.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chai, L.; Kröbel, R.; Janzen, H.H.; Beauchemin, K.A.; McGinn, S.M.; Bittman, S.; Atia, A.; Edeogu, I.; MacDonald, D.; Dong, R. A Regional Mass Balance Model Based on Total Ammoniacal Nitrogen for Estimating Ammonia Emissions from Beef Cattle in Alberta Canada. Atmos. Environ. 2014, 92, 292–302. [Google Scholar] [CrossRef]
- Chai, L.; Kröbel, R.; MacDonald, D.; Bittman, S.; Beauchemin, K.A.; Janzen, H.H.; McGinn, S.M.; Vanderzaag, A. An Ecoregion-Specific Ammonia Emissions Inventory of Ontario Dairy Farming: Mitigation Potential of Diet and Manure Management Practices. Atmos. Environ. 2016, 126, 1–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Shepherd, T.A.; Li, H.; Xin, H. Environmental Assessment of Three Egg Production Systems—Part I: Monitoring System and Indoor Air Quality. Poult. Sci. 2015, 94, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.-Q.; Diehl, C.A.; Chai, L.; Chen, Y.; Heber, A.J.; Lim, T.-T.; Bogan, B.W. Factors and Characteristics of Ammonia, Hydrogen Sulfide, Carbon Dioxide, and Particulate Matter Emissions from Two Manure-Belt Layer Hen Houses. Atmos. Environ. 2017, 156, 113–124. [Google Scholar] [CrossRef]
- Ni, J.-Q.; Liu, S.; Diehl, C.A.; Lim, T.-T.; Bogan, B.W.; Chen, L.; Chai, L.; Wang, K.; Heber, A.J. Emission Factors and Characteristics of Ammonia, Hydrogen Sulfide, Carbon Dioxide, and Particulate Matter at Two High-Rise Layer Hen Houses. Atmos. Environ. 2017, 154, 260–273. [Google Scholar] [CrossRef]
- NRC (National Research Council). Air Emissions from Animal Feeding Operations: Current Knowledge, Future Needs; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- EPA National Emission Inventory. Ammonia Emissions from Animal Husbandry Operations; EPA National Emission Inventory: Washington, DC, USA, 2004; Volume 131. Available online: https://www3.epa.gov/ttnchie1/ap42/ch09/related/nh3inventorydraft_jan2004.pdf (accessed on 30 September 2022).
- Ritz, C.; Mitchell, B.; Fairchild, B.; Czarick III, M.; Worley, J. Improving In-House Air Quality in Broiler Production Facilities Using an Electrostatic Space Charge System. J. Appl. Poult. Res. 2006, 15, 333–340. [Google Scholar] [CrossRef]
- Chai, L.; Ni, J.-Q.; Diehl, C.A.; Kilic, I.; Heber, A.; Chen, Y.; Cortus, E.; Bogan, B.; Lim, T.; Ramirez-Dorronsoro, J.-C. Ventilation Rates in Large Commercial Layer Hen Houses with Two-Year Continuous Monitoring. Br. Poult. Sci. 2012, 53, 19–31. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Wang-Li, L.; Cortus, E.; Bogan, B.W.; Kilic, I.; Liang, W.-Z.; Xiao, C.-H.; Chai, L.-L.; Ni, J.-Q. The National Air Emissions Monitoring Study′s Southeast Layer Site: Part V. Hydrogen Sulfide and Volatile Organic Compounds. Trans. ASABE 2016, 59, 681–693. [Google Scholar]
- WHO (World Health Organization). Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 30 September 2022).
- US EPA. Health and Environmental Effects of Particulate Matter (PM). Available online: https://www.epa.gov/pm-pollution/health-and-environmental-effects-particulate-matter-pm (accessed on 30 September 2022).
- US EPA. NAAQS Table. Available online: https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed on 30 September 2022).
- US EPA. Particulate Matter (PM) Basics. Available online: https://www.epa.gov/pm-pollution/particulate-matter-pm-basics (accessed on 30 September 2022).
- EEA Group 10: Agriculture—European Environment Agency. Available online: https://www.eea.europa.eu/publications/EMEPCORINAIR5/page019.html (accessed on 30 September 2022).
- Wicklin, G.; Czarick, M. Particulate Emissions from Poultry Housing. In Proceedings of the ASAE Annual International Meeting, Minneapolis Convention Center, Minneapolis, MN, USA, 10–14 August 1997; pp. 10–14. [Google Scholar]
- Cambra-López, M.; Hermosilla, T.; Lai, H.T.; Aarnink, A.J.A.; Ogink, N. Particulate Matter Emitted from Poultry and Pig Houses: Source Identification and Quantification. Trans. ASABE 2011, 54, 629–642. [Google Scholar] [CrossRef]
- Ahaduzzaman, M.; Milan, L.; Morton, C.L.; Gerber, P.F.; Walkden-Brown, S.W. Characterization of Poultry House Dust Using Chemometrics and Scanning Electron Microscopy Imaging. Poult. Sci. 2021, 100, 101188. [Google Scholar] [CrossRef]
- Donham, K. Occupational Health Hazards and Recommended Exposure Limits for Workers in Poultry Buildings. In Proceedings of the National Poultry Waste Management Symposium Committee, Birmingham, AL, USA, 28–30 October 2000; pp. 92–109. [Google Scholar]
- Radon, K.; Weber, C.; Iversen, M.; Danuser, B.; Pedersen, S.; Nowak, D. Exposure Assessment and Lung Function in Pig and Poultry Farmers. Occup. Environ. Med. 2001, 58, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Cambra-López, M.; Aarnink, A.J.; Zhao, Y.; Calvet, S.; Torres, A.G. Airborne Particulate Matter from Livestock Production Systems: A Review of an Air Pollution Problem. Environ. Pollut. 2010, 158, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Michiels, A.; Piepers, S.; Ulens, T.; Van Ransbeeck, N.; Sacristán, R.D.P.; Sierens, A.; Haesebrouck, F.; Demeyer, P.; Maes, D. Impact of Particulate Matter and Ammonia on Average Daily Weight Gain, Mortality and Lung Lesions in Pigs. Prev. Vet. Med. 2015, 121, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.; Faísca, V.M.; Dias, H.; Clérigo, A.; Carolino, E.; Viegas, C. Occupational Exposure to Poultry Dust and Effects on the Respiratory System in Workers. J. Toxicol. Environ. Health Part A 2013, 76, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Zhao, Y.; Xin, H.; Wang, T.; Atilgan, A.; Soupir, M.; Liu, K. Reduction of Particulate Matter and Ammonia by Spraying Acidic Electrolyzed Water onto Litter of Aviary Hen Houses: A Lab-Scale Study. Trans. ASABE 2017, 60, 497–506. [Google Scholar]
- Qi, R.; Manbeck, H.; Maghirang, R. Dust Net Generation Rate in a Poultry Layer House. Trans. ASAE 1992, 35, 1639–1645. [Google Scholar] [CrossRef]
- Roumeliotis, T.S.; Dixon, B.J.; Van Heyst, B.J. Characterization of Gaseous Pollutant and Particulate Matter Emission Rates from a Commercial Broiler Operation Part I: Observed Trends in Emissions. Atmos. Environ. 2010, 44, 3770–3777. [Google Scholar] [CrossRef]
- Donham, K.J.; Scallon, L.J.; Popendorf, W.; Treuhaft, M.W.; Roberts, R.C. Characterization of Dusts Collected from Swine Confinement Buildings. Am. Ind. Hyg. Assoc. J. 1986, 47, 404–410. [Google Scholar] [CrossRef]
- Bonifacio, H.F.; Maghirang, R.G.; Trabue, S.L.; McConnell, L.L.; Prueger, J.H.; Bonifacio, E.R. TSP, PM10, and PM2. 5 Emissions from a Beef Cattle Feedlot Using the Flux-Gradient Technique. Atmos. Environ. 2015, 101, 49–57. [Google Scholar] [CrossRef]
- Chai, L.; Xin, H.; Wang, Y.; Oliveira, J.; Wang, K.; Zhao, Y. Mitigating Particulate Matter Generation in a Commercial Cage-Free Hen House. Trans. ASABE 2019, 62, 877–886. [Google Scholar] [CrossRef]
- Lin, X.-J.; Cortus, E.; Zhang, R.; Jiang, S.; Heber, A. Ammonia, Hydrogen Sulfide, Carbon Dioxide and Particulate Matter Emissions from California High-Rise Layer Houses. Atmos. Environ. 2012, 46, 81–91. [Google Scholar] [CrossRef]
- Anderson, N.; Strader, R.; Davidson, C. Airborne Reduced Nitrogen: Ammonia Emissions from Agriculture and Other Sources. Environ. Int. 2003, 29, 277–286. [Google Scholar] [CrossRef]
- Ten Brink, H.; Even, A. Secondary Aerosol in the Netherlands. In Proceedings of the Annual Report of Subproject AEROSOL of Eurotrac-2, Munich, Germany, 1999. [Google Scholar]
- Sethi, P.; Muduli, S.; Aman Mishra, D.; Roul, A.K.; Mishra, A. Poultry Dust and Risks Associated with Public Health. Pharma Innov. 2019, 8, 7725. [Google Scholar]
- Harry, E. Air Pollution in Farm Buildings and Methods of Control: A Review. Avian Pathol. 1978, 7, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Donham, K.J.; Cumro, D.; Reynolds, S. Synergistic Effects of Dust and Ammonia on the Occupational Health Effects of Poultry Production Workers. J. Agromed. 2002, 8, 57–76. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Carolino, E.; Malta-Vacas, J.; Sabino, R.; Viegas, S.; Veríssimo, C. Fungal Contamination of Poultry Litter: A Public Health Problem. J. Toxicol. Environ. Health A 2012, 75, 1341–1350. [Google Scholar] [CrossRef]
- Morgan, R.J.; Wood, D.J.; Van Heyst, B.J. The Development of Seasonal Emission Factors from a Canadian Commercial Laying Hen Facility. Atmos. Environ. 2014, 86, 1–8. [Google Scholar] [CrossRef]
- Knight, R.M.; Tong, X.; Liu, Z.; Hong, S.; Zhao, L. Spatial and Seasonal Variations of PM Concentration and Size Distribution in Manure-Belt Poultry Layer Houses. Trans. ASABE 2019, 62, 415–427. [Google Scholar] [CrossRef]
- Liu, Q.; Cheng, W.; Liu, L.; Hua, Y.; Guo, L.; Nie, W. Research on the Control Law of Dust in the Main Ventilation System in Excavated Tunnels for Cleaner Production. Build. Environ. 2021, 205, 108282. [Google Scholar] [CrossRef]
- Shepherd, T.A.; Zhao, Y.; Li, H.; Stinn, J.P.; Hayes, M.D.; Xin, H. Environmental Assessment of Three Egg Production Systems—Part II. Ammonia, Greenhouse Gas, and Particulate Matter Emissions. Poult. Sci. 2015, 94, 534–543. [Google Scholar] [CrossRef]
- Le Bouquin, S.; Huneau-Salaün, A.; Huonnic, D.; Balaine, L.; Martin, S.; Michel, V. Aerial Dust Concentration in Cage-Housed, Floor-Housed, and Aviary Facilities for Laying Hens. Poult. Sci. 2013, 92, 2827–2833. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Seedorf, J.; Hartung, J. Inhalable and Respirable Dust, Bacteria and Endotoxins in the Air of Poultry Houses. In Proceedings of the 16th International Conference on Engineering Design, Paris, France, 28–31 July 2007. [Google Scholar]
- Kim, K.Y.; Ko, H.J. Field Survey on Concentration and Emission of Dust in Different Types of Poultry Houses of South Korea. Atmosphere 2020, 11, 530. [Google Scholar] [CrossRef]
- Demmers, T.; Saponja, A.; Thomas, R.; Phillips, G.; McDonald, A.; Stagg, S.; Bowry, A.; Nemitz, E. Dust and Ammonia Emissions from UK Poultry Houses. In Proceedings of the 17th World Congress of the International Commission of Agricultural and Biosystems Engineering (CIGR), Québec City, QC, Canada, 13–17 June 2010. [Google Scholar]
- Nimmermark, S.; Lund, V.; Gustafsson, G.; Eduard, W. Ammonia, Dust and Bacteria in Welfare-Oriented Systems for Laying Hens. Ann. Agric. Environ. Med. 2009, 16, 103–113. [Google Scholar] [PubMed]
- Adell, E.; Calvet, S.; Pérez-Bonilla, A.; Jiménez-Belenguer, A.; García, J.; Herrera, J.; Cambra-Lopez, M. Air Disinfection in Laying Hen Houses: Effect on Airborne Microorganisms with Focus on Mycoplasma Gallisepticum. Biosyst. Eng. 2015, 129, 315–323. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Li, B.; Shi, Z.; Zheng, W.; Teng, G. Concentration and Size Distribution of Particulate Matter in a New Aviary System for Laying Hens in China. J. Air Waste Manag. Assoc. 2020, 70, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Xin, H.; Li, H.; Shepherd, T.; Zhao, Y.; Stinn, J. Ammonia, Greenhouse Gas, and Particulate Matter Emissions of Aviary Layer Houses in the Midwestern US. Trans. ASABE 2013, 56, 1921–1932. [Google Scholar]
- Li, Q.-F.; Wang-Li, L.; Wang, K.; Chai, L.; Cortus, E.L.; Kilic, I.; Bogan, B.W.; Ni, J.-Q.; Heber, A.J. The National Air Emissions Monitoring Study′s Southeast Layer Site: Part II. Particulate Matter. Trans. ASABE 2013, 56, 1173–1184. [Google Scholar]
- Li, H.; Xin, H.; Burns, R.T.; Jacobson, L.D.; Noll, S.; Hoff, S.; Harmon, J.; Koziel, J.A.; Hetchler, B. Air Emissions from Tom and Hen Turkey Houses in the US Midwest. Trans. ASABE 2011, 54, 305–314. [Google Scholar] [CrossRef]
- EU Council Directive 1999/74/EC of 19 July 1999 Laying down Minimum Standards for the Protection of Laying Hens. Available online: https://www.legislation.gov.uk/eudr/1999/74/contents (accessed on 30 September 2022).
- Aarnink, A.; Ellen, H. Processes and Factors Affecting Dust Emissions from Livestock Production. How to Improve Air Quality. 2007. Available online: https://www.researchgate.net/profile/Andre-Aarnink/publication/40098613_Processes_and_factors_affecting_dust_emissions_from_livestock_production/links/55adff7508aed9b7dcdb09b3/Processes-and-factors-affecting-dust-emissions-from-livestock-production.pdf (accessed on 10 September 2022).
- Munir, M.; Belloncle, C.; Irle, M.; Federighi, M. Wood-Based Litter in Poultry Production: A Review. World’s Poult. Sci. J. 2019, 75, 5–16. [Google Scholar] [CrossRef]
- Gustafsson, G.; Von Wachenfelt, E. Reducing Airborne Dust in a Loose-Housing System for Laying Hens. J. Agric. Sci. Technol. A 2012, 2, 350. [Google Scholar]
- Van Harn, J.; Aarnink, A.; Mosquera, J.; Van Riel, J.; Ogink, N. Effect of Bedding Material on Dust and Ammonia Emission from Broiler Houses. Trans. ASABE 2012, 55, 219–226. [Google Scholar] [CrossRef]
- Gustafsson, G.; Von Wachenfelt, E. Airborne Dust Control Measures for Floor Housing System for Laying Hens. CIGR J. 2006, VII, 1–13. [Google Scholar]
- Takai, H.; Pedersen, S.; Johnsen, J.O.; Metz, J.; Koerkamp, P.G.; Uenk, G.; Phillips, V.; Holden, M.; Sneath, R.; Short, J. Concentrations and Emissions of Airborne Dust in Livestock Buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 59–77. [Google Scholar] [CrossRef]
- Farghly, M.; Mahrose, K.M.; Cooper, R.; Metwally, K.A.; Abougabal, M.S.; El-Ratel, I. Use of Available Crop By-Products as Alternative Bedding Materials to Wheat Straw for Rearing Broilers. Animal 2021, 15, 100260. [Google Scholar] [CrossRef] [PubMed]
- Samadi, S.; Van Eerdenburg, F.J.; Jamshidifard, A.-R.; Otten, G.P.; Droppert, M.; Heederik, D.J.; Wouters, I.M. The Influence of Bedding Materials on Bio-Aerosol Exposure in Dairy Barns. J. Expo. Sci. Environ. Epidemiol. 2012, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-T.; Heber, A.J.; Ni, J.-Q.; Gallien, J.; Xin, H. Air Quality Measurements at a Laying Hen House: Particulate Matter Concentrations and Emissions; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; p. 249. [Google Scholar]
- Shang, B.; Liu, Y.; Dong, H.; Tao, X.; Yao, H. Particulate Matter Concentrations and Emissions of a Fattening Pig Facility in Northern China. Atmos. Pollut. Res. 2020, 11, 1902–1911. [Google Scholar] [CrossRef]
- Mostafa, E.; Buescher, W. Indoor Air Quality Improvement from Particle Matters for Laying Hen Poultry Houses. Biosyst. Eng. 2011, 109, 22–36. [Google Scholar] [CrossRef]
- Zhou, Z.; Huo, L.; Yang, D.; Zhao, J.; Meng, L.; Bai, Z. Monitoring Particulate Matter Levels and Climate Conditions in Commercial Cage Laying Duck Houses; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2022; p. 1. [Google Scholar]
- Tang, Q.; Huang, K.; Liu, J.; Shen, D.; Dai, P.; Li, Y.; Li, C. Seasonal Variations of Microbial Assemblage in Fine Particulate Matter from a Nursery Pig House. Sci. Total Environ. 2020, 708, 134921. [Google Scholar] [CrossRef]
- Roumeliotis, T.S.; Van Heyst, B.J. Size Fractionated Particulate Matter Emissions from a Broiler House in Southern Ontario, Canada. Sci. Total Environ. 2007, 383, 174–182. [Google Scholar] [CrossRef]
- Winkel, A.; Mosquera, J.; Koerkamp, P.W.G.; Ogink, N.W.; Aarnink, A.J. Emissions of Particulate Matter from Animal Houses in the Netherlands. Atmos. Environ. 2015, 111, 202–212. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, W.; Wei, Y.; Li, B.; Wang, Y.; Zheng, H. Prevention of Particulate Matter and Airborne Culturable Bacteria Transmission between Double-Tunnel Ventilation Layer Hen Houses. Poult. Sci. 2019, 98, 2392–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chai, L.; Richardson, B.; Xin, H. Field Evaluation of an Electrostatic Air Filtration System for Reducing Incoming Particulate Matter of a Hen House. Trans. ASABE 2018, 61, 295–304. [Google Scholar] [CrossRef]
- Almuhanna, E. Characteristics of Air Contaminants in Naturally and Mechanically Ventilated Poultry Houses in Al-Ahsa, Saudi Arabia. Trans. ASABE 2011, 54, 1433–1443. [Google Scholar] [CrossRef]
- Pedersen, S.; Nonnenmann, M.; Rautiainen, R.; Demmers, T.; Banhazi, T.; Lyngbye, M. Dust in Pig Buildings. J. Agric. Saf. Health 2000, 6, 261. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhang, R.; Jiang, S.; El-Mashad, H.; Xin, H. Emissions of Ammonia, Carbon Dioxide and Particulate Matter from Cage-Free Layer Houses in California. Atmos. Environ. 2017, 152, 246–255. [Google Scholar] [CrossRef]
- Chai, L.; Xin, H.; Zhao, Y.; Wang, T.; Soupir, M.; Liu, K. Mitigating Ammonia Emissions from Liquid-Sprayed Litter of Cage-Free Hen House with a Solid Litter Additive; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2017; p. 1. [Google Scholar]
- Winkel, A.; Mosquera, J.; Aarnink, A.J.; Koerkamp, P.W.G.; Ogink, N.W. Evaluation of Oil Spraying Systems and Air Ionisation Systems for Abatement of Particulate Matter Emission in Commercial Poultry Houses. Biosyst. Eng. 2016, 150, 104–122. [Google Scholar] [CrossRef]
- Winkel, A.; Cambra-López, M.; Koerkamp, P.W.G.; Ogink, N.W.; Aarnink, A.J. Abatement of Particulate Matter Emission from Experimental Broiler Housings Using an Optimized Oil Spraying Method. Trans. ASABE 2014, 57, 1853–1864. [Google Scholar]
- Aarnink, A.; van Harn, J.; Van Hattum, T.; Zhao, Y.; Ogink, N. Dust Reduction in Broiler Houses by Spraying Rapeseed Oil. Trans. ASABE 2011, 54, 1479–1489. [Google Scholar] [CrossRef]
- Ogink, N.; van Harn, J.; van Emous, R.; Ellen, H. Top Layer Humidification of Bedding Material of Laying Hen Houses to Mitigate Dust Emissions: Effects of Water Spraying on Dust, Ammonia and Odor Emissions; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012; p. 3. [Google Scholar]
- Yang, X.; Chai, L.; Bist, R.B.; Subedi, S.; Guo, Y. Variation of Litter Quality in Cage-Free Houses during Pullet Production; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2022; p. 1. [Google Scholar]
- Bist, R.B.; Chai, L.; Yang, X.; Subedi, S.; Guo, Y. Air Quality in Cage-Free Houses during Pullets Production; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2022; p. 1. [Google Scholar]
- Vucemilo, M.; Matković, K.; Vinković, B.; Jakšić, S.; Granić, K.; Mas, N. The Effect of Animal Age on Air Pollutant Concentration in a Broiler House. Czech J. Anim. Sci 2007, 52, 170–174. [Google Scholar] [CrossRef]
- Shen, D.; Li, C. Distribution of Particulate Matter and Ammonia in a Mechanically Ventilated Layer House. In Proceedings of the International Symposium Animal Environment and Welfare, Chongqing, China, 23–25 October 2017. [Google Scholar]
- Zhao, Y.; Zhao, D.; Ma, H.; Liu, K.; Atilgan, A.; Xin, H. Environmental Assessment of Three Egg Production Systems—Part III: Airborne Bacteria Concentrations and Emissions. Poult. Sci. 2016, 95, 1473–1481. [Google Scholar] [CrossRef]
- Koren, H.; Bisesi, M. Handbook of Environmental Health, Fourth Edition, Volume II: Pollutant Interactions in Air, Water and Soil; CRC Press: Boca Raton, FL, USA, 2003; Volume 2. [Google Scholar]
- Schwarze, P.; Øvrevik, J.; Låg, M.; Refsnes, M.; Nafstad, P.; Hetland, R.; Dybing, E. Particulate Matter Properties and Health Effects: Consistency of Epidemiological and Toxicological Studies. Hum. Exp. Toxicol. 2006, 25, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.; Caroli, A.; Navarotto, P. Dust Concentration and Mortality Distribution in an Enclosed Laying House. Trans. ASAE 1999, 42, 1127. [Google Scholar] [CrossRef]
- Green, A.R.; Wesley, I.; Trampel, D.W.; Xin, H. Air Quality and Bird Health Status in Three Types of Commercial Egg Layer Houses. J. Appl. Poult. Res. 2009, 18, 605–621. [Google Scholar] [CrossRef]
- Dai, P.; Shen, D.; Tang, Q.; Huang, K.; Li, C. PM2. 5 from a Broiler Breeding Production System: The Characteristics and Microbial Community Analysis. Environ. Pollut. 2020, 256, 113368. [Google Scholar] [CrossRef] [PubMed]
- Roque, K.; Shin, K.-M.; Jo, J.-H.; Kim, H.-A.; Heo, Y. Relationship between Chicken Cellular Immunity and Endotoxin Levels in Dust from Chicken Housing Environments. J. Vet. Sci. 2015, 16, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Madelin, T.M.; Wathes, C. Air Hygiene in a Broiler House: Comparison of Deep Litter with Raised Netting Floors. Br. Poult. Sci. 1989, 30, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, C.; Chen, S.; Shi, L.; Li, D.C.; Lv, N.; Cui, L.; Chen, Y.; Zheng, Y. Particulate Matter 2.5 Induced Developmental Cardiotoxicity in Chicken Embryo and Hatchling. Front. Pharmacol. 2020, 11, 841. [Google Scholar] [CrossRef]
- Shen, D.; Guo, Z.; Huang, K.; Dai, P.; Jin, X.; Li, Y.; Li, C. Inflammation-Associated Pulmonary Microbiome and Metabolome Changes in Broilers Exposed to Particulate Matter in Broiler Houses. J. Hazard. Mater. 2022, 421, 126710. [Google Scholar] [CrossRef]
- Zuskin, E.; Mustajbegovic, J.; Schachter, E.N.; Kern, J.; Rienzi, N.; Goswami, S.; Marom, Z.; Maayani, S. Respiratory Function in Poultry Workers and Pharmacologic Characterization of Poultry Dust Extract. Environ. Res. 1995, 70, 11–19. [Google Scholar] [CrossRef]
- Miller, K.A.; Siscovick, D.S.; Sheppard, L.; Shepherd, K.; Sullivan, J.H.; Anderson, G.L.; Kaufman, J.D. Long-Term Exposure to Air Pollution and Incidence of Cardiovascular Events in Women. N. Engl. J. Med. 2007, 356, 447–458. [Google Scholar] [CrossRef]
- Schikowski, T.; Sugiri, D.; Ranft, U.; Gehring, U.; Heinrich, J.; Wichmann, H.; Krämer, U. Long-Term Air Pollution Exposure and Living Close to Busy Roads Are Associated with COPD in Women. Respir. Res. 2005, 6, 152. [Google Scholar] [CrossRef] [PubMed]
- WHO (World Health Organization). Health Effects of Particulate Matter: Policy Implications for Countries in Eastern Europe, Caucasus and Central Asia; World Health Organization: Geneva, Swizterland, 2013. [Google Scholar]
- Dai, P.; Shen, D.; Li, Y.; Li, C. Analysis of PM2. 5 Distribution in Chicken House and Its Damage on Human Alveolar Epithelial Cells. Anim. Environ. Welf. 2017, 21, e46. [Google Scholar]
- Hagmar, L.; Schütz, A.; Hallberg, T.; Sjöholm, A. Health Effects of Exposure to Endotoxins and Organic Dust in Poultry Slaughter-House Workers. Int. Arch. Occup. Environ. Health 1990, 62, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Guillam, M.-T.; Pédrono, G.; Le Bouquin, S.; Huneau, A.; Gaudon, J.; Leborgne, R.; Dewitte, J.-D.; Ségala, C. Chronic Respiratory Symptoms of Poultry Farmers and Model-Based Estimates of Long-Term Dust Exposure. Ann. Agric. Environ. Med. 2013, 20, 307–311. [Google Scholar]
- Rylander, R. Lung Diseases Caused by Organic Dusts in the Farm Environment. Am. J. Ind. Med. 1986, 10, 221–227. [Google Scholar] [CrossRef]
- Willis, W.L.; Ouart, M.; Quarles, C. Effect of an Evaporative Cooling and Dust Control System on Rearing Environment and Performance of Male Broiler Chickens. Poult. Sci. 1987, 66, 1590–1593. [Google Scholar] [CrossRef]
- Lai, H.T.; Nieuwland, M.G.; Kemp, B.; Aarnink, A.J.; Parmentier, H.K. Effects of Dust and Airborne Dust Components on Antibody Responses, Body Weight Gain, and Heart Morphology of Broilers. Poult. Sci. 2009, 88, 1838–1849. [Google Scholar] [CrossRef]
- Homidan, A.A.; Robertson, J.; Petchey, A. Review of the Effect of Ammonia and Dust Concentrations on Broiler Performance. World′s Poult. Sci. J. 2003, 59, 340–349. [Google Scholar] [CrossRef]
- Banhazi, T.; Seedorf, J.; Laffrique, M.; Rutley, D. Identification of the Risk Factors for High Airborne Particle Concentrations in Broiler Buildings Using Statistical Modelling. Biosyst. Eng. 2008, 101, 100–110. [Google Scholar] [CrossRef]
- US EPA. National Ambient Air Quality Standards (NAAQS) for PM. Available online: https://www.epa.gov/pm-pollution/national-ambient-air-quality-standards-naaqs-pm (accessed on 30 September 2022).
- DES Smoke and Dust Health Action Levels—Environment, Land and Water. Available online: https://apps.des.qld.gov.au/air-quality/health/ (accessed on 30 September 2022).
- OSHA Permissible Exposure Limits—OSHA Annotated Table Z-1—Occupational Safety and Health Administration. Available online: https://www.osha.gov/annotated-pels/table-z-1 (accessed on 30 September 2022).
- WHO (World Health Organization). Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneva, Switzerland, 2006; ISBN 92-890-2192-6. [Google Scholar]
- IOM. Occupational Exposure Limits for Dusts; IOM: Edinburgh, UK, 1984; Volume 23, Available online: http://winnipegsafetycompanies.com/wp-content/uploads/2016/09/dust-overview-john-cherrie.pdf#:~:text=1984%20HSE%20publish%20Guidance%20Note%20EH40%2C%20Occupational%20Exposure,or%205%20mg%2Fm3%20of%20respirable%20dust.%20COSHH%20Regulations (accessed on 3 April 2022).
- Wood, D. The Evaluation of Ammonia and Particulate Matter Control Strategies for Poultry Production Facilities. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2015. [Google Scholar]
- Almuhanna, E.A. Dust Control in Livestock Buildings with Electrostatically-Charged Water Spray; Kansas State University: Manhattan, KS, USA, 2007; ISBN 1-109-97127-3. [Google Scholar]
- Dawkins, M.S.; Donnelly, C.A.; Jones, T.A. Chicken Welfare Is Influenced More by Housing Conditions than by Stocking Density. Nature 2004, 427, 342–344. [Google Scholar] [CrossRef]
- Ellen, H.; Bottcher, R.; Von Wachenfelt, E.; Takai, H. Dust Levels and Control Methods in Poultry Houses. J. Agric. Saf. Health 2000, 6, 275. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Y.; Xin, H.; Gates, R.S.; Li, B.; Zhang, Y.; Soupir, M.L. Airborne Particulate Matter and Culturable Bacteria Reduction from Spraying Slightly Acidic Electrolyzed Water in an Experimental Aviary Laying-Hen Housing Chamber. Trans. ASABE 2014, 57, 229–236. [Google Scholar]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, C.N. Effect of Spraying Biological Additives for Reduction of Dust and Bioaerosol in a Confinement Swine House. Ann. Agric. Environ. Med. 2006, 13, 133–138. [Google Scholar]
- Zhang, Y.; Tanaka, A.; Barber, E.; Feddes, J. Effects of Frequency and Quantity of Sprinkling Canola Oil on Dust Reduction in Swine Buildings. Trans. ASAE 1996, 39, 1077–1081. [Google Scholar] [CrossRef]
- Yang, Y.; Kirychuk, S.P.; Si, Y.; Martel, M.C.; Guo, H.; Predicala, B.Z.; Zhang, L. Reduction of Airborne Particulate Matter from Pig and Poultry Rearing Facilities Using Engineered Water Nanostructures. Biosyst. Eng. 2022, 218, 1–9. [Google Scholar] [CrossRef]
- Zheng, W.; Li, B.; Cao, W.; Zhang, G.; Yang, Z. Application of Neutral Electrolyzed Water Spray for Reducing Dust Levels in a Layer Breeding House. J. Air Waste Manag. Assoc. 2012, 62, 1329–1334. [Google Scholar] [CrossRef]
- Takai, H.; Pedersen, S. A Comparison Study of Different Dust Control Methods in Pig Buildings. Appl. Eng. Agric. 2000, 16, 269. [Google Scholar] [CrossRef]
- Wood, D.J.; Van Heyst, B.J. A Review of Ammonia and Particulate Matter Control Strategies for Poultry Housing. Trans. ASABE 2016, 59, 329–344. [Google Scholar]
- Patterson, P. Management Strategies to Reduce Air Emissions: Emphasis—Dust and Ammonia. J. Appl. Poult. Res. 2005, 14, 638–650. [Google Scholar] [CrossRef]
- Garlinski, E.M.; Mann, D.D. Design and Evaluation of a Horizontal Airflow Biofilter on a Swine Facility; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; p. 1. [Google Scholar]
- Koerkamp, P.G. Review on Emissions of Ammonia from Housing Systems for Laying Hens in Relation to Sources, Processes, Building Design and Manure Handling. J. Agric. Eng. Res. 1994, 59, 73–87. [Google Scholar] [CrossRef]
- Sommer, S.; Hutchings, N. Techniques and Strategies for the Reduction of Ammonia Emission from Agriculture. Water Air Soil Pollut. 1995, 85, 237–248. [Google Scholar] [CrossRef]
- Ullman, J.; Mukhtar, S.; Lacey, R.; Carey, J. A Review of Literature Concerning Odors, Ammonia, and Dust from Broiler Production Facilities: Remedial Management Practices. J. Appl. Poult. Res. 2004, 13, 521–531. [Google Scholar] [CrossRef]
- Melse, R.W.; Mosquera, J. Nitrous Oxide (N2O) Emissions from Biotrickling Filters Used for Ammonia Removal at Livestock Facilities. Water Sci. Technol. 2014, 69, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Ogink, N.W.; Melse, R.W.; Mosquera, J. Multi-Pollutant and One-Stage Scrubbers for Removal of Ammonia, Odor, and Particulate Matter from Animal House Exhaust Air; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2009; p. 37. [Google Scholar]
- Snell, H.; Schwarz, A. Development of an Efficient Bioscrubber System for the Reduction of Emissions; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2003; p. 1. [Google Scholar]
- Seedorf, J.; Hartung, J. Reduction Efficiencies of a Biofilter and a Bio-Scrubber as Bio-Aerosols in Two Piggeries. Berl. Und Munch. Tierarztl. Wochenschr. 1999, 112, 444–447. [Google Scholar]
- Johnson, J.; Zwirzitz, B.; Oladeinde, A.; Milfort, M.; Looft, T.; Chai, L.; Zock, G.; Sommers, M.; Tunim, S.; Aggrey, S.E. Succession patterns of the bacterial community in poultry litter after bird removal and sodium bisulfate application. J. Environ. Qual. 2021, 50, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Gentry, J.; McGlone, J.; Blanton, J., Jr.; Miller, M. Alternative Housing Systems for Pigs: Influences on Growth, Composition, and Pork Quality. J. Anim. Sci. 2002, 80, 1781–1790. [Google Scholar] [CrossRef]
- Asaniyan, E.; Agbede, J.; Laseinde, E. Impact Assessment of Different Litter Depths on the Performance of Broiler Chickens Raised on Sand and Wood Shaving Litters. World J. Zool. 2007, 2, 67–72. [Google Scholar]
- Jones, W.G.; Dennis, J.W.; May, J.J.; Whitmer, M.P.; Siegel, P.D.; Sorenson, W.; Schwegler-Berry, D.; Kullman, G.J. Dust Control during Bedding Chopping. Appl. Occup. Environ. Hyg. 1995, 10, 467–475. [Google Scholar] [CrossRef]
- Melse, R.; Ogink, N.; Bosma, A. Multi-Pollutant Scrubbers for Removal of Ammonia, Odor, and Particulate Matter from Animal House Exhaust Air. In Proceedings of the Exploring the Advantages, Limitations, and Economics of Mitigation Technologies, Des Moines, IW, USA, 19–21 May 2008. [Google Scholar]
- Zhao, Y.; Aarnink, A.; De Jong, M.; Ogink, N.; Koerkamp, P.G. Effectiveness of Multi-Stage Scrubbers in Reducing Emissions of Air Pollutants from Pig Houses. Trans. ASABE 2011, 54, 285–293. [Google Scholar] [CrossRef]
- Ru, Y.; Zhao, L.; Hadlocon, L.J.S.; Zhu, H.; Ramdon, S.K. Laboratory Evaluation of Electrostatic Spray Wet Scrubber to Control Particulate Matter Emissions from Poultry Facilities. Environ. Technol. 2017, 38, 23–33. [Google Scholar] [CrossRef]
- Mosquera, J.; Hol, J.; Melse, R.; Winkel, A.; Nijeboer, G.; Ploegaert, J.; Ogink, N.; Aarnink, A. Dust Emission from Animal Houses: Air Scrubbing Techniques; Wageningen Livestock Research: Wageningen, The Netherlands, 2011; ISSN 1570-8616. [Google Scholar]
- Aarnink, A.; Landman, W.; Melse, R.; Huynh, T. Systems for Eliminating Pathogens from Exhaust Air of Animal Houses; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005; p. 239. [Google Scholar]
- Mitchell, B.; Buhr, R.; Berrang, M.; Bailey, J.; Cox, N. Reducing Airborne Pathogens, Dust and Salmonella Transmission in Experimental Hatching Cabinets Using an Electrostatic Space Charge System. Poult. Sci. 2002, 81, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.; Waltman, W. Reducing Airborne Pathogens and Dust in Commercial Hatching Cabinets with an Electrostatic Space Charge System. Avian Dis. 2003, 47, 247–253. [Google Scholar] [CrossRef]
- Cambra-López, M.; Winkel, A.; Van Harn, J.; Ogink, N.; Aarnink, A. Ionization for Reducing Particulate Matter Emissions from Poultry Houses. Trans. ASABE 2009, 52, 1757–1771. [Google Scholar] [CrossRef]
- Winkel, A.; Mosquera, J.; Ogink, N. Removal Efficiency of a Wire-to-Plate Electrostatic Precipitator for Abatement of Particulate Matter Emission from Poultry Houses; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012; p. 3. [Google Scholar]
- Winkel, A.; Van Riel, J.; Van Emous, R.; Aarnink, A.; Koerkamp, P.G.; Ogink, N. Abatement of Particulate Matter Emission from Experimental Aviary Housings for Laying Hens by Spraying Rapeseed Oil. Poult. Sci. 2016, 95, 2836–2848. [Google Scholar] [CrossRef]
- Manuzon, R.; Zhao, L.; Gecik, C. An Optimized Electrostatic Precipitator for Air Cleaning of Particulate Emissions from Poultry Facilities. ASHRAE Trans. 2014, 120. [Google Scholar]
- Knight, R.M.; Zhao, L.; Zhu, H. Modelling and Optimisation of a Wire-Plate ESP for Mitigation of Poultry PM Emission Using COMSOL. Biosyst. Eng. 2021, 211, 35–49. [Google Scholar] [CrossRef]
- Lim, T.T.; Heber, A.J.; Ni, J.; Zhao, L.; Hanni, S.H. Effects of Electrostatic Space Charge System on Particulate Matter Emission from High-Rise Layer Barn; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2008; p. 1. [Google Scholar]
- Mitchell, B.; Richardson, L.; Wilson, J.; Hofacre, C. Application of an Electrostatic Space Charge System for Dust, Ammonia, and Pathogen Reduction in a Broiler Breeder House. Appl. Eng. Agric. 2004, 20, 87. [Google Scholar] [CrossRef]
- Veenhuizen, M.; Bundy, D. Electrostatic Precipitation Dust Removal System for Swine Housing; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1990; p. 1. [Google Scholar]
- Costa, N.; Accioly, J.; Cake, M. Determining Critical Atmospheric Ammonia Levels for Cattle, Sheep and Goats-a; Brisbane Meat & Livestock Australia Ltd.: Sydney, Australia, 2003; ISBN 1740362969. [Google Scholar]
- Van Buggenhout, S.; Van Brecht, A.; Özcan, S.E.; Vranken, E.; Van Malcot, W.; Berckmans, D. Influence of Sampling Positions on Accuracy of Tracer Gas Measurements in Ventilated Spaces. Biosyst. Eng. 2009, 104, 216–223. [Google Scholar] [CrossRef]
- Calvet, S.; Van den Weghe, H.; Kosch, R.; Estellés, F. The Influence of the Lighting Program on Broiler Activity and Dust Production. Poult. Sci. 2009, 88, 2504–2511. [Google Scholar] [CrossRef]
- Yoder, M.; Van Wicklen, G. Respirable Aerosol Generation by Broiler Chickens. Trans. ASAE 1988, 31, 1510–1517. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).