Featured Application
In our study, transcriptomes of different genders of lizardfish (Saurida elongata) were compared. Interesting findings about sex-related genes for putative future aquaculture applications are reported here. We provide a transcriptome dataset of S. elongata that will be valuable for further research into the reproductive biology of S. elongata and other teleost fishes.
Abstract
Among vertebrates, teleost fishes exhibit the largest array of sex-determining systems, resulting in many reproductive strategies. Screening these fish for sex-related genes could enhance our understanding of sexual differentiation. The lizardfish, Saurida elongata (Temminck & Schlegel, 1846), is a commercially important marine fish in tropical and subtropical seas of the northwest Pacific. However, little genomic information on S. elongata is available. In this study, the transcriptomes of three female and three male S. elongata were sequenced. A total of 49.19 million raw read pairs were generated. After identification and assembly, a total of 59,902 nonredundant unigenes were obtained with an N50 length of 2070 bp. Then, 38,016 unigenes (63.47% of the total) were successfully annotated through multiple public databases. A comparison of the unigenes of different sexes of S. elongata revealed that 22,507 unigenes (10,419 up-regulated in a female and 12,088 up-regulated in a male) were differentially expressed between sexes. Then, numerous candidate sex-related genes were identified, including dmrt2, dmrt4, foxl2, zps and starts. Furthermore, 23,941 simple sequence repeats (SSRs) were detected in SSR-containing sequences. This informative transcriptome analysis provides valuable data to increase the genomic resources of S. elongata.
1. Introduction
Sex determination has received significant research attention in various animals, and numerous genes involved in sex determination have been found in many model species (human, mice, goat, chicken, zebra fish, killifish and cichlid), including sox9, foxl2, wnt4 and dmrt1 [1,2,3]. Facilitated by high conservation, sex-related genes in other species have been identified through homology screening [4,5]. However, the sex-determination mechanisms in fish (especially teleost fishes) are not as well conserved as those in mammals or birds [6,7]. Fishes are extremely diverse in the sex chromosome systems and exhibit a variety of sex-determining systems among vertebrates, resulting in many reproductive strategies [8,9]. Therefore, it is important to screen differentially expressed genes (DEGs) in different sexes and enhance our understanding of sexual differentiation in fishes. Furthermore, growth is often related to gender. Sexual growth dimorphism could be observed in many teleost fish, and these species often show different growth rates between the genders [10,11]. Fish growth is a complex polygenic trait. It is regulated by numerous factors, including the nutrients, energy metabolism, environment and reproductive activity [12]. Thus, elucidating the sex differentiation in fish could help us understand the behavioral, cellular and phenotypic differences between sexes in vertebrates [13].
The lizardfish, Saurida elongata (Temminck & Schlegel, 1846), is a commercially important teleost species that distributes in subtropical and tropical seas of the northwest Pacific, ranging from the coastal waters of Japan to the northern South China Sea (SCS) [14,15]. Lizardfish is primarily used to produce various fish gel products (fish cakes or surimi) due to its high gel-forming ability and meat yield [16]. In addition, it has been reported that lizardfish can be the basic raw material of biologically active peptide production for the treatment of hypertension, heart failure and other cardiovascular disease [17,18]. The lizardfish is abundant, and it has been reported that the production of lizard fish is estimated at more than 120,000 tons in Guangxi of China [17]. As an economic fishery species, there are many studies on the stock resources [19], biology [15], food chemistry [18] and population genetics [20] of S. elongata. However, the biogenetical and molecular studies on S. elongata are insufficient due to limited gene sequence information, as only a few sequences were encountered during searches of public domain nucleotide and protein databases. Moreover, genomic studies on lizardfish are lacking, as these species are difficult to rear. There were only nine records in the SRA (Sequence Read Archive) database of NCBI.
Recently, with rapid advances in next-generation sequencing (NGS) technologies, RNA sequencing has emerged as a useful tool for transcriptome analysis enrichment [9,21]. This technique is ideal for identifying candidate genes and pathways underlying the traits of species whose genome is not yet available [22,23]. Recently, numerous studies have successfully used this approach for genes, single-nucleotide polymorphism (SNP) and simple sequence repeat (SSR) discovery in numerous teleost fishes, such as turbot (Scophthalmus maximus) [24], silver sillago (Sillago sihama) [25] and crimson seabream (Parargyrops edita) [26], among others. Despite the economic importance of lizardfish (Synodontidae), no published genome or transcriptome sequence is currently available for these species. In the present study, the first transcriptome of different tissues (muscle, gonad, liver and heart) in male and female S. elongata was reported. We focused on genes related to sex differentiation of S. elongata. In addition, we also screened numerous simple sequence repeats (SSRs) loci in the transcriptome of S. elongata and developed the SSRs markers. The present study provided valuable genomic information of lizardfish and will facilitate further molecular biology research in teleost.
2. Materials and Methods
2.1. Sample Collection
The three female and three male lizardfish used in the present study were collected from the Beibu Gulf of China (N 21.2793°, E 108.9580°). After acclimating in the tank, the lizardfishes were killed immediately after anesthesia. We collected liver, heart, gonads and muscle tissues from each sample.
2.2. Transcriptome Sequencing
Total RNA was extracted from each tissue using TRIzol reagent. Then, we used Agilent 2100 Bioanalyzer to assess integrity of the RNA. Only the RNA sample with the integrity number (RIN) ≥ 7 was used to subsequent analysis [27]. The RNA of tissues of every sample was pooled in equal amounts. Then, cDNA libraries were constructed using 3 μg of RNA from each sample via a conventional protocol. The prepared cDNA libraries were sequenced on a BGISEQ-500 platform (BGI, Shenzhen, China).
2.3. De Novo Assembly and Functional Annotation
In the present study, we used SOAPnuke v 2.10 to control the raw read quality (https://github.com/BGI-flexlab/SOAPnuke/releases/tag/SOAPnuke2.1.0, accessed on 24 July 2019). Clean data were obtained after raw read trimming by using Trimmomatic v0.35 [28]. The transcriptome was de novo assembled and combined using the clean data based on the Trinity software package (version: 2.0.6) [29]. Then, completeness of the assembly was assessed using BUSCO v 5.0.0 (Benchmarking Universal Single-Copy Orthologs) base on the Actinopterygii reference set [30]. All unigenes were annotated through comparison with databases, including NR (version: Release-20190312) and NT (version: Release-20190312) database of NCBI, Gene Ontology (GO) database (version: Release-20191101), Eukaryotic Orthologous Group (KOG) database (version: Release 201407), Kyoto Encyclopedia of Genes and Genomes (KEGG) database (version: Release 89.1) and Swiss-Prot protein database (version: Release 201902) (BLASTn 2.6.0 and BLASTx 2.5.0 with an E-value threshold of 1 × 10−5).
2.4. Candidate Sex-Related Genes Analysis
The assembled transcriptome was selected as a reference because of the lack of genome information of S. elongata. All clean reads were aligned to the references with Bowtie 2 [31]. Then, RSEM version v1.2.12 was used to normalize the expression levels of the unigenes [32]. R package DEG-seq was used to identify differentially expressed genes (DEGs) [33]. The thresholds of DEGs were defined as |log2 fold change| ≥ 2 and a p value ≤ 0.001. Enrichment analyses (GO and KEGG) of the DEGs were performed based on the hypergeometric distribution test. A significance test was applied, and a Q-value value ≤ 0.05 indicated significantly enriched terms.
2.5. Potential Simple Sequence Repeat (SSR) Marker Detection
In the present study, we used the MIcroSAtellite identification tool (MISA, version 2.1, https://webblast.ipk-gatersleben.de/misa/, accessed on 25 August 2020) to detect SSR loci in the S. elongata transcriptome. In order to detect the useful SSR maker, we referenced the selection criteria of pervious SSR maker devolvement studies [24,25,26]. We used Primer 3 software to design primers for the detected SSR loci [34].
2.6. Quantitative Real-Time PCR (qRT-PCR) Validation
To validate the RNA-seq data, eight DEGs were randomly selected and analyzed via qRT-PCR. The eight selected genes showed significantly different expression between different genders, including four up-regulated genes and four down-regulated genes in the female. According to previous studies, the β-actin gene and 18S gene were proved to be constitutively expressed across different genders in teleost fish species [35,36]. Thus, the two genes were selected as reference genes for internal standardization. We used Primer Premier 6.0 to design the primers (PREMIER Biosoft International, Palo Alto, CA, USA). The amplification reaction system and procedure of qRT-PCR were the same as those described by Lou [23]. We calculated the relative expression levels of eight target unigenes based on the 2−ΔΔCt method (ΔCT = CTtarget unigene − CTreference gene, −ΔΔCT = ΔCTfemale − ΔCTmale), and the log2 fold change was then used for comparison with the log2 fold change of RNA-seq.
3. Results
3.1. Transcriptome Sequencing and De Novo Assembly
In this study, 49.19 million raw read pairs were generated for males and females, respectively. After preprocessing, 44.65 and 45.08 million clean paired-end sequence reads with Q30 percentages of 88.36% and 88.81% were obtained (Table S1). A total of 59,902 unigenes were assembled with an N50 length of 2070 bp and a mean length of 1121 bp (Table S2). The results of BUSCO showed that 94.39% of the genome (73.93% as single genes and 20.46% as duplicated genes) was complete, and 0.01% was missing, indicating the high quality of S. elongata transcriptome (Figure S1).
3.2. Functional Annotation of the S. elongata Transcriptome
In summary, there were only 7627 unigenes (12.73%) annotated to the NT database. Meanwhile, 38,006 were annotated to at least one of the five protein databases (NR, Swiss-Prot, KEGG, KOG, GO) (Table 1). Specifically, 2113 unigenes were annotated with the five protein databases that were searched in the present study (Figure 1).

Table 1.
Summary of unigenes annotations.
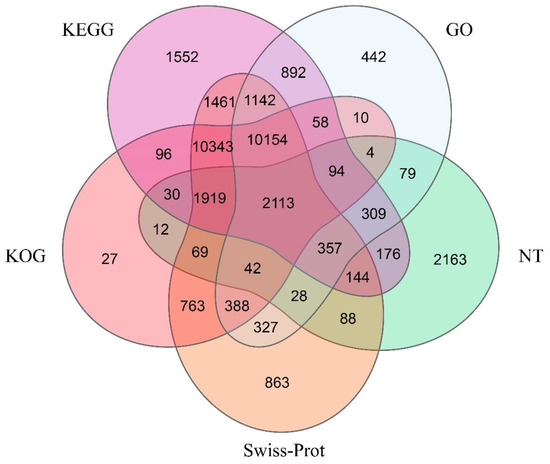
Figure 1.
Venn diagram of the functional annotation.
A total of 30,840 unigenes were annotated to different pathways based on the KEGG pathway analysis; the top two pathways were ‘Human Diseases’ (15,982 unigenes) and ‘Organismal Systems’ (12,845 unigenes). The predominant pathway subcategories were ‘Signal Transduction’ (5188), ‘Immune System’ (3313) and ‘Cancers: Overview’ (3120) (Figure 2). In total, 16,439 unigenes were assigned to three GO categories. As Figure S2 show, the dominant terms were ‘binding’ (8261 unigenes), ‘catalytic activity’ (6036 unigenes) and ‘membrane part’ (4878 unigenes). In addition, 26,122 unigenes were assigned to the KOG. These unigenes were classified into 25 subcategories (Figure 3).
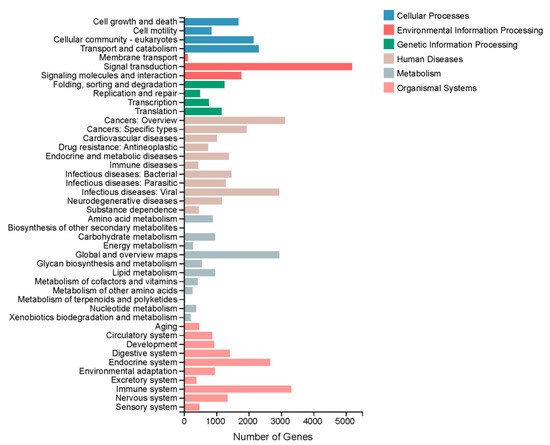
Figure 2.
KEGG pathway classifications of the unigenes.
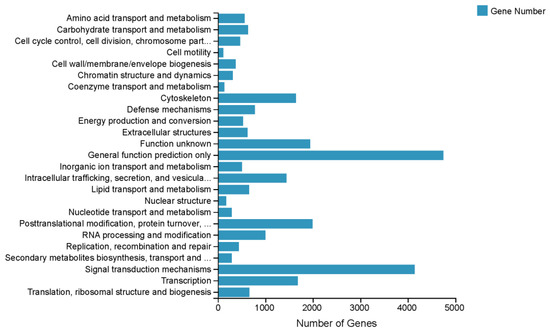
Figure 3.
Clusters of KOG functional classifications.
3.3. Candidate Sex-Related Genes and Functional Enrichment Analysis Results
To detect the potential genes and pathways related to sex, comparative analyses were performed to identify differentially expressed unigenes between genders. In the present study, unigenes with |log2 Fold Change| ≥ 2 and p-value ≤ 0.001 were determined to be differentially expressed genes. After filtering, 22,507 DEGs were identified between the sexes, 10,419 of which were female-biased DEGs, while 12,088 were male-biased DEGs (Figure 4 and Figure S3). Many well-known sex-related genes were detected. For instance, zona pellucida sperm-binding protein genes (zps), StAR-related lipid transfer protein genes (start), spermatogenesis-associated protein genes (spatas), doublesex- and mab-3-related transcription factor (dmtr), P43 5S RNA-binding protein-like (42sp43), follicle stimulating hormone receptor (fshr) and forkhead box protein L2 (foxl2) (Table 2).
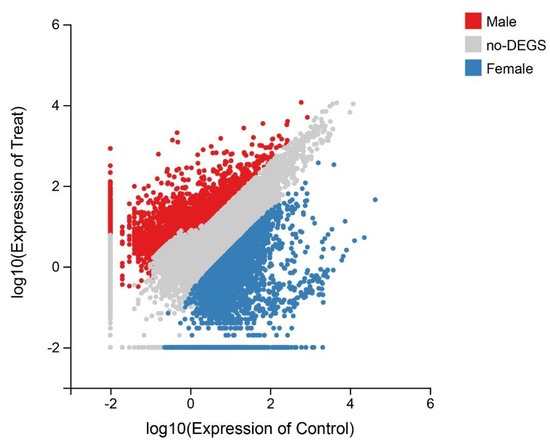
Figure 4.
Scatter plots of Gene expression profiles in female and male S. elongata. Thresholds for inclusion were defined by p-value ≤ 0.01 and |log2 fold change| ≥ 1.

Table 2.
Genes related to the sex differentiation of Saurida elongata.
Furthermore, the results of enrichment analyses of the 22,507 DEGs showed that 7832 unigenes were assigned to 3293 GO terms. The results in GO terms showed that sex-biased genes were predominantly associated with ‘integral component of membrane’ (GO:0016021) (Table S3). In addition, results suggested that the three most-enriched KEGG pathways were ‘Pathways in cancer’ (ko05200), ‘PI3K-Akt signalling pathway’ (ko04151) and ‘Human papillomavirus infection’ (ko05165) (Figure 5). Figure 5 showed the top twenty statistically significant KEGG classifications of the DEGs.
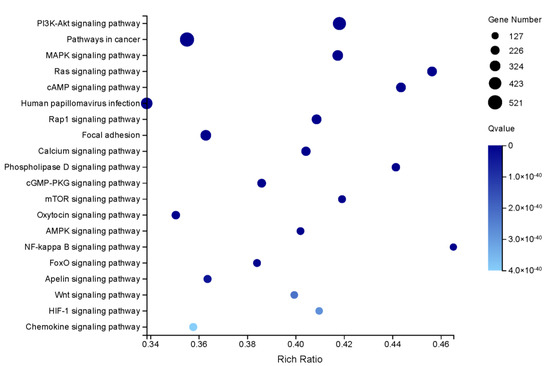
Figure 5.
KEGG pathway enrichment analyses of the DEGs.
3.4. SSR Markers Detection
We identified 23,941 SSRs, of which 10,592 44.24% were di-nucleotide repeats, followed by mononucleotide (8320 SSRs, 34.75%) and trinucleotide (3458 SSRs, 14.44%), and other types SSRs (6.57%). Among these loci, SSRs with six tandem repeats were the most dominant (Figure 6). Furthermore, we gave the primers and other information of these SSR loci.
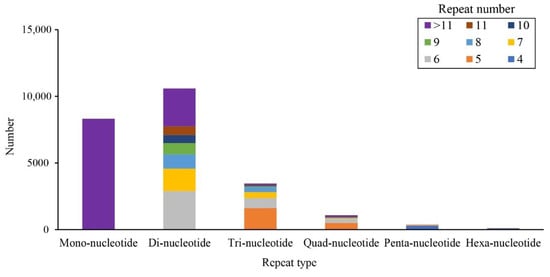
Figure 6.
The distribution of different SSRs.
3.5. Transcriptome Data Validation
To validate the transcriptomic data, quantitative RT-PCR (qRT-PCR) was performed on eight DEGs. Four were up-regulated and four were down-regulated in the female. Specific primers of each gene were listed in Table S4. As shown in Figure 7, these randomly selected genes displayed similar expression patterns in both RNA-seq and qRT-PCR, confirming the reliability of our RNA-seq data.
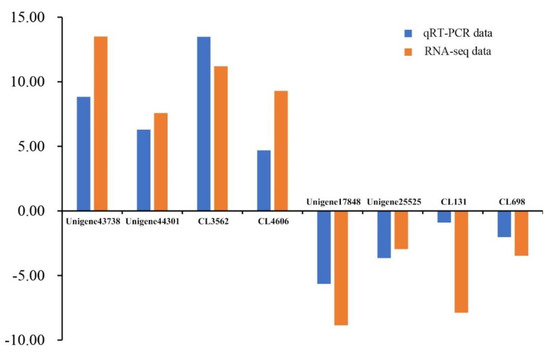
Figure 7.
Expression levels of eight unigenes as determined by transcriptome analysis and qRT-PCR.
4. Discussion
In the present study, 59,902 de novo assembled unigenes were obtained. Moreover, the results of BUSCO showed 94.39% of the genome was complete. These results showed the quality of transcriptome in our study was high, and it was reliable for subsequent analyses. Among the unigenes, 63.45% were annotated to public databases. The match ratio was low because of the lack of genetic data of lizardfish species in the public databases. To date, only three protein sequences have been deposited for species in genus Saurida in the NCBI NR database, explaining the low number of BLAST top hits for lizardfish. The result of main species distribution matched against the NR database showed that 11.99% of the annotated unigenes shared similar sequences with Lates calcarifer, whose draft genome was published in 2015 [37]. Seriola lalandi dorsalis, Larimichthys crocea, Seriola dumerili and Stegastes partitus also shared similar sequences with S. elongata. Thus, unigenes of S. elongata transcriptome matched well to proteins of other teleost fish. It has been reported that the annotation results of the non-model species were highly dependent on the availability of annotated sequence information in the database, and the sizes of their contig sequences [38,39]. Compared to other non-model teleost species, the number of transcripts obtained for S. elongata from this study was at moderate level [24,26,40].
It is evident that genes involved in gonad development and related to sex differentiation play important roles in controlling the sex ratio of teleost fishes [24]. It is crucial to elucidate the mechanisms of sex determination and gonad differentiation. In the present study, we identified numerous DEGs and suspected that these genes may play important roles in the sex differentiation between genders of S. elongata. However, sex determination in teleost fish is an extremely complex process, regulated by numerous genes [9,25]. Due to the limitations of the present study, we could not clarify the exact mechanisms of sex determination or gonad differentiation. The transcriptome data in this study will facilitate further studies of S. elongata. With the annotation results, we identified several well-known genes relate to sex control and gonadal development (zp, dmtr, 42sp43, start, Wee1, foxl2, etc.).
The zona pellucida plays many important biological functions, including postfertilization blockade of polyspermy and relative species-specific binding of sperm to ovulated eggs [41,42]. In fish oocytes, the zona pellucida plays a protective role in sperm binding [43]. It has been reported that zona pellucida protein 3 plays a crucial role in acrosome reaction, and zona pellucida 4 participates in primordial follicle formation [44,45]. In the present study, zp 1-4 showed significantly higher expression in females, showing these genes may play important roles in reproduction and folliculogenesis in S. elongata.
In teleost, dmrt gene families play crucial roles in sexual differentiation and determination [46,47]. It is well known that the dmrt gene family includes many members. These genes encode putative transcription factors with evolutionarily well-conserved Doublesex and Mad-3 (DM) domains [48]. The DM domain is involved in sexual differentiation and development in many species (insects, fish and mammals) [49,50]. Eight dmrt genes (dmrt1 through dmrt 8) have been reported in mammals [51], and some of them have also been detected in teleosts. These include Oreochromis niloticus [52], Gadus morhua [53], Danio rerio [54], Takifugu rubripes [55] and Cynoglossus semilaevis [56]. As an important member, the dmrt2 gene plays a key role in neurogenesis, testicular hypoplasia and even embryonic sex reversal [57,58]. Recently, the dmrt2 gene was found to play a crucial factor in germ cell maturation and gonadal differentiation in male teleost fishes [56]. Consistent with previous studies, the dmrt2 gene showed significantly higher expression in males. Unlike dmrt2, dmrt4 has different expression patterns in different fish. Guan [59] demonstrated that dmrt4 showed female-specific expression pattern in gonads in Oreochromis niloticus, and Cao [60] detected that dmrt4 was expressed in the endbrain, pituitary gland, thalamencephalon and ovary in Oreochromis aureus. However, dmrt4 showed strong expression in the testis but weak expression in the ovary of the adult olive flounder (Paralichthys olivaceus) [61]. In our study, we detected that the dmrt4 gene showed significantly higher expression in female S. elongata. Results showed that the dmrt4 gene may have potentially important roles in ovary development.
Among sex differentiation genes of teleost fish, foxl2 is one of the earliest known markers of ovarian differentiation [62]. The foxl2 is expressed as a transcription regulator of the cytochrome P450 family and maintains cyp19a expression, which could convert androgens into estrogens [63]. Numerous previous studies demonstrated that foxl2 exhibits significant sex-dimorphic expression patterns and plays a key role in fish gonad development and differentiation [64,65]. In our study, foxl2 was highly expressed in females, indicating that foxl2 participates in the development of gonads in S. elongata.
5. Conclusions
In summary, we sequenced the transcriptomes of female and male S. elongata. After assembly, identification and annotation, numerous putative genes related to sex differentiation were detected. Our results will be useful for improving our understanding of sexual differentiation and the molecular mechanism of sex determination in fishes. It will also facilitate future functional analyses of sex-associated genes. Furthermore, SSR loci was detected in the transcriptome of S. elongata, providing valuable markers for future molecular biology on S. elongata. Ours is the first comparative transcriptomic analysis of different genders of S. elongata. The transcriptome in the present study will provide valuable data to enrich the genomic resources of S. elongata.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122211319/s1. Table S1: Summary statistics of clean transcriptome sequencing data from each sample; Table S2: Statistics for the assembled unigenes; Table S3: Top 20 GO pathways; Table S4: Specific primers for the selected unigenes and reference genes; Figure S1: Completeness of the assembly and annotations; Figure S2: GO terms for the DEGs in the biological process, cellular component, and molecular function categories; Figure S3: Venn diagram of common and differential expressed genes between the two genders.
Author Contributions
Conceptualization, B.S. and Y.L.; methodology, B.S. and L.W.; software, B.S. and C.Y.; validation, B.S., Y.L. and C.Y.; formal analysis, B.S. and Y.L.; investigation, B.S. and L.W.; resources, B.S. and M.L.; data curation, Y.L. and C.Y.; writing—original draft preparation, B.S.; writing—review and editing, P.H., C.Y. and D.S.; visualization, B.S.; supervision, M.L. and D.S.; project administration, L.W. and D.S.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Asia Cooperation Fund Project—Modern fishery cooperation between China and neighboring countries around the South China Sea.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of Laboratory Animal Welfare and Ethics of South China Sea Fisheries Research Institute (code: nhdf 2021-01 and date of approval: 10 January 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
All raw reads in this study are archived in the National Center for Biotechnology Information (NCBI) Short Read Archive (SRA) databases under BioProject PRJNA739278, with accession numbers SRR14866270 and SRR14866271. This Transcriptome Shotgun Assembly (TSA) project has been deposited at DDBJ/EMBL/GenBank under the accession GJGB00000000. The version described in this paper is the first version, GJGB00000000.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ottolenghi, C.; Pelosi, E.; Tran, J.; Colombino, M.; Douglass, E.; Nedorezov, T.; Cao, A.; Forabosco, A.; Schlessinger, D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 2007, 16, 2795–2804. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Lavery, R.; Chassot, A.A.; Pauper, E.; Gregoire, E.P.; Klopfenstein, M.; de Rooij, D.G.; Mark, M.; Schedl, A.; Ghyselinck, N.B.; Chaboissier, M.C. Testicular differentiation occurs in absence of R-spondin1 and Sox9 in mouse sex reversals. PLoS Genet. 2012, 8, e1003170. [Google Scholar] [CrossRef]
- Chandler, J.C.; Elizur, A.; Ventura, T.J.H. The decapod researcher’s guide to the galaxy of sex determination. Hydrobiologia 2018, 825, 61–80. [Google Scholar] [CrossRef]
- Tao, W.J.; Chen, J.L.; Tan, D.J.; Yang, J.; Sun, L.N.; Wei, J.; Conte, M.A.; Kocher, T.D.; Wang, D.S. Transcriptome display during tilapia sex determination and differentiation as revealed by RNA-Seq analysis. BMC Genomics 2018, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Barske, L.A.; Capel, B. Blurring the edges in vertebrate sex determination. Curr. Opin. Genet. Dev. 2008, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.W.; Anderson, J.; Bertho, S.; Herpin, A.; Wilson, C.; Postlethwait, J.H.; Schartl, M.; Guiguen, Y. Vertebrate sex-determining genes play musical chairs. C. R. Biol. 2016, 339, 258–262. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nagahama, Y.; Nakamura, M.J.S.D. Diversity and plasticity of sex determination and differentiation in fishes. Sex. Dev. 2013, 7, 115–125. [Google Scholar] [CrossRef]
- Casas, L.; Saborido-Rey, F.; Ryu, T.; Michell, C.; Ravasi, T.; Irigoien, X. Sex Change in Clownfish: Molecular insights from transcriptome analysis. Sci. Rep. 2016, 6, 35461. [Google Scholar] [CrossRef]
- Saillant, E.; Fostier, A.; Menu, B.; Haffray, P.; Chatain, B. Sexual growth dimorphism in sea bass Dicentrarchus labrax. Aquaculture 2001, 202, 371–387. [Google Scholar] [CrossRef]
- Dutney, L.; Elizur, A.; Lee, P. Analysis of sexually dimorphic growth in captive reared cobia (Rachycentron canadum) and the occurrence of intersex individuals. Aquaculture 2017, 468, 348–355. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.K.; Wang, R.Q.; Chen, S.L. Transcriptomics analysis revealing candidate networks and genes for the body size sexual dimorphism of Chinese tongue sole (Cynoglossus semilaevis). Funct. Integr. Genomic. 2018, 18, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Cribbin, K.M.; Quackenbush, C.R.; Taylor, K.; Arias-Rodriguez, L.; Kelley, J.L. Sex-specific differences in transcriptome profiles of brain and muscle tissue of the tropical gar. BMC Genom. 2017, 18, 283. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, A.; Sakai, T.; Tokimura, A.; Horikawa, H.; Matsuyama, M. Age and growth of the lizardfish Saurida sp. 1 in the East China Sea using otolith ring marks. Fish. Res. 2002, 55, 231–238. [Google Scholar] [CrossRef]
- Sakai, T.; Yoneda, M.; Shiraishi, T.; Tokimura, M.; Horikawa, H.; Matsuyama, M. Age and growth of the lizardfish Saurida elongata from the Tsushima/Korea Strait. Fish. Sci. 2009, 75, 895–902. [Google Scholar] [CrossRef]
- Shimizu, Y.; Wendakoon, C.N. Agriculture Effects of maturation and spawning on the gel-forming ability of lizardfish (Saurida elongata) muscle tissues. J. Sci. Food Agr. 1990, 52, 331–338. [Google Scholar] [CrossRef]
- Wu, S.G.; Sun, J.H.; Tong, Z.F.; Lan, X.D.; Zhao, Z.X.; Liao, D.K. Optimization of hydrolysis conditions for the production of angiotensin-I converting enzyme-inhibitory peptides and isolation of a novel peptide from lizard fish (Saurida elongata) muscle protein hydrolysate. Mar. Drugs 2012, 10, 1066–1080. [Google Scholar] [CrossRef]
- Lan, X.D.; Sun, L.X.; Muhammad, Y.; Wang, Z.F.; Liu, H.B.; Sun, J.H.; Zhou, L.Q.; Feng, X.Z.; Liao, D.K.; Wang, S.F. Studies on the interaction between angiotensin-converting enzyme (ACE) and ACE inhibitory peptide from Saurida elongata. J. Agric. Food Chem. 2018, 66, 13414–13422. [Google Scholar] [CrossRef]
- Liu, Y.W.; Zhang, C.L.; Zan, X.X.; Sun, M.; Xu, B.D.; Xue, Y.; Ren, Y.P. Distribution of relative abundance of slender lizardfish and its influencing factors in southern coastal waters of Shandong during autumn. Period. Ocean Univ. China 2020, 50, 45–53. [Google Scholar]
- Tu, Z.; Liu, M.; Wang, Y.P.; Xu, S.Y.; Song, N.; Gao, T.X.; Han, Z.Q. The low mitochondrial diversities in lizardfish Saurida elongata: Recent population expansion and selection. Biochem. Syst. Ecol. 2016, 68, 44–50. [Google Scholar] [CrossRef]
- Lobo, I.K.C.; do Nascimento, A.R.; Yamagishi, M.E.B.; Guiguen, Y.; da Silva, G.F.; Severac, D.; Amaral, A.D.; Reis, V.R.; de Almeida, F.L. Transcriptome of tambaqui Colossoma macropomum during gonad differentiation: Different molecular signals leading to sex identity. Genomics 2020, 112, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Chatchaiphan, S.; Srisapoome, P.; Kim, J.H.; Devlin, R.H.; Na-Nakorn, U. De novo transcriptome characterization and growth-related gene expression profiling of diploid and triploid bighead catfish (Clarias macrocephalus Gunther, 1864). Mar. Biotechnol. 2017, 19, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.R.; Yang, T.Y.; Han, Z.Q.; Gao, T.X. Transcriptome analysis for identification of candidate genes related to sex determination and growth in Charybdis japonica. Gene 2018, 677, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ma, A.; Huang, Z.; Wang, G.N.; Wang, T.; Xia, D.; Ma, B. Transcriptome analysis for identification of genes related to gonad differentiation, growth, immune response and marker discovery in the turbot (Scophthalmus maximus). PLoS ONE 2016, 11, e0149414. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, Z.; Dong, Z.; Huang, Y.; Du, T.; Chen, H.; Jiang, D.; Deng, S.; Zhang, Y.; Wandia, S.; et al. Transcriptome analysis of male and female mature gonads of silver sillago (Sillago sihama). Genes 2019, 10, 129. [Google Scholar] [CrossRef]
- Shan, B.; Liu, Y.; Yang, C.; Zhao, Y.; Sun, D. Comparative transcriptomic analysis for identification of candidate sex-related genes and pathways in Crimson seabream (Parargyrops edita). Sci. Rep. 2021, 11, 1077. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.J. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.W.; Zhang, X.G. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Filby, A.L.; Tyler, C.R. Appropriate ’housekeeping’ genes for use in expression profiling the effects of environmental estrogens in fish. BMC Mol. Biol. 2007, 8, 10. [Google Scholar] [CrossRef]
- Martins, R.S.; Pinto, P.I.; Guerreiro, P.M.; Zanuy, S.; Carrillo, M.; Canário, A.V. Novel galanin receptors in teleost fish: Identification, expression and regulation by sex steroids. Gen. Comp. Endocr. 2014, 205, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Domingos, J.A.; Zenger, K.R.; Jerry, D.R. Whole-genome shotgun sequence assembly enables rapid gene characterization in the tropical fish barramundi, Lates calcarifer. Anim. Genet. 2015, 46, 468–469. [Google Scholar] [CrossRef]
- Schmid, R.; Blaxter, M.L. annot8r: GO, EC and KEGG annotation of EST datasets. BMC Bioinform. 2008, 9, 180. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Rozenfeld, C.; Blanca, J.; Gallego, V.; García-Carpintero, V.; Herranz-Jusdado, J.; Pérez, L.; Asturiano, J.F.; Cañizares, J.; Peñaranda, D.S. De novo European eel transcriptome provides insights into the evolutionary history of duplicated genes in teleost lineages. PLoS ONE 2019, 14, e0218085. [Google Scholar] [CrossRef]
- Wassarman, P.M.J.S. The biology and chemistry of fertilization. Science 1987, 235, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Ringuette, M.J.; Chamberlin, M.E.; Baur, A.W.; Sobieski, D.A.; Dean, J.J.D.B. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev. Biol. 1988, 127, 287–295. [Google Scholar] [CrossRef]
- Dumont, J.N.; Brummett, A.R.J.O. Egg Envelopes in Vertebrates. In Oogenesis; Springer: Berlin/Heidelberg, Germany, 1985; pp. 235–288. [Google Scholar]
- Litscher, E.S.; Wassarman, P.M. Egg extracellular coat proteins: From fish to mammals. Histol. Histopathol. 2007, 22, 337–347. [Google Scholar] [PubMed]
- Meczekalski, B.; Nawrot, R.; Nowak, W.; Czyzyk, A.; Kedzia, H.; Gozdzicka-Jozefiak, A. Study on the zona pellucida 4 (ZP4) gene sequence and its expression in the ovaries of patients with polycystic ovary syndrome. J. Endocrinol. Investig. 2015, 38, 791–797. [Google Scholar] [CrossRef]
- Kikuchi, K.; Hamaguchi, S. Novel sex-determining genes in fish and sex chromosome evolution. Dev. Dyn. 2013, 242, 339–353. [Google Scholar] [CrossRef]
- Zhang, X.B.; Wang, H.; Li, M.H.; Cheng, Y.Y.; Jiang, D.N.; Sun, L.N.; Tao, W.J.; Zhou, L.Y.; Wang, Z.J.; Wang, D.S. Isolation of doublesex- and mab-3-related transcription factor 6 and its involvement in spermatogenesis in tilapia. Biol. Reprod. 2014, 91, 136. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The mammalian doublesex homolog DMRT1 is a transcriptional gatekeeper that controls the mitosis versus meiosis decision in male germ cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef]
- Erdman, S.E.; Burtis, K.C. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 1993, 12, 527–535. [Google Scholar] [CrossRef]
- Krentz, A.D.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev. Biol. 2011, 356, 63–70. [Google Scholar] [CrossRef]
- Veith, A.M.; Klattig, J.; Dettai, A.; Schmidt, C.; Englert, C.; Volff, J.N. Male-biased expression of X-chromosomal DM domain-less Dmrt8 genes in the mouse. Genomics 2006, 88, 185–195. [Google Scholar] [CrossRef]
- Rather, M.A.; Dhandare, B.C.J.B.R. Genome-Wide identification of doublesex and Mab-3-Related transcription factor (DMRT) genes in nile tilapia (Oreochromis niloticus). Biotechnol. Rep. 2019, 24, e00398. [Google Scholar]
- Johnsen, H.; Andersen, O. Sex dimorphic expression of five dmrt genes identified in the Atlantic cod genome. The fish-specific dmrt2b diverged from dmrt2a before the fish whole-genome duplication. Gene 2012, 505, 221–232. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Q.; Lu, H.; Chen, H.; Guo, Y.Q.; Cheng, H.H.; Zhou, R.J. Fish specific duplication of Dmrt2: Characterization of zebrafish Dmrt2b. Biochimie 2008, 90, 878–887. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Lee, K.H.; Fujimoto, H.; Kadomura, K.; Yasumoto, S.; Matsuyama, M. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes. Comp. Biochem. Phys. D 2006, 1, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cui, Z.K.; Yang, Y.M.; Xu, W.T.; Shao, C.W.; Fu, X.Q.; Li, Y.Z.; Chen, S.L. Expression analysis and characterization of dmrt2 in Chinese tongue sole (Cynoglossus semilaevis). Theriogenology 2019, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, R.; Lopes, S.S.; Saude, L. Left-right function of dmrt2 genes is not conserved between zebrafish and mouse. PLoS ONE 2010, 5, e14438. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Nakahara, Y.; Izawa, T.; Ishitani, T.; Tsutsumi, M.; Kuroiwa, A.; Itoh, M.; Kikuchi, Y. Zebrafish Dmrta2 regulates neurogenesis in the telencephalon. Genes Cells 2011, 16, 1097–1109. [Google Scholar] [CrossRef]
- Guan, G.J.; Kobayashi, T.; Nagahama, Y. Sexually dimorphic expression of two types of DM (Doublesex/Mab-3)-domain genes in a teleost fish, the Tilapia (Oreochromis niloticus). Biochem. Biophys. Res. Commun. 2000, 272, 662–666. [Google Scholar] [CrossRef]
- Cao, J.L.; Chen, J.J.; Wu, T.T.; Gan, X.; Luo, Y.J. Molecular cloning and sexually dimorphic expression of DMRT4 gene in Oreochromis aureus. Mol. Biol. Rep. 2010, 37, 2781–2788. [Google Scholar] [CrossRef]
- Wen, A.Y.; You, F.; Tan, X.G.; Sun, P.; Ni, J.; Zhang, Y.Q.; Xu, D.D.; Wu, Z.H.; Xu, Y.L.; Zhang, P.J. Expression pattern of dmrt4 from olive flounder (Paralichthys olivaceus) in adult gonads and during embryogenesis. Fish Physiol. Biochem. 2009, 35, 421–433. [Google Scholar] [CrossRef]
- Nakamoto, M.; Matsuda, M.; Wang, D.S.; Nagahama, Y.; Shibata, N. Molecular cloning and analysis of gonadal expression of Foxl2 in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 2006, 344, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.F.; Zou, Y.X.; Liang, D.D.; Tan, X.G.; Jiao, S.; Wu, Z.H.; Li, J.; Zhang, P.J.; You, F. Roles of forkhead box protein L2 (foxl2) during gonad differentiation and maintenance in a fish, the olive flounder (Paralichthys olivaceus). Reprod. Fert. Develop. 2019, 31, 1742–1752. [Google Scholar] [CrossRef]
- Li, C.G.; Wang, H.; Chen, H.J.; Zhao, Y.; Fu, P.S.; Ji, X.S. Differential expression analysis of genes involved in high-temperature induced sex differentiation in Nile tilapia. Comp. Biochem. Phys. B 2014, 177, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.J.; Gao, T.; Liu, Z.L.; Sun, L.N.; Jiang, X.L.; Chen, L.L.; Wang, D.S. Blockage of androgen and administration of estrogen induce transdifferentiation of testis into ovary. J. Endocrinol. 2017, 233, 65–80. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).