Mechanical Behaviour of Human and Porcine Urethra: Experimental Results, Numerical Simulation and Qualitative Analysis
Abstract
Highlights
- The porcine urethra is a suitable replacement candidate for the human urethra.
- The circumferential urethra should be the object of further investigations.
- The urethra under large deformations may be modelled using FEM and hyperelastic models.
Abstract
1. Introduction
2. Materials and Methods
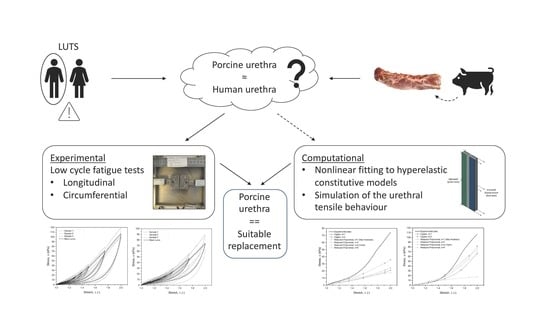
2.1. Methodology Overview
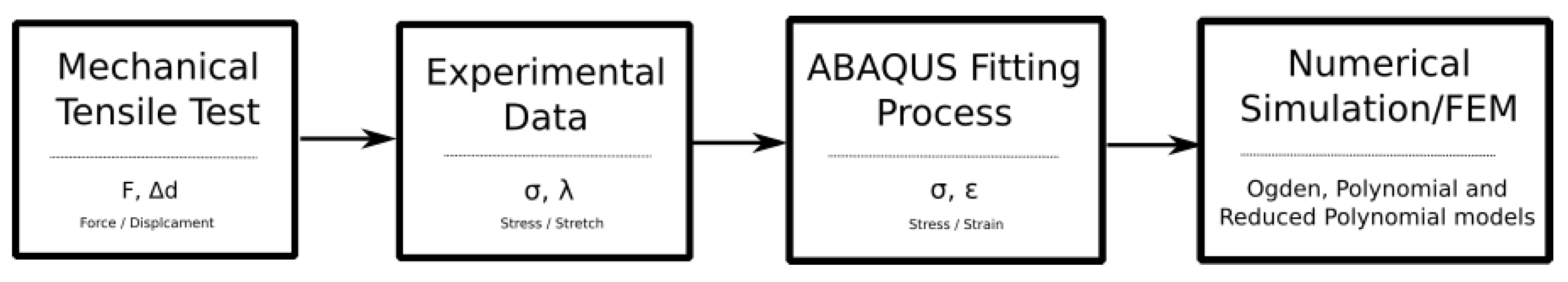
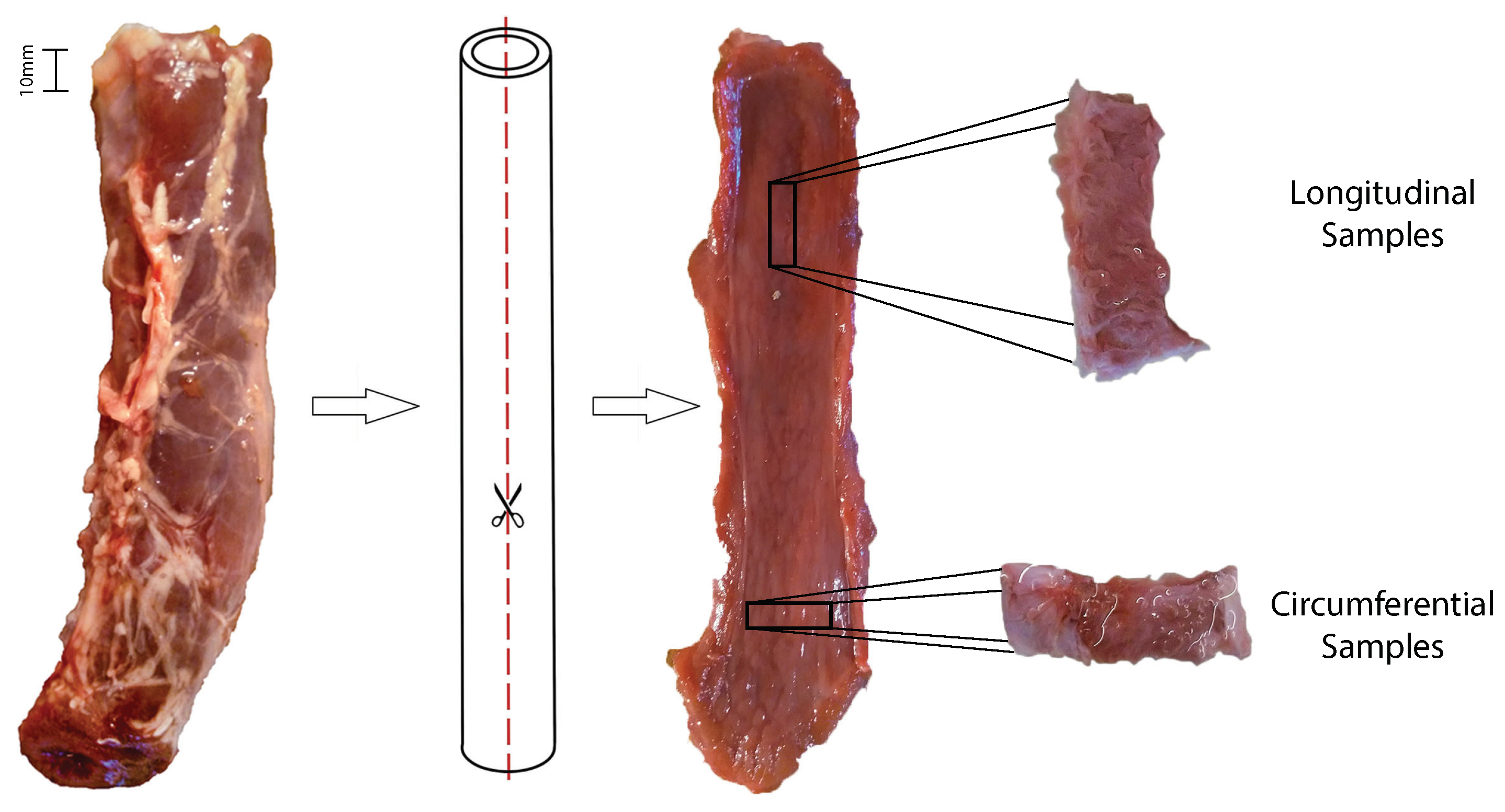
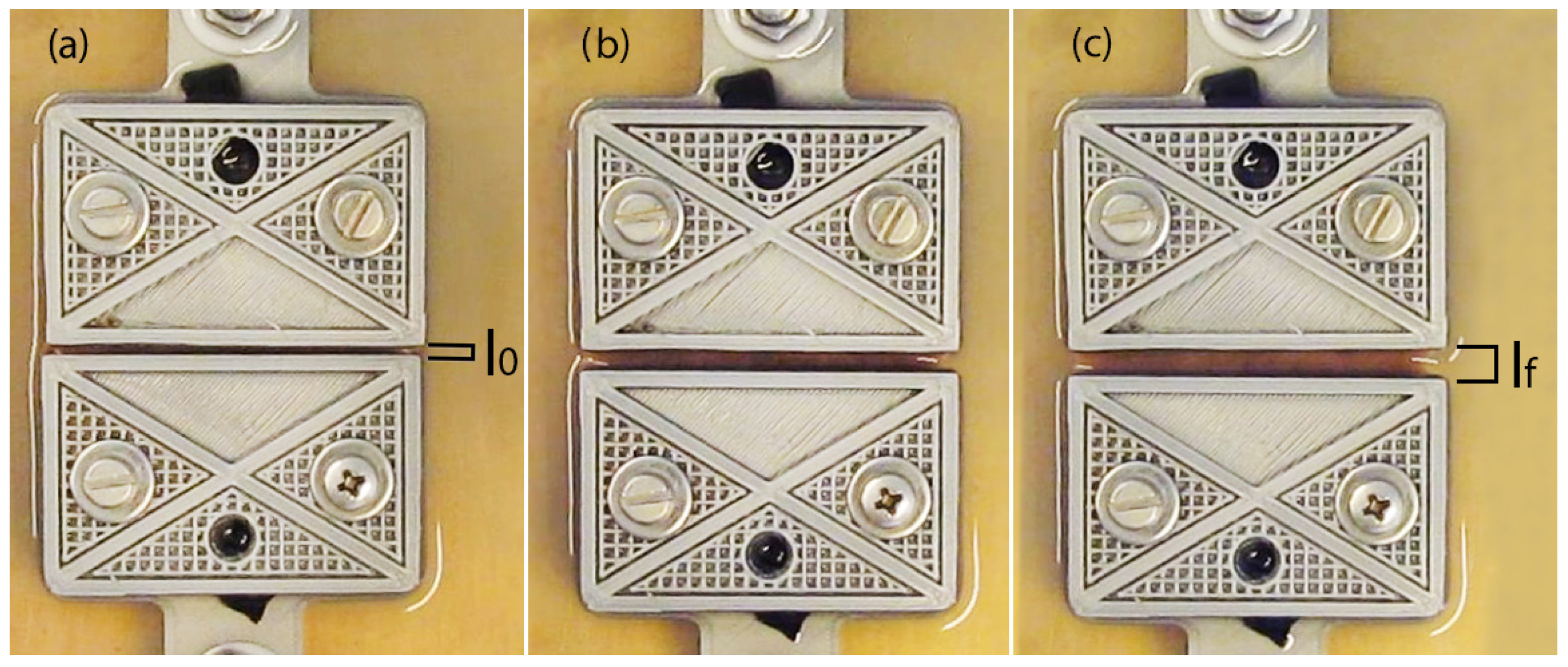
2.2. Mechanical Tests
2.3. Mathematical Analysis
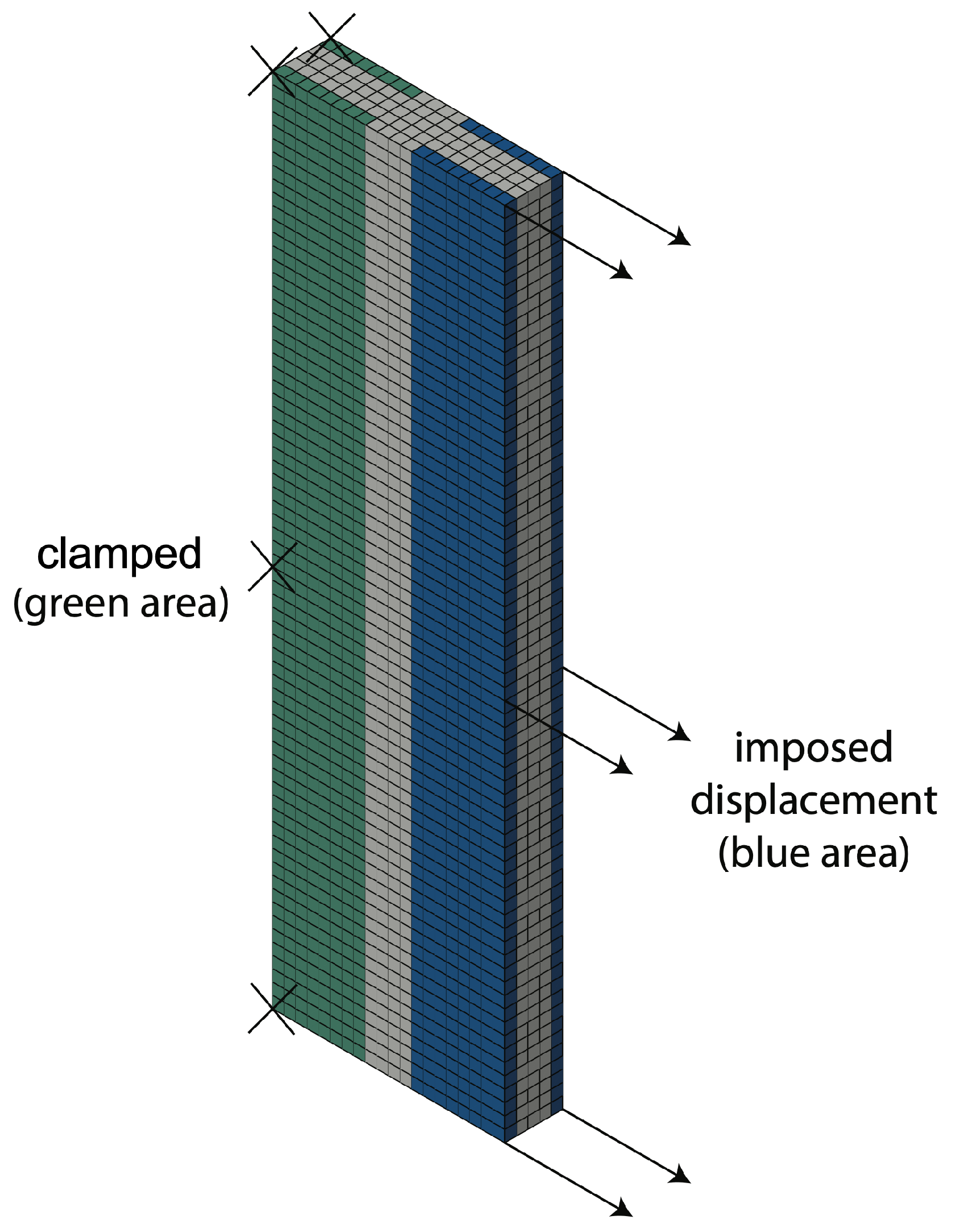
2.4. FEM Simulation
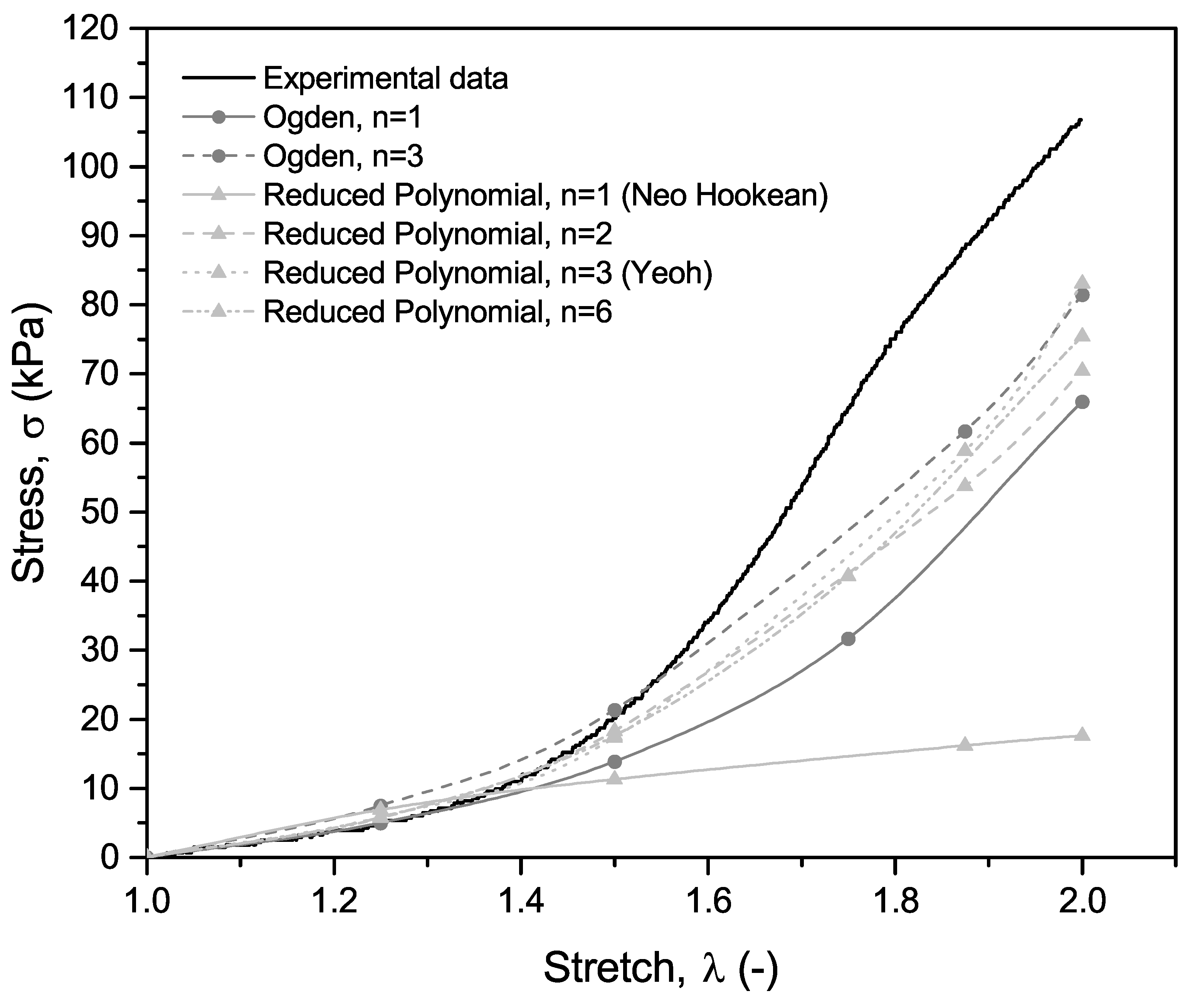
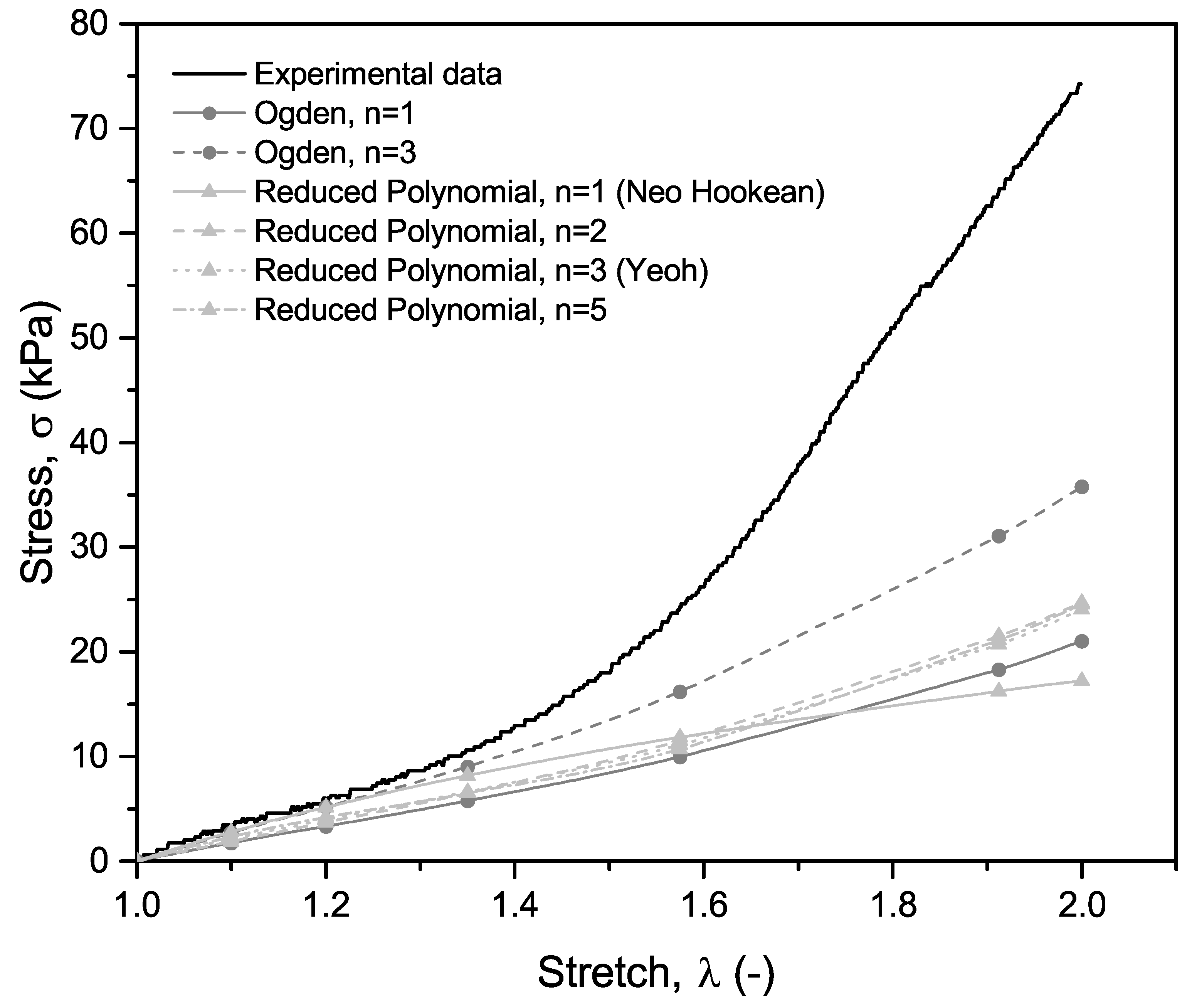
3. Results and Discussion
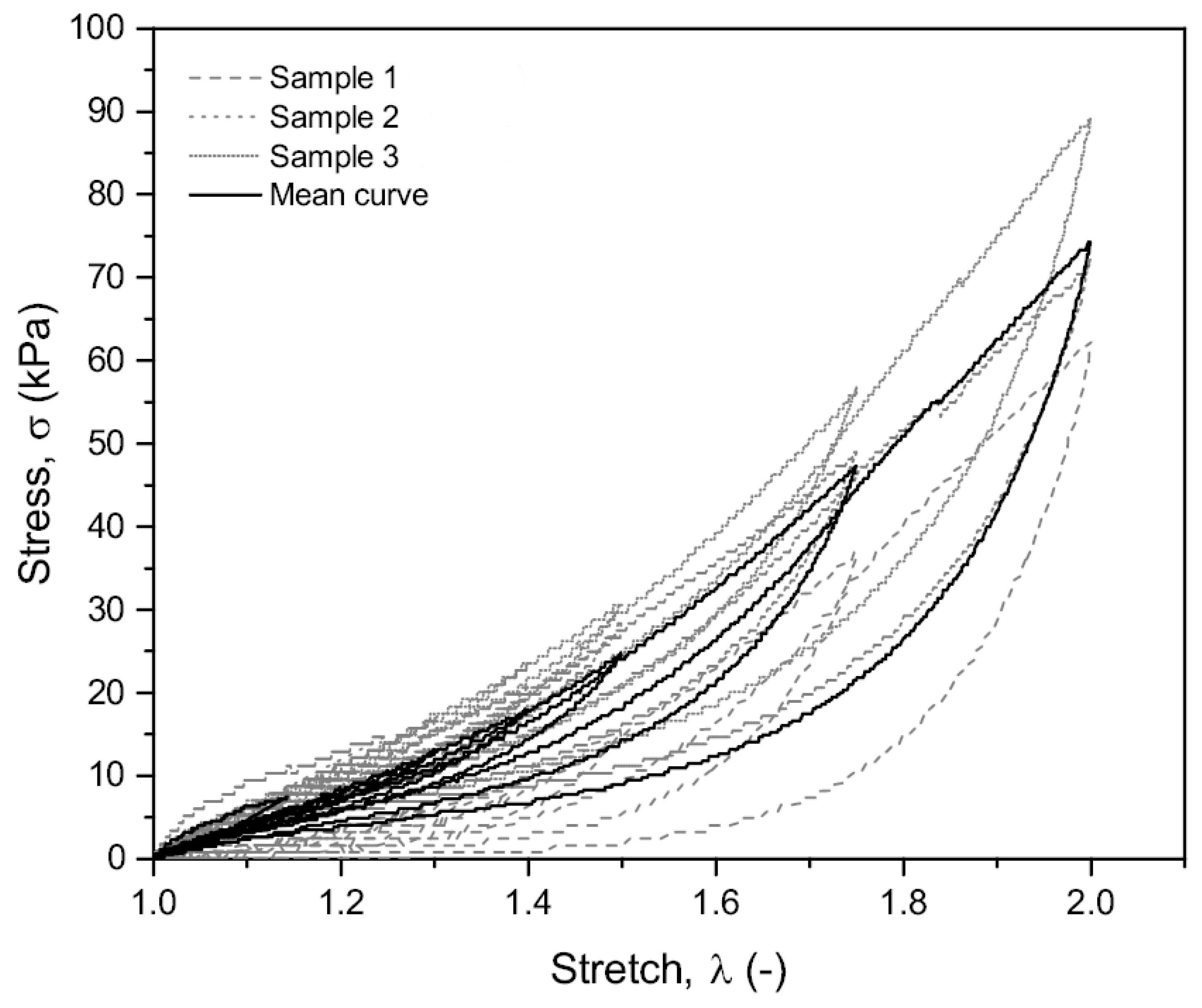
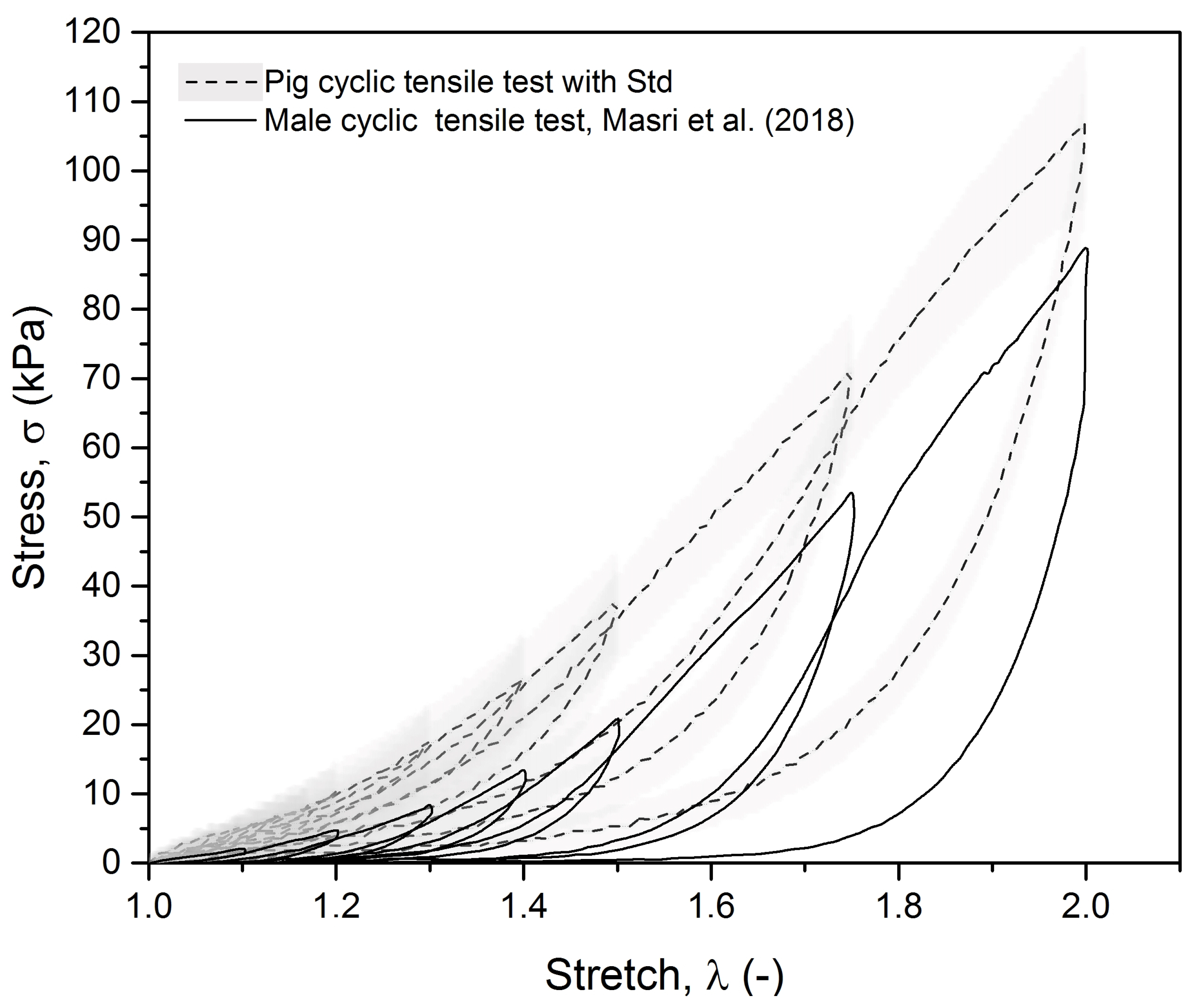
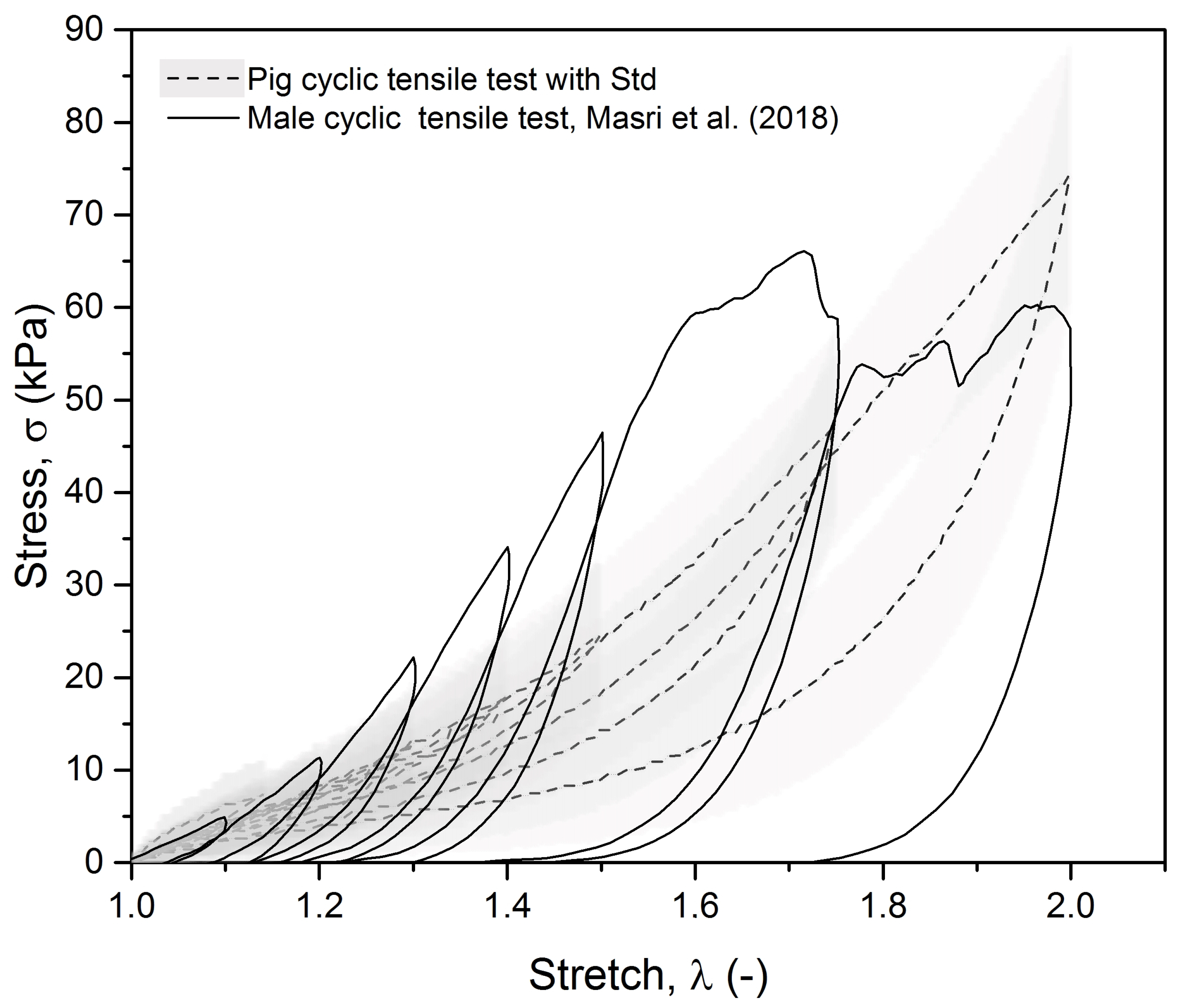
3.1. Experimental Results
3.2. FEM Simulation
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LUTS | Low urinary tract dysfunctions and symptoms |
| UI | Urinary incontinence |
| SUI | Stress urinary incontinence |
| BPH | Benign prostatic hyperplasia |
| PDE5Is | Phosphodiesterase type 5 inhibitors |
| FEM | Finite element method |
| STD | Standard deviation |
| DGAV | Direção-Geral da Alimentação e Veterinária |
| INEGI | Instituto de Ciência e Inovação em Engenharia Mecânica e Engenharia Industrial |
References
- Kim, K.H.; Ahn, B.; Lim, S.K.; Han, W.K.; Kim, J.H.; Rha, K.H.; Kim, J. Indenter Study: Associations between prostate elasticity and lower urinary tract symptoms. Urology 2014, 83, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.B. Epidemiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 148–151. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T. BPH: Epidemiology and comorbidities. Am. J. Manag. Care 2006, 12, S122–S128. [Google Scholar] [PubMed]
- Natali, A.N.; Carniel, E.L.; Frigo, A.; Pavan, P.G.; Todros, S.; Pachera, P.; Fontanella, C.G.; Rubini, A.; Cavicchioli, L.; Avital, Y.; et al. Experimental investigation of the biomechanics of urethral tissues and structures. Exp. Physiol. 2016, 101, 641–656. [Google Scholar] [CrossRef]
- Buckley, B.S.; Lapitan, M.C.M. Prevalence of Urinary Incontinence in Men, Women, and Children—Current Evidence: Findings of the Fourth International Consultation on Incontinence. Urology 2010, 76, 265–270. [Google Scholar] [CrossRef]
- Nitti, V.W. The Prevalence of Urinary Incontinence. Rev. Urol. 2001, 3, 2–6. [Google Scholar]
- Wyndaele, M.; Hashim, H. Pathophysiology of urinary incontinence. Surgery 2017, 6, 287–292. [Google Scholar] [CrossRef]
- Hall, C.D.; Rabin, J.M. Urinary incontinence. Med. Update Psychiatr. 1996, 1, 71–76. [Google Scholar] [CrossRef]
- Fantl, J.A.; Newman, D.; Colling, J. Urinary Incontinence in Adults: Acute and Chronic Management; U.S. Department of Health and Human Services: Washington, DC, USA, 1996.
- Naughton, M.J.; Wyman, J.F. Quality of Lie in Geriatric Patients with Lower Urinary Tract Dysfunction. Am. J. Med. Sci. 1997, 314, 217–227. [Google Scholar] [CrossRef]
- Hornberger, B.; Bollner, M.R. Kidney Stones. Physician Assist. Clin. 2018, 3, 37–54. [Google Scholar] [CrossRef]
- Alelign, T.; Petros, B. Kidney Stone Disease: An Update on Current Concepts. Adv. Urol. 2018, 2018, 3068365. [Google Scholar] [CrossRef] [PubMed]
- Idzenga, T.; Pel, J.J.; Mastrigt, R.V. A Biophysical Model of the Male Urethra: Comparing Viscoelastic Properties of Polyvinyl Alcohol Urethras to Male Pig Urethras. Urol. Nephrol. 2006, 25, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Keplen, S.A.; McVary, K.T. Male Lower Urinary Tract Symptoms and Benign Prostatic Hyperplasia; Wiley Blackwell: Hoboken, NJ, USA, 2014. [Google Scholar]
- Roehrborn, C.G.; McConnell, J.D.; Saltzman, B.; Bergner, D.; Gray, T.; Narayan, P.; Cook, T.J.; Johnson-Levonas, A.O.; Quezada, W.A.; Waldstreicher, J.; et al. Storage (Irritative) and Voiding (Obstructive) Symptoms as Predictors of Benign Prostatic Hyperplasia Progression and Related Outcomes. Eur. Urol. 2002, 42, 1–6. [Google Scholar] [CrossRef]
- Coyne, K.S.; Wein, A.J.; Tubaro, A.; Sexton, C.C.; Thompson, C.L.; Kopp, Z.S.; Aiyer, L.P. The burden of lower urinary tract symptoms: Evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009, 103, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.A.; Wei, J.T. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr. Urol. Rep. 2006, 7, 272–281. [Google Scholar] [CrossRef]
- Peyronnet, B.; Brucker, B.M.; Michel, M.C. Lower Urinary Tract Symptoms: What’s New in Medical Treatment? Eur. Urol. Focus 2018, 4, 17–24. [Google Scholar] [CrossRef]
- Chong, J.T.; Simma-Chiang, V. A historical perspective and evolution of the treatment of male urinary incontinence. Neurol. Urodyn. 2017, 37, 1169–1175. [Google Scholar] [CrossRef]
- Natali, A.N.; Carniel, E.L.; Fontanella, C.G.; Frigo, A.; Todros, S.; Rubini, A.; De Benedictis, G.M.; Cerruto, M.A.; Artibani, W. Mechanics of the urethral duct: Tissue constitutive formulation and structural modeling for the investigation of lumen occlusion. Biomech. Model. Mechanobiol. 2017, 16, 439–447. [Google Scholar] [CrossRef]
- Natali, A.N.; Carniel, E.L.; Frigo, A.; Fontanella, C.G.; Rubini, A.; Avital, Y.; De Benedictis, G.M. Experimental investigation of the structural behavior of equine urethra. Comput. Methods Programs Biomed. 2017, 141, 35–41. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Li, Z.; Zhang, M.; Yao, J.; Sheng, N.; Lu, M.; Wang, H.; Chen, S. Urethra-inspired biomimetic scaffold: A therapeutic strategy to promote angiogenesis for urethral regeneration in a rabbit model. Acta Biomater. 2016, 16, 247–258. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Y.M.; Fu, Q.; Zhu, W.D.; Cui, L.; Chen, J. Evaluation of the biocompatibility and mechanical properties of naturally derived and synthetic scaffolds for urethral reconstruction. J. Biomed. Mater. Res. 2010, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, C.V.; Croghan, S.M.; Walsh, M.T.; Cunnane, E.M.; Davis, N.F.; Flood, H.D.; Mulvihill, J.J. Cryopreservation of porcine urethral tissue: Storage at −20 °C preserves the mechanical, failure and geometrical properties. J. Mech. Behav. Biomed. Mater. 2021, 119, 104516. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, E.M.; Davis, N.F.; Cunnane, C.V.; Lorentz, K.L.; Ryan, A.J.; Hess, J.; Weinbaum, J.S.; Walsh, M.T.; O’Brien, F.J.; Vorp, D.A. Mechanical, compositional and morphological characterisation of the human male urethra for the development of a biomimetic tissue engineered urethral scaffold. Biomaterials 2021, 269, 120651. [Google Scholar] [CrossRef] [PubMed]
- Masri, C.; Chagnon, G.; Favier, D.; Sartelet, H.; Girard, E. Experimental characterization and constitutive modeling of the biomechanical behavior of male urethra tissues validated by histological observations. Biomech. Model. Mechanobiol. 2018, 17, 939–950. [Google Scholar] [CrossRef]
- ABAQUS Theory Manual. Available online: https://classes.engineering.wustl.edu/2009/spring/mase5513/abaqus/docs/v6.6/books/stm/default.htm (accessed on 13 July 2022).
- Ogden, R.W. Non-Linear Elastic Deformations; Dover Publications Inc.: New York, NY, USA, 1997. [Google Scholar]
- Dassault Systemes. ABAQUS Analysis User’s Guide. Available online: http://130.149.89.49:2080/v2016/books/usb/default.htm (accessed on 10 June 2022).
- Esmail, J.F.; Mohamedmeki, M.Z.; Ajeel, A.E. Using the uniaxial tension test to satisfy the hyperelastic material simulation in ABAQUS. IOP Conf. Ser. Mater. Sci. Eng. 2020, 888, 012065. [Google Scholar] [CrossRef]
- Seibert, D.J.; Schoche, N. Direct Comparison of Some Recent Rubber Elasticity Models. Rubber Chem.-Techonol.-Rubber Rev. 2000, 73, 366–384. [Google Scholar] [CrossRef]
- Müller, B.; Schulz, G.; Herzen, J.; Mushkolaj, S.; Bormann, T.; Beckmann, F.; Püschel, K. Morphology of urethral tissues. In Proceedings of the Developments in X-ray Tomography VII. International Society for Optics and Photonics, San Diego, CA, USA, 20 September 2010; Volume 7804, p. 78040D. [Google Scholar] [CrossRef]
- Lim, S.H.; Wang, T.J.; Tseng, G.F.; Lee, Y.F.; Huang, Y.S.; Chen, J.R.; Cheng, C.L. The distribution of muscles fibers and their types in the female rat urethra: Cytoarchitecture and three-dimensional reconstruction. Anat. Rec. 2013, 296, 1640–1649. [Google Scholar] [CrossRef]
- Cone, S.G.; Warren, P.B.; Fisher, M.B. Rise of the Pigs: Utilization of the Porcine Model to Study Musculoskeletal Biomechanics and Tissue Engineering During Skeletal Growth. Tissue Eng. Part Methods 2017, 23, 763–780. [Google Scholar] [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef]
- Gasser, T.C.; Ogden, R.W.; Holzapfel, G.A. Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 2006, 3, 15–35. [Google Scholar] [CrossRef]
- Rynkevic, R.; Ferreira, J.a.; Martins, P.; Parente, M.; Fernandes, A. Linking hyperelastic theoretical models and experimental data of vaginal tissue through histological data. J. Biomech. 2019, 82, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.S.; Rynkevic, R.; Martins, P.A.L.S.; Parente, M.P.L.; Famaey, N.M.; Deprest, J.; Fernandes, A.A. Predicting the mechanical response of the vaginal wall in ball burst tests based on histology. J. Biomed. Mater. Res. Part Appl. Biomater. 2019, 108, 1925–1933. [Google Scholar] [CrossRef] [PubMed]


| Models | Longitudinal | Circumferential | ||||
|---|---|---|---|---|---|---|
| Ogden | k | k | ||||
| 1 | 1 | |||||
| 1 | 2.14665 | −4.01108 | 1 | 1.22825 | −1.27365 | |
| 2 | 2.61517 | 1.98579 | 2 | 2.06915 | 0.60762 | |
| 3 | 1.64414 | 2.03311 | 3 | 0.25084 | 0.68139 | |
| Reduced Polynomial | k | k | ||||
| 1 | 1 | |||||
| 1 | 1 | |||||
| 2 | 2 | |||||
| 1 | 1 | |||||
| 2 | 2 | |||||
| 3 | 3 | |||||
| 1 | - | 1 | ||||
| 2 | - | 2 | ||||
| 3 | - | 3 | ||||
| 4 | - | 4 | ||||
| 5 | - | 5 | ||||
| 1 | 1 | - | ||||
| 2 | 2 | - | ||||
| 3 | 3 | - | ||||
| 4 | 4 | - | ||||
| 5 | 5 | - | ||||
| 6 | 6 | - | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, A.D.; Areias, B.; Teixeira, A.M.; Pinto, S.; Martins, P. Mechanical Behaviour of Human and Porcine Urethra: Experimental Results, Numerical Simulation and Qualitative Analysis. Appl. Sci. 2022, 12, 10842. https://doi.org/10.3390/app122110842
André AD, Areias B, Teixeira AM, Pinto S, Martins P. Mechanical Behaviour of Human and Porcine Urethra: Experimental Results, Numerical Simulation and Qualitative Analysis. Applied Sciences. 2022; 12(21):10842. https://doi.org/10.3390/app122110842
Chicago/Turabian StyleAndré, António Diogo, Bruno Areias, Ana Margarida Teixeira, Sérgio Pinto, and Pedro Martins. 2022. "Mechanical Behaviour of Human and Porcine Urethra: Experimental Results, Numerical Simulation and Qualitative Analysis" Applied Sciences 12, no. 21: 10842. https://doi.org/10.3390/app122110842
APA StyleAndré, A. D., Areias, B., Teixeira, A. M., Pinto, S., & Martins, P. (2022). Mechanical Behaviour of Human and Porcine Urethra: Experimental Results, Numerical Simulation and Qualitative Analysis. Applied Sciences, 12(21), 10842. https://doi.org/10.3390/app122110842