1. Introduction
Dental implant application is a broadly used modality for the rehabilitation of partial/complete edentulism with high success rates. Alveolar bone with sufficient quantity and quality is mandatory for successful dental implantation.
Sufficient volume of alveolar bone is a key factor in obtaining correct positioning of dental implants and providing long-term biomechanical stability outcomes [
1]. As is known, different traumatic events may result in alveolar bone loss, such as tooth loss, periodontal disease, dental and facial trauma, tumors and cysts, sinus pneumatization, and many other conditions [
2]. It is well known that bone resorption occurs as a result of tooth loss, particularly regarding the alveolar process. Within the first year following tooth loss, resorption of the alveolar ridge may take place involving 50% of the existing bucco-lingual dimension [
3].
A variety of surgical techniques may be performed to provide more bone volume to the horizontally deficient alveolar ridges and consequently allow for implant placement coordinated with a prosthodontic treatment plan. Alveolar bone loss can be reconstructed using different strategies, such as GBR, inlay and onlay grafting, free vascularized autografts, distraction osteogenesis, and ridge splitting. The amount and configuration of osseous defects determine the type, magnitude, and prognosis of the bone-augmentation modality [
4].
GBR is a reliable technique for hard tissue augmentation. It allows for reconstruction of horizontal and vertical defects, providing sufficient bone volume for osseointegrated dental implants to occur. Horizontal ridge augmentation via GBR with the combination of particulate bone graft materials and resorbable collagen membrane is a frequently re-ported surgery in the literature [
5]. Autogenous bone graft is considered the gold standard for use in hard tissue augmentation procedures, providing critical minerals, proteins, and vital bone-related cells to the recipient site for bone healing, resulting in the improved success of bone augmentation. By contrast, the use of autogenous bone might induce critical morbidity at the donor site, such as postsurgical pain, increased time/costs, and unpredictable resorption rate [
6]. ABB is an osteoconductive bone graft material that can provide a sufficient scaffold for new bone growth; moreover, the use of 50% ABB minimizes the need for a large quantity of harvested host bone. The combination of ABB and autogenous bone graft alters the resorption rate of the autogenous bone during the healing process, whereas particulate autogenous bone enhances osteogenic factors [
7].
One of the most important steps in a successful hard tissue augmentation is the preclusion of bone graft movement. Sufficient stability and minimal stress exposure are necessary to allow for new bone formation since a new vascular network is prone to deterioration caused by mechanical conditions [
8]. CS is a human-derived allogeneic cancellous block that is produced of osseous structure of femoral heads. CS is a stable and rigid plate which plays a critical role in the ‘’shell technique’’ for hard tissue augmentation. The shell technique creates space between the host bone and the cortical plate and can thus be filled with a variety of different particulate bone grafting materials. Two screws are recommended to fix CS to the residual bone to prevent rotation [
9].
Cone-beam computed tomography (CBCT) images are approved for their capability to assess hard tissue augmented sites and their contours. While CBCT scans allow for nearly the same exact measurement of alveolar bone volume as CT and micro-CT scans, CBCT also offers some advantages, such as reduced radiation dose, lower cost, smaller device size, and high resolution [
10].
The principal objective of this retrospective study was to evaluate volumetric changes by means of CBCT following horizontal alveolar ridge augmentation, either by using CS with a combination of particulate autogenous bone and bovine bone minerals covered by a resorbable collagen membrane (test group), or by using a mixture of an inorganic bovine bone substitute and particulate autogenous bone with resorbable collagen membrane as GBR (control group). Additionally, complications and insertion torques between groups were recorded. The null hypothesis was that there would be a difference between the two groups.
2. Materials and Methods
2.1. Study Design and Sample Selection
This study included patients with horizontally deficient alveolar ridges in anterior or posterior regions of the maxilla where the incisors and canine region were considered as anterior, whereas the premolar and molar teeth regions were considered as posterior. Ridges were treated with CS or GBR between January 2019 and 2020 at Istanbul University, Istanbul Medeniyet University, and Sourasky Medical Center. Inclusion criteria applied to this clinical study were as follows: the presence of residual bone width of <4 mm and sufficient bone height, which could be treated with two-stage horizontal hard tissue augmentation for implant placement; availability of CBCT data acquired prior to surgery, 2 weeks post-operation, and 7 months after healing. Exclusion criteria were as follows: ASA III and IV patients (American Society of Anesthesiologists III and IV), smoking, pregnancy, alcohol abuse, use of bisphosphonates, and radiotherapy. The study protocol is in full accordance with the Helsinki Declaration and was approved by Istanbul Medeniyet University’s ethical committee (Protocol number: 2022/0283). Written informed consent to the procedures was obtained from all patients.
2.2. Surgical Methods
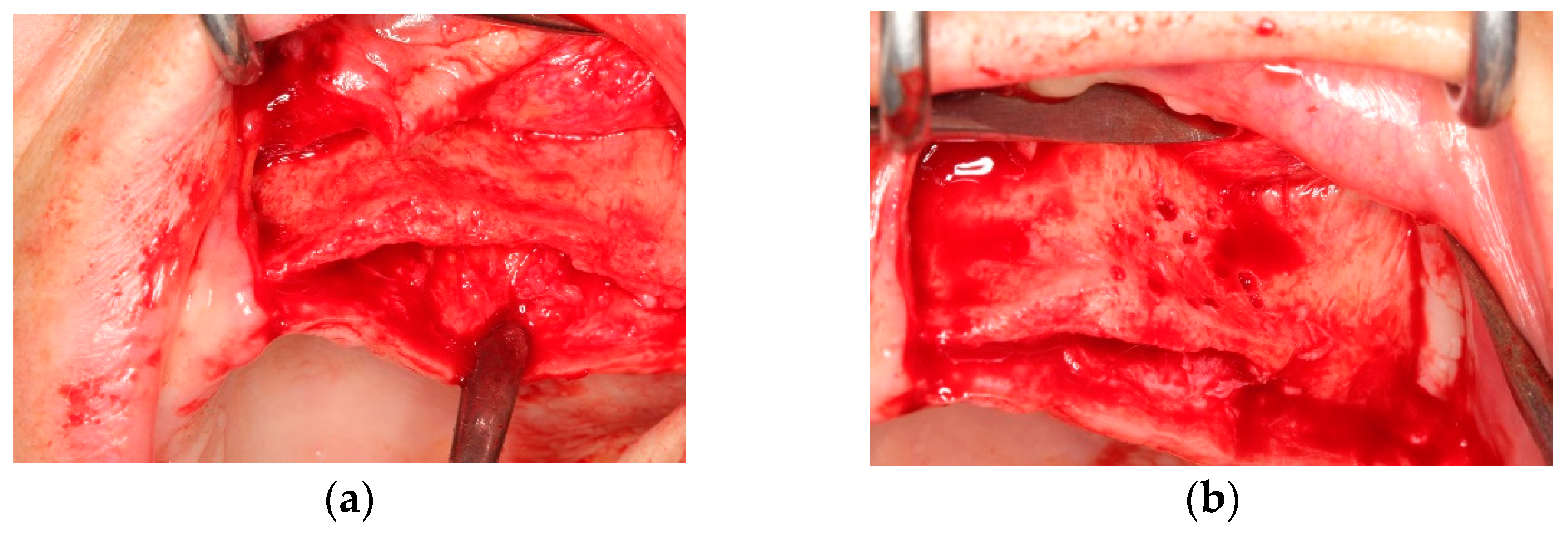
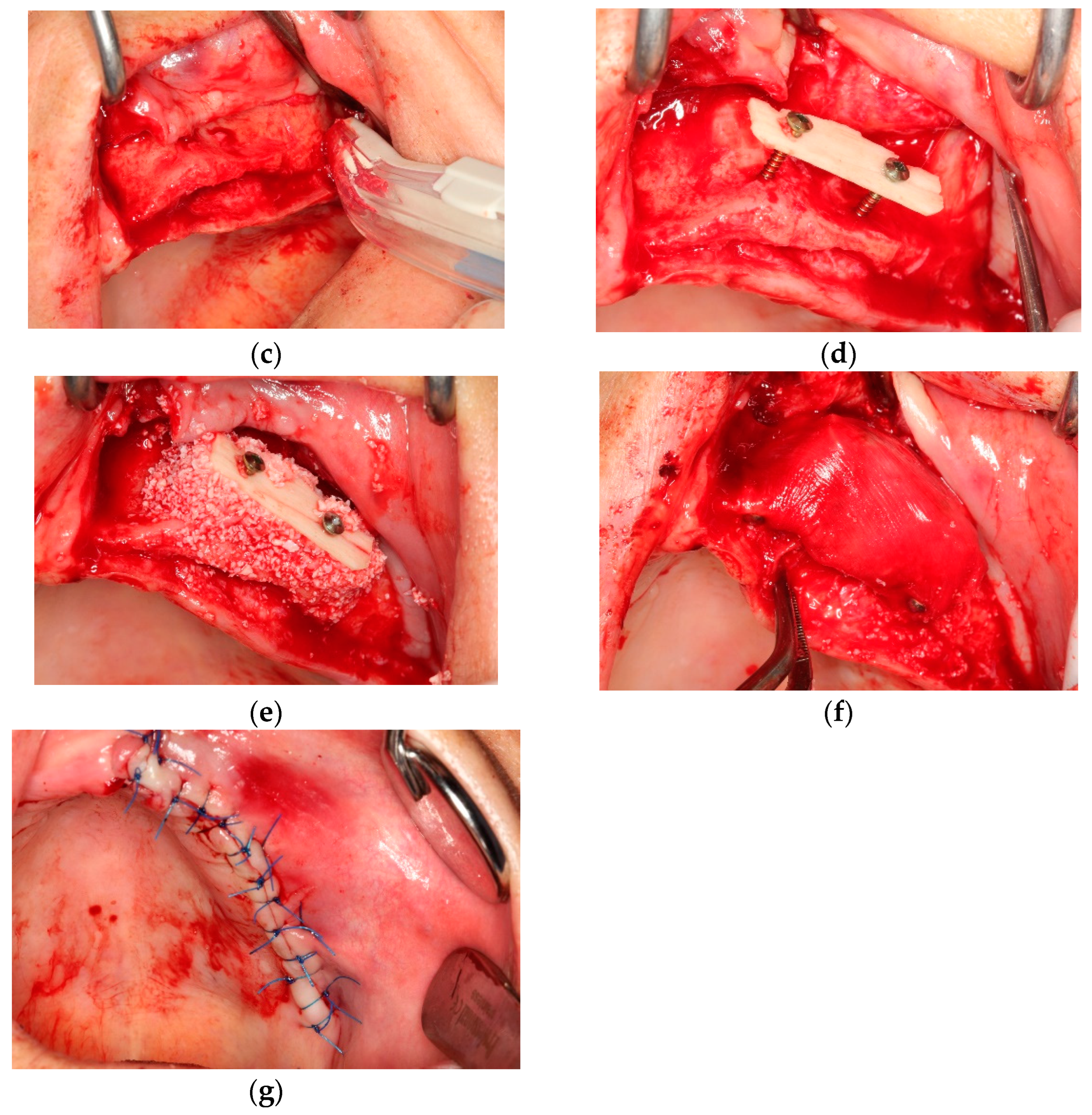
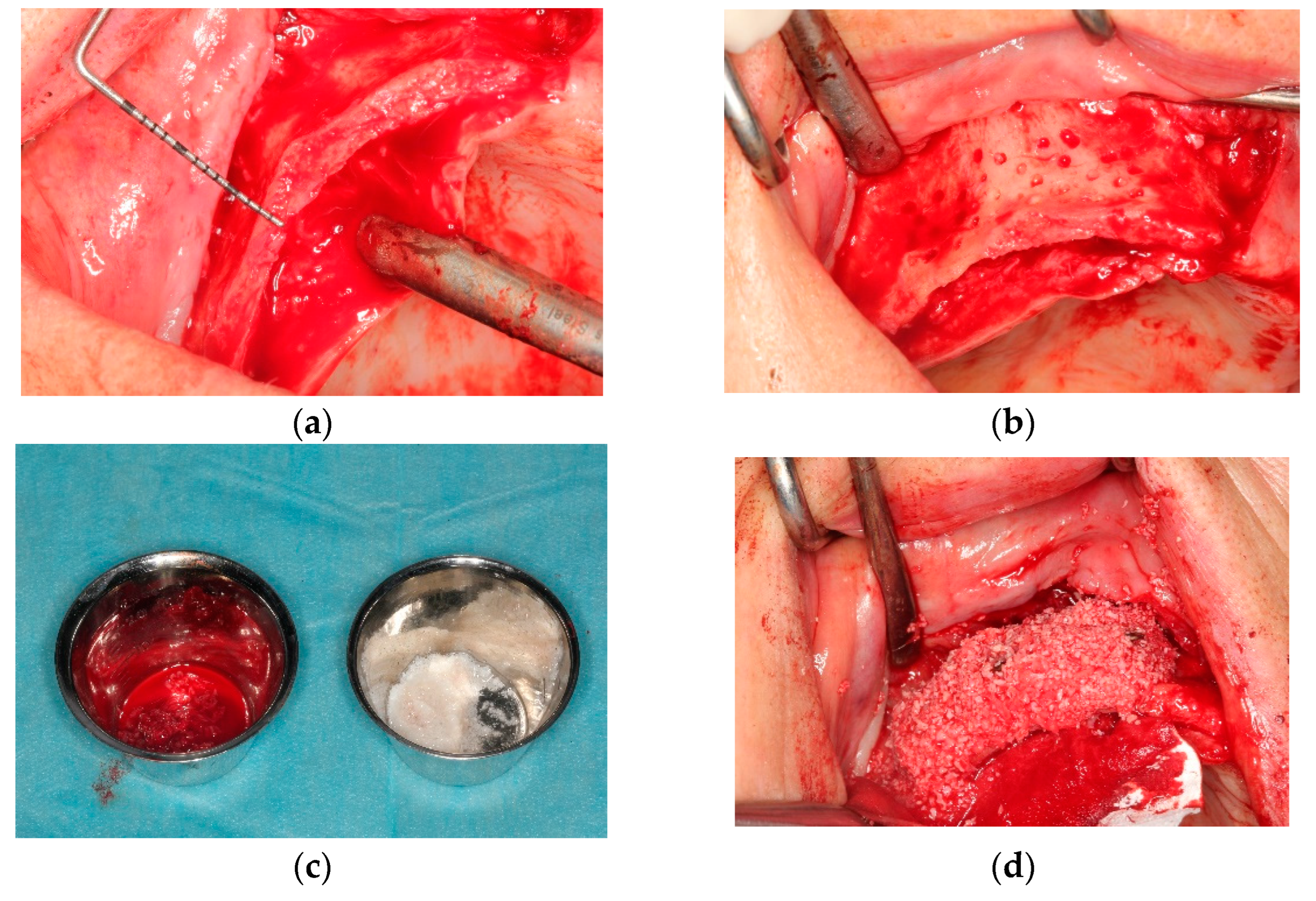
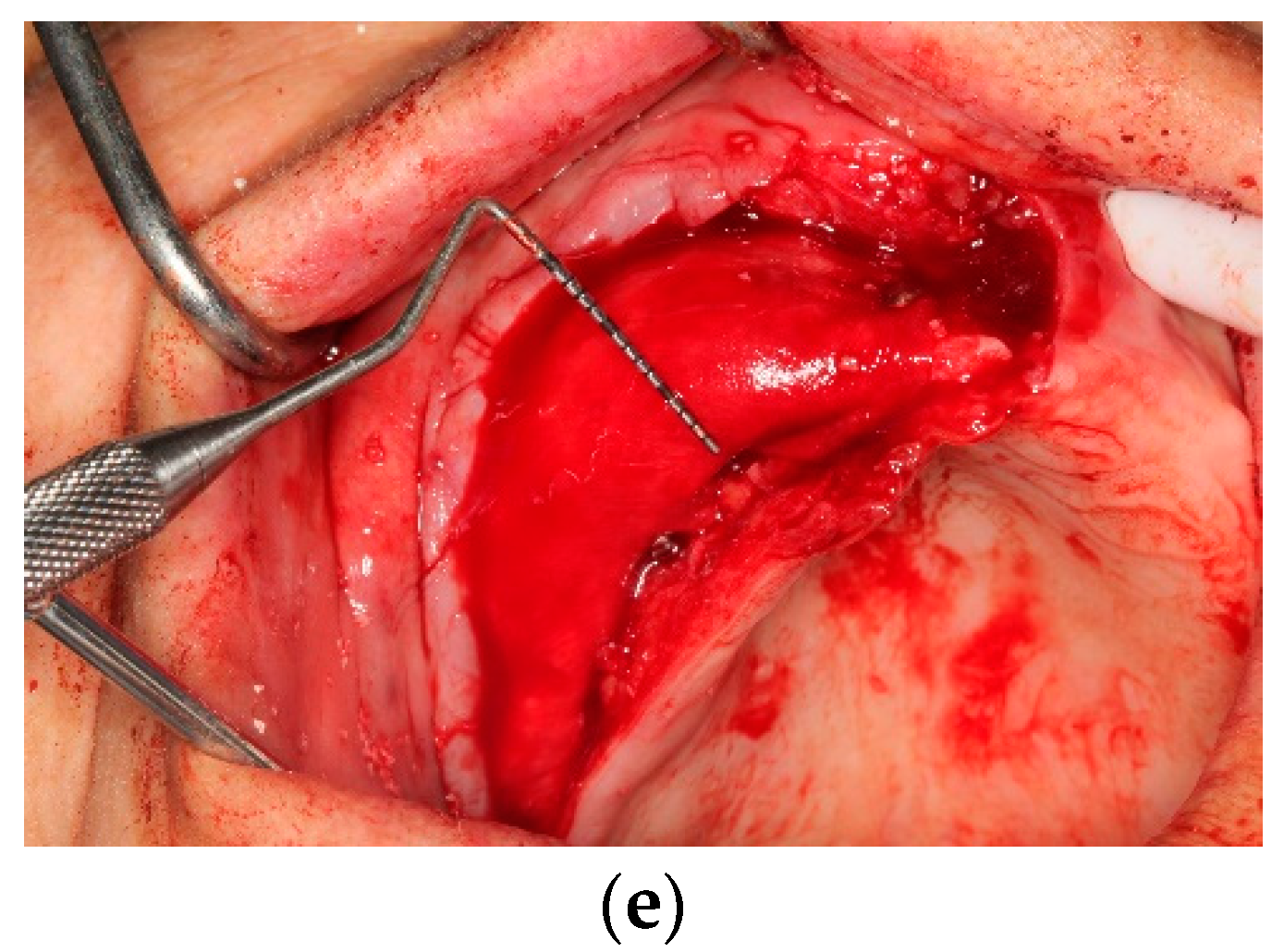
All operations were fulfilled under local anesthesia. Before surgery, 0.2% chlorhexidine mouthwash (Klorhex, Drogsan Pharma, Ankara, Turkey) was recommended to all patients to rinse their mouths for 1 min. For both groups, mid-crestal and vertical incisions were made. Next, a full-thickness flap was elevated completely to allow observation of the surrounding bone and the hard tissue deficiency. The native bone was decorticated to enhance healing, both by advancing bleeding and by allowing blood vessels and progenitor cells to attain the augmented site more readily. The residual bone was cleaned with curettes to remove any soft tissue remnants that could affect hard tissue healing negatively. Autogenous bone particles were harvested from a region close to the recipient site with the help of a bone scraper (Safe scraper, META, Italy), and then added to a bone graft mixing bowl cup to be mixed with ABB (Cerabone, botiss biomaterials, particle size, 0.25–1.0 mm); in a ratio of approximately 1:1 to form the mixture. In the test group, CS (Maxgraft
® cortico, botiss biomaterials, GmbH, Zossen, Germany) was soaked in sterile 0.9% saline solution for approximately 20 min before use. The blocks’ corners were smoothed to avoid undesirable exposure and CS was fixed onto native bone with two osteosynthesis screws to create a shell for autogenous and ABB graft combination. Resorbable collagen membrane (Jason
® membrane, botiss biomaterials GmbH, Zossen, Germany) was aligned, according to the shape of the augmented site, and then applied. After grafting, the tacks (Pinfix, Sedenta, Istanbul, Turkey) were used at buccal and palatinal sites to immobilize the resorbable membrane and attain the stability needed for the particulates. For tension-free closure of the flaps, periosteal-releasing incisions were performed, and the flaps were loosely reapproximated for tight closure with non-resorbable sutures, using interrupted and mattress suture techniques (
Figure 1 and
Figure 2).
Antibiotics (1000 mg amoxicillin and clavulanic acid, two times a day for one week), analgesics (600 mg ibuprofen), and 0.2% chlorhexidine mouthwash (twice a day) were prescribed to all patients as postsurgical medications. Dexamethasone (4 mg per day for 2 days) and ice compression was administered to minimize postoperative swelling and edema. Sutures were removed 2 weeks after operation. Patients in the CS and GBR groups were allowed to heal for 7 months. None of the patients were allowed to wear any kind of prosthetic device during the healing period. Thereafter, patients received fixed porcelain-fused-to-metal or zirconia crowns and bridges. At the second-stage surgery, implant placement procedures were carried out using a tapered implant system with SLA-treated surface and a macro-design possessing distinctive aggressive threads (DE|TECH Implant System®).
2.3. Study Variables
The primary outcome and predictor variables were the augmentation technique (CS or GBR) and the resorption rate at the grafted site, whereas the secondary study variables included complications and implant stability.
2.4. Clinical Assessment
Patients in both the test and control groups were evaluated. Any complications, including inflammation at the augmented site, graft and/or collagen exposure, loss of bone particles, infection, and insufficient augmented bone volume were evaluated. At the second-stage surgery, implant insertion torque values (ITV) were used to assess clinical success of implant placement in the graft site. Final insertion torques (< or ≥25 Ncm) of implants were recorded with the help of a physiodispenser (W&H ImplantMed, Burmoos, Austria). Clinical evaluation of the presence of pain and/or deep periodontal probing depth were performed.
2.5. Radiographic Assessment
Three-dimensional volumetric radiological evaluations were performed using CBCT at the augmented sites. Images were taken preoperatively, within 2 weeks (V1), and 7 months postoperatively (V2) for both the test and control groups. Sample analysis was performed with the assistance of the i-CAT 3D imaging system (Imaging Sciences International Inc., Hatfield, PA, USA), with a voxel size of 0.25 and a field of view extended over 12 × 8 cm. The hard tissue augmentation area was traced. Imaging data of the augmented sites were evaluated with commercially available software (MIMICS 14.0, Materialise, Leuven, Belgium), where the volumetric alteration in the grafts was analyzed. The augmented sites were modeled in 3D to evaluate postsurgical volumetric changes at two different time points (V1 and V2) (
Figure 3). For certain reproducibility of volumetric measurements at various CBCT scans, screws, fixation tacks, and anatomical landmarks were used as points of reference for graft site selection. Native bone, tacks, and resorbable membranes were screened at the regions of interest in the augmented sites during reconstruction of the grafted areas and included in volumetric measurement. All radiographic measurements were recorded by the same examiner under identical conditions in an effort to avoid bias and certify excellent reliability.
2.6. Statistical Analysis
Number Cruncher Statistical System 2007 (NCSS) was used to perform the statistical analysis (Kaysville, UT, USA). Mum, frequency, and percentages presented the descriptive statistical values used in this statistical evaluation. To test the differences in quantitative variables between the control and test groups (two independent groups), independent sample t-tests were used. The differences in qualitative variables were tested with the help of Yates’ continuity correction. Pearson’s correlation test was used to perform the analysis of the correlation among variables. To analyze the possible risk factors of any changes in volume (V1-V2), linear regression analysis was carried out. In addition, p values < 0.05 were considered statistically significant.
4. Discussion
In this retrospective study, a comparison was made to evaluate the efficacy of CS and GBR techniques in bone augmentation of maxilla with insufficient bone thickness needed for dental implant application. Autogenous and inorganic bovine bone graft materials of particle forms were used in both the control and test groups. The grafting procedure was supplemented with a resorbable collagen membrane coverage and flap closure. Following a 7-month healing period, pre- and post-surgical complications were estimated. Additionally, evaluation of volumetric changes at the implantation phase was achieved using CBCT. Moreover, implant insertion torque was measured for comparison between groups. As a result, a lower amount of bone volume loss was assessed to be statistically significant in the CS-augmented group, compared with the GBR-augmented group. However, no significant difference between the two groups was noticed in terms of complication incidence and implant placement torque.
Among hard tissue augmentation techniques, immobilization of the applied graft materials is considered one of the most substantial factors affecting the success of the treatment. The graft materials of particulate form applied during an augmentation procedure require physical resistance to prevent any potential collapse. In order to achieve this, efforts are being made to increase the success rate by using block autogenous and allogeneic bone plates combined with various particulate graft materials applied into the spaces created by these block grafts. Use of the shell technique has shown positive results [
11].
Autogenous block graft has been used successfully in hard tissue augmentation for many years. Due to its osteogenic, osteoinductive, and osteoconductive effects, it is considered the gold standard graft; thus, it is preferred to any other alternative graft materials, which have unpredictable resorption and can induce donor-site morbidities, such as formation of a second wound area, risk of post-procedure pain, edema, and sensory damage, in addition to their inability to obtain the desired result in some cases [
7,
12].
Although autogenous block bone applications are frequently preferred, especially in vertical bone augmentation, resorption rates of 21–25% are reported in the literature [
13,
14].
Presently, the utilization of allogeneic block graft in hard tissue augmentation is increasing, which can be stabilized to the existing osseous defect with the help of tenting screws; resulting in mechanical resistance for the particulate graft, which, in turn, can be condensed to the block-constructed area with the shell technique. In this manner, bone volume can be preserved by minimizing the risk of mobilization or collapse of a bone graft during the healing process. In the present study, it is suggested that the main reason to adopt the shell technique is to obtain significantly less bone volume loss in the CS group, compared with the GBR group.
Numerous bone defects, such as horizontal and vertical bone augmentation [
15], cleft lip and palate [
16], and maxillary sinus augmentation [
17,
18], can be reconstructed successfully with allogeneic bone grafting.
Many studies have confirmed the use of allogeneic grafting in particulate and block forms. However, few studies focused on the effectiveness of allogeneic block application using the shell technique [
18]. In the present study, the effectiveness of the shell technique performed with the help of allogeneic block graft was evaluated, and a volume loss of 8.26 ± 1.60% was observed after 7-month recovery period.
In the literature, numerous horizontal bone augmentation techniques have been reported with varying success rates. Urban et al. reconstructed a hard tissue deficient region by mixing autogenous bone and xenograft particles at a ratio of 1:1 and covering with a resorbable membrane using pins. In this technique, the benefits of osteogenic and osteoinductive properties of autogenous bone occur. Additionally, the osteoconductive structure of xenografts is used in an attempt to prevent unpredictable resorption, which can be seen in autogenous bone. In the present study, autogenous and xenograft particles in a ratio of 1:1 were utilized as graft material in both groups [
19]. There are four major principles to accomplish successful GBR, which are described by Wang et al. as PASS: primary closure of wound area, angiogenesis to accelerate new bone formation, space creation to avoid the collapse of bone grafts, and stability of the blood clot. Such requirements reduce the incidence of adverse effects and enable better results [
20].
Autogenous block grafts have been widely and extensively used for many years to create a space and maintain sufficient stability in it until the bone graft is integrated with the host bone. Since autogenous blocks can be used on their own, they are fixed to the recipient area using the shell technique. Subsequently, the created space is filled with bone graft particulates [
21].
In a published systemic review, a comparable amount of regenerative bone was obtained using either allogenic graft or autogenous block graft [
22]. In the literature, there are many studies in which autogenous or allogeneic blocks are applied alone for hard tissue augmentation, but they have shown varying amounts of resorption.
Silva et al. have applied allogenous block grafts alone in the mandible for hard tissue augmentation. As a result, 31% resorption was observed following a 6-month healing period after dental implantation, with an additional 10% resorption 1 year after implant loading [
23], whereas Stricker et al. observed an average of 43.7% resorption of the autogenous block grafts used in horizontal augmentation after a 12-month follow-up [
24]. Lee et al. also reported an average of 25.4% resorption of autogenous block grafts 4–6 months after the procedure [
25].
The possible reason proposed for the higher rate of resorption observed in studies where only autogenous and block allografts were applied for horizontal hard tissue augmentation, in comparison to the present study, is the role of the xenograft particulates which filled the space that was created between the fixed block allograft material and the recipient site in the augmented region and performed using the shell technique.
Apart from the disadvantages of autogenous block augmentation, such as the difficulty of harvesting autogenous block graft, the inability to obtain the desired amount in some cases, and the risk of nerve damage and patient morbidity; the excess amount of resorption is also encountered, which has provoked the question of whether it is possible for allogeneic grafts to be an alternative to autogenous blocks. Kloss et al. compared the efficacy in their study of both autogenous and allogeneic blocks in hard tissue augmentation, and consequently reported 12.5% and 14.4% resorption, respectively, after 12 months of follow up [
26].
Gultekin et al. [
27] compared the efficacy of GBR and autogenous block graft for bone reconstruction in maxillary regions with insufficient thickness. They observed an average of 12.48% bone resorption at the end of 6–7 months of the healing period where GBR procedure using particulate autogenous bone, xenograft, and collagen membrane was applied (control group), whereas only 7.20% bone resorption was observed at the end of 4 months where autogenous block was used in the procedure (test group). Since the results of the studies mentioned earlier are somewhat similar to those of the present study, CS protocol is suggested as a successful alternative to autogenous block grafts.
There are few studies in the literature concerned with allogeneic block grafts application in horizontal augmentation with the help of the shell technique. Our study is the only one in the literature where the success of the allogeneic block graft was evaluated volumetrically. Tunkel et al. [
28] used autogenous and allogeneic blocks for horizontal bone augmentation using the shell technique and only particulate autogenous bone graft was applied between the block and the host bone. They observed a 5% loss in the autogenous block group and a 2% loss in the allogeneic block group in the linear measurements carried out during the implantation phase after 5 months of augmentation recovery. The reasons for the lower amount of resorption in the Tunkel study, compared to the present study, may be attributed to the relatively shorter waiting time of that study, in addition to the different radiological measurement techniques used in both of them.
One of the limitations presented in this study is the lack of histological and histomorphometric samples harvested from the augmentation areas for detailed evaluation. Only the three-dimensional volumetric change in the newly formed bone was examined after a healing period of 7 months, with no information available on parameters such as status of newly forming bone, remaining graft, or connective tissue in the augmentation area. In this study, the efficacy of block allogeneic and GBR techniques were compared for horizontal bone augmentation in maxillary regions where insufficient thickness was found. Compared to the mandible, vascularization and nutrition were higher in the maxilla; thus, similar studies on the mandible are needed to fully determine the effectiveness of the augmentation materials. Moreover, the relatively short follow-up period is considered another limitation of this study. After 7 months of healing, volumetric changes were examined by comparing CBCT images taken before implantation with images taken immediately after bone augmentation. Long-term radiological evaluation is needed to assess possible continuous resorption that might continue in the augmented zone.