Featured Application
Introduction of lactic acid solution treatment of game meat carcasses as a relevant critical control point (CCP) in the HACCP plan of game handling establishment, according to EC regulations.
Abstract
Solutions of lactic acid 2% and aromatic vinegar were investigated for microbial load reduction on the surfaces of wild boar carcasses. The animals were hunted and processed according to production specifications to obtain the best hygiene for carcasses. The solutions were sprayed on carcass surfaces after skinning, and sites of 5 × 5 cm were sampled 2 h and 48 h post-treatment, with the carcasses under refrigeration conditions. The values of the microbial loads were lower for lactic-acid-treated sites, compared with control, after both 2 and 48 h. Nonetheless, the differences in the microbial loads were only higher than 1 Log/CFU 25 cm2 for the aerobic colony count. The aromatic vinegar resulted in lower values than in controls only after 48 h for the aerobic colony count, Staphylococcus count, and Lactobacillus count, with values always below 1 Log/CFU 25 cm2. The implementation of a lactic acid solution could represent a valuable strategy to reduce microbial growth on wild boar carcasses, thus becoming a relevant critical control point in this peculiar and niche meat production process.
1. Introduction
The European Union policy on meat safety is based on the implementation of good hygienic practices and the HACCP system to prevent the contamination of meat and, therefore, microbial growth. According to EC regulation, indeed, if the procedures during slaughtering are correctly adopted, the hygienic level of the carcasses would be high, and there would be no need for decontamination strategies [1].
It is clear that the EU approach, reflected in the regulatory framework, is based on the prevention of meat contamination limiting further treatments. EC Regulation 853/2004 states that food business operators (FBOs) shall not use any substance other than potable water on food surfaces and, therefore, also on carcasses [1]. Nonetheless, the European legislation establishes that post-slaughtering treatments with substances other than water could be performed only after EU Commission’s specific approval. The use of an approved substance shall not affect the FBOs’ duty to comply with hygienic production requirements [1].
In this context, the EFSA indicates that weak acid solutions could be considered for carcass treatment after slaughter without safety issues for consumers [2,3]. The opinions about lactic acid solutions specifically referred to bovine [2], pigs [4], and recently also to goat, sheep, kangaroo, and wild pigs carcasses and meat [5]. The EFSA also recommends that FBOs should validate the antimicrobial efficacy of such treatments under their specific processing conditions [6].
Only the use of 2–5% of lactic acid solution sprayed on bovine carcasses was approved by the Commission, following the EFSA opinion, and is now admitted in Europe [6]. This treatment is not permitted if it causes irreversible physical modification of the meat as well as in carcasses with visible fecal contamination. The lactic acid treatment could be, therefore, considered in the HACCP system as a relevant CCP to be monitored. The possible use of weak acid solutions in other species is not yet allowed, even if the EFSA provides a positive opinion on the use of peroxyacetic acids in poultry and red meat [3]. There are no indications and recommendations on hunted game meat so far.
Game meat in industrialized countries is considered a niche production of highly valuable food [7,8]. The interest of consumers in this type of meat is debatable: There are ethical doubts due to hunting practices or meat consumption [9], while there is also increasing awareness of the nutritional quality of game meat and the sustainability that characterizes its production, compared with meat from intensively farmed animals [10,11,12]. In Europe, the number of some wild species has been rising dramatically in recent decades due to several anthropic and non-anthropic factors, such as the availability of widely abandoned or marginally rural areas; the increase in protected areas lacking in monitoring and managing invasive species; the introduction of both typical and alien species for recreational hunting purposes, and other environmental and animal-related factors [13,14,15,16,17]. The rise in wild populations, in particular large ungulates, is generating impacts on agronomic, economic, environmental (i.e., biodiversity), and public health (i.e., the spread of infectious disease) aspects. Wild boar is probably the best example of this phenomenon in Europe, and it became a relevant issue to be managed by different proponents (i.e., politicians, hunters, and animal rights activists) [18]. In the Umbria Region, central Italy, the population of wild boar is enormously increased, with more than 70,000 subjects and over 20,000 hunted animals in 2021, over a surface of 8500 km2 [19]. In this region, hunting is the main strategy for population control, but to date, more than 95% of the meat obtained is intended for self-consumption by hunters and/or partially directly sold in small quantities to local restaurants. Both these conditions do not provide the implementation of specific hygienic rules set by EU regulation [1]. This generates poor attention to meat hygiene and quality and a black market for meat without a comprehensive control procedure by local authorities. For this reason, there is a strong interest in creating specific certificated production chains that could increase consumer demand for high-quality meat, give economic incentives to hunters, and guarantee proper controls by official authorities during the pre-harvest and post-harvest phases [7]. These certified chains are fully respondent to EC regulation [1] and are based on specific procedures designed to obtain good hygienic levels of the carcasses. These procedures must be implemented by hunters, in the harvest phase; operators of the collection centers where animals are eviscerated and refrigerated without skinning; and operators of game meat establishments where wild boars are properly dressed and refrigerated. In these certified chains, further improvement to wild boar carcass hygiene could be obtained with the use of weak acid solutions applied by the operators of game meat establishments under the supervision of competent authorities. No studies are yet available, to the best of our knowledge, on the use of organic acid on wild-hunted ungulate carcasses in general and on wild boar in particular.
The aim of this study is to evaluate, in a real production environment, the effects of spraying selected organic acids solutions on the surfaces of wild boar carcasses. The hypothesis of this study is that the use of a low dose of weak organic acids will improve the hygienic level of wild boar carcasses, without any irreversible physical modification of the carcass surface. To comply with the FBOs’ duty in terms of hygienic procedures, the trial was conducted exclusively on the carcasses obtained under best hunting and post-harvest management procedures.
2. Materials and Methods
2.1. Sampling of the Carcasses
Sampling was performed on the external surfaces of wild boar carcasses obtained from animals hunted with the “still hunting” method [20] during the winter of 2020–2021 and 2021–2022 in the Umbria Region (central Italy). The hunted animals were selected according to specific pre-harvest and post-harvest procedures to obtain the best hygienic level of the carcasses. Specifically, the factors considered were animal weight between 45 and 55 kg (non-eviscerated); environmental temperature during hunting below 15 °C; the absence of rainfall during hunting; shot-to-kill position in head or heart, without damaging the intestine and the carcass; the time between killing, bleeding, and evisceration less than 1 h; the time before the refrigeration of the carcasses less than 2 h; refrigeration temperature in the collection center below 7 °C in the deep muscle; and maximum 2 days of refrigerated storage under the skin in the collection center. The carcasses were then transferred under refrigerated conditions to a local slaughterhouse, which serves as a game meat establishment, for further skinning and storage. A total of 54 carcasses were transferred to the game meat establishment during hunting seasons but only 10 of them (6 male and 4 female) fitted with all the defined and aforementioned factors and were, therefore, taken into consideration. After skinning, for each carcass, 6 sites were defined for microbial surface sampling purposes (the lateral part of the thigh, the flank, and the thorax, on the left and the right sides of the carcasses; Figure 1).
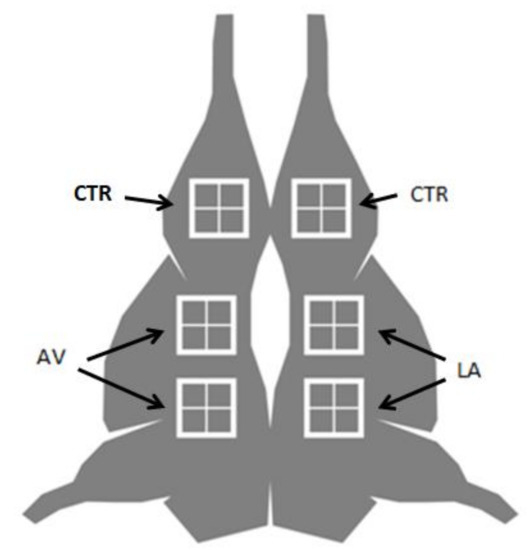
Figure 1.
Sampling areas of wild boar external carcass surfaces sprayed with 2% lactic acid solution (LA), aromatic vinegar (AV), or untreated (CTR).
These sampling sites were chosen to avoid different levels of contamination, as preliminary studies proved no difference in their microbial loads (Table S1).
The carcasses were already refrigerated at 7 °C before skinning and maintained at the same temperature until sampling was performed. Two sites (the lateral surface of the tights of both the right and left sides of the carcass) were considered as a control (CTR) and not treated with solutions or sterile water; two sites (the lateral surface of the thorax of the right side of the carcass) were treated with 2 mL of fine sprayed 2% lactic acid solution at 15 °C (LA-Todini chemicals, Milan, Italy, pH 2.4); two sites (the lateral surface of the thorax of the left side of the carcass) were treated with 2 mL fine sprayed food grade aromatic vinegar at 15 °C (aromatic vinegar GPI 6.2—Lazzari Equipment and Packaging, Settimo di Pescantina, VR. Italy, characterized by a pH of 6.02, acetic acid 5%, and a total phenolic count of 2.6 mg gallic acid equivalent/mL). Fine spraying was performed using a one-hand sprayer (hand nebulizer ECON Stocker s.r.l., Lana (BZ), Italy—1 bar pressure) at 50 cm from the surface. The number of solutions, as well as the pressure adopted, were not sufficient to define it as a rinse or a wash; for this reason, no sprayed sterile water was considered as a control. Other authors adopted the same approach for control samples when antimicrobial solutions were tested [21].
From each of the sites, 4 sampling areas of 5 × 5 cm were randomly sampled after 2 h (T2), and 4 other sampling areas of the same dimensions were sampled after 48 h (T48). The sampling time was chosen to allow for surface drying after the treatment (T2) and when the carcasses were still available in the game meat establishment (T48), before their transfer to cutting plants. The total number of samples was, therefore, 240 (10 carcasses, 3 treatments, 4 sampling areas of 25 cm2, 2 sampling times). This sampling protocol was defined in order to have a high number of samples from a limited number of wild boar carcasses obtained in the same optimal conditions, as the consistency of pre-harvest and harvest conditions represents a general problem in game meat sampling [8,13]. After the first treatments and sampling, the carcasses were kept under refrigerated conditions (7 ± 1 °C). The samples were obtained with the wet and dry swab method [22], and both swabs, belonging to one sampling site, were put in a vial containing 9 mL of sterile solutions (NaCl 0.9% solution, Oxoid, Basingstoke, UK) and kept under refrigerated conditions (isothermal box) until microbiological determination.
2.2. Microbiological Determination
The samples were, therefore, vortexed, and serial decimal dilutions (NaCl 0.9% solution—Oxoid) were performed. The dilutions were used for the following determinations:
- Aerobic colony count (ACC) [23] on plate count agar (PCA-Bio-RAD Laboratories, Marnes-la-Coquette, France) aerobically incubated at 30 °C for 48 h;
- Psychotropic colony count (PCC) [24] on PCA (Bio-RAD Laboratories) aerobically incubated at 7 °C for 10 days;
- Enterobacteriaceae count (EC) [25] on Vilet Red Bile Glucose Agar (VRBGA-Bio-RAD Laboratories) aerobically incubated at 37 °C for 24 h;
- Staphylococcus spp. count (SC) on Mannitol Salt Agar (MSA-Biolife Italiana s.r.l., Milan, Italy) incubated at 37 °C for 48 h;
- Lactobacillus spp. count (LABC) on De Man Rogosa Sharp Agar (MRSA-Oxoid) anaerobically incubated at 30 °C for 48 h;
The colonies were, therefore, counted, and the results were converted into Log colony forming units (CFUs)/25 cm2. When no colonies were counted in the lower dilution plates, a middle bound level of detection (LOD) approach was used to manage the left censored data, taking into account the lowest sample dilution (1:10) and the quantity of the sample (1 mL or 0.1 mL) included or spread onto the plates. In particular, for ACC, PCC, and Enterobacteriaceae counts, a value of 0.7 Log CFU/25 cm2 was assigned for non-detectable colonies in the sample, while for Staphylococcus and Lactobacillus counts, 1.7 Log CFU/25 cm2 were assigned. Foodborne pathogens were not investigated because no experimental contamination could be performed in the game meat establishment.
2.3. Same–Different Test Analysis
To evaluate if the treatments irreversibly affected the surface characteristics, the same 10 carcasses sampled after 24 h underwent visual examination by 8 trained assessors to confirm if there was a perceivable difference between the treated and untreated surfaces. Each of the 8 judges performed 4 same–different tests over 10 experimental sessions (1 for each carcass) according to the following scheme: A = control, B = LA, or AV. The differences between the samples were recorded considering modification of odor, discoloration, and surface appearance. The same–different test was conducted as follows: Each assessor was asked to analyze a pair of different square surfaces of the carcass and was asked to determine if there was a perceptible similarity or difference with the following sample sequence: <AA>, <BB>, <AB>, and <BA>, in random order. For each pair of samples, the judges were asked to answer the question if the samples were the “same” (S) or “different” (D). The same–different test was separately conducted both for LA and AV treatment versus control.
2.4. Statistical Analyses
Data were analyzed using the GLM procedure of SAS [26] to define descriptive statistics (mean and standard errors). Furthermore, the effect of treatment and time on the different microbial populations considered was determined with an ANOVA model with treatments (CTR, LA, and AV) and time (T2 and T48) as fixed variables. Post hoc Tukey tests were, therefore, used to compare the least square means, and the significance was set at p < 0.05. For the same–different test performed on the carcasses, an χ2 test was carried out [27]. A type I error of 5% with α = 0.05 was chosen. The critical value to define the similarity of the treatment was calculated with chi-squared distribution with a one-tailed test and one degree of freedom (n 1) and fixed to 3.84.
3. Results
The results of the microbial loads (MLs) are presented in Figure 2.
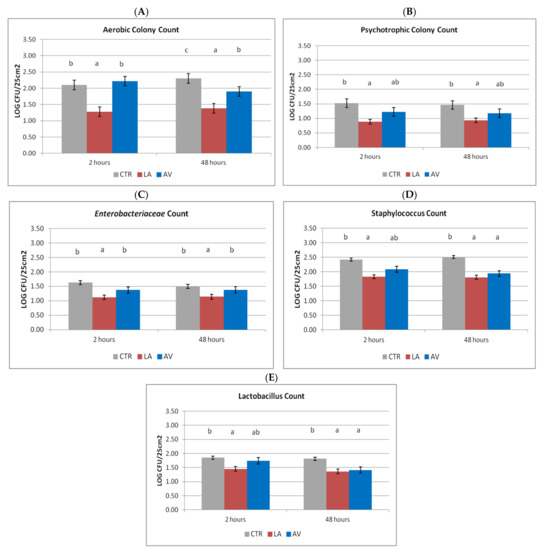
Figure 2.
Aerobic colony count (A), Enterobacteriaceae count (B), psychotrophic colony counts (C), Staphylococcus count (D), and Lactobacillus count (E) on external surfaces of wild boar carcasses sprayed with 2% Lactic acid solution (LA), aromatic vinegar (AV), and untreated (CTR) after 2 and 48 h from the treatments. Different letters on the bars describe statistically different mean values (p < 0.05).
Some samples had ML values below the LOD, in particular LA samples (Table 1).

Table 1.
Number of samples below the limit of detection for the microbial load considered in treated and untreated surfaces of wild boar carcasses at different storage times.
The ACC counts at T2 reveal lower average values for LA than the other groups, and at T48 the highest values were detected for CTR, followed by AV and LA samples. The PCC values were lower for LA than CTR in both of the times considered, without differences with AV. Regarding EC, the LA group differed from the other two groups both at T2 and T48. Similar results were detected for SC and LABC, with the main difference between CTR and LA groups at T2 and CTR and LA and AV at T48. In general, the treatment effect was always statistically significant, while the time effect was not detected. Only ACC increased in CTR samples during the storage time.
Regarding the results of the same–different test, the calculated χ2 statistic was below 3.84, indicating that no significant differences between the compared two surfaces (CTR versus AV and CTR versus LA, respectively) were recorded by assessors.
4. Discussion
The effect of lactic acid applied on the surfaces of wild boar carcasses was evident on all the microbial loads, compared with untreated carcasses. Aromatic vinegar showed less effective activity because it exerted effects only 48 h after the treatment in some of the microbial parameters tested (ACC, SC, and LABC).
The results of the activity of lactic acid solutions against the microorganisms that grow on the carcasses are in line with other authors’ findings, even if several factors of those studies differ from the present study and, therefore, only an attempt of comparison could be made: the percentage and temperature of the solution used; the application procedures of the solution on the carcasses (or on the meat); the time and site of sampling; sampling methods; the microorganism investigated; experimentally contaminated samples; and animal species [21,28,29]. Lactic acid is active against microbial loads and specific populations of both Gram-negative and Gram-positive bacteria, in vitro and on meat [30,31]. This outcome has been registered also in the present study, where the application of lactic acid solution affected both EC and SC. The effects of lactic acid on LAB highlighted in this study depend on the specific LAB population and on different factors, such as the concentration of lactic acid and the presence of other organic acids. For instance, Vermuelen et al. (2007) [32] observed higher effects of lactic acid addition to sauces on L. plantarum than on L. fructivorans, and the growth probability for both strains is related to the buffering capacity of the media after the treatment (pKa 3.8).
The reduction in the MLs between CTR and LA samples was higher than 1 Log only for ACC at T48, while it was lower than 1 Log in the other parameters and times tested (Figure 2). The high hygienic level of the carcasses detected in the present study could not allow for an evaluation of higher ML reductions. Similar effects on MLs (ACC and EC) are reported in beef carcasses by Han et al. (2020) [33] with lactic acid solution up to 4%, even though samples were collected only after 45 min. Nonetheless, there is no consensus on the microbial quantitative reduction values when lactic acid solutions are applied on carcass surfaces. A reduction of 1 Log was recorded at 24 h by Rodriguez-Melcon et al. [34] in ACC and PCC on beef carcasses using from 2% to 5% LA solution. Reduction values decreased only after 72 h. Moreover, Castiglio et al. [35] reported a higher efficiency of the treatment on beef carcasses, with a reduction in ACC of up to 3 Log CFU but using a larger amount of a 4% solution of LA (500 mL), while lower effects (mainly < 1 Log) were reported for coliforms. Residual growth inhibitions were even reported by Carpenter et al. [36] that could explain the similar level recorded for MLs at T2 and T48.
The antimicrobial activity of organic acids can be exerted by different biochemical pathways, even though the specific mechanisms are still not entirely understood [37]. Many authors indicated that organic acids tend to modify the pH of the surface to an unacceptable level for most microorganisms [28]. Some studies showed that lactic acid is more effective than other organic acids probably due to its higher acidity; in fact, it is documented that acids with lower pH values usually have higher antimicrobial efficacy [38,39]. Week organic acids have a lower pKa value than the pH of the cell cytoplasm, and when the undissociated acid enters into the cell and dissociates, with the release of protons (H+), a consequent reduction in intracellular pH value is produced [33,40]. This dissociation of the acids also produces and accumulates anions, which determine homeostatic stress and metabolic perturbation of bacteria [21,39]. Furthermore, organic acids are hydrophilic, and this trait enhances their antimicrobial activity since bacteria also tend to suspend in the water phase [41]. Another way to exert the antimicrobial action of weak acids is the promotion of oxidative stress, which changes and disrupts cell regulation and produces free radicals, leading to cell death [21,40].
Regarding AV, the effects of acetic or peroxyacetic acid solutions sprayed on beef carcasses were reported by different authors on ACC, coliforms, and some staphylococcus strains [33,41,42]. As previously reported, in the present study, the values of MLs differed between CTR and AV mainly after T48 for some of the parameters considered. However, when compared with LA at the same sampling time, the AV values were similar to LA ones only for PCC, SC, and LABC. Sallam et al. [42] recorded comparable ML values between the carcasses treated with 2% lactic acid solution and 2% acetic acid solution. The similar values of microbial growth registered at T2 and T48 for the carcasses treated with AV suggest a bacteriostatic activity of this solution, as already reported on different bacterial populations in other types of food [43]. Furthermore, the effect of AV on the microbial population depends not only on organic acids (acetic acid) but also on polyphenols [44,45]. These latter compounds could affect microbial growth by increasing the duration of the lag phase (λ, h) and decreasing the maximum growth rate (μmax, Log CFU/mL/h) values [46]. Indeed, the antimicrobial activity of polyphenols is influenced by the compounds’ structure, their concentration, and how they enter and modify the bacterial cell membrane [45,47]. The use of organic acids in decontamination strategies could represent a useful tool to reduce the level of MLs and also to inhibit several pathogens that could grow on wild boar carcasses [36].
Some authors in the literature assume that acids would have the potential to accelerate the oxidation of myoglobin and impart acidic odors or flavors to meat, but the literature abounds with disparity, and this could be attributable to the extent of variability in treatments [48]. Smulder et al. [48], in agreement with this study, reported that the decontamination of red meat carcasses with 1% to 2% of lactic or acetic acid had no impact on the sensory characteristics. The absence of sensory modification on the treated surface was also attributed to the fact that the lactic acid solution temporarily reduces the pH of the meat surface. However, due to the buffering capacity of the meat, the pH quickly returns to near-previous levels [5,49].
Taking into account the definition of CCP as “a step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level” [50], in small slaughterhouses, such as the game handling establishments considered in trials, there are still controversies on the presence of production steps that would prevent, eliminate, or reduce the likely occurrence of a biological hazard to an acceptable level [51,52]. For this reason, the use of 2% LA solution on carcass surfaces should be considered in game meat establishments, as it could represent the only real CCP in this certified chain. The validation of this process, according to EC regulations, must be performed by adopting the aerobic colony count and Enterobacteriaceae count as microbiological hygiene criteria [53]. Both of these counts were considered in this study and were reduced by treating carcasses with a 2% LA solution. Furthermore, the positive effects of LA solutions on food-borne pathogens have already been reported [54,55].
5. Conclusions
The results of the present study suggest that the adoption of LA could be considered a valuable strategy to improve the hygienic level of carcasses in hunted game meat production and could represent a suitable candidate for EU Commission approval. By contrast, AV was proven less effective than LA for carcass treatment.
The treatment with LA, as well as the other procedures adopted in the present study for pre-harvest and post-harvest phases, could lay down the basis for the definition and implementation of wild boar meat certified production chain able to ensure high quality and good hygienic standards to consumers.
Further studies could investigate their potential use on game meat food-borne pathogens in situ, as well as the effects on game meat shelf-life.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122010419/s1, Table S1: Preliminary evaluation of the aerobic colony count (ACC) and Enterobacteriaceae count (EC) in the three areas considered for sampling after skinning.
Author Contributions
Conceptualization, D.R. and F.C.; methodology, D.R. and R.R.; formal analysis, R.R., C.A., and L.C.; investigation, A.C., L.C., and C.A.; data curation, R.B.; writing—original draft preparation, R.R. and D.R.; writing—review and editing, R.B., S.P., A.V., and A.C.; supervision, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed are available from the corresponding author.
Acknowledgments
The authors would like to thank Monacelli Alessandro at Serra Brunamonti S.r.l. for his significant support in the organization of the research, the wild boar collection, and the assistance in the sampling. The study has been developed in the context of the research project: the Study of “green” strategies to ensure the hygiene and safety of prepared and processed foods of animal origin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32004R0853 (accessed on 1 September 2022).
- European Food Safety Authority. Scientific Opinion on the Evaluation of the Safety and Efficacy of Lactic Acid for the Removal of Microbial Surface Contamination of Beef Carcasses, Cuts and Trimmings. EFSA J. 2011, 9, 2317. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the evaluation of the safety and efficacy of peroxyacetic acid solutions for reduction of pathogens on poultry carcasses and meat. EFSA J. 2014, 12, 3599. [Google Scholar] [CrossRef][Green Version]
- European Food Safety Authority. Scientific Opinion on the evaluation of the safety and efficacy of lactic and acetic acids to reduce microbiological surface contamination on pork carcasses and pork cuts. EFSA J. 2018, 16, 5482. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on the evaluation of the safety and efficacy of lactic acid to reduce microbiological surface contamination on carcases from kangaroos, wild pigs, goats and sheep. EFSA J. 2022, 20, 7265. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 101/2013 of 4 February 2013 Concerning the Use of Lactic Acid to Reduce Microbiological Surface Contamination on Bovine Carcases. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32013R0101 (accessed on 1 September 2022).
- Marescotti, M.E.; Caputo, V.; Demartini, E.; Gaviglio, A. Discovering market segments for hunted wild game meat. Meat Sci. 2019, 149, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomska, K.; Kosicka-Gębska, M.; Gębski, J.; Gutkowska, K.; Jeżewska-Zychowicz, M.; Sułek, M. Game Meat Consumption—Conscious Choice or Just a Game? Foods 2020, 9, 1357. [Google Scholar] [CrossRef] [PubMed]
- Czarniecka-Skubina, E.; Stasiak, D.M.; Latoch, A.; Owczarek, T.; Hamulka, J. Consumers’ Perception and Preference for the Consumption of Wild Game Meat among Adults in Poland. Foods 2022, 11, 830. [Google Scholar] [CrossRef] [PubMed]
- Demartini, E.; Vecchiato, D.; Tempesta, T.; Gaviglio, A.; Viganò, R. Consumer preferences for red deer meat: A discrete choice analysis considering attitudes towards wild game meat and hunting. Meat Sci. 2018, 146, 168–179. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Wiklund, E. Game and venison—Meat for the modern consumer. Meat Sci. 2006, 74, 197–208. [Google Scholar] [CrossRef]
- Soriano, A.; Sánchez-García, C. Nutritional Composition of Game Meat from Wild Species Harvested in Europe. In Meat and Nutrition; Ranabhat, C.L., Ed.; IntechOpen: London, UK, 2021; pp. 77–100. [Google Scholar]
- Branciari, R.; Onofri, A.; Cambiotti, F.; Ranucci, D. Effects of Animal, Climatic, Hunting and Handling Conditions on the Hygienic Characteristics of Hunted Roe Doer (Capreolus capreolus L.). Foods 2020, 9, 1076. [Google Scholar] [CrossRef]
- Orsoni, F.; Romeo, C.; Ferrari, N.; Bardasi, L.; Merialdi, G.; Barbani, R. Factors affecting the microbiological load of Italian hunted wild boar meat (Sus scrofa). Meat Sci. 2020, 160, 107967. [Google Scholar] [CrossRef] [PubMed]
- Roila, R.; Branciari, R.; Primavilla, S.; Miraglia, D.; Vercillo, F.; Ranucci, D. Microbial, physico-chemical and sensory characteristics of salami produced from wild boar (Sus scrofa). Potravin. J. Food Sci. 2021, 15, 475–483. [Google Scholar] [CrossRef]
- Sales, J.; Kotrba, R. Meat from wild boar (Sus scrofa L.): A review. Meat Sci. 2013, 94, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Tirloni, E.; Castelli, E.; Colombo, F.; Bernardi, C. Microbiological evaluation of carcasses of wild boar hunted in a hill area of northern Italy. J. Food Prot. 2018, 81, 1519–1525. [Google Scholar] [CrossRef]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozolinš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest. Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Regione Umbria. Caccia al Cinghiale 2021. Available online: https://www.regione.umbria.it/turismo-attivita-sportive/caccia/caccia-al-cinghiale (accessed on 1 September 2022).
- Ranucci, D.; Roila, R.; Onofri, A.; Cambiotti, F.; Primavilla, S.; Miraglia, D.; Andoni, E.; Di Cerbo, A.; Branciari, R. Improving hunted wild boar carcass hygiene: Roles of different factors involved in the harvest phase. Foods 2021, 10, 1548. [Google Scholar] [CrossRef]
- Manzoor, A.; Jaspal, M.H.; Yaqub, T.; Haq, A.U.; Nasir, J.; Avais, M.; Asghar, B.; Badar, I.H.; Ahmad, S.; Yar, M.K. Effect of lactic acid spray on microbial and quality parameters of buffalo meat. Meat Sci 2020, 159, 107923. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 17604:2014; Microbiology of the Food Chain—Carcass Sampling for Microbiological Analysis. International Organization for Standardization: Geneva, Switzerland, 2014.
- International Organization for Standardization. ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- International Organization for Standardization. ISO 17410:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2001.
- International Organization for Standardization. ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2017.
- Statistical Analysis System. JMP Statistics and Graphics Guide; Version 4; SAS Institute Inc.: Cary, NC, USA, 2001. [Google Scholar]
- Kim, I.A.; Yoon, J.Y.; Lee, H.S. Measurement of consumers’ sensory discrimination and preference: Efficiency of preference-difference test utilizing the 3-point preference test precedes the same-different test. Food Sci. Biotechnol. 2015, 24, 1355–1362. [Google Scholar] [CrossRef]
- Nkosi, D.V.; Bekker, J.L.; Hoffman, L.C. The Use of Organic Acids (Lactic and Acetic) as a Microbial Decontaminant during the Slaughter of Meat Animal Species: A Review. Foods 2021, 10, 2293. [Google Scholar] [CrossRef]
- Loretz, M.; Stephan, R.; Zweifel, C. Antibacterial activity of decontamination treatments for cattle hides and beef carcasses. Food Control 2011, 22, 347–359. [Google Scholar] [CrossRef]
- King, D.A.; Lucia, L.M.; Castillo, A.; Acuff, G.R.; Harris, K.B.; Savell, J.W. Evaluation of peroxyacetic acid as a post-chilling intervention for control of Escherichia coli O157: H7 and Salmonella Typhimurium on beef carcass surfaces. Meat Sci. 2005, 69, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Saad, S.M.; Hassanin, F.S.; Salem, A.M.; Saleh, E.A.E. Efficiency of some organic acids as decontaminants in sheep carcasses. Benha Med. J. 2020, 38, 116–119. [Google Scholar] [CrossRef]
- Vermeulen, A.; Devlieghere, F.; Bernaerts, K.; Van Impe, J.; Debevere, J. Growth/no growth models describing the influence of pH, lactic and acetic acid on lactic acid bacteria developed to determine the stability of acidified sauces. Int. J. Food Microbiol. 2007, 119, 258–269. [Google Scholar] [CrossRef]
- Han, J.; Luo, X.; Zhang, Y.; Zhu, L.; Mao, Y.; Dong, P.; Yang, X.; Liang, R.; Hopkins, D.L.; Zhang, Y. Effects of spraying lactic acid and peroxyacetic acid on the bacterial decontamination and bacterial composition of beef carcasses. Meat Sci. 2020, 164, 108104. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Lactic acid concentrations that reduce microbial load yet minimally impact colour and sensory characteristics of beef. Meat Sci. 2017, 129, 169–175. [Google Scholar] [CrossRef]
- Castillo, A.; Lucia, L.M.; Mercado, I.; Acuff, G.R. In-plant evaluation of a lactic acid treatment for reduction of bacteria on chilled beef carcasses. J. Food Prot. 2001, 64, 738–740. [Google Scholar] [CrossRef]
- Carpenter, C.E.; Smith, J.V.; Broadbent, J.R. Efficacy of washing meat surfaces with 2% levulinic, acetic, or lactic acid for pathogen decontamination and residual growth inhibition. Meat Sci. 2011, 88, 256–260. [Google Scholar] [CrossRef]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. J. 2003, 82, 632–639. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.; Shah, A.J. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Hirshfield, I.N.; Terzulli, S.; O’Byrne, C. Weak organic acids: A panoply of effects on bacteria. Sci. Prog. 2003, 86, 245–270. [Google Scholar] [CrossRef]
- Boomsma, B.; Bikker, E.; Lansdaal, E.; Stuut, P. L-Lactic Acid-A Safe Antimicrobial for Home-and Personal Care Formulations. Sofw. J. 2015, 141, 2–5. [Google Scholar]
- Van Ba, H.; Seo, H.W.; Pil-Nam, S.; Kim, Y.S.; Park, B.Y.; Moon, S.S.; Kang, S.J.; Choi, Y.M.; Kim, J.H. The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Sci. 2018, 137, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Abd-Elghany, S.M.; Hussein, M.A.; Imre, K.; Morar, A.; Morshdy, A.E.; Sayed-Ahmed, M.Z. Microbial decontamination of beef carcass surfaces by lactic acid, acetic acid, and trisodium phosphate sprays. Biomed. Res. Int. 2020, 2020, 2324358. [Google Scholar] [CrossRef] [PubMed]
- Di Toro, J.; Branciari, R.; Roila, R.; Altissimi, S.; Jang, H.; Zhou, K.; Perucci, S.; Codini, M.; Ranucci, D. Eficacy of an aromatic vinegar in reducing psychrotrophic bacteria and biogenic amines in salmon fillets (Salmo salar) stored in modified atmosphere packaging. Pol. J. Food Nutr. Sci. 2019, 69, 397–405. [Google Scholar] [CrossRef]
- Bakir, S.; Devecioglu, D.; Kayacan, S.; Toydemir, G.; Korbancioglu-Guler, F.; Capanoglu, E. Investigating the antioxidant and antimicrobial activities of different vinegars. Eur. Food Res. Technol. 2017, 243, 2083–2094. [Google Scholar] [CrossRef]
- Chen, H.; Chen, T.; Giudici, P.; Chen, F. Vinegar function on health: Constituents, sources, and formation mechanisms. Compr. Rev. Food Sci. 2016, 15, 1124–1138. [Google Scholar] [CrossRef]
- Roila, R.; Valiani, A.; Ranucci, D.; Ortenzi, R.; Servili, M.; Veneziani, G.; Branciari, R. Antimicrobial efficacy of a polyphenolic extract from olive oil by-product against “Fior di latte” cheese spoilage bacteria. Int. J. Food Microbiol. 2019, 295, 49–53. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Smulders, F.J.M.; Greer, G.G. Integrating microbial decontamination with organic acids in HACCP programmes for muscle foods: Prospects and controversies. Int. J. Food Microbiol. 1998, 44, 149–169. [Google Scholar] [CrossRef]
- Grajales-Lagunes, A.; Rivera-Bautista, C.; Ruiz-Cabrera, M.; Gonzalez-Garcia, R.; Ramirez-Telles, J.; Abud-Archila, M. Effect of lactic acid on the meat quality properties and the taste of pork Serratus ventralis muscle. Agric. Food Sci. 2012, 21, 171–181. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Recommended International Code of Practice—General Principles of Food Hygiene—CAC/RCP 1-1969, Rev.4-2003; FAO: Rome, Italy, 1969. [Google Scholar]
- European Food Safety Authority. Scientific opinion on hazard analysis approaches for certain small retail establishments in view of the application of their food safety management systems. EFSA J. 2017, 15, 4697. [Google Scholar] [CrossRef][Green Version]
- Howlett, B.; Bolton, D.J.; O’Sullivan, C. Development of Pre-Requisite Programmes and HACCP Principles for Irish Beef SLAUGHTERHOUSES; Teagasc—The National Food Centre: Wexford, PA, USA, 2005. [Google Scholar]
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 1 September 2022).
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Epling, L.K.; Carpenter, J.A.; Blankenship, L.C. Prevalence of Campylobacter spp. and Salmonella spp. on pork carcasses and the reduction effected by spraying with lactic acid. J. Food Prot. 1993, 56, 536–537. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).