Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
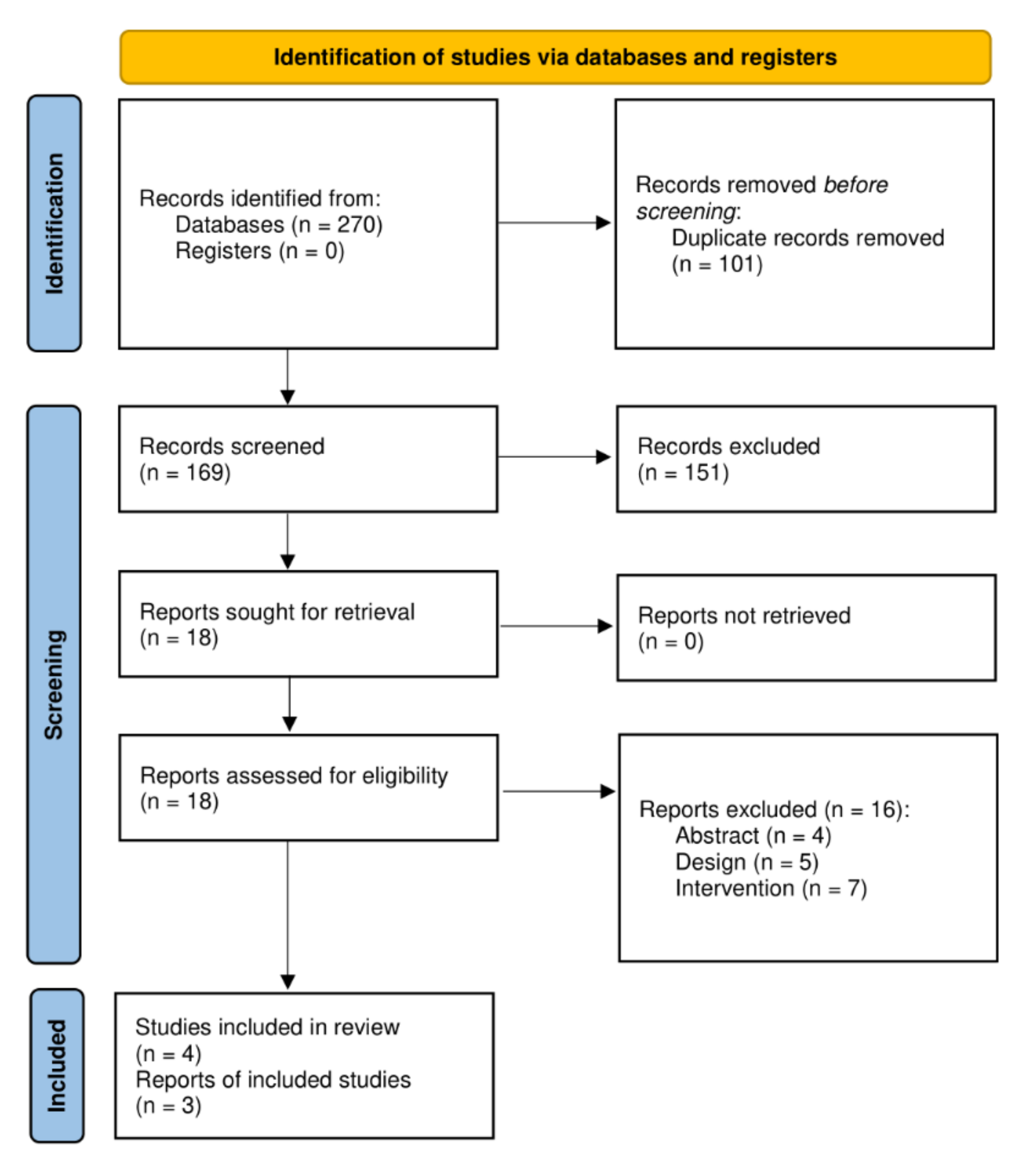
2.3. Study Selection
2.4. Data Extraction and Quality Assessment
2.5. Data Synthesis
3. Results
3.1. Study Characteristics
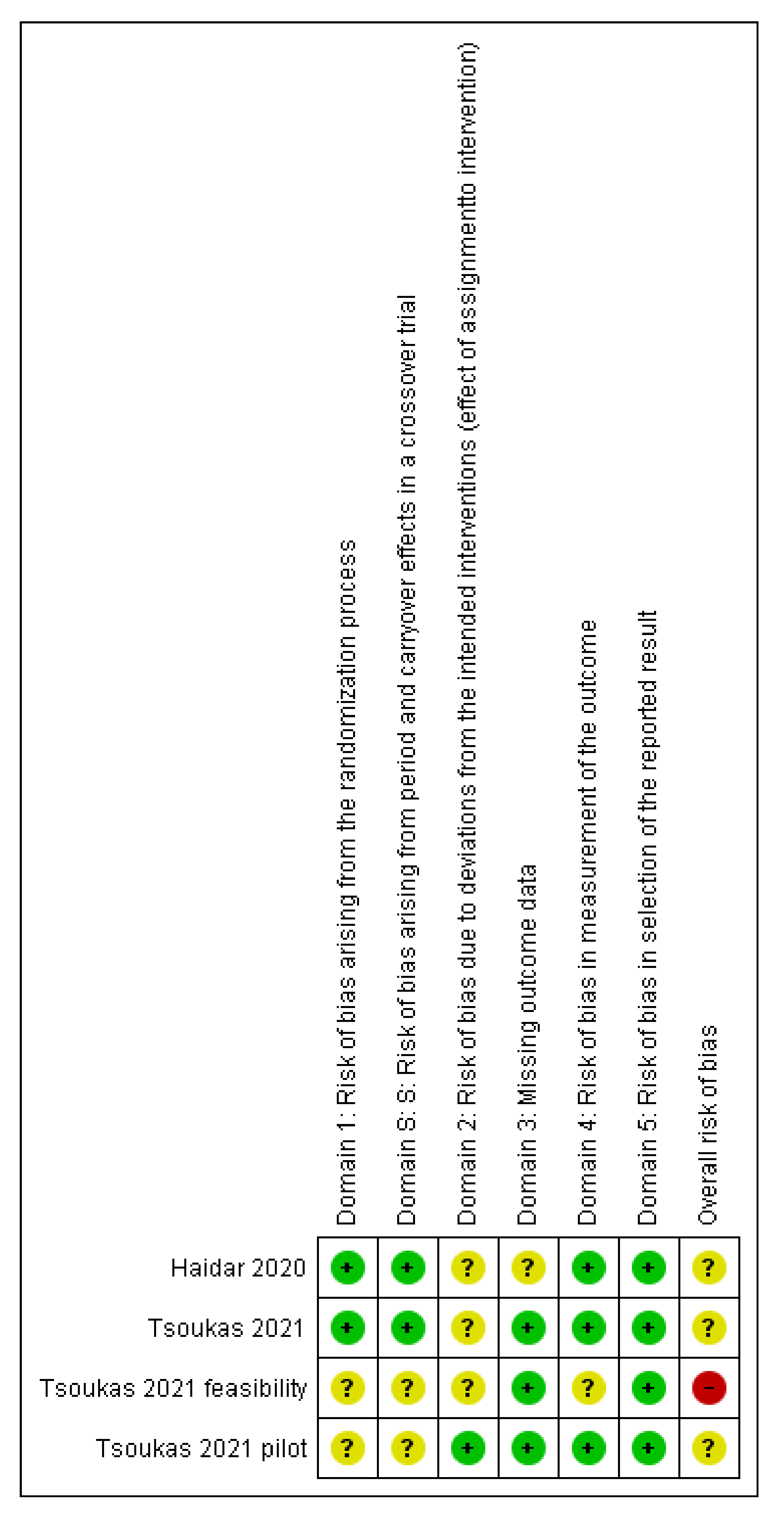
3.2. Quality Assessment
3.3. Main Results
3.3.1. Rapid Insulin-and-Pramlintide (CC) vs. Rapid Insulin-Alone (CC) vs. Regular Insulin-and-Pramlintide (CC)
Effectiveness
- Clinical results. In Haidar et al. (2020) [8], the dual system with rapid insulin was better than rapid insulin alone for time in target range (84% vs. 74%, p = 0.001), time > 180 mg/dL (12% vs. 22%, p < 0.001), average glucose (133 mg/dL vs. 144 mg/dL p = 0.001) and coefficient of variation of the glucose level (26.8% vs. 30.3%, p = 0.035). The results were only significant during the daytime period; at night, they were only significantly favorable to the intervention in terms of the standard deviation of glucose levels (36 mg/dL vs. 45 mg/dL p = 0.002). There were no significant differences in time in hypoglycemia or the number of insulin boluses. In the overnight period, the dual system with regular insulin was significantly worse than rapid insulin alone in time in target range (83% vs. 94%, p = 0.002), time < 70 mg/dL (<1% vs. <1%, p = 0.006) and >180 mg/dL (<1% vs. <1%, p = 0.013), and also for the coefficient of variation of the glucose level (24.6% vs. 15.9%, p = 0.001). During the 24 h, the dual system required more insulin boluses (25.5 vs. 22.6 units, p = 0.002) and basal insulin (27.5 vs. 23.8 units, p = 0.048).
Safety
3.3.2. FiASP-and-Pramlintide System with SMA vs. FiASP System with CC vs. FiASP System with SMA
Effectiveness
- Clinical results. The crossover feasibility trial (n = 7) and the pilot study (n = 4) by Tsoukas et al. (2021) [24] did not perform statistical contrasts, given the small sample sizes. According to the feasibility study, the dual system with SMA had a shorter time under 70 mg/dL than the FiASP with CC (2.1% vs. 4.1%), a slightly longer time in target range (84% vs. 81%) and less use of boluses (8.4 vs. 18.1). In the pilot study, the time in hypoglycemia was longer with the dual system (1.4% vs. 1.0%), which also used fewer boluses (13.0 vs. 18.4). This study also included a phase of FiASP alone with SMA, which obtained clearly worse results than the other two interventions (results not shown in the table).
- Quality of life. In the pilot study, the dual system obtained lower diabetes-related stress scores (Diabetes Distress Scale, range 1–6) than FiASP alone (1.8 vs. 2.4) but slightly worse values in fear of hypoglycemia (1.6 vs. 1.4, Hypoglycemia Fear Survey-II, range 1–5).
- Satisfaction with treatment. The dual system scored slightly worse than FiASP alone (4.1 vs. 4.3, INSPIRE questionnaire, range 1–5).
Safety
3.3.3. FiASP-and-Pramlintide System with no Meal Input (Fully Artificial Pancreas) vs. FiASP-Alone System with Precise CC (Hybrid Artificial Pancreas)
Effectiveness
- Clinical results. In the study by Tsoukas et al. (2021b) [25] (n = 28), the dual-release system was found to be non-inferior (within 6% of the time) to the hybrid system (without pramlintide) for time in target range (74.3% vs. 78.1%, p = 0.28). There were no significant differences in time in hypoglycemia and hyperglycemia or glycemic variability for the 24-h period in the superiority contrasts. For the AP system with pramlintide, results during the daytime (8:00–22:00) were significantly worse in terms of time in range (66.1% vs. 78.6%, p = 0.016), time above 180 mg/dL (32.7% vs. 20.8%, p = 0.009), and glucose level (160 vs. 150 mg/dL, p = 0.018) (data not shown in the table). The differences were not significant during the night. During the intervention phase, fewer insulin boluses were used (18.8 vs. 27.9, p = 0.010).
Safety
3.3.4. Ongoing Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Elbalshy, M.; Haszard, J.; Smith, H.; Kuroko, S.; Galland, B.; Oliver, N.; Shah, V.; de Bock, M.I.; Wheeler, B.J. Effect of divergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabet. Med. 2022, 39, e14854. [Google Scholar] [CrossRef]
- Teo, E.; Hassan, N.; Tam, W.; Koh, S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: A systematic review of randomised controlled trials and meta-analysis. Diabetologia 2022, 65, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Quispe, B.V.; Frías, M.M.; Martín, M.B.R.; Valverde, R.Y.; Gómez, M.Á.Á.; Castellanos, R.B. Effectiveness of MiniMed 640G with SmartGuard® System for prevention of hypoglycemia in pediatric patients with type 1 diabetes mellitus. Endocrinol. Diabetes Nutr. 2017, 64, 198–203. Available online: https://pubmed.ncbi.nlm.nih.gov/28417874/ (accessed on 28 December 2021). [CrossRef] [PubMed]
- American Diabetes Association. Diabetes Technology: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S77–S88. [Google Scholar] [CrossRef]
- Quintal, A.; Messier, V.; Rabasa-Lhoret, R.; Racine, E. A critical review and analysis of ethical issues associated with the artificial pancreas. Diabetes Metab. 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Farrington, C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: A review. Diabet. Med. 2018, 35, 436–449. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/dme.13567 (accessed on 22 August 2022). [CrossRef] [PubMed]
- Haidar, A.; Tsoukas, M.A.; Bernier-Twardy, S.; Yale, J.F.; Rutkowski, J.; Bossy, A.; Pytka, E.; Fathi, A.E.; Strauss, N.; Legault, L. A Novel Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care 2020, 43, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Boughton, C.K.; Hovorka, R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet. Med. 2019, 36, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Weisman, A.; Bai, J.W.; Cardinez, M.; Kramer, C.K.; Perkins, B.A. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: A systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017, 5, 501–512. Available online: https://pubmed.ncbi.nlm.nih.gov/28533136/ (accessed on 14 July 2022). [CrossRef]
- Bekiari, E.; Kitsios, K.; Thabit, H.; Tauschmann, M.; Athanasiadou, E.; Karagiannis, T.; Haidich, A.-B.; Hovorka, R.; Tsapas, A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018, 361, 1310. Available online: https://www.bmj.com/content/361/bmj.k1310 (accessed on 27 September 2022). [CrossRef]
- Moon, S.J.; Jung, I.; Park, C.Y. Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence. Diabetes Metab. J. 2021, 45, 813–839. Available online: https://pubmed.ncbi.nlm.nih.gov/34847641/ (accessed on 27 September 2022). [CrossRef] [PubMed]
- Zeng, B.; Jia, H.; Gao, L.; Yang, Q.; Yu, K.; Sun, F. Dual-hormone artificial pancreas for glucose control in type 1 diabetes: A meta-analysis. Diabetes Obes. Metab. 2022, 24, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Thabit, H.; Tauschmann, M.; Allen, J.M.; Leelarathna, L.; Hartnell, S.; Wilinska, M.E.; Acerini, C.L.; Dellweg, S.; Benesch, C.; Heinemann, L.; et al. Home Use of an Artificial Beta Cell in Type 1 Diabetes. N. Engl. J. Med. 2015, 373, 2129–2140. [Google Scholar] [PubMed]
- Bergenstal, R.M.; Garg, S.; Weinzimer, S.A.; Buckingham, B.A.; Bode, B.W.; Tamborlane, W.V.; Kaufman, F.R. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA 2016, 316, 1407–1408. [Google Scholar]
- Lawton, J.; Blackburn, M.; Rankin, D.; Allen, J.; Campbell, F.; Leelarathna, L.; Tauschmann, M.; Thabit, H.; Wilinska, M.E.; Hovorka, R.; et al. The impact of using a closed-loop system on food choices and eating practices among people with Type 1 diabetes: A qualitative study involving adults, teenagers and parents. Diabet. Med. 2019, 36, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Haidar, A.; Farid, D.; St-Yves, A.; Messier, V.; Chen, V.; Xing, D.; Brazeau, A.-S.; Duval, C.; Boulet, B.; Legault, L.; et al. Post-breakfast closed-loop glucose control is improved when accompanied with carbohydrate-matching bolus compared to weight-dependent bolus. Diabetes Metab. 2014, 40, 211–214. Available online: https://pubmed.ncbi.nlm.nih.gov/24656963/ (accessed on 28 December 2021).
- Riddle, M.C. Rediscovery of the Second β-Cell Hormone: Co-replacement With Pramlintide and Insulin in Type 1 Diabetes. Diabetes Care 2020, 43, 518–521. Available online: https://diabetesjournals.org/care/article/43/3/518/35673/Rediscovery-of-the-Second-Cell-Hormone-Co (accessed on 27 September 2022).
- P.R. Vademecum. Pramlintida. Available online: https://ar.prvademecum.com/principio-activo/pramlintida-6086/ (accessed on 20 October 2021).
- Colburn, W.A.; Gottlieb, A.B.; Koda, J.; Kolterman, O.G. Pharmacokinetics and pharmacodynamics of AC137 (25,28,29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J. Clin. Pharmacol. 1996, 36, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kolterman, O.G.; Schwartz, S.; Corder, C.; Levy, B.; Klaff, L.; Peterson, J.; Gottlieb, A. Effect of 14 days’ subcutaneous administration of the human amylin analogue, pramlintide (AC137), on an intravenous insulin challenge and response to a standard liquid meal in patients with IDDM. Diabetologia 1996, 39, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 14 July 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 4898. Available online: https://pubmed.ncbi.nlm.nih.gov/31462531/ (accessed on 28 December 2021). [CrossRef] [PubMed]
- Tsoukas, M.A.; Cohen, E.; Legault, L.; von Oettingen, J.E.; Yale, J.F.; Vallis, M.; Odabassian, M.; Fathi, A.E.; Rutkowski, J.; Jafar, A.; et al. Alleviating carbohydrate counting with a FiASP-plus-pramlintide closed-loop delivery system (artificial pancreas): Feasibility and pilot studies. Diabetes Obes. Metab. 2021, 23, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Majdpour, D.; Yale, J.F.; Fathi, A.E.; Garfield, N.; Rutkowski, J.; Rene, J.; Legault, L.; Haidar, A. A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: A single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit. Health 2021, 7500, 1–10. [Google Scholar] [CrossRef]

| 1 | Diabetes Mellitus, Type 1/ | 78,504 |
| 2 | exp Diabetic Ketoacidosis/ | 6745 |
| 3 | (diabet$ adj3 (britt$ or juvenil$ or pediatric or pediatric or child$ or early or keto$ or labil$ or acidos$ or autoimmun$ or auto immun$ or sudden onset or typ$ 1 or typ$ I)).ti,ab,hw. | 116,952 |
| 4 | (insulin depend$ or insulindepend$ or insulin-depend$).ti,ab,kw. | 29,716 |
| 5 | (IDDM or T1DM or T1D or dm1 or dm 1 or dmt1 or dm t1 or t1 dm).ti,ab,kw. | 24,116 |
| 6 | 1 or 2 or 3 or 4 or 5 | 136,479 |
| 7 | exp Diabetes Insipidus/ | 8025 |
| 8 | diabet$ insipidus.tw. | 8905 |
| 9 | 7 or 8 | 11174 |
| 10 | 6 not 9 | 135,897 |
| 11 | Pancreas, Artificial/ | 842 |
| 12 | artificial pancreas.ti,ab. | 1292 |
| 13 | 11 or 12 | 1626 |
| 14 | pramlintide.ti,ab. | 359 |
| 15 | 13 and 14 | 10 |
| 16 | ((single or dual) adj3 hormon*).ti,ab. | 1122 |
| 17 | 13 and 16 | 54 |
| Author (Year), Country | Study Design | Population | Intervention | Duration | Comparator | Outcome | Conflict of Interest |
|---|---|---|---|---|---|---|---|
| Haidar (2020) [8], Canada | RCT (crossover) | N = 24 T1DM | AP with rapid insulin-and-pramlintide | Three 24-h inpatient visits Each visit was preceded by an outpatient hormonal open-loop run-in period of 10–14 d | AP with regular insulin-and-pramlintide AP with rapid insulin alone | Time in target range (70–180 mg/dL) Time in hypoglycemia Time in hyperglycemia Mean glucose level (mg/dL) Glucose variability Insulin units | The authors received research support/consulting fees from different pharmaceutical industries. |
| Tsoukas (2021) [25], Canada | RCT (crossover) | N = 24 T1DM | AP with FiASP-and-pramlintide | Two 27-h inpatient visits | AP with FiASP alone | Time in target range (70–180 mg/dL) Time in hypoglycemia Time in hyperglycemia Mean glucose level (mg/dL) Glucose variability Insulin units | The authors received research support/consulting fees from medical industries (Lily, Eli, Adocia, Aga Matrix, Novo Nordisk, Boeringher Ingelheim, Janssen and AstraZeneca) and insulin pumps, glucose sensors, and monitors from Dexcom, Tandem, and Medtronic. |
| Tsoukas (2021) [24], Canada | Feasibility study | N = 7 (4 adults and 3 adolescents) T1DM | AP with FiASP-and-pramlintide + SMA | 24h inpatient visits | AP with FiASP + FCC | Gastrointestinal symptoms | The authors received research support/consulting fees from different pharmaceutical industries (Eli Lilly, Novo Nordisk, Boehringer Ingelheim, Janssen and AstraZeneca) and received consulting fees from Dexcom and Insulet. One author has pending patents in the artificial pancreas area. |
| Tsoukas (2021) [24], Canada | Pilot study | N = 4 T1DM | AP with FiASP-and-pramlintide + SMA | 12 d | AP with FiASP-and-placebo + FCC AP with FiASP-and-placebo + SMA | Time in target range (70–180 mg/dL) Time in hypoglycemia Time in hyperglycemia Mean glucose level (mg/dL) Glucose variability Insulin units DDS HFS-w INSPIRE DBSQ |
| Time in Target Range (70–180 mg/dL) | Time in Hypoglycemia | Time in Hyperglycemia | Mean Glucose Level (mg/dL) | Glucose Variability | Insulin Units | |
|---|---|---|---|---|---|---|
| Haidar, (2020) [8] | RAI + PR (CC) vs. RAI (CC) 84% vs. 74% p = 0.001 | RAI + PR (CC) vs. RAI (CC) <70 mg/dL 0% vs. 1.2% p = 0.43 <60 mg/dL 0% vs. 0% p = 0.78 | RAI + PR (CC) vs. RAI (CC) >180 mg/dL 12% vs. 22% p <0.001 >250 mg/dL 0% vs. 0% p = 0.002 | RAI + PR (CC) vs. RAI (CC) 133 vs. 144 p = 0.001 | RAI + PR (CC) vs. RAI (CC) SD (mg/dL) 36 vs. 45 p = 0.002 VC% 25.6 vs. 29.3 p = 0.017 | RAI + PR (CC) vs. RAI (CC) Total basal 15.3 vs. 15.3 p = 0.79 Total boluses 22.6 vs. 23.1 p = 0.35 |
| REGI + PR (CC) vs. RAI (CC) 69% vs. 74% p = 0.22 | REGI + PR (CC) vs. RAI (CC) <70 mg/dL 7.3% vs. 1.2% p = 0.008 <60 mg/dL 1.2% vs. 0% p = 0.027 | REGI + PR (CC) vs. RAI (CC) >180 mg/dL 24% vs. 22% p = 0.49 >250 mg/dL 1% vs. 0% p = 0.37 | REGI + PR (CC) vs. RAI (CC) 144 vs. 144 p = 0.95 | REGI + PR (CC) vs. RAI (CC) (mg/dL) 45 vs. 45 p = 0.81 VC% 28.7 vs. 29.3 p = 1.00 | REGI + PR (CC) vs. RAI (CC) Total basal 19.4 vs. 15.3 p = 0.016 Total boluses 25.5 vs. 23.1 p = 0.002 | |
| Tsoukas, (2021) [24] | FiASP + PR (SMA) vs. FiASP (CC) Feasibility study 84% vs. 81% Pilot study 70% vs. 70% | FiASP + PR (SMA) vs. FiASP (CC) <70 mg/dL Feasibility study 2.1% vs. 4.1% Pilot study 1.4% vs. 1.1% | FiASP + PR (SMA) vs. FiASP (CC) >180 mg/dL Feasibility study 14% vs. 13% Pilot study 28% vs. 28% | FiASP + PR (SMA) vs. FiASP (CC) Feasibility study 135 vs. 131 Pilot study 158 vs. 157 | FiASP + PR (SMA) vs. FiASP (CC) SD (mg/dL) Feasibility study 43 vs. 40 Pilot study 58 vs. 54 VC% Feasibility study 31.1 vs. 29.7 Pilot study 35.5 vs. 33.9 | FiASP + PR (SMA) vs. FiASP (CC) Total basal Feasibility study 28.7 vs. 27.4 Pilot study 33.0 vs. 30.9 Total boluses Feasibility study 8.4 vs. 18.1 Pilot study 13.0 vs. 18.4 |
| Tsoukas, (2021b) [25] | FiASP + PR (CLS) vs. FiASP (CC) 74.3% vs. 78.1% p = 0.28 (Non-inferiority contrast) | FiASP + PR (CLS) vs. FiASP (CC) <70 mg/dL 0% vs. 1.8% p = 0.058 >60 mg/dL 0% vs. 0% p = 0.32 | FiASP + PR (CLS) vs. FiASP (CC) >180 mg/dL 24.3% vs. 19.8% p = 0.093 >234 mg/dL 2.4% vs. 0.4% p = 0.56 | FiASP + PR (CLS) vs. FiASP (CC) 148 vs. 142 p = 0.060 | FiASP + PR (CLS) vs. FiASP (CC) SD (mg/dL) 49 vs. 45 p = 0.44 VC% 31.4 vs. 33.1 p = 0.95 | FiASP + PR (CLS) vs. FiASP (CC) Total basal 30.5 vs. 30.7 p = 0.42 Total boluses 18.8 vs. 27.9 p = 0.010 |
| Adverse Events | |
|---|---|
| Haidar, (2020) [8] | RAI: 11 hypoglycemic episodes (HE) requiring oral treatment (1 HE every 2.5 d); gastrointestinal symptoms (GIS) 0% after (0 of 112) meals). RAI + PR: 12 HE (1HE every 2.3 d); GIS: 6% (6 out of 108 meals); 3 mild and 3 moderate, all were transient. REGI + PR: 18 HE (1 HE every 1.4 d); GIS: 11% (11 of 104 meals); 2 mild, 6 moderate and 3 moderate to severe. There were no elevated ketones (>18 mg/dl) in any administration of the artificial pancreas; non-inferiority contrast |
| Tsoukas, (2021b) [25] | 8 participants (33%) had at least one hypoglycemia event (<60 mg/dL) with the closed artificial pancreas vs. 14 (58%) participants with the hybrid system; 3 participants (13%) reported non-mild nausea and 1 participant (4%) with non-mild bloating with the artificial pancreas closed; no participant presented these events in the hybrid system. |
| Tsoukas, (2021) [24] | Feasibility study: RAI + PR with SMA: 3 participants experienced gastrointestinal symptoms. Pilot study: Frequency and severity of gastrointestinal symptoms measured with a Likert scale from 1 to 6: FIASP + placebo (CC) Frequency: 1.1 ± 0.4 Severity: 1.0 ± 0.2 FIASP + PR (SMA): Frequency: 1.3 ± 0.7 Severity: 1.3 ± 0.6 1 participant reported moderate abdominal pain FIASP + placebo con (SMA): Frequency: 1.3 ± 0.5 Severity: 1.3 ± 0.6 1 participant reported moderate abdominal pain and moderate nausea. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Castaño, A.; Rivero-Santana, A.; Perestelo-Pérez, L.; Duarte-Díaz, A.; Abt-Sacks, A.; Ramos-García, V.; Álvarez-Pérez, Y.; Wäagner, A.M.; Rigla, M.; Serrano-Aguilar, P. Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review. Appl. Sci. 2022, 12, 10262. https://doi.org/10.3390/app122010262
Torres-Castaño A, Rivero-Santana A, Perestelo-Pérez L, Duarte-Díaz A, Abt-Sacks A, Ramos-García V, Álvarez-Pérez Y, Wäagner AM, Rigla M, Serrano-Aguilar P. Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review. Applied Sciences. 2022; 12(20):10262. https://doi.org/10.3390/app122010262
Chicago/Turabian StyleTorres-Castaño, Alezandra, Amado Rivero-Santana, Lilisbeth Perestelo-Pérez, Andrea Duarte-Díaz, Analia Abt-Sacks, Vanesa Ramos-García, Yolanda Álvarez-Pérez, Ana M. Wäagner, Mercedes Rigla, and Pedro Serrano-Aguilar. 2022. "Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review" Applied Sciences 12, no. 20: 10262. https://doi.org/10.3390/app122010262
APA StyleTorres-Castaño, A., Rivero-Santana, A., Perestelo-Pérez, L., Duarte-Díaz, A., Abt-Sacks, A., Ramos-García, V., Álvarez-Pérez, Y., Wäagner, A. M., Rigla, M., & Serrano-Aguilar, P. (2022). Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Systematic Review. Applied Sciences, 12(20), 10262. https://doi.org/10.3390/app122010262