Abstract
The artificial pancreas (AP) is equipped with a glucose monitoring sensor, an insulin pump and an integrated mathematical algorithm that determines insulin infusion based on the glucose levels detected by the sensor. Research has shown that AP can help patients with type-1 Diabetes Mellitus (T1DM) to improve the control of their glucose levels, but the occurrence of postprandial hyperglycemia is still considerable. The addition of pramlintide (a synthetic derivative analog of amylin) in a dual-hormone AP could improve postprandial glycemic control. This systematic review aims to evaluate and synthesize the evidence on the safety, efficacy and cost-effectiveness of the dual insulin- and pramlintide-releasing AP. The electronic databases MEDLINE, Embase, Web of Science and ClinicalTrials.gov were consulted up to 6 June 2021. We identified four small crossover studies (n = 59) and two ongoing crossover trials, all of them carried out by the same research group. The four studies observed more gastrointestinal adverse effects with the dual system. One study found that the dual system improved outcomes compared to insulin alone, with precise carbohydrate counting (CC) in both groups. Another study showed that a fully closed-loop system (without CC) was equivalent to an insulin-alone AP (with CC) on time in the target range but performed worse in hyperglycemia during the daytime. These preliminary results suggest that the control of postprandial hyperglycemia remains a challenge.
1. Introduction
Successful management of type 1 Diabetes Mellitus (T1DM) requires that patients learn to plan daily activities such as physical exercise and diet and to measure their glucose levels regularly in order to avoid acute and long-term complications, which produce a high burden on patients’ quality of life [1]. Monitoring devices and insulin pumps developed in the last decades with the functionality to continuously measure interstitial glucose and adjust insulin infusion are helpful in improving glucose control clinical outcomes [2,3].
The artificial pancreas (AP) represents the most recent evolution of these devices, aimed at simplifying and improving care for patients with T1DM. The AP combines a glucose monitoring sensor (which is attached to the arm or abdomen and measures interstitial glucose concentrations), a transmitter that sends these data to an insulin pump and an integrated mathematical algorithm mounted on smartphones or tablets that processes and models continuous glucose monitoring (CGM) data to provide the appropriate insulin infusion based on the glucose levels detected by the sensor. In addition to suspending insulin delivery when glucose levels fall below a predetermined threshold, as in previous devices that integrated CGM and insulin pumps (i.e., sensor-augmented pump) [2,4], the AP also increases insulin dosing when hyperglycemic levels are reached to mimic the functioning of the biological pancreas [5]. Single-hormone versions only deliver insulin, whereas dual-hormone versions also include the infusion of glucagon (requiring an additional catheter and infusion pump) [6,7,8,9].
Research has shown that AP increases time in the glucose target range and reduces time in hypo- and hyperglycemic levels compared to insulin pump treatment or sensor-augmented pumps [10,11,12], although the quality of this evidence is limited due to the short follow-up of studies carried out to date. Compared to single-hormone AP, dual-hormone systems have shown a slightly lower time in hypoglycemia, but no differences in the time in the target range and more gastrointestinal symptoms [13]
Most versions of currently developed APs are not fully automated closed-loop systems; they still require the patient to provide information on physical activities and meals in order to adjust the insulin infusion (hybrid systems). Despite the benefits mentioned above, studies show that significant durations of hyperglycemia (5–8 h/day above 10 mmol/L [180 mg/dL]) are still reported, particularly after meals [14,15]. Therefore, manual adjustments and carbohydrate counting (CC) are still required to calculate insulin doses for each meal. The need for accurate CC can be a barrier for patients, making them feel restrained or anxious or influencing dietary choices in favor or pre-packaged processed foods [16]. An alternative to CC in the use of AP is the “simple meal announcement” (SMA), whereby the system delivers partial boluses of insulin regardless of the carbohydrate content to be ingested. However, this has resulted in higher levels of postprandial hyperglycemia compared to CC [17].
Pramlintide is a synthetic derivative analog of amylin, a hormone released by the beta cells of the pancreas that is co-secreted with insulin after a meal in healthy individuals but is deficient in people with T1DM [8]. Three effects of this hormone are highly relevant to the treatment of diabetes: (1) modulation of gastric emptying, which can be abnormally rapid in diabetes; (2) suppression of glucagon, which is excessively secreted in diabetes, especially after meals; (3) development of satiety shortly after starting to eat. Together with insulin, these effects limit post-meal hyperglycemia and prevent calorie intake [18]. Pramlintide is injected with meals because it lowers postprandial glycemia, allowing patients to reduce insulin doses [19,20,21].
The use of an AP with dual infusion of insulin and pramlintide could represent a helpful resource in the control of postprandial blood glucose levels in complex diabetic patients. This systematic review, therefore, aims to explore the available evidence about the effectiveness and safety of the use of an AP with dual release of insulin and pramlintide for the treatment of people with type 1 diabetes.
2. Materials and Methods
A systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [22]. The detail of the PRISMA checklist can be found in Supplementary Table S1. The systematic review was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number CRD42022290673.
2.1. Search Strategy
First, a preliminary manual search was carried out to locate possible health technology assessment (HTA) reports and/or previous systematic reviews on the subject that could provide background information. Secondly, Medline (Ovid SP), Embase (Elsevier) and Web of Science (Clarivate Analytics) were searched for potentially eligible articles published up to 6 June 2021. An overall search strategy was developed using subject headings and free text terms and then adapted for each database to ensure sensitivity. As an example, the MEDLINE search strategy is shown in Table 1. Search strategies for the other two electronic databases are available in Supplementary Table S2. No language or publication year restrictions were applied to limit the search strategy. In addition, a manual search was performed at the ClinicalTrials.gov website in October 2021 for a complete identification of ongoing studies. Details are also available in Supplementary Table S3.

Table 1.
Medline search strategy.
2.2. Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: (1) participants of all ages with Type 1 Diabetes Mellitus, (2) treated with dual-hormone insulin-and-pramlintide AP; (3) insulin-alone AP as control; and (4) designed as systematic reviews, randomized controlled trials (RCT), non-randomized controlled trials (NRCT) or observational studies (both prospective and retrospective). Studies with the following characteristics were excluded: (1) disease other than type 1 diabetes, (2) diabetes management devices other than AP, and APs that did not have dual release of insulin and pramlintide. (3) other outcome measures not related to the effectiveness and safety of the AP, (4) designed as narrative reviews, editorials, letters to the editor, opinions, qualitative studies, or conference abstracts.
2.3. Study Selection
The citations retrieved from the electronic databases were imported into a standardized Microsoft Excel data sheet and duplicates were removed. First, all titles and abstracts were screened in order to pre-select those meeting the inclusion criteria. Full texts for all the potentially relevant articles were retrieved. Then, the full texts of these studies were analyzed in depth. The study selection process was conducted independently by two authors, and any disagreement was solved through discussion and consensus or through consultations with a third reviewer if disagreement persisted. The bibliographic references were stored using the Reference Manager Version 10® (Thomson Scientific, Philadelphia, PA, USA).
2.4. Data Extraction and Quality Assessment
The following items were extracted from all included studies using a pre-specified data extraction form in Microsoft Excel: first author, year of publication, participants’ characteristics, intervention and comparator details, outcome measures and conflict of interest. Data extraction was performed by one reviewer and checked by another, and the possible discrepancies were resolved through discussion. The risk of bias in the included studies was assessed using version 2 of the Cochrane risk-of-bias tool for randomized trials (ROB-2) [23]. Quality assessment was undertaken by two independent reviewers, and disagreements were solved by discussion and consensus or after consulting a third reviewer.
2.5. Data Synthesis
A narrative synthesis of the results of each individual study was conducted.
3. Results
In total, 270 publications were identified from the literature search, including 57 from Medline, 112 from EMBASE, and 101 from Web of Science; 101 of these were duplicates and were removed. Hence, 169 unique articles were identified, and after reviewing titles and abstracts, 18 were selected for full-text review. Three of these articles met the inclusion criteria and were finally included in this review. Figure 1 shows the PRISMA flowchart of the study selection process.
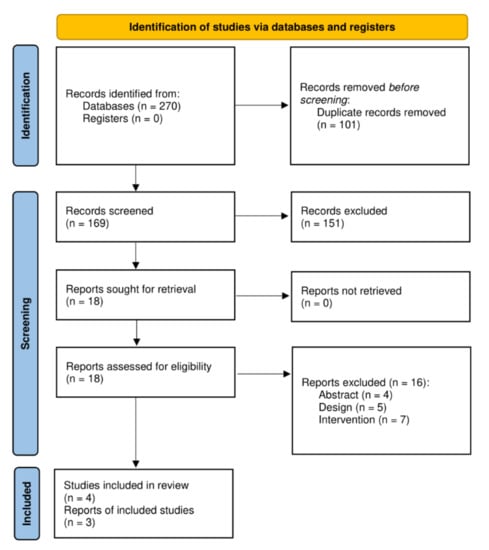
Figure 1.
PRISMA flowchart of the study selection process.
3.1. Study Characteristics
The characteristics of the four included studies are shown in Table 2. All of them were crossover RCTs conducted by the same research group. Haidar et al. (2020) [8] (n = 24) compared an AP with rapid insulin and pramlintide, AP with regular insulin and pramlintide, and AP with rapid insulin alone (in all cases with CC) over one hospital day. Tsoukas et al. (2021) [24] included two small studies. The first was a feasibility study (n = 7) comparing a dual system with Faster acting insulin Aspart (FiASP) and pramlintide (with SMA) versus FiASP alone (with CC), also over one hospital day. The second was a pilot study (n = 7) that compared the same interventions as in the feasibility study over three 12-day phases. However, the FiASP alone phase also included a placebo, and there was a third phase of FiASP with placebo and SMA. Finally, Tsoukas et al. (2021b) [25] (n = 28) compared FiASP and pramlintide during one day of hospitalization with FiASP alone with CC.

Table 2.
Characteristics of the included studies.
All four studies included a washout period between interventions of 14–45 days. In the study by Haidar et al. (2020) [8], pramlintide was administered at a fixed rate of 6 μg/insulin unit, whereas 10 μg/insulin unit was used in the remaining studies (since no CC was performed in these studies, the bolus was smaller, and therefore, the ratio was increased to administer an amount similar to pramlintide at meals). Pramlintide was administered by means of an independent infusion pump to that of insulin.
3.2. Quality Assessment
An assessment of the risk of bias of these studies was undertaken using the Cochrane Risk of Bias (RoB-2) tool [23], which is shown in detail in Supplementary Table S4. Overall, four studies were rated at unclear risk of bias. The reason for this is related to lack of blinding, both for participants and for those administering the intervention, and lack of information on protocol deviations. Furthermore, missing data exceeded 5% of the total. For the feasibility study by Tsoukas et al. [24], the main concerns were a lack of information about the randomization procedure and the number of participants in each intervention. Figure 2 shows the risk of bias summary. Figure 3 shows each reviewer’s assessment in relation to each item presented as percentages across all included studies.

Figure 2.
Risk of bias summary [8,24].
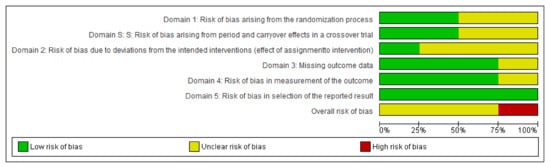
Figure 3.
Authors’ judgments about each item.
3.3. Main Results
3.3.1. Rapid Insulin-and-Pramlintide (CC) vs. Rapid Insulin-Alone (CC) vs. Regular Insulin-and-Pramlintide (CC)
Effectiveness
- Clinical results. In Haidar et al. (2020) [8], the dual system with rapid insulin was better than rapid insulin alone for time in target range (84% vs. 74%, p = 0.001), time > 180 mg/dL (12% vs. 22%, p < 0.001), average glucose (133 mg/dL vs. 144 mg/dL p = 0.001) and coefficient of variation of the glucose level (26.8% vs. 30.3%, p = 0.035). The results were only significant during the daytime period; at night, they were only significantly favorable to the intervention in terms of the standard deviation of glucose levels (36 mg/dL vs. 45 mg/dL p = 0.002). There were no significant differences in time in hypoglycemia or the number of insulin boluses. In the overnight period, the dual system with regular insulin was significantly worse than rapid insulin alone in time in target range (83% vs. 94%, p = 0.002), time < 70 mg/dL (<1% vs. <1%, p = 0.006) and >180 mg/dL (<1% vs. <1%, p = 0.013), and also for the coefficient of variation of the glucose level (24.6% vs. 15.9%, p = 0.001). During the 24 h, the dual system required more insulin boluses (25.5 vs. 22.6 units, p = 0.002) and basal insulin (27.5 vs. 23.8 units, p = 0.048).
Table 3 shows the effectiveness of the included studies.

Table 3.
Effectiveness results among the included studies.
Safety
With the dual system with rapid insulin and with insulin alone, the rate of patients with at least one hypoglycemic event (<60 mg/dL) that required treatment was similar (30% vs. 32%, respectively; no statistical contrasts were performed), as well as the number of events (12 vs. 11). In the dual system with regular insulin, the results were poorer (50% of patients had 18 events). During the phase of rapid insulin alone, no patient suffered gastrointestinal adverse events, but with the dual system with rapid insulin, there were 7% mild and 11% moderate events. In the dual system phase with regular insulin, 4% had mild gastrointestinal symptoms, 12% had moderate symptoms, and 8% had moderate-severe symptoms.
3.3.2. FiASP-and-Pramlintide System with SMA vs. FiASP System with CC vs. FiASP System with SMA
Effectiveness
- Clinical results. The crossover feasibility trial (n = 7) and the pilot study (n = 4) by Tsoukas et al. (2021) [24] did not perform statistical contrasts, given the small sample sizes. According to the feasibility study, the dual system with SMA had a shorter time under 70 mg/dL than the FiASP with CC (2.1% vs. 4.1%), a slightly longer time in target range (84% vs. 81%) and less use of boluses (8.4 vs. 18.1). In the pilot study, the time in hypoglycemia was longer with the dual system (1.4% vs. 1.0%), which also used fewer boluses (13.0 vs. 18.4). This study also included a phase of FiASP alone with SMA, which obtained clearly worse results than the other two interventions (results not shown in the table).
- Quality of life. In the pilot study, the dual system obtained lower diabetes-related stress scores (Diabetes Distress Scale, range 1–6) than FiASP alone (1.8 vs. 2.4) but slightly worse values in fear of hypoglycemia (1.6 vs. 1.4, Hypoglycemia Fear Survey-II, range 1–5).
- Satisfaction with treatment. The dual system scored slightly worse than FiASP alone (4.1 vs. 4.3, INSPIRE questionnaire, range 1–5).
Safety
During the FiASP-alone phase of the feasibility study, there were no complaints of gastrointestinal symptoms after any meal, whereas with the dual system, mild symptoms occurred in 12.9% of meals, moderate symptoms in 6.5%, and moderate-severe symptoms in 6.5% (none serious). In the pilot study, the phase with the dual system had slightly higher scores in frequencies (1.3 vs. 1.1) and severity (1.3 vs. 1.0) on the Diabetes Bowel Symptom Questionnaire (range 1–5). During the pramlintide phase, two patients showed skin irritation in the area of the infusion of this hormone, and another patient showed lipodystrophy in the area of the infusion of both hormones.
3.3.3. FiASP-and-Pramlintide System with no Meal Input (Fully Artificial Pancreas) vs. FiASP-Alone System with Precise CC (Hybrid Artificial Pancreas)
Effectiveness
- Clinical results. In the study by Tsoukas et al. (2021b) [25] (n = 28), the dual-release system was found to be non-inferior (within 6% of the time) to the hybrid system (without pramlintide) for time in target range (74.3% vs. 78.1%, p = 0.28). There were no significant differences in time in hypoglycemia and hyperglycemia or glycemic variability for the 24-h period in the superiority contrasts. For the AP system with pramlintide, results during the daytime (8:00–22:00) were significantly worse in terms of time in range (66.1% vs. 78.6%, p = 0.016), time above 180 mg/dL (32.7% vs. 20.8%, p = 0.009), and glucose level (160 vs. 150 mg/dL, p = 0.018) (data not shown in the table). The differences were not significant during the night. During the intervention phase, fewer insulin boluses were used (18.8 vs. 27.9, p = 0.010).
Safety
In the intervention phase, fewer participants experienced hypoglycemia (<60 mg/dL), although this difference was not significant (33% vs. 58%, p = 0.15), and similar results were found for hypoglycemia that required carbohydrate intake (11 vs. 21 events, no statistical comparison was made). There were more participants in the intervention phase who presented with some intestinal symptoms (29% versus 8%, no statistical comparison was made), one of them severe (nausea). There were no serious adverse effects or ketosis. One participant in the intervention phase showed skin irritation at the pramlintide insertion site. Table 4 shows the adverse effects observed among the included studies.

Table 4.
Adverse events observed among the included studies.
3.3.4. Ongoing Trials
We found two ongoing small crossover trials carried out by the same research group, with estimated completion dates of August (NCT05199714) and November 2022 (NCT04243629). They are described in Supplementary Table S3.
4. Discussion
People with T1DM require lifelong glucose monitoring and insulin replacement therapy, and several technological devices have been developed over the last decades to help them in the self-management of this disease, such as insulin pumps or continuous glucose monitoring devices [1,12]. The AP is the latest evolution of these technologies, with the main aim of enabling a fully closed-loop system to provide a completely automatized insulin administration, mimicking the biological pancreas [12]. However, current closed-loop systems still require that users enter the carbohydrate content of upcoming meals to determine the adequate dosing of pre-prandial insulin [6]. The incorporation of pramlintide within the AP system could help to delay mealtime insulin requirements by delaying gastric emptying, suppressing nutrient-stimulated glucagon secretion and increasing [18]. This may be of particular interest to those patients who do not achieve the expected clinical results with conventional insulin therapy.
The results of this review show that the scientific evidence on the efficacy and safety of this device is still very scarce, consisting of four small studies carried out in a 24-h inpatient setting (except one of them, with an outpatient follow up of 12 days but only 4 patients) by the same research group. Haidar et al. (2020) [8] found that the dual system outperformed the insulin-alone system when both were applied along CC, while the results of Tsoukas et al. (2021b) [25] showed that the fully closed-loop dual system (without CC or SMA), although showed non-inferiority in time in therapeutic glucose range compared to the insulin-alone system with CC, obtained significantly worse outcomes during the daytime (relative increase of 57% of time above 180 mg/dL). When the dual system was accompanied by SMA in the two studies of Tsoukas et al. (2021) [24], results were very similar to that obtained by the insulin-alone system with CC.
Regarding safety, the three studies showed gastrointestinal adverse effects associated with the dual system, although they were non-serious in all cases. Previous studies about pramlintide administration have shown that nausea, the most common of these effects, is transient and dissipates within days to weeks.
In summary, the identified evidence is very limited, carried out by the same research group, with a total of 59 patients studied in a 24-h inpatient intervention (except for four patients). The review highlights the still-emerging nature of this technology and the need for additional technological research and development before conducting more externally valid outpatient studies, including pump chamber redesign and improvements in meal detection algorithms. Future studies should compare different pramlintide-to-insulin ratios, as well as develop optimal co-formulations of insulin and pramlintide, allowing the infusion of both hormones through the same conventional single-chamber insulin pump. Efficacy trials with longer follow-ups in outpatient settings are warranted, as well as the evaluation of the safety profile of the device. While the aim of AP is to improve glycemic control and reduce the need for decision-making regarding insulin infusion, the results show that control of postprandial hyperglycemia remains a challenge.
5. Conclusions
The current evidence on the effectiveness of a dual-hormone AP with insulin and pramlintide is very scarce and preliminary. The results of 24-h inpatient interventions suggest that it could outperform the single-hormone system when it is accompanied by an accurate CC before meals, but it could increase hyperglycemia during the daytime when no CC or SMA is made. Further, it could increase the occurrence of gastrointestinal adverse effects. Research in a real-world setting with longer follow-ups is warranted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app122010262/s1, Table S1: PRISMA check-list, Table S2: Search strategy, Table S3: Ongoing studies, Table S4: Risk of bias of crossover RCTs.
Author Contributions
Conceptualization, supervision, writing: A.T.-C.; original draft preparation, —reviewing and editing, A.D.-D.; formal analysis, data curation: A.R.-S.; conceptualization, writing—reviewing and editing: L.P.-P., A.A.-S., V.R.-G., Y.Á.-P.; conceptualization, writing—reviewing and editing: A.M.W., M.R., P.S.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by the Spanish Ministry of Health in the framework of activities developed by the Spanish Network of Agencies for Health Technology Assessment for the National Health Service.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank Carlos Gonzalez Rodriguez and Leticia Rodriguez Rodriguez for their support as documentalists.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Elbalshy, M.; Haszard, J.; Smith, H.; Kuroko, S.; Galland, B.; Oliver, N.; Shah, V.; de Bock, M.I.; Wheeler, B.J. Effect of divergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabet. Med. 2022, 39, e14854. [Google Scholar] [CrossRef]
- Teo, E.; Hassan, N.; Tam, W.; Koh, S. Effectiveness of continuous glucose monitoring in maintaining glycaemic control among people with type 1 diabetes mellitus: A systematic review of randomised controlled trials and meta-analysis. Diabetologia 2022, 65, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Quispe, B.V.; Frías, M.M.; Martín, M.B.R.; Valverde, R.Y.; Gómez, M.Á.Á.; Castellanos, R.B. Effectiveness of MiniMed 640G with SmartGuard® System for prevention of hypoglycemia in pediatric patients with type 1 diabetes mellitus. Endocrinol. Diabetes Nutr. 2017, 64, 198–203. Available online: https://pubmed.ncbi.nlm.nih.gov/28417874/ (accessed on 28 December 2021). [CrossRef] [PubMed]
- American Diabetes Association. Diabetes Technology: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S77–S88. [Google Scholar] [CrossRef]
- Quintal, A.; Messier, V.; Rabasa-Lhoret, R.; Racine, E. A critical review and analysis of ethical issues associated with the artificial pancreas. Diabetes Metab. 2019, 45, 1–10. [Google Scholar] [CrossRef]
- Farrington, C. Psychosocial impacts of hybrid closed-loop systems in the management of diabetes: A review. Diabet. Med. 2018, 35, 436–449. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/dme.13567 (accessed on 22 August 2022). [CrossRef] [PubMed]
- Haidar, A.; Tsoukas, M.A.; Bernier-Twardy, S.; Yale, J.F.; Rutkowski, J.; Bossy, A.; Pytka, E.; Fathi, A.E.; Strauss, N.; Legault, L. A Novel Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care 2020, 43, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Boughton, C.K.; Hovorka, R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet. Med. 2019, 36, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Weisman, A.; Bai, J.W.; Cardinez, M.; Kramer, C.K.; Perkins, B.A. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: A systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017, 5, 501–512. Available online: https://pubmed.ncbi.nlm.nih.gov/28533136/ (accessed on 14 July 2022). [CrossRef]
- Bekiari, E.; Kitsios, K.; Thabit, H.; Tauschmann, M.; Athanasiadou, E.; Karagiannis, T.; Haidich, A.-B.; Hovorka, R.; Tsapas, A. Artificial pancreas treatment for outpatients with type 1 diabetes: Systematic review and meta-analysis. BMJ 2018, 361, 1310. Available online: https://www.bmj.com/content/361/bmj.k1310 (accessed on 27 September 2022). [CrossRef]
- Moon, S.J.; Jung, I.; Park, C.Y. Current Advances of Artificial Pancreas Systems: A Comprehensive Review of the Clinical Evidence. Diabetes Metab. J. 2021, 45, 813–839. Available online: https://pubmed.ncbi.nlm.nih.gov/34847641/ (accessed on 27 September 2022). [CrossRef] [PubMed]
- Zeng, B.; Jia, H.; Gao, L.; Yang, Q.; Yu, K.; Sun, F. Dual-hormone artificial pancreas for glucose control in type 1 diabetes: A meta-analysis. Diabetes Obes. Metab. 2022, 24, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Thabit, H.; Tauschmann, M.; Allen, J.M.; Leelarathna, L.; Hartnell, S.; Wilinska, M.E.; Acerini, C.L.; Dellweg, S.; Benesch, C.; Heinemann, L.; et al. Home Use of an Artificial Beta Cell in Type 1 Diabetes. N. Engl. J. Med. 2015, 373, 2129–2140. [Google Scholar] [PubMed]
- Bergenstal, R.M.; Garg, S.; Weinzimer, S.A.; Buckingham, B.A.; Bode, B.W.; Tamborlane, W.V.; Kaufman, F.R. Safety of a Hybrid Closed-Loop Insulin Delivery System in Patients With Type 1 Diabetes. JAMA 2016, 316, 1407–1408. [Google Scholar]
- Lawton, J.; Blackburn, M.; Rankin, D.; Allen, J.; Campbell, F.; Leelarathna, L.; Tauschmann, M.; Thabit, H.; Wilinska, M.E.; Hovorka, R.; et al. The impact of using a closed-loop system on food choices and eating practices among people with Type 1 diabetes: A qualitative study involving adults, teenagers and parents. Diabet. Med. 2019, 36, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Haidar, A.; Farid, D.; St-Yves, A.; Messier, V.; Chen, V.; Xing, D.; Brazeau, A.-S.; Duval, C.; Boulet, B.; Legault, L.; et al. Post-breakfast closed-loop glucose control is improved when accompanied with carbohydrate-matching bolus compared to weight-dependent bolus. Diabetes Metab. 2014, 40, 211–214. Available online: https://pubmed.ncbi.nlm.nih.gov/24656963/ (accessed on 28 December 2021).
- Riddle, M.C. Rediscovery of the Second β-Cell Hormone: Co-replacement With Pramlintide and Insulin in Type 1 Diabetes. Diabetes Care 2020, 43, 518–521. Available online: https://diabetesjournals.org/care/article/43/3/518/35673/Rediscovery-of-the-Second-Cell-Hormone-Co (accessed on 27 September 2022).
- P.R. Vademecum. Pramlintida. Available online: https://ar.prvademecum.com/principio-activo/pramlintida-6086/ (accessed on 20 October 2021).
- Colburn, W.A.; Gottlieb, A.B.; Koda, J.; Kolterman, O.G. Pharmacokinetics and pharmacodynamics of AC137 (25,28,29 tripro-amylin, human) after intravenous bolus and infusion doses in patients with insulin-dependent diabetes. J. Clin. Pharmacol. 1996, 36, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Kolterman, O.G.; Schwartz, S.; Corder, C.; Levy, B.; Klaff, L.; Peterson, J.; Gottlieb, A. Effect of 14 days’ subcutaneous administration of the human amylin analogue, pramlintide (AC137), on an intravenous insulin challenge and response to a standard liquid meal in patients with IDDM. Diabetologia 1996, 39, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. Available online: https://www.bmj.com/content/372/bmj.n71 (accessed on 14 July 2022).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, 4898. Available online: https://pubmed.ncbi.nlm.nih.gov/31462531/ (accessed on 28 December 2021). [CrossRef] [PubMed]
- Tsoukas, M.A.; Cohen, E.; Legault, L.; von Oettingen, J.E.; Yale, J.F.; Vallis, M.; Odabassian, M.; Fathi, A.E.; Rutkowski, J.; Jafar, A.; et al. Alleviating carbohydrate counting with a FiASP-plus-pramlintide closed-loop delivery system (artificial pancreas): Feasibility and pilot studies. Diabetes Obes. Metab. 2021, 23, 2090–2098. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Majdpour, D.; Yale, J.F.; Fathi, A.E.; Garfield, N.; Rutkowski, J.; Rene, J.; Legault, L.; Haidar, A. A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: A single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit. Health 2021, 7500, 1–10. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).