Effects of Humic Acids on Size and Species Composition of Phytoplankton in a Eutrophic Temperate Estuary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Experimental Designs
2.2. Measurement of Nutrients and Phytoplankton Community
2.3. Statistical Analysis
3. Results
3.1. Physical, Chemical, and Phytoplankton Properties (In Situ)
3.2. Response of Phytoplankton Size and Taxonomic Classes to HA Addition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiken, G.R.; McKnight, D.M.; Wershaw, R.L.; MacCarthy, P. An introduction to humic substances in soils, sediments and waters. In Humic Substances in Soil, Sediment, and Water: Geochemistry, Isolation, and Characterization; Aiken, G.R., McKnight, D.M., Wershaw, R.L., MacCarthy, P., Eds.; Wiley: New York, NY, USA, 1985; pp. 1–9. [Google Scholar]
- Thurman, E.M. Aquatic humic substances. In Organic Geochemistry of Natural Waters; Thurman, E.M., Ed.; Martinus Nijhof/Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1985; pp. 273–361. [Google Scholar]
- Carlsson, P.; Granéli, E.; Tester, P.; Boni, L. Influences of riverine humic substances on bacteria, protozoa, phytoplankton, and copepods in a coastal plankton community. Mar. Ecol. Prog. Ser. 1995, 127, 213–221. [Google Scholar] [CrossRef]
- Carlsson, P.; Edling, H.; Béchemin, C. Interactions between a marine dinoflagellate (Alexandrium catenella) and a bacterial community utilizing riverine humic substances. Aquat. Microb. Ecol. 1998, 16, 65–80. [Google Scholar] [CrossRef]
- Carlsson, P.; Granéli, E.; Segatto, A.Z. Cycling of biologically available nitrogen in riverine humic substances between marine bacteria, a heterotrophic nanoflagellate and a photosynthetic dinoflagellate. Aquat. Microb. Ecol. 1999, 18, 23–36. [Google Scholar] [CrossRef]
- Steinberg, C.E.W.; Meinelt, T.; Timofeyev, M.A.; Bittner, M.; Menzel, R. Humic substances. Part 2: Interactions with organisms. Environ. Sci. Pollut. Res. 2008, 15, 128–135. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Höfle, M.G. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl. Environ. Microbiol. 1987, 53, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Tranvik, L.J.; Sieburth, J.M.N. Effects of flocculated humic matter on free and attached pelagic microorganisms. Limnol. Oceanogr. 1989, 34, 688–699. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E. Humic substances, their microbial interactions and effects on biological transformations of organic pollutants in water and soil: A review. Chemosphere 2018, 202, 420–437. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.H.; Shin, Y.S.; Lee, B.C.; Chang, N.I.; Cho, J.; Kim, S.D. Effect of dissolved organic matter on the growth of algae, Pseudokirchneriella subcapitata, in Korean lakes: The importance of complexation reactions. Ecotoxicol. Environ. Saf. 2009, 72, 335–343. [Google Scholar] [CrossRef] [PubMed]
- See, J.H.; Bronk, D.A.; Lewitus, A.J. Uptake of Spartina-derived humic nitrogen by estuarine phytoplankton in nonaxenic and axenic culture. Limnol. Oceanogr. 2006, 51, 2290–2299. [Google Scholar] [CrossRef]
- Malazarte, J.M.; Lee, H.; Kim, H.; Sin, Y. Spatial and temporal dynamics of potentially toxic cyanobacteria in the riverine region of a temperate estuarine system altered by weirs. Water. 2017, 9, 819. [Google Scholar] [CrossRef]
- Yi, S.; Sin, Y.; Jang, N.; Kim, J.; Kim, H.; Cho, Y.; Jeong, J. Trophic state and water quality in major lakes of the Sumjin and Youngsan river systems. Korean J. Limnol. 2006, 39, 296–309. [Google Scholar]
- Sin, Y.; Hyun, B.; Jeong, B.; Soh, H.Y. Impacts of eutrophic freshwater inputs on water quality and phytoplankton size structure in a temperate estuary altered by a sea dike. Mar. Environ. Res. 2013, 85, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sin, Y.; Lee, E.; Lee, Y.; Shin, K. The river–estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuarine Coast. Shelf Sci. 2015, 163, 279–289. [Google Scholar] [CrossRef]
- Sin, Y.; Jeong, B. Short-term variations of phytoplankton communities in response to anthropogenic stressors in a highly altered temperate estuary. Estuarine Coast. Shelf Sci. 2015, 156, 83–91. [Google Scholar] [CrossRef]
- Murrell, M.C.; Hagy, J.D., III; Lores, E.M.; Greene, R.M. Phytoplankton production and nutrient distributions in a sub-tropical estuary: Importance of freshwater flow. Estuar. Coast. 2007, 30, 390–402. [Google Scholar] [CrossRef]
- Saeck, E.A.; Hadwen, W.L.; Rissik, D.; O’Brien, K.R.; Burford, M.A. Flow events drive patterns of phytoplankton distribution along a rivereestuaryebay continuum. Mar. Freshwater Res. 2013, 64, 655–670. [Google Scholar] [CrossRef]
- Shin, Y.; Yu, H.; Lee, H.; Lee, D.; Park, G. The Change in patterns and conditions of algal blooms resulting from construction of weirs in the Yeongsan River: Long-term data analysis. Korean J. Environ. Ecol. 2015, 48, 238–252. [Google Scholar] [CrossRef]
- Nürnberg, G.K. Trophic state of clear and colored, soft- and hardwater lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake Reserv. Manag. 1996, 12, 432–447. [Google Scholar] [CrossRef]
- Yoon, B. A Study of Nutrient Limitation in Estuary Using Bioassay Experiment. Master’s Thesis, Mokpo National Maritime University, Mokpo, Korea, 2012. [Google Scholar]
- Steinberg, C.E.W. Ecology of Humic Substances in Freshwaters. In Determinants from Geochemistry to Ecological Niches; Springer: Berlin, Germany, 2003. [Google Scholar]
- Francko, D.A. Epilimnetic phosphorus cycling: Influence of humic materials and iron on coexisting major mechanisms. Can. J. Fish. Aquat. Sci. 1986, 43, 302–310. [Google Scholar] [CrossRef]
- Salonen, K.; Jones, R.I.; De Haan, H. Phosphorus Transformations in the Epilimnion of Humic Lakes: Abiotic Interactions Between Dissolved Humic Materials and Phosphate. Freshw. Biol. 1988, 19, 357–369. [Google Scholar]
- Martin, M.V.; Gebühr, C.; Mártire, D.O.; Wiltshire, K.H. Characterization of a humic acid extracted from marine sediment and its influence on the growth of marine diatoms. J. Mar. Biol. Assoc. 2014, 94, 895–906. [Google Scholar] [CrossRef]
- Legrand, C.; Carlsson, P. Uptake of high molecular weight dextran by the dinoflagellate Alexandrium catenella. Aquat. Microb. Ecol. 1998, 16, 81–86. [Google Scholar] [CrossRef]
- Lewitus, A.J. Osmotrophy in marine microalgae. In Algal Cultures, Analogues of Blooms and Applications; Subba Rao, D.V., Ed.; Science Publishers: Enfield, CT, USA, 2006; pp. 343–383. [Google Scholar]
- Jones, R.I. Phytoplankton, primary production and nutrient cycling. In Aquatic Humic Substances-Ecological Studies; Hessen, D.O., Tranvik, L.J., Eds.; Springer: New York, NY, USA, 1998. [Google Scholar]
- Eloranta, P. Light penetration in water. In Limnology of Humic Waters; Keskitalo, J., Eloranta, P., Eds.; Backhuys Publishers: Kerkwerve, The Netherlands, 1999; pp. 61–63. [Google Scholar]
- Rodríguez, P.; Pizarro, H. Phytoplankton productivity in a highly colored shallow lake of a South American floodplain. Wetlands 2007, 27, 1153–1160. [Google Scholar] [CrossRef]
- Gilbert, M.; Bährs, H.; Steinberg, C.E.W.; Wilhelm, C. The artificial humic substance HS1500 does not inhibit photosynthesis of the green alga Desmodesmus armatus in vivo but interacts with the photosynthetic apparatus of isolated spinach thylakoids in vitro. Photosynth. Res. 2018, 137, 403–420. [Google Scholar] [CrossRef]
- Sin, Y.; Lee, H. Changes in hydrology, water quality, and algal blooms in a freshwater system impounded with engineered structures in a temperate monsoon river estuary. J. Hydrol. Reg. Stud. 2020, 32, 100744. [Google Scholar] [CrossRef]
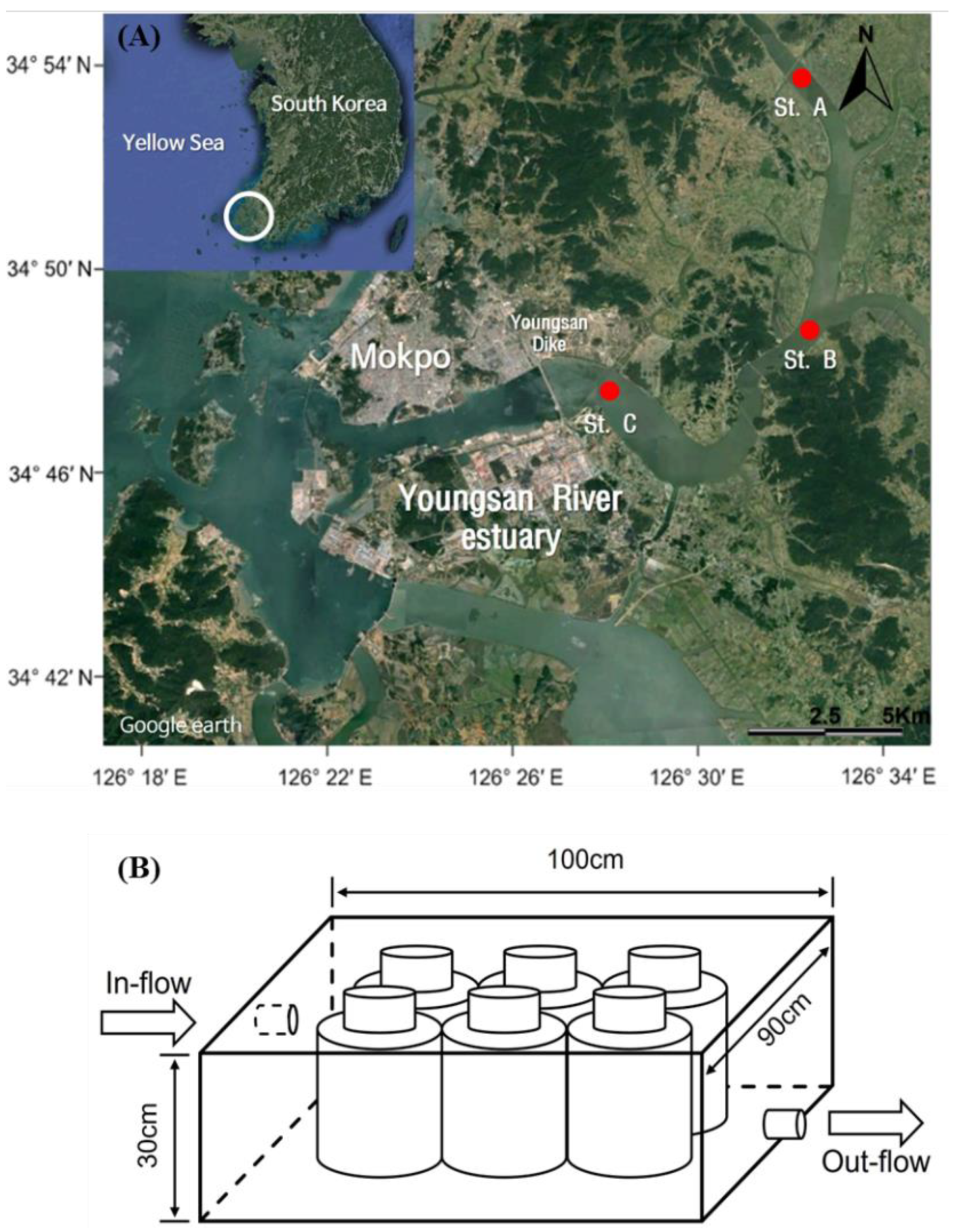
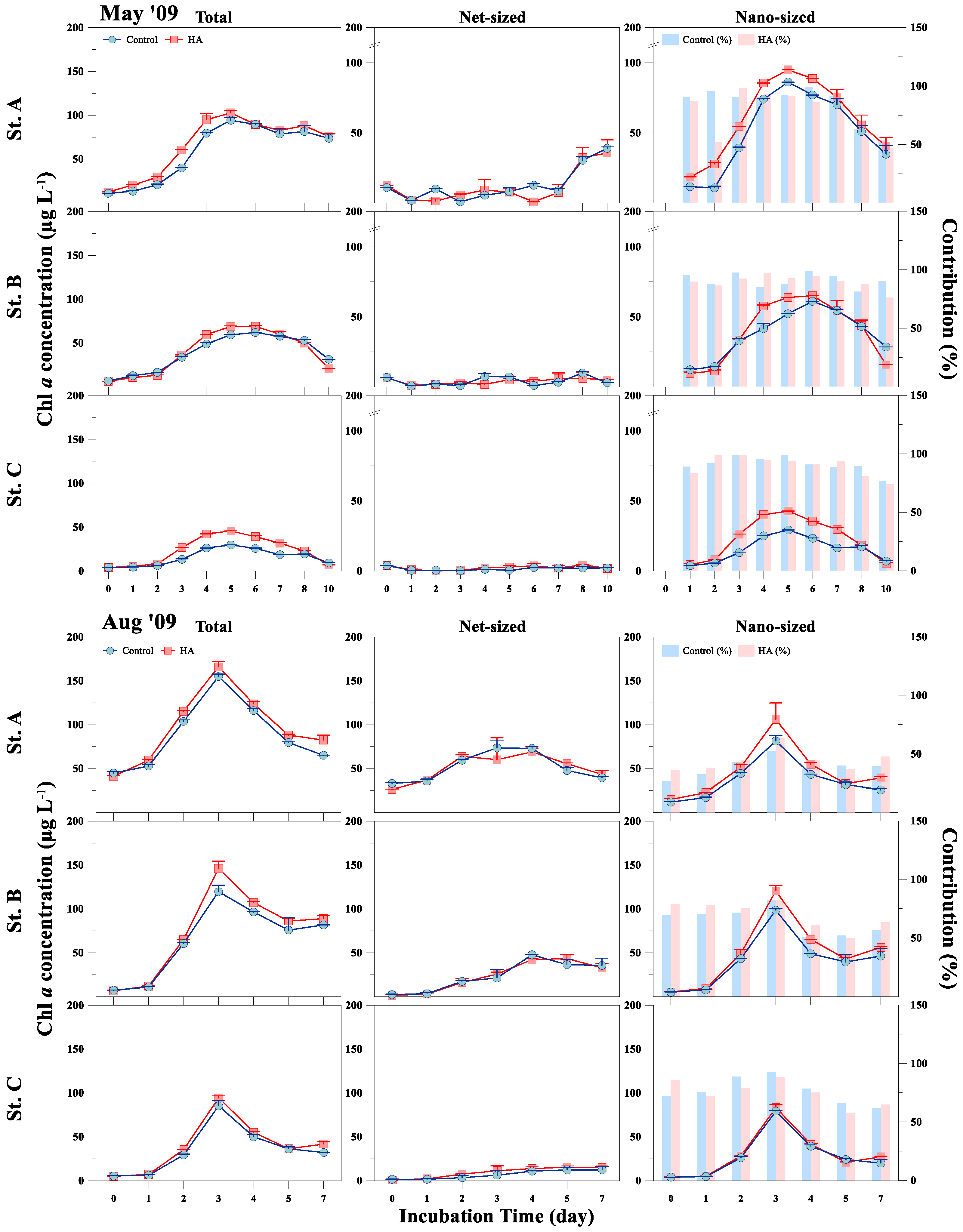


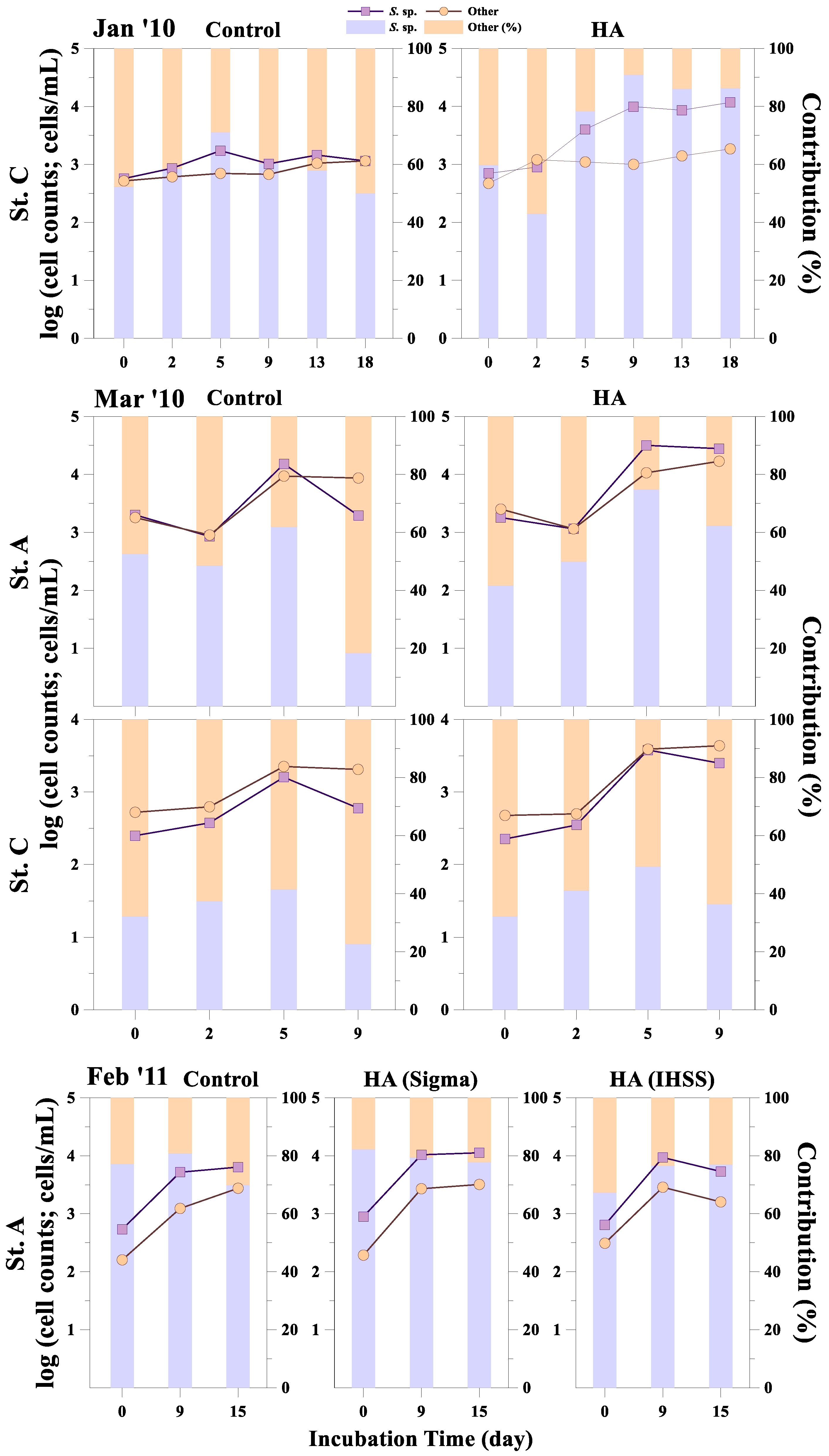
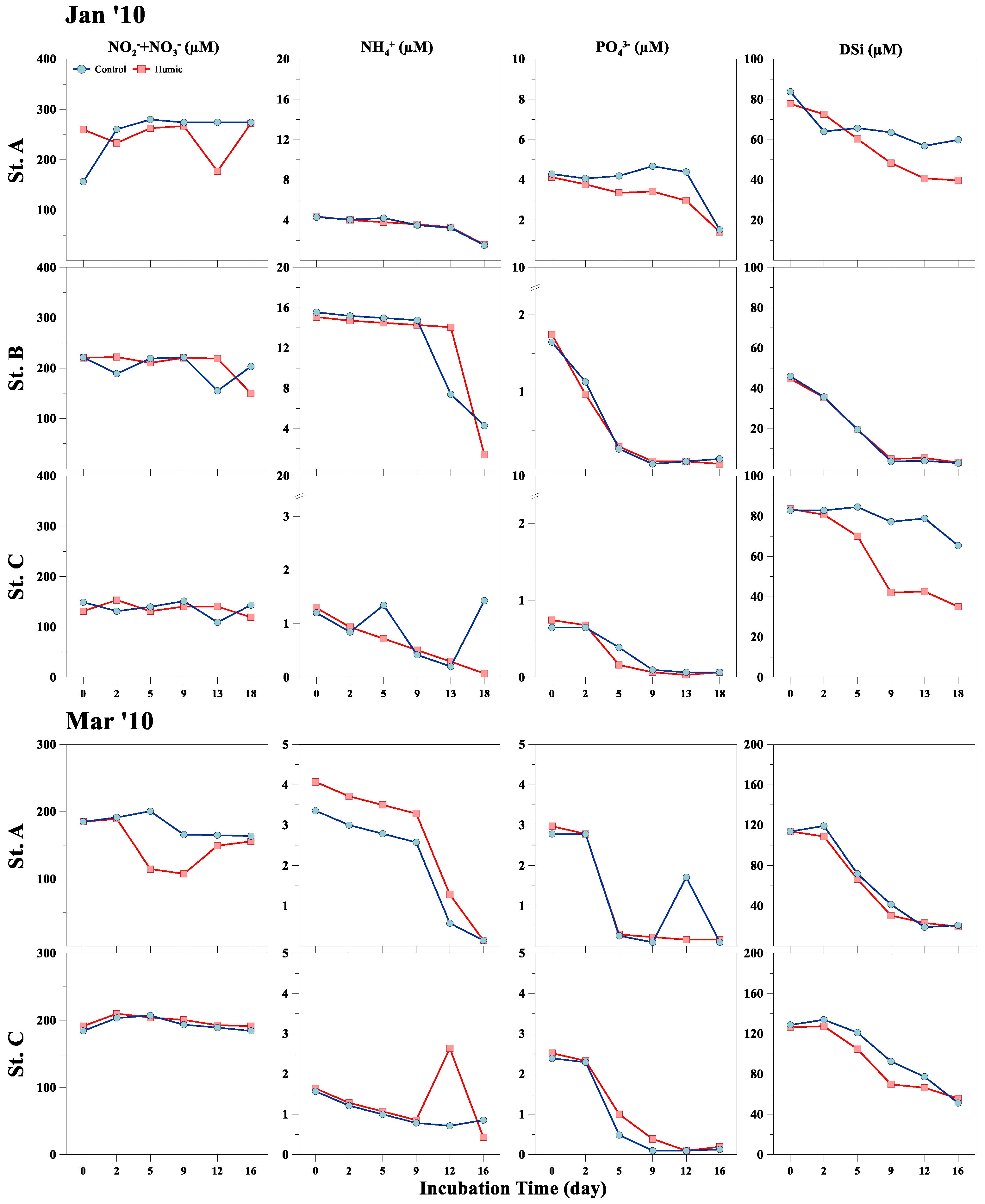
| Sampling Date | Station | Temp (°C) | Turb (NTU) | PAR (μmol m−2 s−1) | DIN (µM) | DIP (µM) | DSi (µM) | DIN/DIP | DIN/DSi |
|---|---|---|---|---|---|---|---|---|---|
| May 2009 | A | 19.76 | 12.30 | 38.01 | 272.63 | 0.31 | 23.23 | 888.76 | 11.74 |
| B | 19.64 | 8.90 | 219.80 | 214.45 | 0.40 | 20.47 | 531.33 | 10.48 | |
| C | 19.08 | 6.50 | 224.40 | 203.57 | 0.19 | 15.93 | 1050.75 | 12.78 | |
| Aug 2009 | A | 26.68 | 36.20 | 1.07 | 205.48 | 4.20 | 124.17 | 48.95 | 1.65 |
| B | 27.60 | 23.10 | 52.38 | 150.05 | 3.33 | 168.96 | 45.12 | 0.89 | |
| C | 27.07 | 15.30 | 146.53 | 137.92 | 3.07 | 148.02 | 44.96 | 0.93 | |
| Nov 2009 | A | 9.45 | 14.70 | 20.41 | 255.43 | 6.13 | 116.06 | 41.63 | 2.20 |
| B | 10.22 | 114.90 | 78.33 | 155.88 | 1.74 | 131.72 | 89.40 | 1.18 | |
| C | 11.06 | 12.50 | 272.70 | 119.47 | 1.16 | 127.95 | 102.77 | 0.93 | |
| Jan 2010 | A | 3.69 | 11.40 | 6.36 | 297.66 | 4.52 | 80.42 | 65.85 | 3.70 |
| B | 3.62 | 9.20 | 25.22 | 251.74 | 1.78 | 38.59 | 141.75 | 6.52 | |
| C | 3.41 | 4.70 | 363.40 | 157.82 | 0.90 | 84.69 | 174.56 | 1.86 | |
| Mar 2010 | A | 9.64 | 11.80 | 23.04 | 219.04 | 3.00 | 120.72 | 72.94 | 1.81 |
| C | 8.94 | 8.70 | 98.78 | 216.09 | 2.52 | 131.47 | 85.80 | 1.64 | |
| Feb 2011 | A | 3.56 | 9.80 | 7.25 | 329.41 | 2.26 | 231.22 | 145.74 | 1.42 |
| Sampling Date | Station | Total | Net | Nano | Diatoms | Chloro | Cyano | Crypto | Other | Dominant Species |
|---|---|---|---|---|---|---|---|---|---|---|
| May 2009 | A | 10.84 | 0.76 (7.0) | 10.08 (93.0) | 370 (24.3) | 510 (33.6) | 170 (11.2) | 260 (17.1) | 210 (13.8) | Coelastrum microporum (18.4) |
| B | 6.70 | 0.10 (1.5) | 6.60 (98.5) | 60 (11.3) | 100 (18.9) | 120 (22.6) | 250 (47.2) | 0 | Teleaulax acuta (34.0) | |
| C | 4.10 | 2.72 (66.3) | 1.38 (33.7) | 65 (21.3) | 45 (14.8) | 65 (21.3) | 130 (42.6) | 0 | Microcystis aeruginosa (21.3) | |
| Aug 2009 | A | 14.36 | 3.96 (27.6) | 10.40 (72.4) | 149 (46.9) | 22 (6.9) | 120 (37.7) | 0 | 27 (8.5) | Microcystis aeruginosa (21.1) |
| B | 6.50 | 1.06 (16.3) | 5.44 (83.7) | 63 (66.3) | 19 (20.0) | 0 | 0 | 13 (13.7) | Discostella stelligera (formerly Cyclotella stelligera) (24.2) | |
| C | 4.68 | 1.66 (35.3) | 3.03 (64.7) | 60 (55.0) | 6 (5.5) | 40 (36.7) | 0 | 3 (2.8) | Microcystis sp. (36.7) | |
| Nov 2009 | A | 13.24 | 1.36 (10.3) | 11.88 (89.7) | 57 (17.0) | 0 | 0 | 153 (45.5) | 126 (37.5) | Teleaulax acuta (35.7) |
| B | 5.54 | 0.16 (2.9) | 5.38 (97.1) | 40 (21.9) | 0 | 0 | 130 (71.0) | 13 (7.1) | Teleaulax acuta (50.8) | |
| C | 3.26 | 0.18 (5.6) | 3.08 (94.4) | 73 (39.5) | 3 (1.6) | 3 (1.6) | 103 (55.7) | 3 (1.6) | Cryptomonas erosa (37.8) | |
| Jan 2010 | A | 109.04 | 9.44 (8.7) | 99.60 (91.3) | 2550 (94.4) | 0 | 0 | 110 (4.1) | 40 (1.5) | Stephanodiscus sp. (84.1) |
| B | 50.96 | 1.60 (3.1) | 49.36 (96.9) | 1240 (91.2) | 10 (0.7) | 0 | 80 (5.9) | 30 (2.2) | Stephanodiscus sp. (35.3) | |
| C | 7.08 | 0.95 (13.4) | 6.12 (86.6) | 1720 (91.5) | 20 (1.1) | 0 | 130 (6.9) | 10 (0.5) | Stephanodiscus sp. (42.0) | |
| Mar 2010 | A | 26.12 | 4.88 (18.7) | 21.24 (81.3) | 1310 (78.9) | 70 (4.2) | 0 | 250 (15.1) | 30 (1.8) | Stephanodiscus sp. (35.5) |
| C | 8.08 | 1.26 (15.6) | 6.82 (84.4) | 230 (36.5) | 80 (12.7) | 10 (1.6) | 180 (28.6) | 130 (20.6) | Stephanodiscus sp. (17.5) | |
| Feb 2011 | A | 8.28 | 1.48 (17.9) | 6.80 (82.1) | 580 (66.7) | 25 (2.9) | 0 | 205 (23.5) | 60 (6.9) | Stephanodiscus sp. (29.9) |
| Sampling Date | Station | Total | Net-sized | Nanosized | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time | Trt | T*Trt | Time | Trt | T*Trt | Time | Trt | T*Trt | ||
| May 2009 | A | 0.002 | 0.028 | 0.178 | 0.014 | 0.645 | 0.205 | 0.003 | 0.026 | 0.279 |
| B | <0.001 | 0.016 | 0.019 | 0.089 | 0.734 | 0.199 | 0.003 | 0.168 | 0.060 | |
| C | <0.001 | 0.001 | 0.004 | 0.073 | 0.021 | 0.281 | <0.001 | <0.001 | 0.005 | |
| Aug 2009 | A | 0.001 | 0.023 | 0.133 | 0.052 | 0.716 | 0.430 | 0.011 | 0.014 | 0.296 |
| B | 0.003 | 0.018 | 0.227 | 0.017 | 0.974 | 0.445 | 0.003 | 0.049 | 0.157 | |
| C | 0.001 | 0.019 | 0.200 | 0.052 | 0.194 | 0.514 | 0.001 | 0.133 | 0.165 | |
| Nov 2009 | A | <0.001 | 0.006 | 0.039 | 0.130 | 0.001 | 0.424 | 0.001 | 0.066 | 0.169 |
| B | 0.006 | 0.015 | 0.139 | 0.130 | 0.827 | 0.446 | 0.001 | 0.001 | 0.016 | |
| C | 0.008 | 0.587 | 0.317 | 0.123 | 0.976 | 0.391 | 0.009 | 0.223 | 0.388 | |
| Jan 2010 | A | 0.003 | 0.062 | 0.165 | 0.026 | 0.096 | 0.122 | 0.006 | 0.034 | 0.223 |
| B | 0.002 | 0.009 | 0.178 | 0.068 | 0.985 | 0.426 | 0.037 | 0.161 | 0.401 | |
| C | 0.002 | <0.001 | 0.006 | 0.073 | 0.098 | 0.196 | 0.003 | <0.001 | 0.007 | |
| Mar 2010 | A | 0.001 | <0.001 | 0.017 | 0.066 | 0.381 | 0.541 | <0.001 | <0.001 | 0.011 |
| C | <0.001 | <0.001 | 0.002 | 0.050 | 0.031 | 0.354 | <0.001 | <0.001 | 0.004 | |
| Feb 2011 | A | 0.022 | 0.090 | 0.093 | 0.261 | 0.956 | 0.371 | <0.001 | 0.052 | 0.077 |
| Phytoplankton Group | Jan 2010 (St. C) | Mar 2010 (St. A) | Mar 2010 (St. C) | Feb 2011 (St. A) | ||||
|---|---|---|---|---|---|---|---|---|
| Time | Trt | Time | Trt | Time | Trt | Time | Trt | |
| Total abundance | 0.378 | 0.042 | 0.031 | 0.173 | 0.025 | 0.185 | 0.019 | 0.412 |
| Diatoms | 0.389 | 0.045 | 0.046 | 0.184 | 0.037 | 0.209 | 0.021 | 0.431 |
| Chlorophytes | 0.032 | 0.222 | 0.006 | 0.248 | 0.007 | 0.380 | 0.029 | 0.391 |
| Cyanobacteria | 0.121 | 0.809 | NA | NA | 0.497 | 0.381 | NA | NA |
| Cryptophytes | 0.005 | 0.363 | 0.608 | 0.983 | 0.541 | 0.203 | 0.016 | 0.161 |
| Others | 0.727 | 0.782 | 0.594 | 0.975 | 0.728 | 0.311 | 0 | 0 |
| Stephanodiscus sp. | 0.445 | 0.049 | 0.081 | 0.188 | 0.093 | 0.230 | 0.023 | 0.374 |
| NO2− + NO3− | 0.788 | 0.893 | 0.017 | 0.080 | 0.166 | 0.127 | ND | ND |
| NH4+ | 0.336 | 0.324 | <0.001 | 0.004 | 0.013 | 0.417 | ND | ND |
| DIP (PO43−) | <0.001 | 0.571 | <0.001 | 0.582 | <0.001 | 0.884 | ND | ND |
| DSi | 0.147 | 0.034 | <0.001 | 0.461 | <0.001 | 0.032 | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sin, Y.; Kim, S. Effects of Humic Acids on Size and Species Composition of Phytoplankton in a Eutrophic Temperate Estuary. Appl. Sci. 2022, 12, 10223. https://doi.org/10.3390/app122010223
Sin Y, Kim S. Effects of Humic Acids on Size and Species Composition of Phytoplankton in a Eutrophic Temperate Estuary. Applied Sciences. 2022; 12(20):10223. https://doi.org/10.3390/app122010223
Chicago/Turabian StyleSin, Yongsik, and Sunghwan Kim. 2022. "Effects of Humic Acids on Size and Species Composition of Phytoplankton in a Eutrophic Temperate Estuary" Applied Sciences 12, no. 20: 10223. https://doi.org/10.3390/app122010223
APA StyleSin, Y., & Kim, S. (2022). Effects of Humic Acids on Size and Species Composition of Phytoplankton in a Eutrophic Temperate Estuary. Applied Sciences, 12(20), 10223. https://doi.org/10.3390/app122010223









