Biospeckle Activity of Highbush Blueberry Fruits Infested by Spotted Wing Drosophila (Drosophila suzukii Matsumura)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
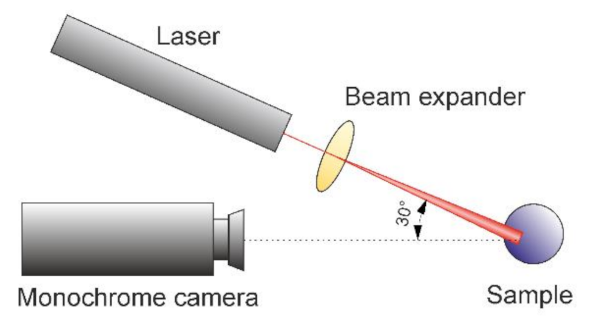
2.2. Experimental Setup
2.3. Biospeckle Activity Measurement
2.4. Statistical Analysis
3. Results and Discussion
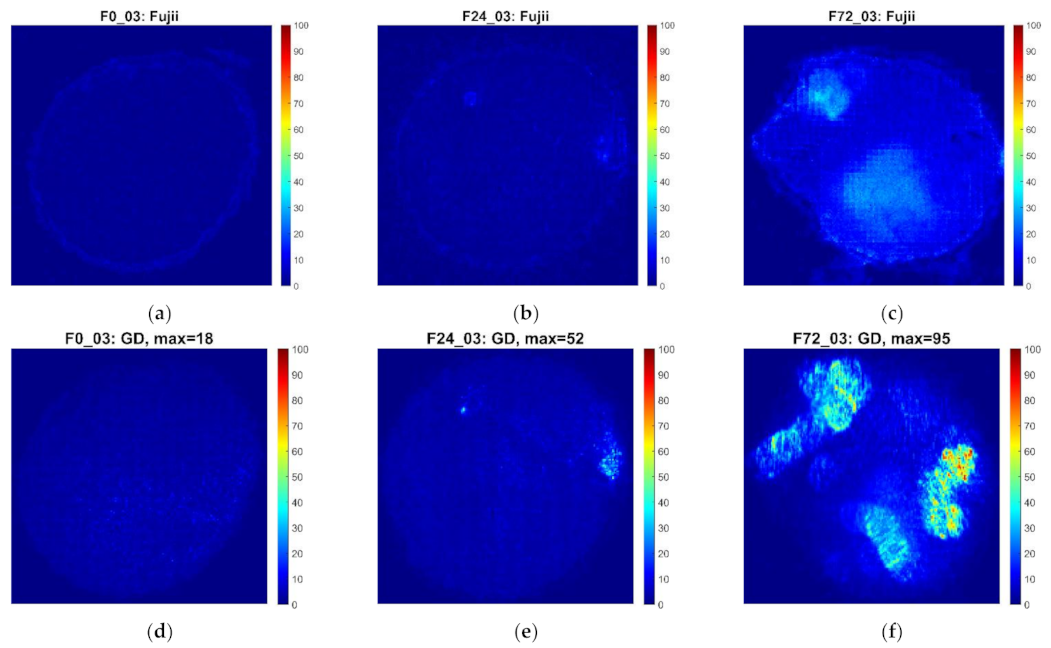
3.1. Qualitative Description of Biospeckle Activity
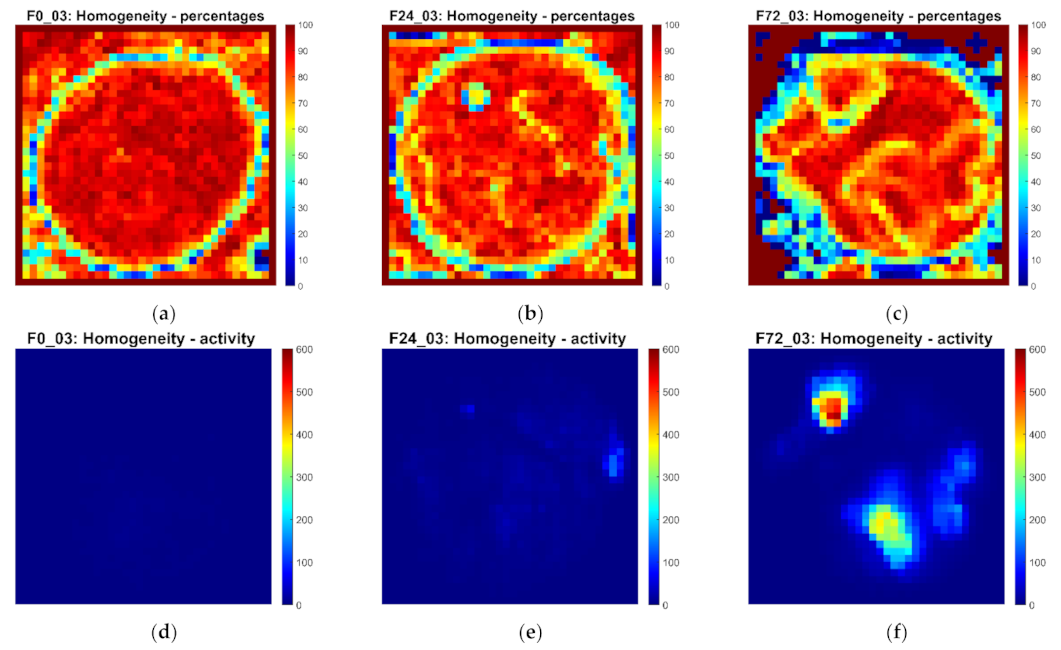
3.2. Spatial Homogeneity of Biospeckle Activity
3.3. Quantitative Analysis of Biospeckle Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arizaga, R.; Trivi, M.; Rabal, H. Speckle Time Evolution Characterization by the Co-Occurrence Matrix Analysis. Opt. Laser Technol. 1999, 31, 163–169. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Nirala, A.K. Assessment of Bio-Activity Using the Methods of Inertia Moment and Absolute Value of the Differences. Optik 2013, 124, 512–516. [Google Scholar] [CrossRef]
- Catalano, M.D.; Rivera, F.P.; Braga, R.A. Viability of Biospeckle Laser in Mobile Devices. Optik 2019, 183, 897–905. [Google Scholar] [CrossRef]
- Briers, J.D. Wavelength Dependence of Intensity Fluctuations in Laser Speckle Patterns from Biological Specimens. Opt. Commun. 1975, 13, 324–326. [Google Scholar] [CrossRef]
- Braga, R.A.; Dupuy, L.; Pasqual, M.; Cardoso, R.R. Live Biospeckle Laser Imaging of Root Tissues. Eur. Biophys. J. 2009, 38, 679–686. [Google Scholar] [CrossRef]
- Kurenda, A.; Adamiak, A.; Zdunek, A. Temperature Effect on Apple Biospeckle Activity Evaluated with Different Indices. Postharvest Biol. Technol. 2012, 67, 118–123. [Google Scholar] [CrossRef]
- Pajuelo, M.; Baldwin, G.; Rabal, H.; Cap, N.; Arizaga, R.; Trivi, M. Bio-Speckle Assessment of Bruising in Fruits. Opt. Lasers Eng. 2003, 40, 13–24. [Google Scholar] [CrossRef]
- Rabelo, G.F.; Enes, A.M.; Braga Junior, R.A.; Dal Fabbro, I.M. Frequency Response of Biospeckle Laser Images of Bean Seeds Contaminated by Fungi. Biosyst. Eng. 2011, 110, 297–301. [Google Scholar] [CrossRef]
- Adamiak, A.; Zdunek, A.; Kurenda, A.; Rutkowski, K. Application of the Biospeckle Method for Monitoring Bull’s Eye Rot Development and Quality Changes of Apples Subjected to Various Storage Methods—Preliminary Studies. Sensors 2012, 12, 3215–3227. [Google Scholar] [CrossRef] [PubMed]
- Enes, A.M.; Fracarolli, J.A.; Fabbro, I.M.D.; Rodrigues, S. Biospeckle Supported Fruit Bruise Detection. Int. J. Nutr. Food Eng. 2012, 6, 889–891. [Google Scholar]
- Sutton, D.B.; Punja, Z.K. Investigating Biospeckle Laser Analysis as a Diagnostic Method to Assess Sprouting Damage in Wheat Seeds. Comput. Electron. Agric. 2017, 141, 238–247. [Google Scholar] [CrossRef]
- Gao, Y.; Rao, X. Blackspot Bruise in Potatoes: Susceptibility and Biospeckle Activity Response Analysis. J. Food Meas. Charact. 2019, 13, 444–453. [Google Scholar] [CrossRef]
- Wu, A.; Zhu, J.; Ren, T. Detection of Apple Defect Using Laser-Induced Light Backscattering Imaging and Convolutional Neural Network. Comput. Electr. Eng. 2020, 81, 106454. [Google Scholar] [CrossRef]
- Alves, R.A.; Oliveira Silva, B.; Rabelo, G.; Marques Costa, R.; Machado Enes, A.; Cap, N.; Rabal, H.; Arizaga, R.; Trivi, M.; Horgan, G. Reliability of Biospeckle Image Analysis. Opt. Lasers Eng. 2007, 45, 390–395. [Google Scholar] [CrossRef]
- Amaral, I.C.; Braga, R.A.; Ramos, E.M.; Ramos, A.L.S.; Roxael, E.A.R. Application of Biospeckle Laser Technique for Determining Biological Phenomena Related to Beef Aging. J. Food Eng. 2013, 119, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Zdunek, A.; Adamiak, A.; Pieczywek, P.M.; Kurenda, A. The Biospeckle Method for the Investigation of Agricultural Crops: A Review. Opt. Lasers Eng. 2014, 52, 276–285. [Google Scholar] [CrossRef]
- Bellamy, D.E.; Sisterson, M.S.; Walse, S.S. Quantifying Host Potentials: Indexing Postharvest Fresh Fruits for Spotted Wing Drosophila, Drosophila Suzukii. PLoS ONE 2013, 8, e61227. [Google Scholar] [CrossRef] [Green Version]
- Ioriatti, C.; Walton, V.; Dalton, D.; Anfora, G.; Grassi, A.; Maistri, S.; Mazzoni, V. Drosophila Suzukii (Diptera: Drosophilidae) and Its Potential Impact to Wine Grapes During Harvest in Two Cool Climate Wine Grape Production Regions. J. Econ. Entomol. 2015, 108, 1148–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Ros, G.; Grassi, A.; Pantezzi, T. Recent Trends in the Economic Impact of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020; ISBN 9783030626914. [Google Scholar] [CrossRef]
- De Ros, G.; Concia, S.; Pantezzia, T.; Savinib, G. The economic impact of invasive pest Drosophila suzukii on berry production in the Province of Trento, Italy. J. Berry Res. 2015, 5, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Mazzi, D.; Bravin, E.; Meraner, M.; Finger, R.; Kuske, S. Economic Impact of the Introduction and Establishment of Drosophila suzukii on Sweet Cherry Production in Switzerland. Insects 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benito, N.P.; Lopes-da-Silva, L.; Sivori Silva dos Santos, R. Potential spread and economic impact of invasive Drosophila suzukii in Brazil. Pesqui. Agropecuária Bras. 2016, 51, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila Suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding Its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Grassi, A.; Giongo, L.; Palmieri, L. Drosophila (Sophophora) Suzukii (Matsumura), New Pest of Soft Fruits in Trentino (North-Italy) and in Europe. IOBC/WPRS Bull. 2011, 70, 121–128. [Google Scholar]
- Ioriatti, C.; Guzzon, R.; Anfora, G.; Ghidoni, F.; Mazzoni, V.; Villegas, T.R.; Dalton, D.T.; Walton, V.M. Drosophila Suzukii (Diptera: Drosophilidae) Contributes to the Development of Sour Rot in Grape. J. Econ. Entomol. 2018, 111, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.A.; Rivera, F.P. Junio Moreira A Practical Guide to Biospeckle Laser Analysis: Theory and Software; Editora UFLA: Lavras, Brazil, 2016; ISBN 9788581270517. [Google Scholar]
- Bio-Speckle Laser Tool Library. Available online: http://www.nongnu.org/bsltl/ (accessed on 20 October 2021).
- Fujii, H.; Nohira, K.; Yamamoto, Y.; Ikawa, H.; Ohura, T. Evaluation of Blood Flow by Laser Speckle Image Sensing. Part 1. Appl. Opt. 1987, 26, 5321–5325. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mayookha, V.P.; Kothakota, A.; Ramesh, S.V.; Thirumdas, R.; Juvvi, P. Biospeckle Laser Technique—A Novel Non-Destructive Approach for Food Quality and Safety Detection. Trends Food Sci. Technol. 2020, 97, 1–13. [Google Scholar] [CrossRef]
- Arizaga, R.A.; Cap, N.L.; Rabal, H.J.; Trivi, M. Display of Local Activity Using Dynamical Speckle Patterns. Opt. Eng. 2002, 41, 287–294. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, SMC-3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, R.R.; Braga, R.A. Enhancement of the Robustness on Dynamic Speckle Laser Numerical Analysis. Opt. Lasers Eng. 2014, 63, 19–24. [Google Scholar] [CrossRef]
- Braga, R.A.; Nobre, C.M.B.; Costa, A.G.; Sáfadi, T.; da Costa, F.M. Evaluation of Activity through Dynamic Laser Speckle Using the Absolute Value of the Differences. Opt. Commun. 2011, 284, 646–650. [Google Scholar] [CrossRef]
- Reis, R.O.; Rabal, H.J.; Braga, R.A. Light Intensity Independence during Dynamic Laser Speckle Analysis. Opt. Commun. 2016, 366, 185–193. [Google Scholar] [CrossRef]
- Xu, Z.; Joenathan, C.; Khorana, B.M. Temporal and Spatial Properties of the Time-Varying Speckles of Botanical Specimens. Opt. Eng. 1995, 34, 1487–1502. [Google Scholar] [CrossRef]
- Ansari, M.D.Z.; Nirala, A.K. Biospeckle Activity Measurement of Indian Fruits Using the Methods of Cross-Correlation and Inertia Moments. Optik 2013, 124, 2180–2186. [Google Scholar] [CrossRef]
- Oulamara, A.; Tribillon, G.; Duvernoy, J. Biological Activity Measurement on Botanical Specimen Surfaces Using a Temporal Decorrelation Effect of Laser Speckle. J. Mod. Opt. 1989, 36, 165–179. [Google Scholar] [CrossRef]
- Papoulis, A.; Pillai, S.U. Probability, Random Variables, and Stochastic Processes; McGraw-Hill: New York, NY, USA, 1984; ISBN 9780070486584. [Google Scholar]
- Braga, R.A.; Dal Fabbro, I.M.; Borem, F.M.; Rabelo, G.; Arizaga, R.; Rabal, H.J.; Trivi, M. Assessment of Seed Viability by Laser Speckle Techniques. Biosyst. Eng. 2003, 86, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Braga, R.; Cardoso, R.R.; Bezerra, P., Jr.; Wouters, F.; Sampaio, G.; Varaschin, M. Biospeckle Numerical Values over Spectral Image Maps of Activity. Opt. Commun. 2012, 285, 553–561. [Google Scholar] [CrossRef]
- Zdunek, A.; Muravsky, L.I.; Frankevych, L.; Konstankiewicz, K. New Nondestructive Method Based on Spatial-Temporal Speckle Correlation Technique for Evaluation of Apples Quality during Shelf-Life. Int. Agrophys. 2007, 21, 305–310. [Google Scholar]
- Ansari, M.Z.; Minz, P.D.; Nirala, A.K. Fruit Quality Evaluation Using Biospeckle Techniques. In Proceedings of the 2012 1st International Conference on Recent Advances in Information Technology (RAIT), Dhanbad, India, 15–17 March 2012; pp. 873–876. [Google Scholar]
- Szymanska-Chargot, M.; Adamiak, A.; Zdunek, A. Pre-Harvest Monitoring of Apple Fruits Development with the Use of Biospeckle Method. Sci. Hortic. 2012, 145, 23–28. [Google Scholar] [CrossRef]
- Mollazade, K.; Arefi, A. Optical Analysis Using Monochromatic Imaging-Based Spatially-Resolved Technique Capable of Detecting Mealiness in Apple Fruit. Sci. Hortic. 2017, 225, 589–598. [Google Scholar] [CrossRef]


| Activity Indicator | F0 | F24 | F72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean Rank | Mean | Median | Mean Rank | Mean | Median | Mean Rank | |
| AVD | 0.994 | 0.917 | 14.000 a | 1.445 | 1.165 | 18.733 a | 4.122 | 2.720 | 36.267 b |
| IM | 3.848 | 2.943 | 12.667 a | 13.263 | 4.614 | 20.067 a | 98.974 | 37.922 | 36.267 b |
| RVD | −0.002 | −0.002 | 24.733 a | −0.001 | −0.002 | 24.000 a | −0.004 | −0.007 | 20.267 a |
| NAD | 0.009 | 0.009 | 13.133 a | 0.012 | 0.010 | 18.600 a | 0.033 | 0.027 | 37.267 b |
| BAI | 0.003 | 0.003 | 9.133 a | 0.014 | 0.013 | 22.333 b | 0.075 | 0.070 | 37.533 c |
| Activity Indicator | F0 | F24 | F72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean Rank | Mean | Median | Mean Rank | Mean | Median | Mean Rank | |
| ROI of 5 × 5 pixels | |||||||||
| AVD | 2.100 | 2.056 | 12.571 a | 3.193 | 2.347 | 18.286 a | 10.614 | 6.491 | 33.643 b |
| IM | 12.306 | 8.422 | 12.357 a | 34.804 | 12.913 | 18.786 a | 430.228 | 96.285 | 33.357 b |
| RVD | −0.028 | −0.020 | 21.071 a | 0.009 | −0.055 | 20.929 a | 0.011 | −0.004 | 22.500 a |
| NAD | 0.010 | 0.009 | 13.000 a | 0.013 | 0.011 | 17.714 a | 0.043 | 0.030 | 33.786 b |
| BAI | 0.008 | 0.005 | 9.429 a | 0.025 | 0.026 | 19.929 b | 0.072 | 0.068 | 35.143 c |
| ROI of 9 × 9 pixels | |||||||||
| AVD | 1.947 | 2.024 | 12.643 a | 2.987 | 2.268 | 18.143 a | 9.821 | 6.241 | 33.714 b |
| IM | 10.312 | 8.197 | 12.286 a | 30.042 | 12.390 | 18.714 a | 358.510 | 87.934 | 33.500 b |
| RVD | −0.018 | −0.004 | 20.214 a | 0.010 | −0.024 | 21.000 a | 0.026 | 0.008 | 23.286 a |
| NAD | 0.010 | 0.009 | 12.929 a | 0.014 | 0.012 | 17.857 a | 0.043 | 0.033 | 33.714 b |
| BAI | 0.008 | 0.005 | 9.143 a | 0.023 | 0.019 | 20.071 b | 0.081 | 0.075 | 35.286 c |
| ROI of 13 × 13 pixels | |||||||||
| AVD | 1.904 | 1.942 | 12.643 a | 2.896 | 2.314 | 18.071 a | 9.267 | 6.156 | 33.786 b |
| IM | 9.805 | 7.743 | 12.286 a | 29.655 | 12.710 | 18.500 a | 320.123 | 84.297 | 33.714 b |
| RVD | −0.016 | −0.006 | 19.571 a | 0.009 | −0.012 | 21.571 a | 0.023 | 0.003 | 23.357 a |
| NAD | 0.010 | 0.010 | 12.786 a | 0.014 | 0.011 | 18.143 a | 0.043 | 0.035 | 33.571 b |
| BAI | 0.007 | 0.005 | 9.214 a | 0.021 | 0.019 | 20.071 b | 0.080 | 0.079 | 35.214 c |
| ROI of 17 × 17 pixels | |||||||||
| AVD | 1.832 | 1.884 | 12.429 a | 2.829 | 2.382 | 18.071 a | 8.936 | 6.000 | 34.000 b |
| IM | 9.100 | 7.370 | 12.071 a | 28.217 | 13.226 | 18.714 a | 299.208 | 81.160 | 33.714 b |
| RVD | −0.013 | −0.010 | 19.286 a | −0.003 | −0.014 | 21.214 a | 0.017 | 0.007 | 24.000 a |
| NAD | 0.010 | 0.010 | 12.000 a | 0.015 | 0.012 | 19.000 a | 0.043 | 0.036 | 33.500 b |
| BAI | 0.007 | 0.004 | 9.429 a | 0.019 | 0.016 | 19.571 b | 0.081 | 0.078 | 35.500 c |
| ROI of 21 × 21 pixels | |||||||||
| AVD | 1.796 | 1.832 | 12.429 a | 2.802 | 2.460 | 18.214 a | 8.515 | 5.854 | 33.857 b |
| IM | 8.768 | 7.012 | 12.000 a | 30.337 | 13.852 | 18.571 a | 272.390 | 77.266 | 33.929 b |
| RVD | −0.008 | −0.003 | 19.429 a | −0.006 | −0.011 | 18.714 a | 0.019 | 0.018 | 26.357 a |
| NAD | 0.010 | 0.009 | 12.071 a | 0.015 | 0.012 | 18.857 a | 0.043 | 0.036 | 33.571 b |
| BAI | 0.007 | 0.004 | 9.286 a | 0.019 | 0.016 | 19.714 b | 0.082 | 0.076 | 35.500 c |
| ROI of 25 × 25 pixels | |||||||||
| AVD | 1.782 | 1.792 | 12.500 a | 2.743 | 2.474 | 18.214 a | 8.265 | 5.757 | 33.786 b |
| IM | 8.624 | 6.774 | 11.929 a | 29.851 | 13.605 | 18.714 a | 258.270 | 73.311 | 33.857 b |
| RVD | −0.008 | −0.005 | 19.929 a | −0.007 | −0.011 | 19.286 a | 0.015 | 0.020 | 25.286 a |
| NAD | 0.010 | 0.009 | 12.000 a | 0.015 | 0.012 | 19.000 a | 0.043 | 0.036 | 33.500 b |
| BAI | 0.006 | 0.004 | 9.143 a | 0.018 | 0.015 | 19.857 b | 0.080 | 0.072 | 35.500 c |
| Activity Indicator | F0 | F24 | F72 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Mean Rank | Mean | Median | Mean Rank | Mean | Median | Mean Rank | |
| AVD | 1.776 | 1.906 | 14.400 a | 2.534 | 2.152 | 18.333 a | 8.421 | 5.660 | 36.267 b |
| IM | 8.260 | 7.496 | 13.400 a | 24.279 | 12.010 | 19.267 a | 272.559 | 77.261 | 36.333 b |
| RVD | −0.004 | −0.003 | 26.800 a | 0.001 | −0.005 | 21.533 a | 0.013 | 0.006 | 20.667 a |
| NAD | 0.010 | 0.010 | 12.933 a | 0.015 | 0.011 | 20.533 a | 0.041 | 0.033 | 35.533 b |
| BAI | 0.004 | 0.003 | 9.467 a | 0.014 | 0.014 | 22.267 b | 0.076 | 0.071 | 37.267 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janaszek-Mańkowska, M.; Ratajski, A.; Słoma, J. Biospeckle Activity of Highbush Blueberry Fruits Infested by Spotted Wing Drosophila (Drosophila suzukii Matsumura). Appl. Sci. 2022, 12, 763. https://doi.org/10.3390/app12020763
Janaszek-Mańkowska M, Ratajski A, Słoma J. Biospeckle Activity of Highbush Blueberry Fruits Infested by Spotted Wing Drosophila (Drosophila suzukii Matsumura). Applied Sciences. 2022; 12(2):763. https://doi.org/10.3390/app12020763
Chicago/Turabian StyleJanaszek-Mańkowska, Monika, Arkadiusz Ratajski, and Jacek Słoma. 2022. "Biospeckle Activity of Highbush Blueberry Fruits Infested by Spotted Wing Drosophila (Drosophila suzukii Matsumura)" Applied Sciences 12, no. 2: 763. https://doi.org/10.3390/app12020763
APA StyleJanaszek-Mańkowska, M., Ratajski, A., & Słoma, J. (2022). Biospeckle Activity of Highbush Blueberry Fruits Infested by Spotted Wing Drosophila (Drosophila suzukii Matsumura). Applied Sciences, 12(2), 763. https://doi.org/10.3390/app12020763






