Clinical Comparison of the Glomerular Filtration Rate Calculated from Different Renal Depths and Formulae
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
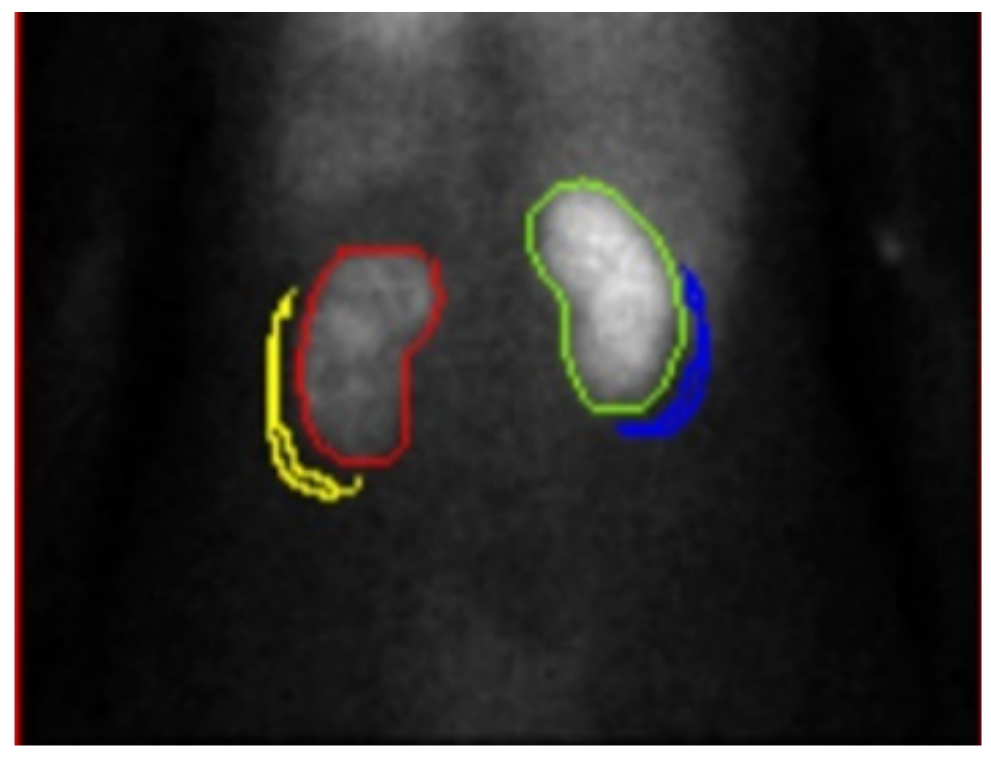
2.2. Renal Dynamic Image
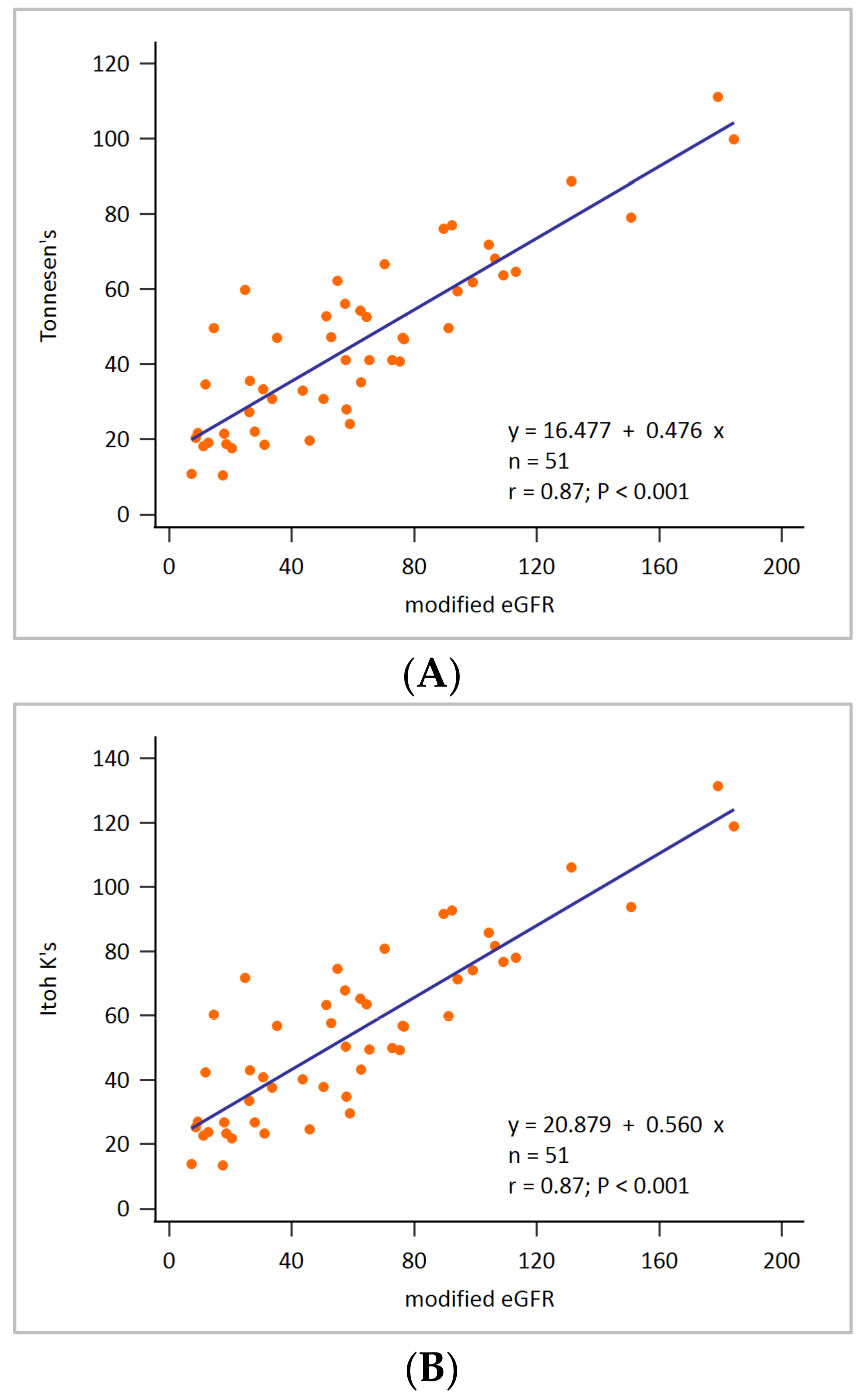
2.3. Assessment of Renal Depth by Tonnesen’ s Formula
2.4. Assessment of Renal Depth by Itoh K’s Formula
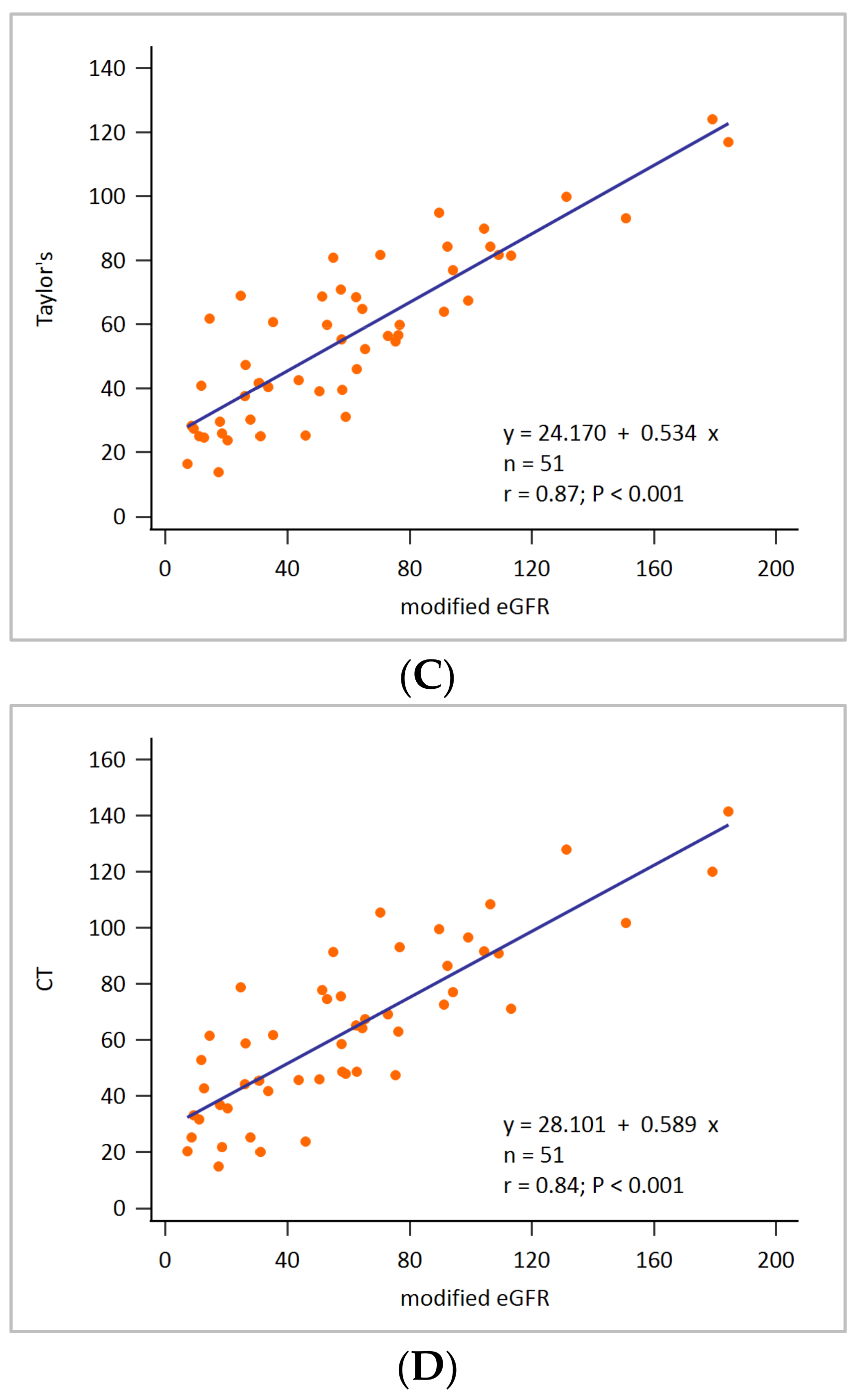
2.5. Assessment of Renal Depth by Taylor’s Formula
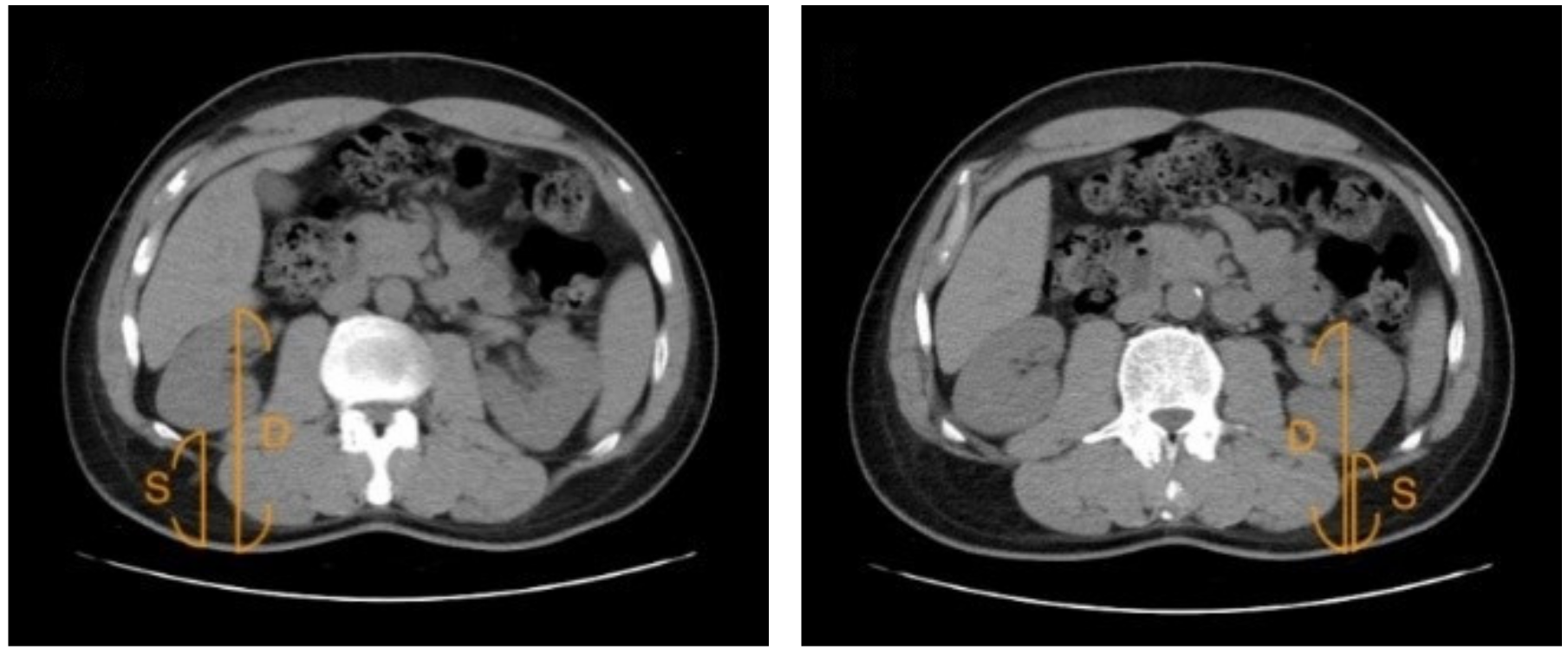
2.6. Assessment of Renal Depth by CT
2.7. Estimated GFR (eGFR)
2.8. Modified Estimated GFR (Modified eGFR)
2.9. Statistical Analysis
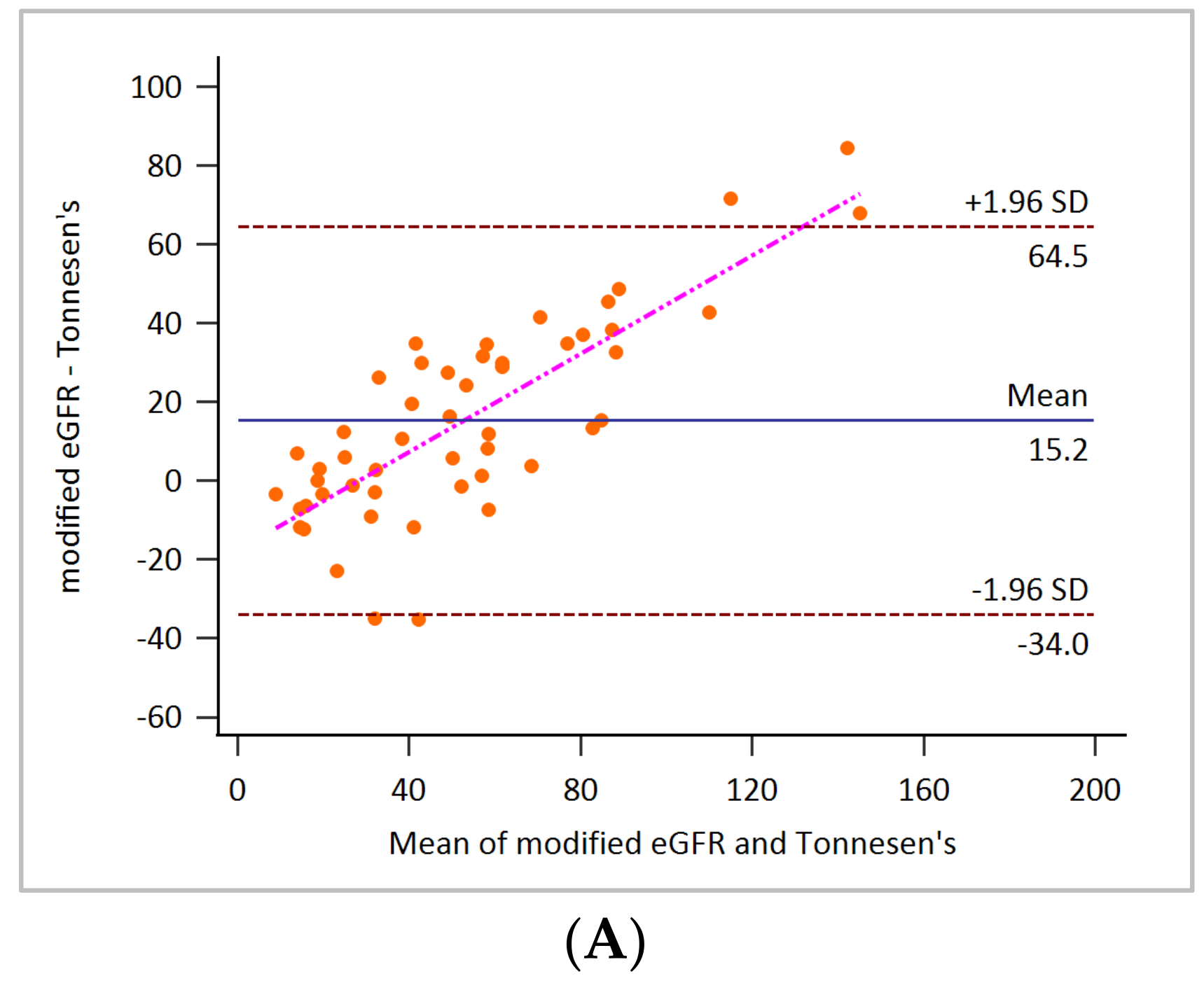
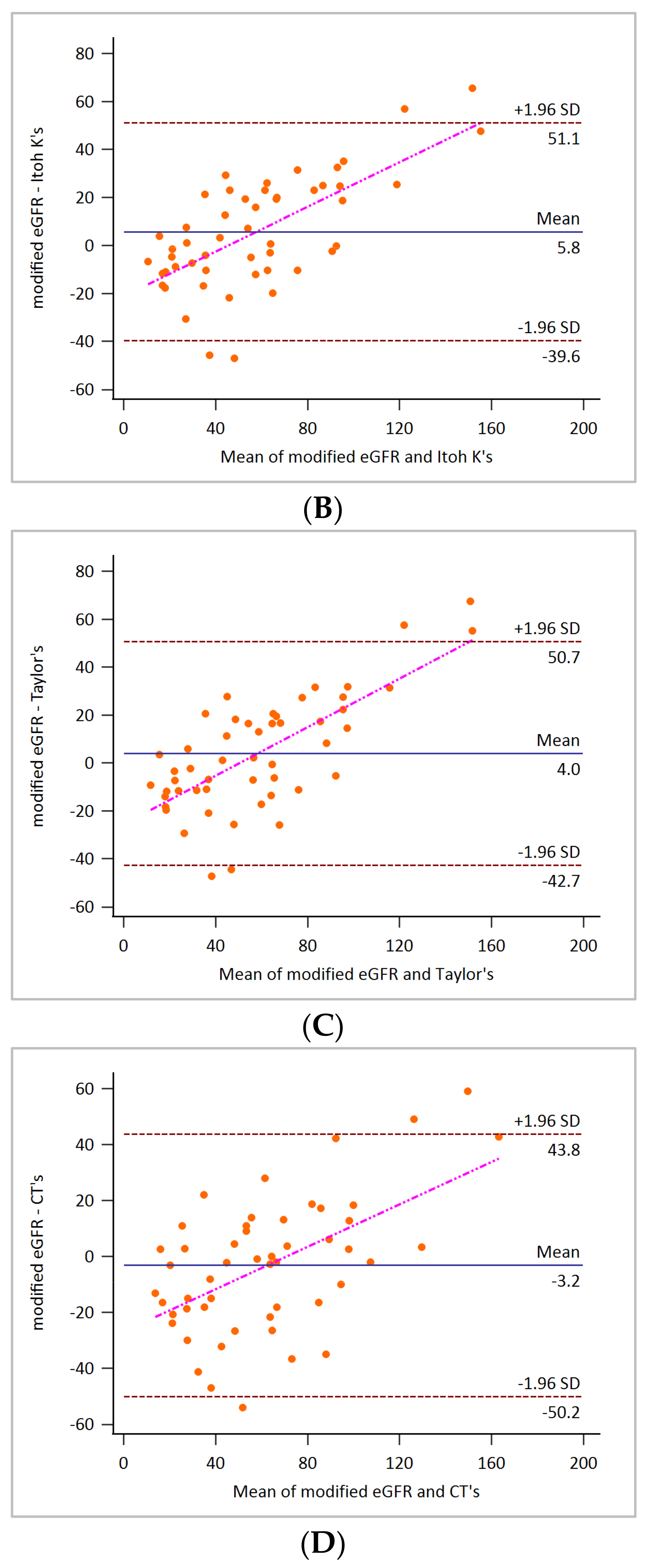
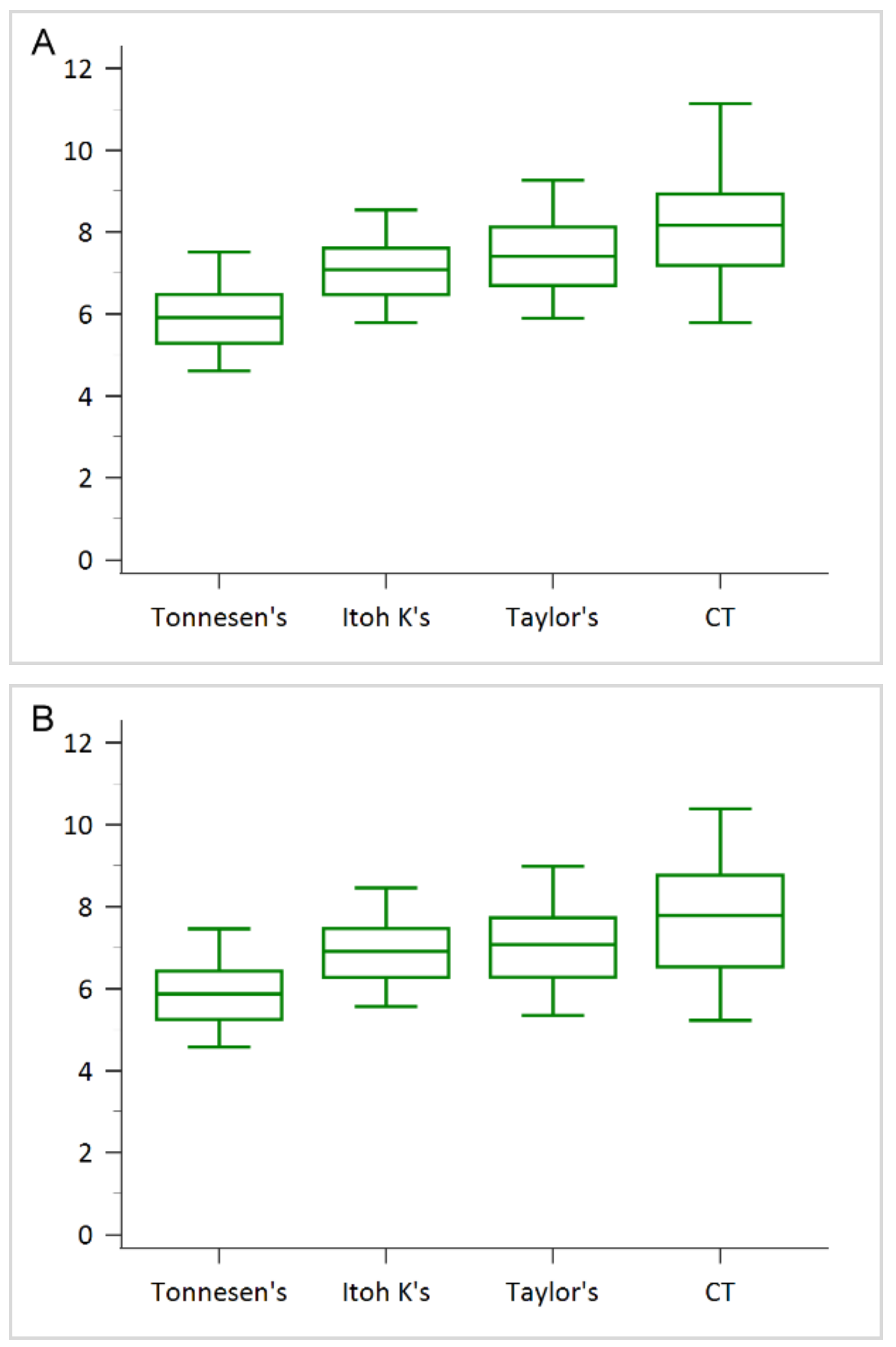
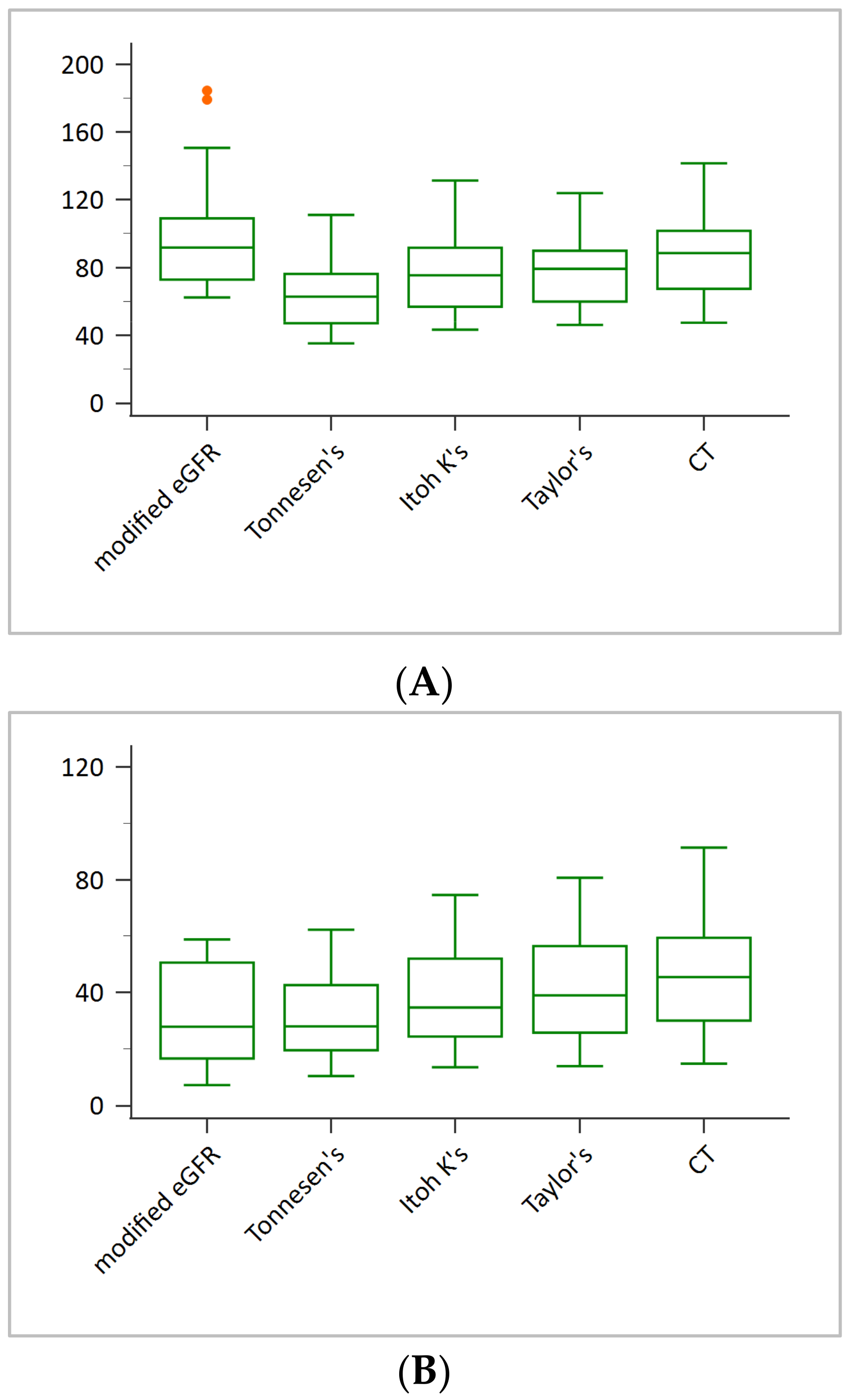
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transpl. 2019, 34, 1803–1805. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Al-Aly, Z.; Bello, A.K.; Bellorin-Font, E.; Carlini, R.G.; Fabian, J.; Garcia-Garcia, G.; Iyengar, A.; Sekkarie, M.; van Biesen, W. Sustainable Development Goals relevant to kidney health: An update on progress. Nat. Rev. Nephrol. 2021, 17, 15–32. [Google Scholar] [CrossRef]
- Price, M. Comparison of creatinine clearance to inulin clearance in the determination of glomerular filtration rate. J. Urol. 1972, 107, 339–340. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef]
- Levey, A.S.; Bosch, J.P.; Lewis, J.B.; Greene, T.; Rogers, N.; Roth, D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann. Intern. Med. 1999, 130, 461–470. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Chen, L.-I.; Kuo, M.-C.; Hwang, S.-J.; Chen, Y.-W.; Wu, K.-D.; Chen, H.-C. Comparisons of technetium-99m diethylenetriaminepentaacetic acid plasma clearance and renal dynamic imaging with inulin clearance. Am. J. Kidney Dis. 2011, 58, 1043–1045. [Google Scholar] [CrossRef]
- De Santo, N.G.; Anastasio, P.; Cirillo, M.; Santoro, D.; Spitali, L.; Mansi, L.; Celentano, L.; Capodicasa, D.; Cirillo, E.; Del Vecchio, E. Measurement of Glomerular FiltrationRate by the 99mTc-DTPA Renogram Is Less Precise than Measured and Predicted Creatinine Clearance. Nephron 1999, 81, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Gates, G.F. Glomerular filtration rate: Estimation from fractional renal accumulation of 99mTc-DTPA (stannous). Am. J. Roentgenol. 1982, 138, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Granerus, G.; Moonen, M. Effects of extra-renal background subtraction and kidney depth correction in the measurement of GFR by gamma camera renography. Nucl. Med. Commun. 1991, 12, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Maneval, D.C.; Magill, H.L.; Cypess, A.M.; Rodman, J.H. Measurement of skin-to-kidney distance in children: Implications for quantitative renography. J. Nucl. Med. 1990, 31, 287–291. [Google Scholar]
- Tonnesen, K. Influence on the radiorenogram of variation in skin to kidney distance and the clinical importance hereof. In Radionuclides in Nephrology; Thieme: Stuttgart, Germany, 1975; pp. 79–86. [Google Scholar]
- Itoh, K.; Arakawa, M. Re-estimation of renal function with 99m Tc-DTPA by the Gates’ method. Kaku Igaku 1987, 24, 389–396. [Google Scholar] [PubMed]
- Taylor, A.; Lewis, C.; Giacometti, A.; Hall, E.C.; Barefield, K.P. Improved formulas for the estimation of renal depth in adults. J. Nucl. Med. 1993, 34, 1766–1769. [Google Scholar] [PubMed]
- Ma, Y.-C.; Zuo, L.; Chen, J.-H.; Luo, Q.; Yu, X.-Q.; Li, Y.; Xu, J.-S.; Huang, S.-M.; Wang, L.-N.; Huang, W.; et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J. Am. Soc. Nephrol. 2006, 17, 2937–2944. [Google Scholar] [CrossRef]
- DuBois, D. A formula to estimate the approximate surface area if height and body mass be known. Arch. Intern. Med. 1916, 17, 863–871. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, A.; Lu, X.; Wang, Q.; Yang, L.; Zhang, Y.; Yan, A.; Tian, P.; Xue, J. Renal depth measured by CT optimize the glomerular filtration rate using the Gates method in living donor kidney transplantation. Chin. J. Organ Transpl. 2019, 12, 195–199. [Google Scholar]
- Xue, J.; Deng, H.; Jia, X.; Wang, Y.; Lu, X.; Ding, X.; Li, Q.; Yang, A. Establishing a new formula for estimating renal depth in a Chinese adult population. Medicine 2017, 96, e5940. [Google Scholar] [CrossRef]
- Sugawara, S.; Ishii, S.; Kojima, Y.; Ito, H.; Suzuki, Y.; Oriuchi, N. Feasibility of gamma camera-based GFR measurement using renal depth evaluated by lateral scan of 99mTc-DTPA renography. Ann. Nucl. Med. 2020, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Awdeh, M.; Kouris, K.; Hassan, I.M.; Abdel-Dayem, H.M. Factors affecting the Gates’ measurement of glomerular filtration rate. Am. J. Physiol. Imaging 1990, 5, 36–41. [Google Scholar]
- Gruenewald, S.; Collins, L.; Fawdry, R. Kidney depth measurement and its influence on quantitation of function from gamma camera renography. Clin. Nucl. Med. 1985, 10, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Saad, A.; Herrmann, S.M.; Textor, S.C.; Lerman, L.O. Determination of single-kidney glomerular filtration rate in human subjects by using CT. Radiology 2015, 276, 490–498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- You, S.; Ma, X.W.; Zhang, C.Z.; Li, Q.; Shi, W.W.; Zhang, J.; Yuan, X.D. Determination of single-kidney glomerular filtration rate (GFR) with CT urography versus renal dynamic imaging Gates method. Eur. Radiol. 2018, 28, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Buckley, D.L.; Shurrab, A.E.; Cheung, C.M.; Jones, A.P.; Mamtora, H.; Kalra, P.A. Measurement of single kidney function using dynamic contrast-enhanced MRI: Comparison of two models in human subjects. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2006, 24, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Tipirneni-Sajja, A.; Loeffler, R.B.; Oesingmann, N.; Bissler, J.; Song, R.; McCarville, B.; Jones, D.P.; Hudson, M.; Spunt, S.L.; Hillenbrand, C.M. Measurement of glomerular filtration rate by dynamic contrast-enhanced magnetic resonance imaging using a subject-specific two-compartment model. Physiol. Rep. 2016, 4, e12755. [Google Scholar] [CrossRef]
| Variable | Values a |
|---|---|
| Age | 60.5 ± 13.3 |
| Sex | |
| Male | 21 (41) |
| Female | 30 (59) |
| Height (cm) | 160.6 ± 8.3 |
| Weight (kg) | 63.4 ± 10.9 |
| BMI (kg/m2) | 24.5 ± 3.5 |
| Plasma creatinine (mg/dL) | 1.8 ± 1.3 |
| Modified eGFR (ml/min) | 60.5 ± 42.7 |
| Tonnesen’s GFR (ml/min) | 45.3 ± 23.3 |
| Itoh K’s GFR (ml/min) | 54.7 ± 27.5 |
| Taylor’s GFR (ml/min) | 56.5 ± 26.3 |
| CT GFR (ml/min) | 63.7 ± 30.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, W.-L.; Chang, S.-M.; Chang, C.-C. Clinical Comparison of the Glomerular Filtration Rate Calculated from Different Renal Depths and Formulae. Appl. Sci. 2022, 12, 698. https://doi.org/10.3390/app12020698
Hsu W-L, Chang S-M, Chang C-C. Clinical Comparison of the Glomerular Filtration Rate Calculated from Different Renal Depths and Formulae. Applied Sciences. 2022; 12(2):698. https://doi.org/10.3390/app12020698
Chicago/Turabian StyleHsu, Wen-Ling, Shu-Min Chang, and Chin-Chuan Chang. 2022. "Clinical Comparison of the Glomerular Filtration Rate Calculated from Different Renal Depths and Formulae" Applied Sciences 12, no. 2: 698. https://doi.org/10.3390/app12020698
APA StyleHsu, W.-L., Chang, S.-M., & Chang, C.-C. (2022). Clinical Comparison of the Glomerular Filtration Rate Calculated from Different Renal Depths and Formulae. Applied Sciences, 12(2), 698. https://doi.org/10.3390/app12020698






