Abstract
Traumatic brain injury (TBI) is currently a problematic issue of public health due to its frequency, and many of the mild cases often remain undiagnosed despite the possible predisposition to prolonged or persistent post-concussive symptomatology. It was shown here that the severity and persistence of grey matter (GM) changes following TBI could predict disease outcomes. Our aim was to conduct a voxel-wise meta-analysis to detect significant GM changes following mild TBI (mTBI) and to investigate whether these changes are associated with the duration and severity of post-concussion syndrome (PCS). A voxel-wise meta-analysis was conducted regarding the GM and white matter (WM) changes in mTBI adult patients versus healthy controls, and Seed-based d Mapping was used to correlate the data. Standard meta-analysis statistical processing was used to assess heterogeneity and publication bias. Our analysis showed significant GM volume increases in the left medial cingulate/paracingulate gyri, the middle frontal gyrus, and the right caudate nucleus of the mTBI patients and significant volume loss in the thalamus, the frontal lobe, and the temporal lobe. These changes could potentially be associated with PCS that some mTBI later patients develop as a result to the injury or other compensatory changes. Additional studies considering long-term GM changes in mTBI patients and their potential relationship to PCS could provide further insight into the pathophysiological similarities and correlations between mTBI and PCS.
1. Introduction
Traumatic brain injury (TBI) is a severe public health issue affecting more than 69 million patients per year worldwide [1]. The diagnosis of TBIs had been traditionally based on the Glasgow Coma Scale (GCS); however, the Mayo classification system is now widely used [2]. The Mayo classification system divides TBIs into possible mild, probable mild, and definite moderate/severe based on the presence of loss of consciousness, post-traumatic amnesia, the GCS within the first 24 h, suggestive brain computed tomography (CT) and magnetic resonance imaging (MRI) scans, and the development of additional neurological symptoms [2]. Approximately 75% of TBIs are mild [3] and do not impose severe chronic consequences on the patients. However, 11–82% of the patients will develop post-concussion symptoms, including headaches, nausea, dizziness, impaired vision, auditory impairment, fatigue, and behavioral changes, while the symptoms will persist over time in 10–25% of the cases [4,5,6,7].
The diagnosis of post-concussion syndrome (PCS) is based on the ICD-10 criteria or on the DSM-IV criteria [8,9]. Although typical brain imaging (including CT and MRI) is, by definition, normal in mild TBIs (mTBIs), growing evidence showed that white matter (WM) and grey matter (GM) changes could be used in mTBIs diagnosis in the absence of significant symptomatology and/or could predict the outcomes, including persistent symptom development and PCS [10,11,12]. Moreover, the few mTBI patients the clinics and studies are currently reporting are extremely heterogeneous in terms of cause, age, recovery time span, and outcomes. Thus, a complex tool for considering the differences and analyzing the quantifiable markers of mTBIs outcomes (being the brain matter volumes provided by voxel-wise screening) could be a step forward in providing additional information and predictions regarding the possible negative outcomes of mTBI, including PCS and chronic traumatic encephalopathy [3].
Thus, our aim was to conduct a voxel-wise meta-analysis to detect significant GM changes following mTBI and to investigate whether these changes are associated with the duration and severity of PCS.
2. Materials and Methods
Main scientific databases (PubMed, Cochrane Library, and Web of Science) and BrainMap were screened using keywords, such as [“mild traumatic brain injury” OR “mTBI” OR “concussion”] AND [“voxel-based” OR “morphometry” OR “VBM” OR “volumetric changes”]. PRISMA guidelines were used to conduct the screening and selection processes. The inclusion criteria were: (1) English-language studies providing full-text versions; (2) that compare GM and WM changes in mTBI adult patients and healthy controls; (3) having their results reported in the Montreal Neurological Institute (MNI) or Talairach space. The exclusion criteria formulated for the current study were: (1) duplicates; (2) studies written in other languages or not available as full-text versions; (3) not providing detailed coordinates for volumetric changes; (4) not comparing with healthy individuals (sex and age matched-controls); or (5) not providing information on the demographic description of the population samples. Data screening and selection was performed by two different investigators. For each study, the number of participants, demographic description, symptomatology, and peak coordinates were extracted.
The meta-analysis was carried out using the SDM software package (Seed-based d Mapping software, version 6.21 for 64-bit Windows PC) [13,14,15] and compared the differences of regional volume changes between the two groups. For each study included in our analysis, we prepared a file with the coordinates, effect size, and sample size, following the guidelines provided by the developers [13,14,15], with full width at half-maximum set at 20 mm and a statistical significance threshold of p < 0.005 [16]. These values have been suggested in previous studies as exhibiting the best balance between sensitivity and specificity and the best control for false-positive rates [17].
The statistical analysis was entirely carried out by the software including data extraction and effect-size mapping of the regional differences recreation. The mean map was then produced through a meta-analytical random-effects model with the weight of each study being calculated by the sample size, intra-study variability, and between-study heterogeneity (according to the formulas presented in the developer’s software manual and the theoretical description of the methods [18]).
The statistical significance was established with standard randomization tests. Default kernel size and statistical thresholds were used to optimize the recreation of the effect-size maps and, at the same time, to preserve robustness, as described by the method developers [19].
In addition to the above, we conducted Jack-knife sensitivity analyses to assess the robustness of the findings by repeating the mean analysis excluding one study each time and recalculating the SMD. We carried out a heterogeneity analysis to determine significant unexplained between-study variability within the results using a random effects model with Q statistics [20,21], and the publication bias was calculated with visual inspection of the funnel plots and using Egger’s test within the SDM software, Cochrane Risk of Bias Tool, and the Robvis tool [21].
3. Search Results and Study Selection Process
Our initial search yielded 102 studies. The initial screening steps (duplicates, language) excluded 34 studies. Abstract screening was thus conducted on 68 studies, of which 30 were retrieved for full-text evaluation, and 18 studies were excluded (not screening adults, not comparing mTBI adult patients and healthy controls, not providing information on the demographic description of the population samples). During the data extraction process, 12 studies were excluded (not reporting in the Montreal Neurological Institute (MNI) or Talairach space or not providing detailed coordinates for volumetric changes). Thus, the present voxel-wise meta-analysis was finally conducted on five relevant studies [22,23,24,25,26].
All the studies used structural T1 MRI for their brain imaging analyses.
PRISMA flowchart was constructed to describe the selection process (Figure 1).
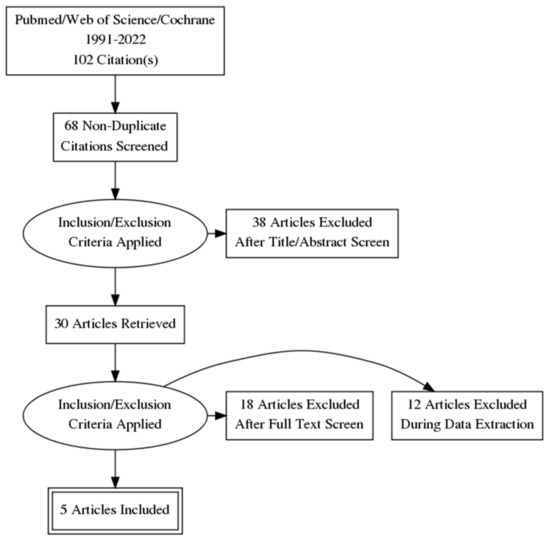
Figure 1.
Screening and selection process—PRISMA flowchart.
4. Qualitative Characteristics of the Studies
Gale et al. [22] used voxel-based morphometry (VBM) to evaluate changes in GM concentration following traumatic brain injury. Nine patients with a history of TBI, ranging from mild to severe and nine age- and gender-matched controls were recruited, and cognitive testing was used to determine the correlations between regional GM concentration, attention, and initial injury severity. The results showed decreased GM concentrations in multiple brain regions (frontal and temporal cortices, cingulate gyrus, subcortical GM, and cerebellum) in TBI patients compared with controls. Significant direct correlations between GM, attention, and GCS were reported [22].
Niu et al. [23] investigated peripheral inflammation and cortical volume changes in mTBI and acute headache patients. Significantly increased GM volume in the right dorsal anterior cingulate cortex and dorsal posterior cingulate cortex was observed in the 77 mTBI patients compared with the 42 controls. Persistently increasing volumetric changes were reported in mTBI patients with chronic headache, at 3 months following initial injury [23].
Significant differences in the regional volume of the right precuneus and medial orbitofrontal gyrus in two mTBIs patients were reported by Wilke et al. [24]. Accelerated hippocampal volume loss was seen in older participants with recurrent mTBI [24], a difference not considered in the meta-analysis calculations.
GM volume changes were also investigated by Burrowes et al. [25] in 50 mTBI patients, of whom 19 were experiencing post-traumatic headaches, and 21 healthy controls. It was reported the mTBI patients with post-traumatic headaches showed decreased GM volume in the right anterior-parietal and left temporal-opercular areas, in contrast with the non-post-traumatic headaches mTBI group, who exhibited decreased volume in the left thalamus. The post-traumatic headache patients showed decreased GM volume in the left-temporal-opercular, temporal-parietal, superior frontal gyrus, and right middle frontal/superior frontal gyrus, as well as in the anterior-parietal gyrus [5].
Wu et al. [26] investigated the grey matter density in the thalamus in 18 patients with mTBI and diffuse axonal injury in comparison with 18 healthy controls and reported significantly decreased GM density in bilateral thalami correlated with motor dysfunction in mTBI patients [26].
5. Quantitative Results
A total of 5 studies were included in the quantitative voxel-wise meta-analysis, with 188 TBI patients and 108 controls.
Although there were some concerns about patients’ randomization and selection in some of the studies (Figure 2A), the overall risk of bias was low (Figure 2B).
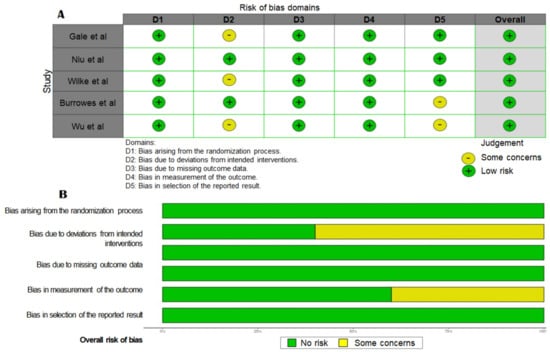
Figure 2.
(A) Risk of bias assessment for individual studies (refs. [22,23,24,25,26]) included in the meta-analysis and (B) summary of the risk of bias of the selected studies.
6. GM Volume Increase
Our analysis showed increased GM volume in the left median cingulate/paracingulate gyri (Brodmann field 23, MNI: −2, −32,36, SDM-Z 1.909, p < 0.0001, voxels: 1259), the right middle frontal gyrus (Brodmann field 9, MNI: 28, 30, 42, SDM-Z 1.895, p < 0.0001, voxels: 282), and the right caudate nucleus (MNI: 8, 6, 4, SDM-Z 1.869, p < 0.0001, voxels: 206) (Figure 3A–F).
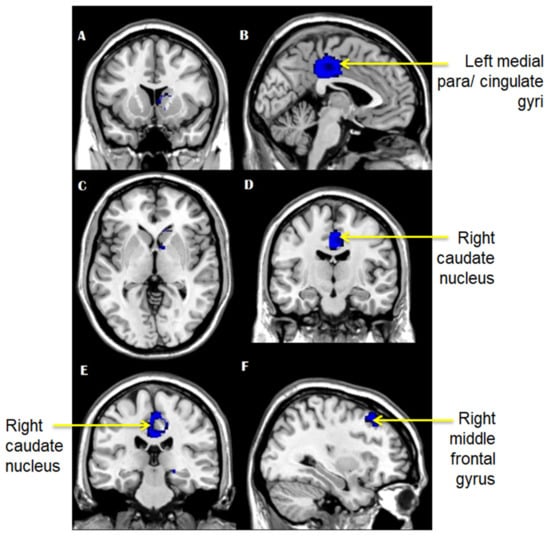
Figure 3.
(A–F): Brain areas that showed increased grey matter volume in mTBI patients compared with controls.
7. GM Volume Decrease
Voxel-wise meta-analysis showed decreased GM volume in TBI patients compared with controls in the right thalamus (MNI: 4, −16, 6, SDM-Z −1.025, p < 0.0001, voxels: 322), the left superior frontal gyrus (Brodmann field 11, MNI: −14, 64, −4, SDM-Z −1.007, p < 0.0001, voxels: 292), the corpus callosum (MNI: 16, −64, 40, SDM-Z −1.010, p < 0.0001, voxels: 208), the right middle frontal gyrus (Brodmann field 45, MNI: 44, 44, 22, SDM-Z −1.024, p < 0.0001, voxels: 125), the left superior frontal gyrus (Brodmann field 10, MNI: −16, −60, 24, SDM-Z −1.020, p < 0.0001, voxels: 87), and the left middle temporal gyrus (Brodmann field 21, MNI: −68, −22, −18, SDM-Z −1.024, p < 0.0001, voxels: 78) (Figure 4A–F).
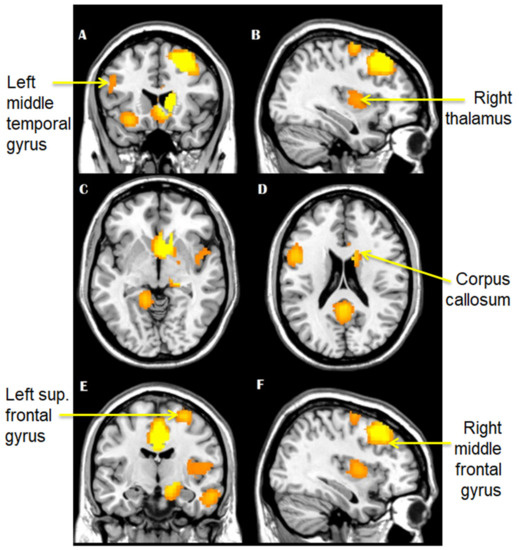
Figure 4.
(A–F): Brain areas that showed decreased grey matter volume in mTBI patients, as compared to controls.
8. Sensitivity Analysis
We conducted a Jack-knife analysis for a complete assessment of the robustness of the results by omitting one study every time and recalculating the SMD-Z. Sensitivity analysis confirmed that the results were robust as there were no significant changes to the overall results.
9. Qualitative Mini Review of Other Studies Regarding the GM Volume Changes in mTBI Patients
Although some studies did not fully meet the inclusion criteria, their results were worth considering in the discussion of the voxel-wise meta-analysis for providing a better understanding of the clinical relevance of our findings; Thus, we here summarize them in a short systematic review.
9.1. Longitudinal Changes in GM Volume in mTBI Patients
Killgore et al. [27] employed voxel-based morphometry in a cross-sectional sample of 26 patients with mTBI and 12 healthy controls by assessing brain morphometrics at 2 weeks, 1 month, 3 months, 6 months, and 1-year post-injury. Larger GM volume within the ventromedial prefrontal cortex and right fusiform gyrus were correlated with longer duration of time since injury, while the ventromedial prefrontal cortex was positively correlated with neurocognitive performance. Additionally, significantly larger GM volume was reported in more chronic individuals, in contrast with healthy controls [27].
Porto et al. [28] investigated the WM changes in adult survivors of childhood TBIs. Detectable axonal injury or chronic contusion on late conventional MRI was reported. VBM on 12 adults with a history of childhood mild or moderate to severe TBI demonstrated higher mean diffusivity in the right cerebral WM, bilaterally in the forceps major and in the body of the splenium of the corpus callosum, while VBM showed bilaterally reduced WM volumes, mainly along the callosum splenium. No GM volumetric changes were identified in this study [28].
Bendlin et al. [29] used high-resolution T1-weighted imaging and diffusion tensor imaging (DTI) to evaluate regional changes. Neuropsychological tests and MRIs were performed at approximately 2 and 12.7 months post-injury in 46 TBI patients and 36 matched controls. Significant neuropsychological function improvement was reported from visit 1 to visit 2 and GM and WM changes in all major fiber bundles in the brain including the corpus callosum, cingulum, superior and inferior longitudinal fascicules, uncinate fasciculus and brain stem, thalamus, and bilateral pallidum were observed during imaging. Mild TBI patients also showed small regions of decreased volume in the cingulum, right post-central gyrus, supplementary motor area, right precentral gyrus, and bilateral putamen. In this way, Bendlin et al. [29] showed that both WM and GM degeneration is a significant contributor to brain volume loss in the post-injury period.
GM and WM changes have been also reported by Muller et al. [30] in their study on 48 uncomplicated mTBI patients and 37 orthopedic controls. Using MRI and VBM, significant GM decrease was observed predominantly in the right hemisphere along GM and CSF border in the lateral and medial portions of the sensorimotor cortex extending into the Rolandic operculum, middle frontal gyrus, insula, and temporal pole. In addition, significant WM decrease in the right hemisphere and more specifically the superior fasciculus longitudinalis, arcuate fasciculus, and cortical-pontine tracts, as well as a significant WM increase in the left arcuate fasciculus and left external capsule were reported [30].
Ledig et al. [31] calculated regional volume and asymmetry features at the acute and subacute stages of mild to severe traumatic brain injuries in 67 patients and found that there are small structural volume changes in the acute stage that can be strong predictors of unfavorable outcomes. Furthermore, patients with unfavorable outcomes showed increased atrophy [31].
The longitudinal subcortical volumetric changes in mTBI patients have been investigated by Zhou et al. [32] in 56 patients and 34 healthy matched controls. A reduction in thalamic and hippocampal volume at 1 month following injury was reported in mTBI patients. The thalamic volume changes persisted for up to 6 months and were correlated with post-concussion symptoms and functional outcomes in mTBI patients [32].
In a prospective cohort study of 25 mTBI patients and 18 matched controls, da Costa et al. [33] reported no significant difference in brain volumes between the two groups, but significant decrease in GM volume in mTBI patients between the first visit, which took place at a mean of 63 days, and second visit, at 180 days post-injury. Also, GM volume loss was positively correlated to patients’ performance on Sport Concussion Assessment Tool 2 (SCAT2) tests [33].
9.2. GM Changes, Cognitive Performance, and Psychiatric Symptoms
On the other hand, Depue et al. [34] used voxel-based morphometry and surface-based morphometry to determine whether comorbid post-traumatic stress disorder (PTSD)/mTBI is characterized by brain structure alterations in the same regions as observed in singular diagnostic PTSD or TBI. In that study, 20 male combat veterans with comorbid PTSD/mTBI diagnosis and 14 male veterans without PTSD or mTBI were evaluated and, volumetric reductions in the bilateral anterior amygdala were observed in the comorbid samples compared with controls. Increased volumetric reduction in the amygdala that predicted poorer inhibitory modulation, increased self-reported impulsivity, and greater symptoms associated with PTSD was reported [34].
Similarly, Chen et al. [35] reported decreased GM density in the dorsolateral prefrontal cortex, striatum, medial frontal, and temporal regions and reduced activation in 24 athletes with concussion and depressive symptoms, as compared to 16 controls and 16 athletes with a concussion, but not depressive symptoms [35].
Livny et al. [10] recruited 50 adult TBI patients and 35 controls and showed a significant positive correlation between cognitive performance testing scores and GM volume in left and right insular cortices using voxel-based regression [10].
9.3. Structural Covariance
Despite these nonstandard approaches to brain morphological change screening in mTBI patients, structural covariance analysis could provide significant evidence regarding the disturbances occurring in the interconnections between the brain networks, rather than between the focal regions [36].
Song et al. [36] analyzed the structural covariance in the acute and chronic phases of mTBI in comparison to controls. Mild TBI patients showed decreased structural covariance in the left supplementary motor area, the left frontal inferior orbital cortex, the left and right insular cortex, and the right supplementary temporal cortex. Chronic mTBI patients showed decreased structural covariance in the right middle occipital cortex, left fusiform cortex, right middle temporal and cingulum, left middle cingulum, left hippocampus, and left supplementary temporal cortex and increased structural covariance in the right precentral and postcentral cortex, right supplementary motor area, left medial frontal supplementary area, left middle and supplementary cortex, and right postcentral and right supplementary parietal cortex [36]. These findings could shed more light on the possible morphological landscapes preserved or changed by the mTBI effects and better explain the sources of the reported mild cognitive and emotional impairments. Also, Song et al. suggested that the structural covariance analysis of the bilateral prefrontal cortices and the right insula could be a potential candidate for mTBI diagnosis and prognosis biomarker.
10. Discussion
Conventionally, while brain MRI is an imaging method showing the brain activity based on increased blood flow, DTI is a special MRI based on the water molecules flow through the neuronal fibers, mainly used to monitor the white matter pathways [37,38]. Due to its specificity to white matter imaging, DTI is currently preferred in mTBI study, diagnosis, and outcome prognosis [39,40]. Despite that, several studies investigated the volumetric changes of brain grey matter using T1 MRI. While the brain has high lipid content and fat has a shorter longitudinal magnetization realignment period than water, T1 MRI was considered a reliable method to evaluate the possibly subtle volume changes of the grey matter previously reported in mTBI cases [22,23,24,25,26]. A recent study published in the Nature Group journal Pediatric Research suggested that T1 and T2 MRI scanning could be sensitive to detecting minuscule hypoxia brain damage in newborns, thus, to assist to mild hypoxic-ischemic encephalopathy diagnosis [41].
Our meta-analysis, as well as the quantitate mini-review of the studies not providing voxel-wise parameters, showed that some brain structures damages are redundant (Table 1). Considering that the main scope of medical imaging in clinical practice is to assist the clinical diagnosis and provide recovery prognosis, these findings could lead to promising imaging biomarkers which could be followed throughout the patient’s recovery process for further assistance in treatment and reinstatement (for example, in sports and military service) [3]. In this way, if a pattern of GM changes could be seen in mTBI patients in a reproducible manner, and if the duration and severity of the consequent symptoms (PCS-dependent or independent), proper management of the mTBI and PCS patients could be performed. In other words, in clinical practice the association between visible symptomatology (mild traumatic brain injury-associated short term or chronic symptoms, including PCS) and organ damage (i.e., brain changes in structure or composition) could mean that the patient evolution could be indirectly correlated with either one. Infections and other diseases could also lead to GM and WM volume changes, as a result to inflammatory response or neuronal loss (as typically seen in Alzheimer’s disease). However, mTBI and the observation of acute and/or chronic symptoms evolution are guided by specific differential diagnosis criteria. The overlap between the two very distinct causes in terms of similar brain matter changes could be an interesting perspective in a future study.

Table 1.
Summary of the main findings and characteristics of the studies included in the present review and meta-analysis.
In our analysis, suggested that the frontal and temporal cortices seem to be mainly affected, showing a significant decrease in GM volume in mTBI patients. Since the frontal and temporal cortices are implicated in cognitive processes, such as attention, prospective memory, and cognitive flexibility, and stimuli perception, emotional processing, and memory formation, respectively, it could be suggested that the general negative outcomes of mTBIs could be associated with cognitive impairments regarding the mentioned processes. In this way, the most frequent condition following mTBIs is the post-concussion syndrome, characterized by headaches, postural and balance impairments, and attention and concentration deficits [3], all of which are short-termed and fully reversible.
Frontal and temporal lobes (Brodmann areas: 11, 45, 10, 21) showed significant volume loss, while 23 Brodmann areas that correspond to cingulate/paracingulate gyri and 9 that corresponds to the middle frontal gyrus, as well as the caudate nucleus, showed significant GM volume increase. Other areas that showed reduced GM in TBI patients were the thalamus and the amygdala. In this context, thalamic atrophy was correlated with persistent post-traumatic headaches and with motor dysfunction in mTBI patients, while frontal and temporal lobe changes have been associated with cognitive impairment, memory and concentration issues, and behavioral changes that mTBI patients could develop. Changes in the amygdala volumes could be correlated with deficits in inhibitory control in patients who develop PTSD following mTBI [34].
However, based on the brain injury span and location, some of the symptoms could persist over time, never to remit, or even progress to more serious long-termed negative outcome, such as the chronic traumatic encephalopathy [3]. This could be the reason why the cerebellum, the basal ganglia, the hippocampus, the amygdala, and the thalamus also showed reduced GM volume following mTBIs. Stimuli perception and response, memory and learning, and emotion processing are also described as impaired in recurrent mTBI patients who later developed chronic traumatic encephalopathy [42].
A percentage of 40–80% of mTBI patients were shown to develop PCS with symptoms including headache, fatigue, irritability, dizziness, balance issues, affected sleep, poor memory and concentration, and increased sensitivity to light and noise. The symptoms were usually occurring shortly following brain injury and could persist for weeks or months [43]. Approximately 15% of patients developed persistent symptoms for more than 6 months or 1 year. Until now, PCS was characterized by the absence of objective findings on examination and on brain imaging [43].
Furthermore, the recent studies reported that the already mentioned brain structure alterations could lead to chain reactions throughout the other brain structure with which they are closely interacting. For an instance, the entire pathway of stimuli and emotional processing was described as possibly impaired following mTBI events, as the patients are reporting confusion, and haziness [44,45]. In some severe cases, mTBI patients are incapable to recognize objects and familiar individuals, meaning that recognition memory could be impaired [46]. Thus, relative to the mentioned cognitive functions, our analyses showed that the cingulate cortex, the prefrontal cortex, and fusiform gyrus have shown a significant increase in GM volume in mTBI patients.
Furthermore, several recent studies suggested that the vasculature structure and ramification could also suffer significant changes following mTBI events. Since most of the total voxel overlap for some of the areas in which GM changes were reported was observed in VBM analysis, it could be suggested that the other changes could be related to vasculature structure and ramification. For an instance, frequent vascular injury following TBI were reported as in many cases the brain vessels suffer from ruptures and tearing resulting in micro or macro-bleeding and cerebral hypoperfusion, ischemia, hypoxia, hemorrhage, blood–brain barrier disruption, and edema [47,48]. In specific animal model studies, this phenomenon was extensively observed and significant changes in brain vasculature after mTBI episodes were reported. Gama Sosa et al. [49] saw chronic vascular remodeling and perivascular astrocytic degeneration in association with vascular neuroinflammation in a rat model of mTBI (low-level blast exposure model). Additionally, while the acute rarefication of the vascular network was reported in a rat model of mTBI [50], Farajzadeh Khosroshahi et al. [51] suggested that the alignment of the direction of the blast with the line of brain vasculature could furthermore potentiate the blast effect.
All in all, our meta-analysis showed that significant GM volume increase was typically seen in the left medial cingulate/paracingulate gyri, the middle frontal gyrus, and the right caudate nucleus of the mTBI patients, while significant volume loss in numerous brain areas, including the thalamus, the frontal lobe, and the temporal lobe was repeatedly reported (Table 1). These results suggest that despite the initially mild brain damaging outcome of the traumatic event, the actual effects on the brain structures and cognitive processes are wider and could persist over time.
11. Limitations
The facts that our search was restricted to English-written studies, the number of selected studies that provided MNI coordinated data was relatively low, and the differences in methodology are inevitable in meta-analyses could be considered the limitations of our study. This could be the reason why some of the statistical parameters could not be properly calculated and reported.
Furthermore, considering the specific design of the analyzed studies, the current study could not provide the relative and/or the quantitative difference between GM and WM in the TBI patients, an additional perspective for analysis.
12. Conclusions
The current study brought additional evidence suggesting that mTBI is associated with grey matter volumetric changes in the frontal and temporal lobes, basal ganglia, amygdala, and thalamus. These changes could potentially be linked to PCS that some mTBI patients later develop as a result to the brain injury or to compensatory changes. Additional studies considering the long-term grey matter changes in mTBI patients and their potential relationship to post-concussion symptoms could provide further insight into the pathology and pathophysiology of mTBI and PCS.
Author Contributions
Conceptualization, methodology, investigation, I.M. and S.C.; software, validation, formal analysis, and data curation, I.M. and S.C.; writing—original draft preparation, I.M., S.C. and I.-M.B.; writing—review and editing, I.-M.B., A.C., R.C., A.I. and A.-C.L.; visualization, I.M. and S.C.; supervision, I.M., A.C. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
TBI—traumatic brain injury, GM—grey matter, WM—white matter, mTBI—mild traumatic brain injury, PCS—post-concussion syndrome, GCS—Glascow Coma Scale, CT—computer tomography, MRI—magnetic resonance imaging, ICD-10—International Classification of Diseases, 10th edition, DSM-IV—Diagnostic and Statistical Manual of Mental Disorders, 4th edition, MNI—Montreal Neurological Institute, SDM—Seed-based d Mapping, PRISMA—Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PROSPERO—International Prospective Register of Systematic Reviews, VBM—voxel-based morphometry, DTI—diffusion tensor imaging MRI, PTSD—post-traumatic stress disorder.
References
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. JNS 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Malec, J.F.; Brown, A.W.; Leibson, C.L.; Flaada, J.T.; Mandrekar, J.N.; Diehl, N.N.; Perkins, P.K. The Mayo Classification System for Traumatic Brain Injury Severity. J. Neurotrauma 2007, 24, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, I.; Kazis, D.; Chowdhury, R.; Petridis, F.; Costa, V.; Balmus, I.M.; Ciobica, A.; Luca, A.C.; Radu, I.; Dobrin, R.P.; et al. Post-Concussion Syndrome and Chronic Traumatic Encephalopathy: Narrative Review on the Neuropathology, Neuroimaging and Fluid Biomarkers. Diagnostics 2022, 12, 740. [Google Scholar] [CrossRef]
- Hiploylee, C.; Dufort, P.A.; Davis, H.S.; Wennberg, R.A.; Tartaglia, M.C.; Mikulis, D.; Hazrati, L.N.; Tator, C.H. Longitudinal Study of Postconcussion Syndrome: Not Everyone Recovers. J. Neurotrauma 2017, 34, 1511–1523. [Google Scholar] [CrossRef]
- Ruff, R.M. Mild traumatic brain injury and neural recovery: Rethinking the debate. NeuroRehabilitation 2011, 28, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Caplain, S.; Blancho, S.; Marque, S.; Montreuil, M.; Aghakhani, N. Early detection of poor outcome after mild traumatic brain injury: Predictive factors using a multidimensional approach a pilot study. Front. Neurol. 2017, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.D.; Cancelliere, C.; Carroll, L.J.; Côté, P.; Hincapié, C.A.; Holm, L.W.; Hartvigsen, J.; Donovan, J.; Nygren-de Boussard, C.; Borg, J.; et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: Results of the international collaboration on mild traumatic brain injury prognosis. Arch. Phys. Med. Rehabil. 2014, 95 (Suppl. S3), S132–S151. [Google Scholar] [CrossRef]
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; DSM-IV; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Livny, A.; Biegon, A.; Kushnir, T.; Harnof, S.; Hoffmann, C.; Fruchter, E.; Weiser, M. Cognitive Deficits Post-Traumatic Brain Injury and Their Association with Injury Severity and Gray Matter Volumes. J. Neurotrauma 2017, 34, 1466–1472. [Google Scholar] [CrossRef]
- Grossman, E.J.; Ge, Y.; Jensen, J.H.; Babb, J.S.; Miles, L.; Reaume, J.; Silver, J.M.; Grossman, R.I.; Inglese, M. Thalamus and cognitive impairment in mild traumatic brain injury: A diffusional kurtosis imaging study. J. Neurotrauma 2012, 29, 2318–2327. [Google Scholar] [CrossRef]
- Kasahara, K.; Hashimoto, K.; Abo, M.; Senoo, A. Voxel- and atlas-based analysis of diffusion tensor imaging may reveal focal axonal injuries in mild traumatic brain injury—Comparison with diffuse axonal injury. Magn. Reson. Imaging 2012, 30, 496–505. [Google Scholar] [CrossRef]
- Radua, J.; Mataix-Cols, D. Meta-analytic methods for neuroimaging data explained. Biol. Mood Anxiety Disord. 2012, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Müller, V.I.; Cieslik, E.C.; Laird, A.R.; Fox, P.T.; Radua, J.; Mataix-Cols, D.; Tench, C.R.; Yarkoni, T.; Nichols, T.E.; Turkeltaub, P.E.; et al. Ten simple rules for neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2018, 84, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Radua, J.; van den Heuvel, O.A.; Surguladze, S.; Mataix-Cols, D. Meta-analytical comparison of voxel-based morphometry studies in obsessive compulsive disorder vs other anxiety disorders. Arch. Gen. Psychiatry 2010, 67, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Albajes-Eizagirre, A.; Solanes, A.; Fullana, M.A.; Ioannidis, J.P.; Fusar-Poli, P.; Torrent, C.; Solé, B.; Bonnín, C.M.; Vieta, E.; MataixCols, D.; et al. Meta-analysis of Voxel-Based Neuroimaging Studies using Seed-based d Mapping with Permutation of Subject Images (SDMPSI). J. Vis. Exp. 2019, 153, e59841. [Google Scholar] [CrossRef]
- Kazis, D.; Petridis, F.; Chatzikonstantinou, S.; Karantali, E.; Jamali, R.; Chowdhury, R.; Duta, R.; Luca, A.C.; Ciobica, A.; Mavroudis, I. Gray Matter Changes in Juvenile Myoclonic Epilepsy. A Voxel-Wise Meta-Analysis. Medicina 2021, 57, 1136. [Google Scholar] [CrossRef] [PubMed]
- Albajes-Eizagirre, A.; Solanes, A.; Vieta, E.; Radua, J. Voxel-based meta-analysis via permutation of subject images (PSI): Theory and implementation for SDM. NeuroImage 2019, 186, 174. [Google Scholar] [CrossRef]
- Radua, J.; Rubia, K.; Canales-Rodriguez, E.J.; Pomarol-Clotet, E.; Fusar-Poli, P.; Mataix-Cols, D. Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front. Psychiatry 2014, 5, 13. [Google Scholar] [CrossRef]
- Pan, P.L.; Song, W.; Shang, H.F. Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson’s disease. Eur. J. Neurol. 2012, 19, 199–206. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Gale, S.D.; Baxter, L.; Roundy, N.; Johnson, S.C. Traumatic brain injury and grey matter concentration: A preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry 2005, 76, 984–988. [Google Scholar] [CrossRef]
- Niu, X.; Bai, L.; Sun, Y.; Wang, Y.; Bai, G.; Yin, B.; Wang, S.; Gan, S.; Jia, X.; Liu, H. Mild traumatic brain injury is associated with effect of inflammation on structural changes of default mode network in those developing chronic pain. J. Headache Pain 2020, 21, 135. [Google Scholar] [CrossRef] [PubMed]
- Wilke, S.; Prehn, K.; Taud, B.; List, J.; Flöel, A. Multimodal Assessment of Recurrent mTBI across the Lifespan. J. Clin. Med. 2018, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Burrowes, S.A.B.; Rhodes, C.S.; Meeker, T.J.; Greenspan, J.D.; Gullapalli, R.P.; Seminowicz, D.A. Decreased grey matter volume in mTBI patients with post-traumatic headache compared to headache-free mTBI patients and healthy controls: A longitudinal MRI study. Brain Imaging Behav. 2020, 14, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, F.; Zhang, Y.; Li, J.; Kuang, H.; Zhan, J.; Peng, D.; He, L.; Zeng, X.; Gong, H. Thalamic atrophy and dysfunction in patients with mild-to-moderate traumatic diffuse axonal injury: A short-term and mid-term MRI study. NeuroReport 2018, 29, 1282–1287. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D.S.; Singh, P.; Kipman, M.; Pisner, D.; Fridman, A.; Weber, M. Gray matter volume and executive functioning correlate with time since injury following mild traumatic brain injury. Neurosci. Lett. 2016, 612, 238–244. [Google Scholar] [CrossRef]
- Porto, L.; Jurcoane, A.; Margerkurth, J.; Althaus, J.; Zanella, F.; Hattingen, E.; Kieslich, M. Morphometry and diffusion MR imaging years after childhood traumatic brain injury. Eur. J. Paediatr. Neurol. 2011, 15, 493–501. [Google Scholar] [CrossRef]
- Bendlin, B.B.; Ries, M.L.; Lazar, M.; Alexander, A.L.; Dempsey, R.J.; Rowley, H.A.; Sherman, J.E.; Johnson, S.C. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage 2008, 42, 503–514. [Google Scholar] [CrossRef]
- Muller, A.M.; Panenka, W.J.; Lange, R.T.; Iverson, G.L.; Brubacher, J.R.; Virji-Babul, N. Longitudinal changes in brain parenchyma due to mild traumatic brain injury during the first year after injury. Brain Behav. 2021, 11, e2410. [Google Scholar] [CrossRef]
- Ledig, C.; Kamnitsas, K.; Koikkalainen, J.; Posti, J.P.; Takala, R.S.K.; Katila, A.; Frantzén, J.; Ala-Seppälä, H.; Kyllönen, A.; Maanpää, H.R.; et al. Regional brain morphometry in patients with traumatic brain injury based on acute- and chronic-phase magnetic resonance imaging. PLoS ONE 2017, 12, e0188152. [Google Scholar] [CrossRef]
- Zhou, Y.; Kierans, A.; Kenul, D.; Ge, Y.; Rath, J.; Reaume, J.; Grossman, R.I.; Lui, Y.W. Mild traumatic brain injury: Longitudinal regional brain volume changes. Radiology 2013, 267, 880–890. [Google Scholar] [CrossRef]
- da Costa, L.; van Niftrik, C.B.; Crane, D.; Fierstra, J.; Bethune, A. Temporal profile of cerebrovascular reactivity impairment, gray matter volumes, and persistent symptoms after mild traumatic head injury. Front. Neurol. 2016, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Depue, B.E.; Olson-Madden, J.H.; Smolker, H.R.; Rajamani, M.; Brenner, L.A.; Banich, M.T. Reduced amygdala volume is associated with deficits in inhibitory control: A voxel- and surface-based morphometric analysis of comorbid PTSD/mild TBI. BioMed. Res. Int. 2014, 2014, 691505. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-K.; Johnston, K.M.; Petrides, M.; Ptito, A. Neural Substrates of Symptoms of Depression Following Concussion in Male Athletes with Persisting Postconcussion Symptoms. Arch. Gen. Psychiatry 2008, 65, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, J.; Chen, L.; Lu, X.; Zheng, S.; Yang, Y.; Cao, B.; Weng, Y.; Chen, Q.; Ding, J.; et al. Altered gray matter structural covariance networks at both acute and chronic stages of mild traumatic brain injury. Brain Imaging Behav. 2021, 15, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- MRI Basics. Available online: https://my-ms.org/mri_basics.htm (accessed on 4 August 2022).
- Brain Mapping: Functional MRI and DTI. Available online: https://www.mayfieldclinic.com/pe-fmri_dti.htm (accessed on 4 August 2022).
- Douglas, D.B.; Iv, M.; Douglas, P.K.; Anderson, A.; Vos, S.B.; Bammer, R.; Zeineh, M.; Wintermark, M. Diffusion Tensor Imaging of TBI: Potentials and Challenges. Top. Magn. Reson. Imaging 2015, 24, 241–251. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Kim, M.; Keser, Z.; Ibrahim, L.; Singh, S.K.; Ahmad, M.J.; Hasan, O.; Kamali, A.; Hasan, K.M.; Schulz, P.E. Diffusion Tensor Imaging Correlates of Concussion Related Cognitive Impairment. Front. Neurol. 2021, 12, 639179. [Google Scholar] [CrossRef]
- Li, Y.; Wisnowski, J.L.; Chalak, L.; Mathur, A.M.; McKinstry, R.C.; Licona, G.; Mayock, D.E.; Chang, T.; Van Meurs, K.P.; Wu, T.W.; et al. Mild hypoxic-ischemic encephalopathy (HIE): Timing and pattern of MRI brain injury. Pediatr. Res. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Turner, R.C.; Logsdon, A.F.; Bailes, J.E.; Huber, J.D.; Rosen, C.L. Linking traumatic brain injury to chronic traumatic encephalopathy: Identification of potential mechanisms leading to neurofibrillary tangle development. J. Neurotrauma 2014, 31, 1129–1138. [Google Scholar] [CrossRef]
- Spinos, P.; Sakellaropoulos, G.; Georgiopoulos, M.; Stavridi, K.; Apostolopoulou, K.; Ellul, J.; Constantoyannis, C. Postconcussion syndrome after mild traumatic brain injury in Western Greece. J. Trauma 2010, 69, 789–794. [Google Scholar] [CrossRef]
- Lumba-Brown, A.; Teramoto, M.; Bloom, O.J.; Brody, D.; Chesnutt, J.; Clugston, J.R.; Collins, M.; Gioia, G.; Kontos, A.; Lal, A.; et al. Concussion Guidelines Step 2: Evidence for Subtype Classification. Neurosurgery 2020, 86, 2–13. [Google Scholar] [CrossRef]
- Sahler, C.S.; Greenwald, B.D. Traumatic brain injury in sports: A review. Rehabil. Res. Pract. 2012, 2012, 659652. [Google Scholar] [CrossRef] [PubMed]
- Qubty, D.; Glazer, S.; Schreiber, S.; Rubovitch, V.; Pick, C.G. Chapter 22—Mild Traumatic Brain Injuries and Object Recognition. In Handbook of Behavioral Neuroscience; Ennaceur, A., de Souza Silva, M.A., Eds.; Academic Press, Elsevier: London, UK, 2018; Volume 27, pp. 331–339. [Google Scholar] [CrossRef]
- Salehi, A.; Zhang, J.H.; Obenaus, A. Response of the cerebral vasculature following traumatic brain injury. J. Cereb. Blood Flow Metab. 2017, 37, 2320–2339. [Google Scholar] [CrossRef] [PubMed]
- Monson, K.; Converse, M.; Manley, G. Cerebral blood vessel damage in traumatic brain injury. Clin. Biomech. 2018, 64, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Gama Sosa, M.A.; De Gasperi, R.; Pryor, D.; Perez Garcia, G.S.; Perez, G.M.; Abutarboush, R.; Kawoos, U.; Hogg, S.; Ache, B.; Elder, G.A.; et al. Low-level blast exposure induces chronic vascular remodeling, perivascular astrocytic degeneration and vascular-associated neuroinflammation. Acta Neuropathol. Commun. 2021, 9, 167. [Google Scholar] [CrossRef]
- Obenaus, A.; Ng, M.; Orantes, A.M.; Kinney-Lang, E.; Rashid, F.; Hamer, M.; DeFazio, R.A.; Tang, J.; Zhang, J.H.; Pearce, W.J. Traumatic brain injury results in acute rarefication of the vascular network. Sci. Rep. 2017, 7, 239. [Google Scholar] [CrossRef]
- Farajzadeh Khosroshahi, S.; Yin, X.; KDonat, C.; McGarry, A.; Yanez Lopez, M.; Baxan, N.; Sharp, D.J.; Sastre, M.; Ghajari, M. Multiscale modelling of cerebrovascular injury reveals the role of vascular anatomy and parenchymal shear stresses. Sci. Rep. 2021, 11, 12927. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).