Recent Development of Heavy Atom-Free Triplet Photosensitizers for Photodynamic Therapy
Abstract
1. Introduction
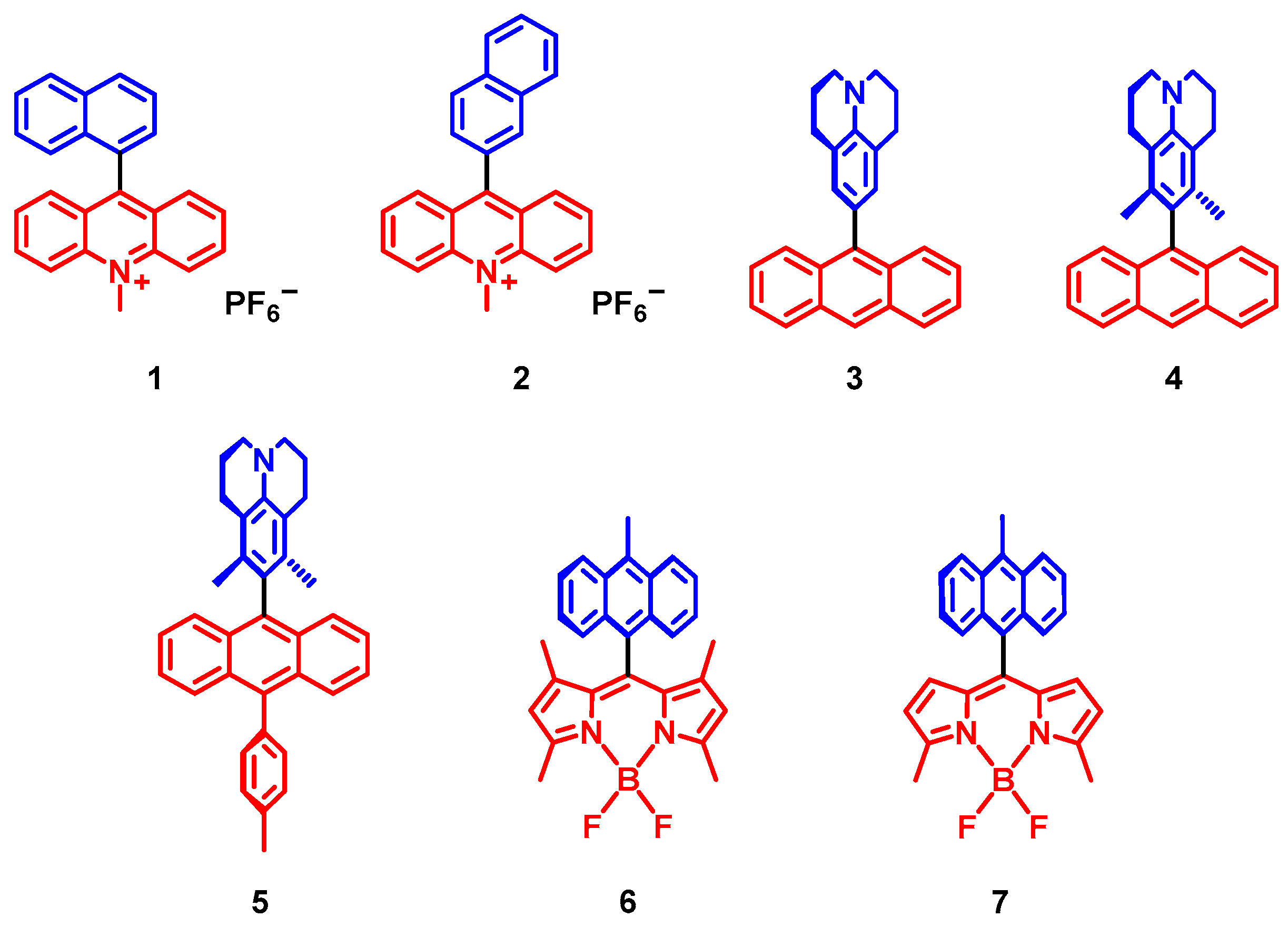
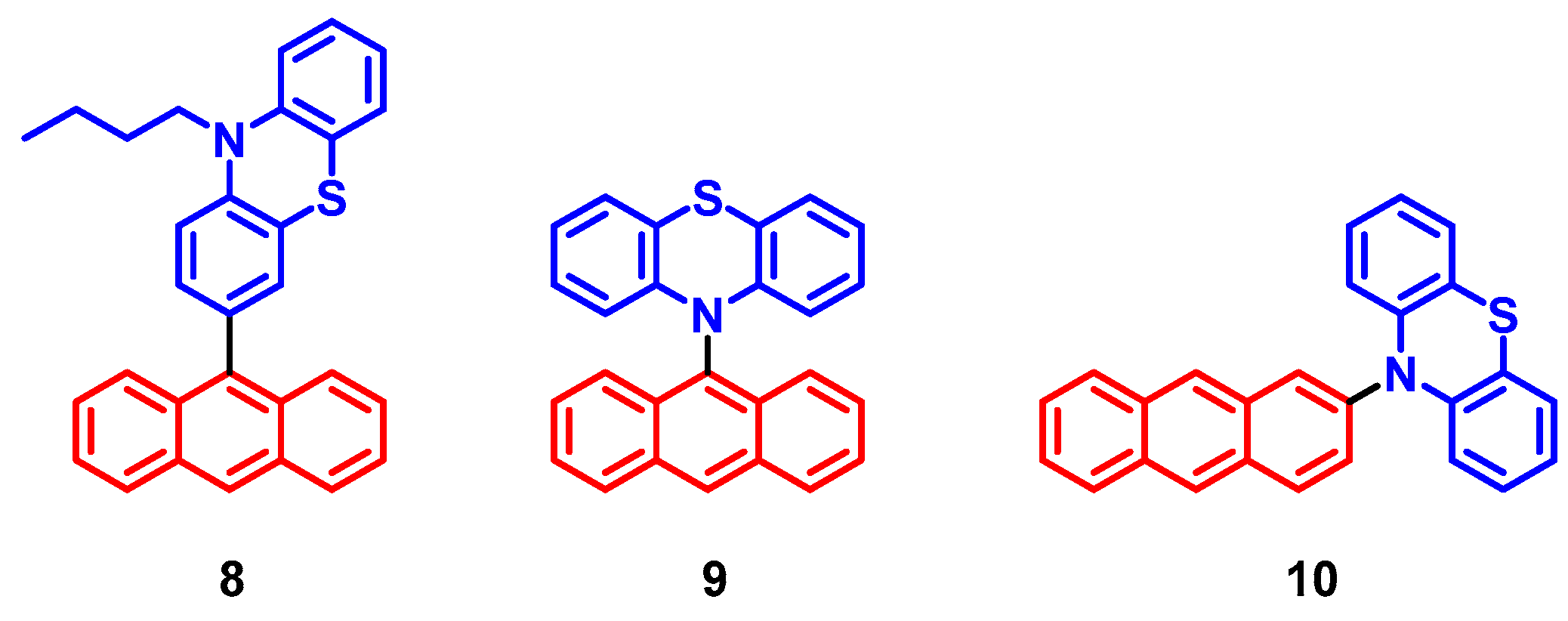
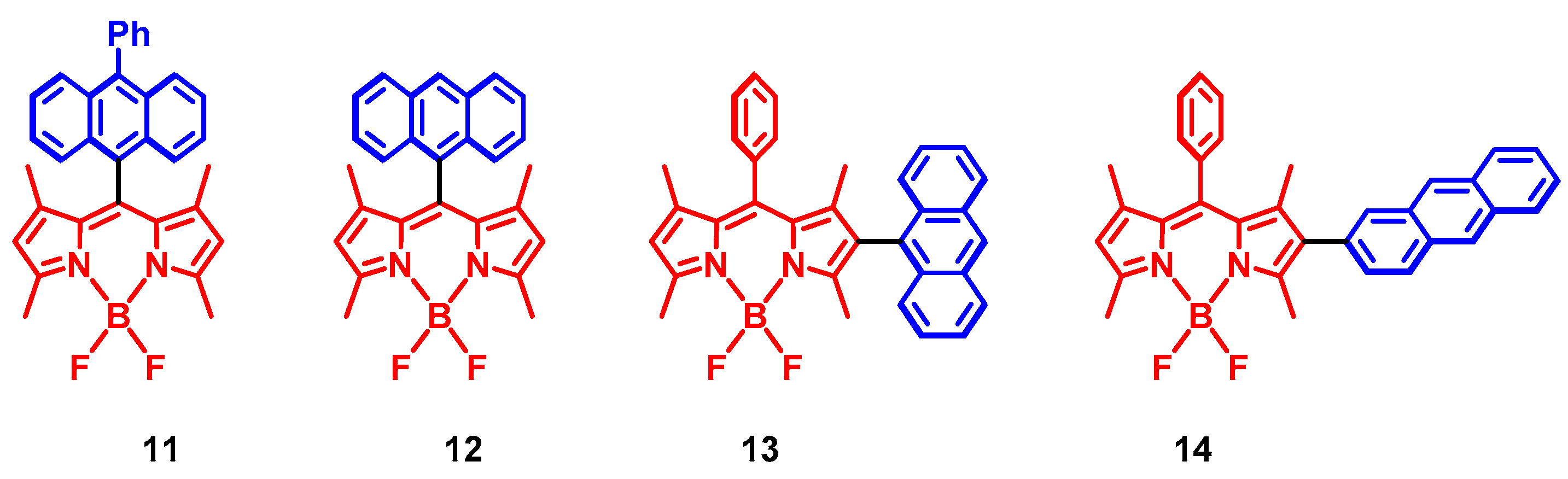
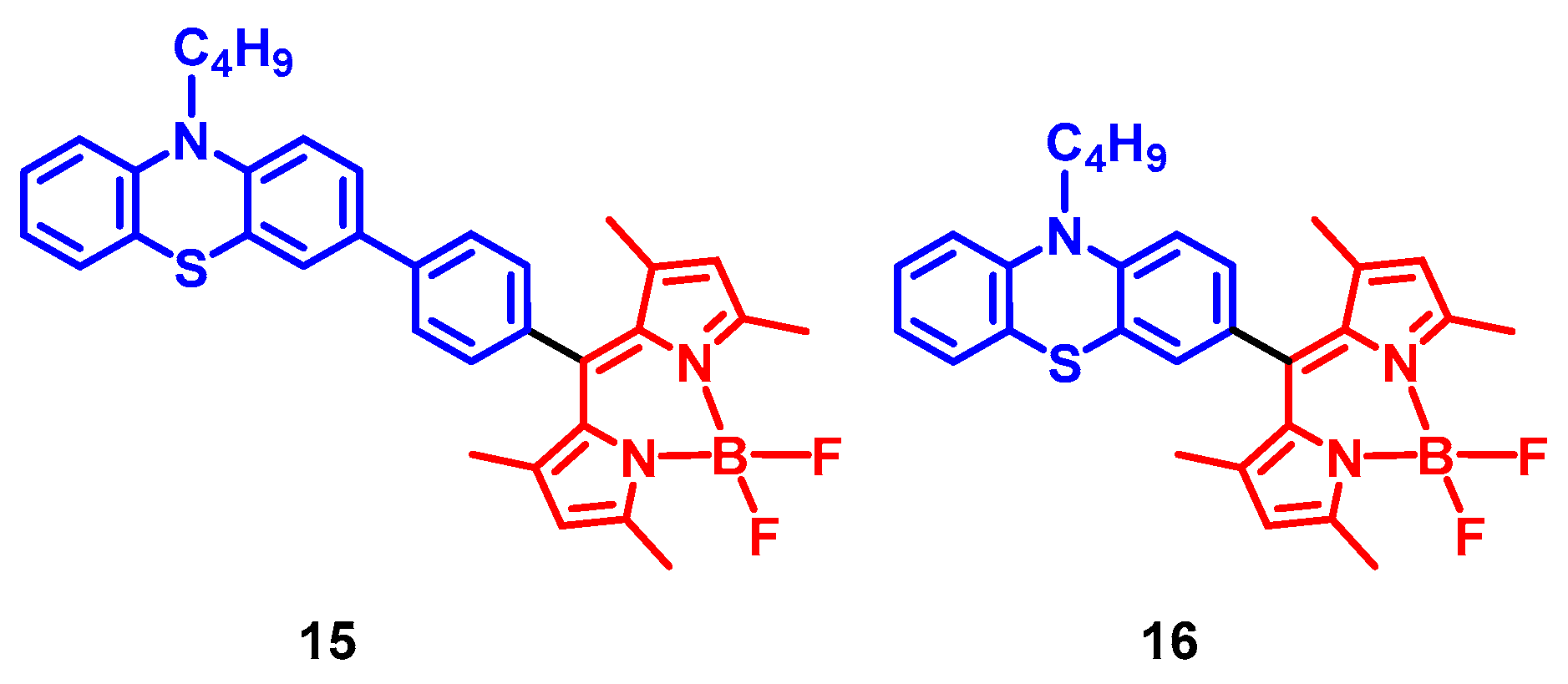
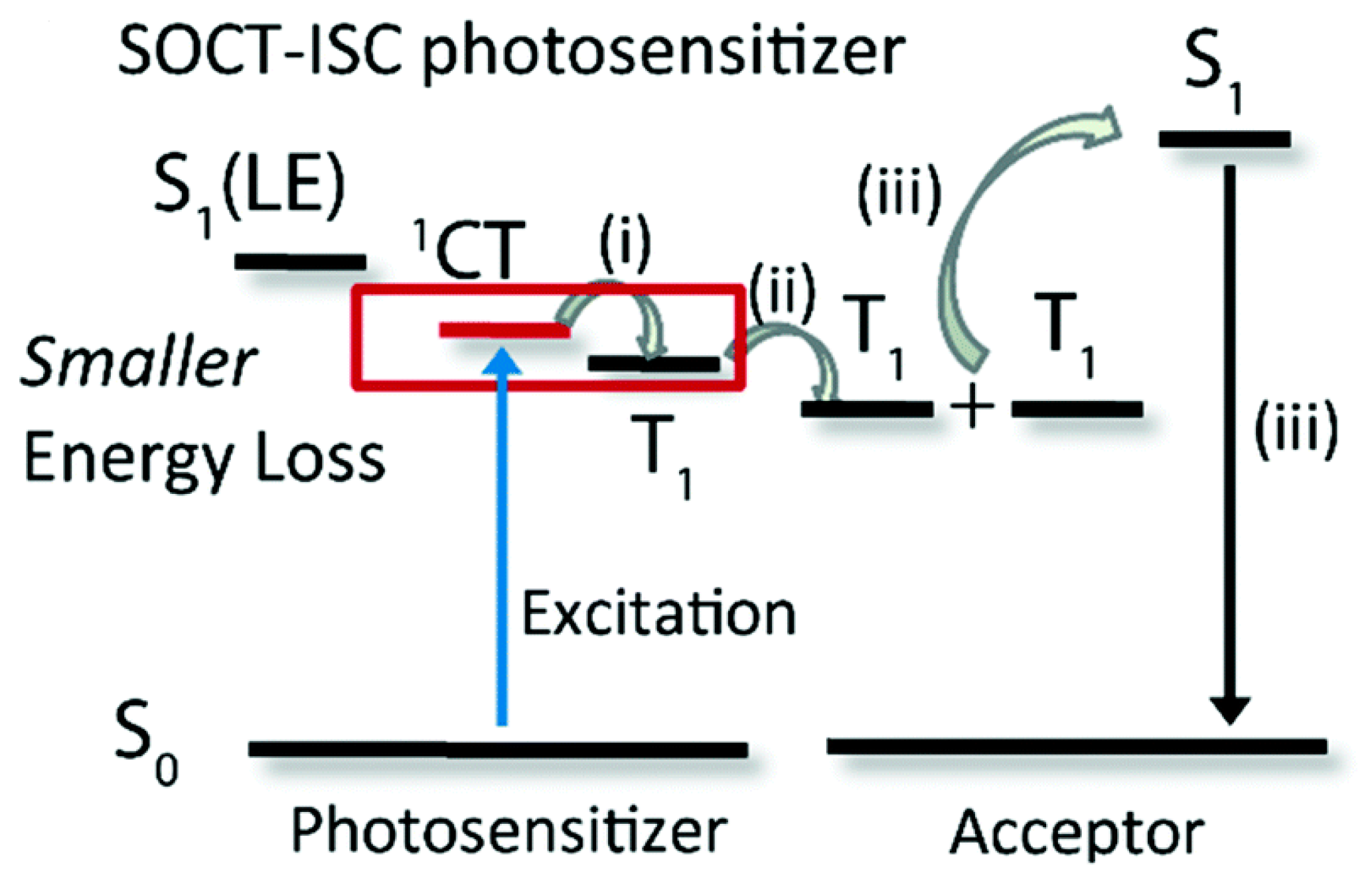
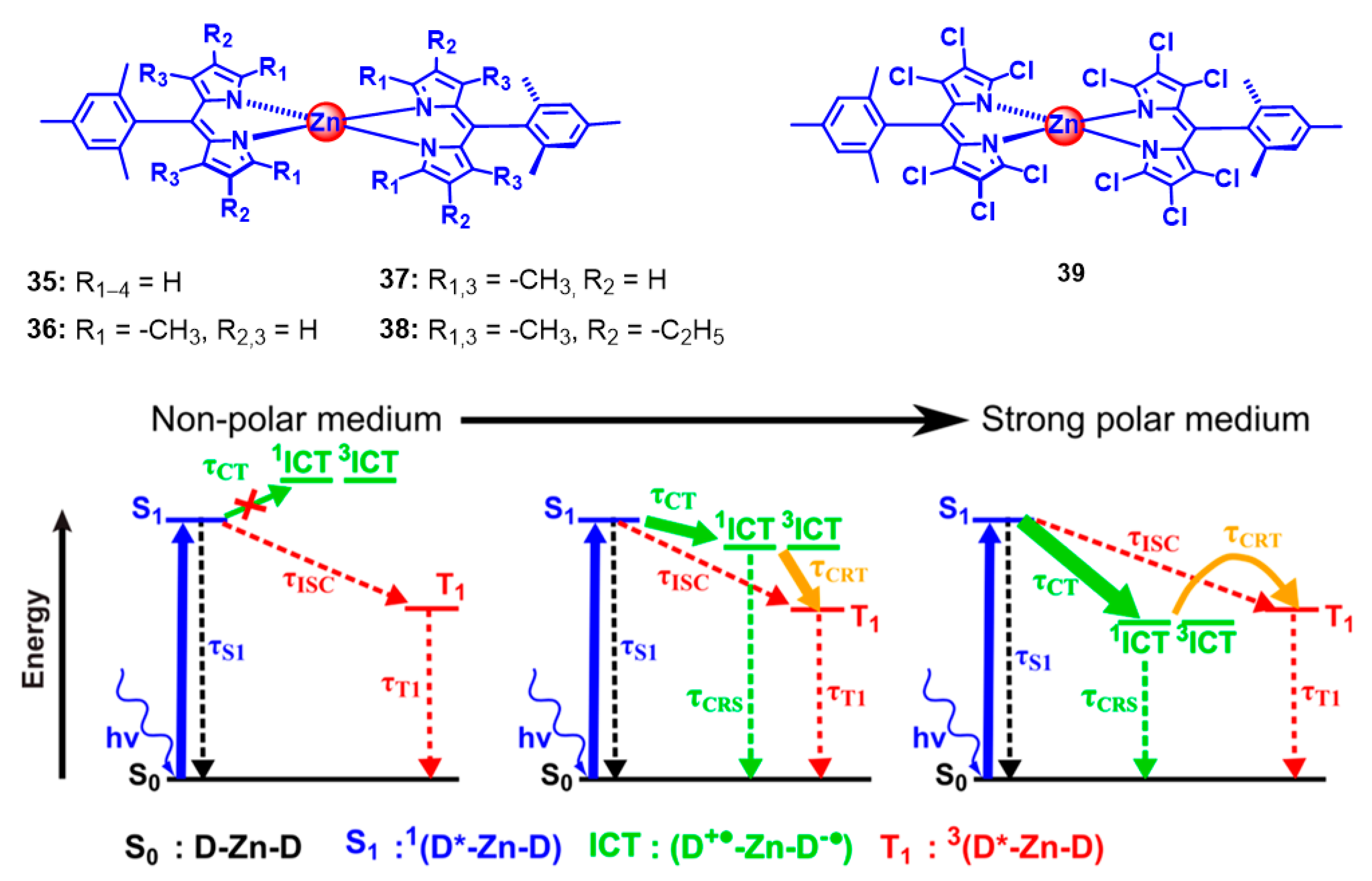
2. Spin-Orbit Charge Transfer Intersystem Crossing (SOCT-ISC)
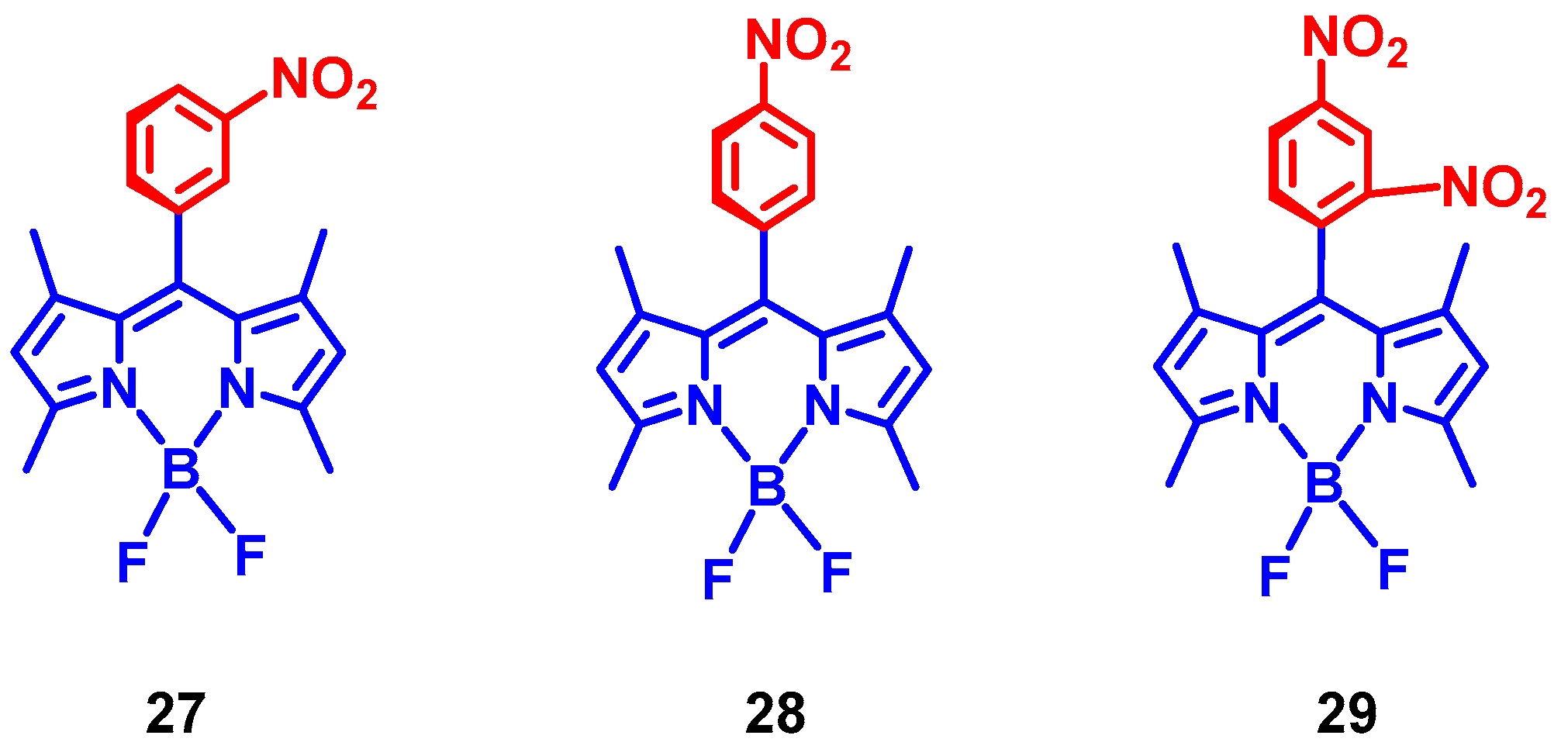
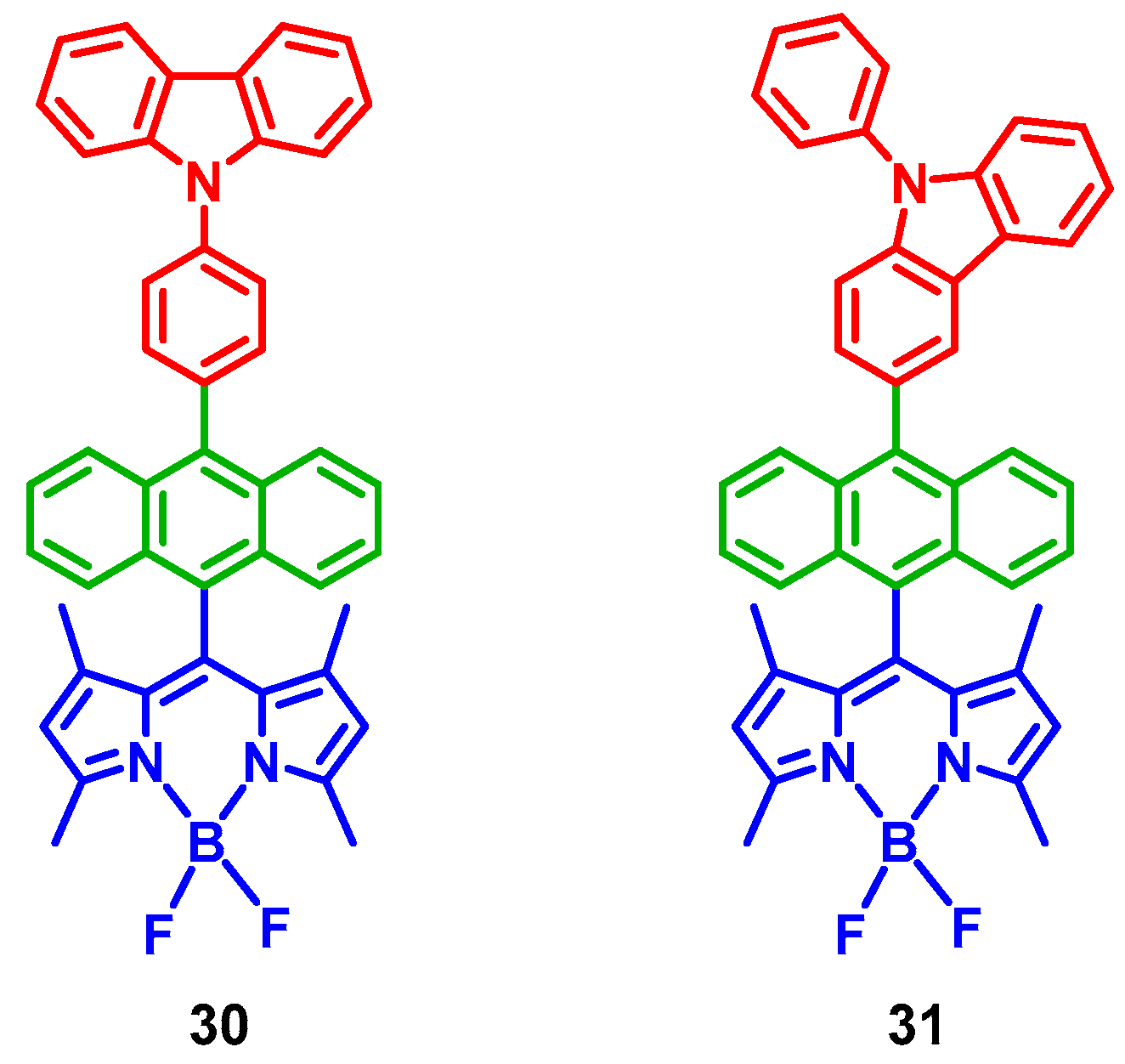
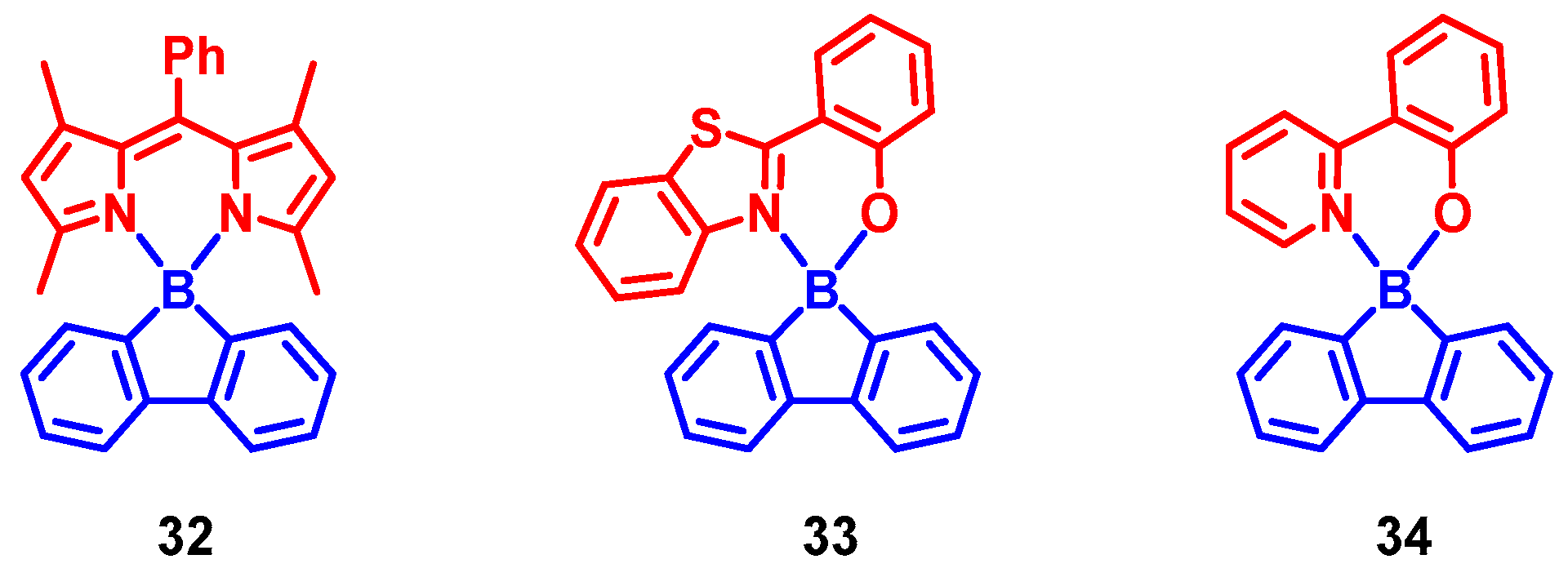
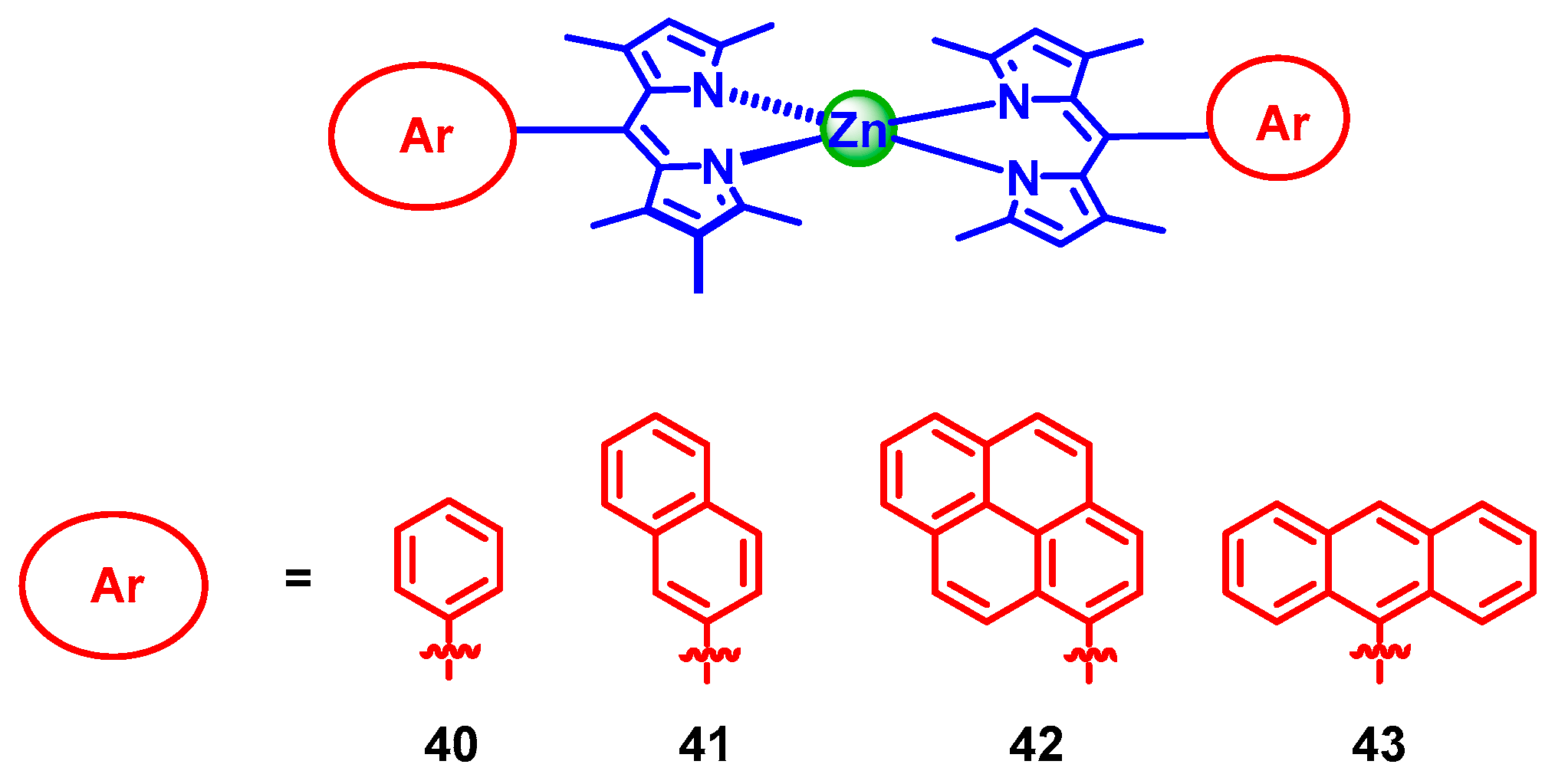
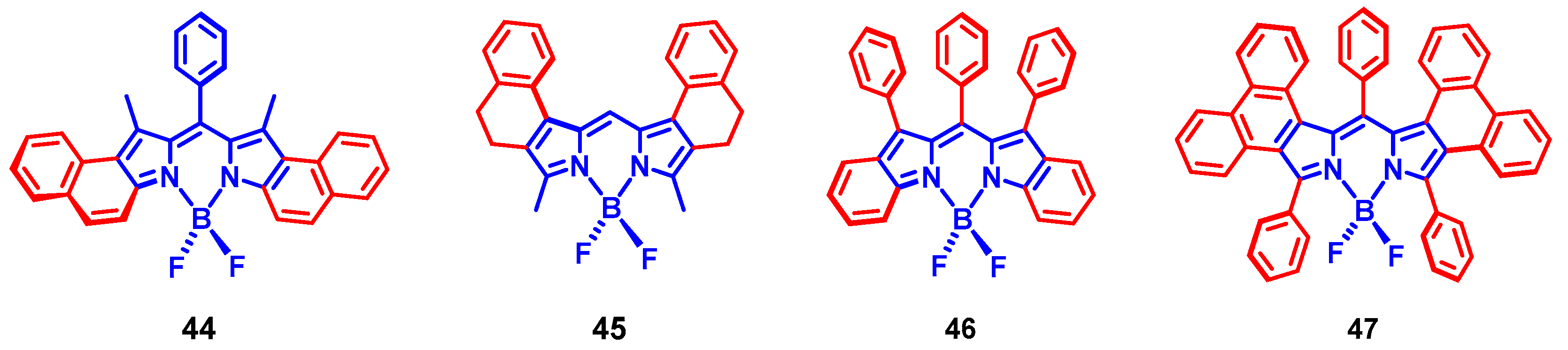
3. Twisted π-Conjugation Framework-Induced ISC
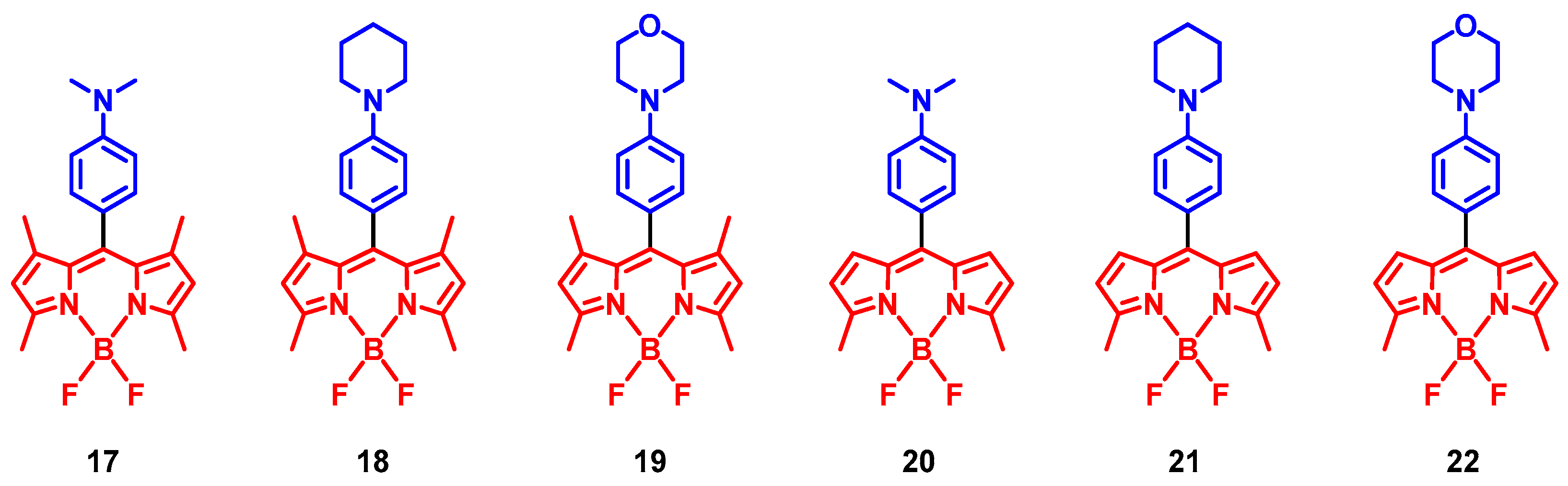
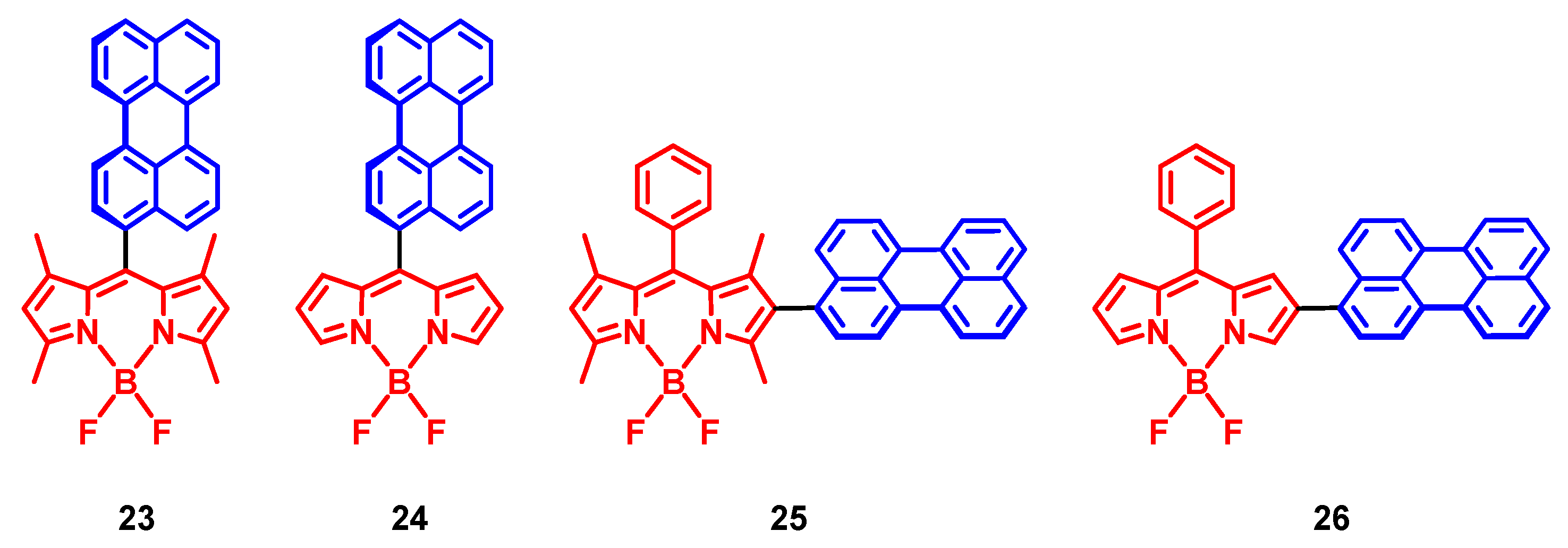
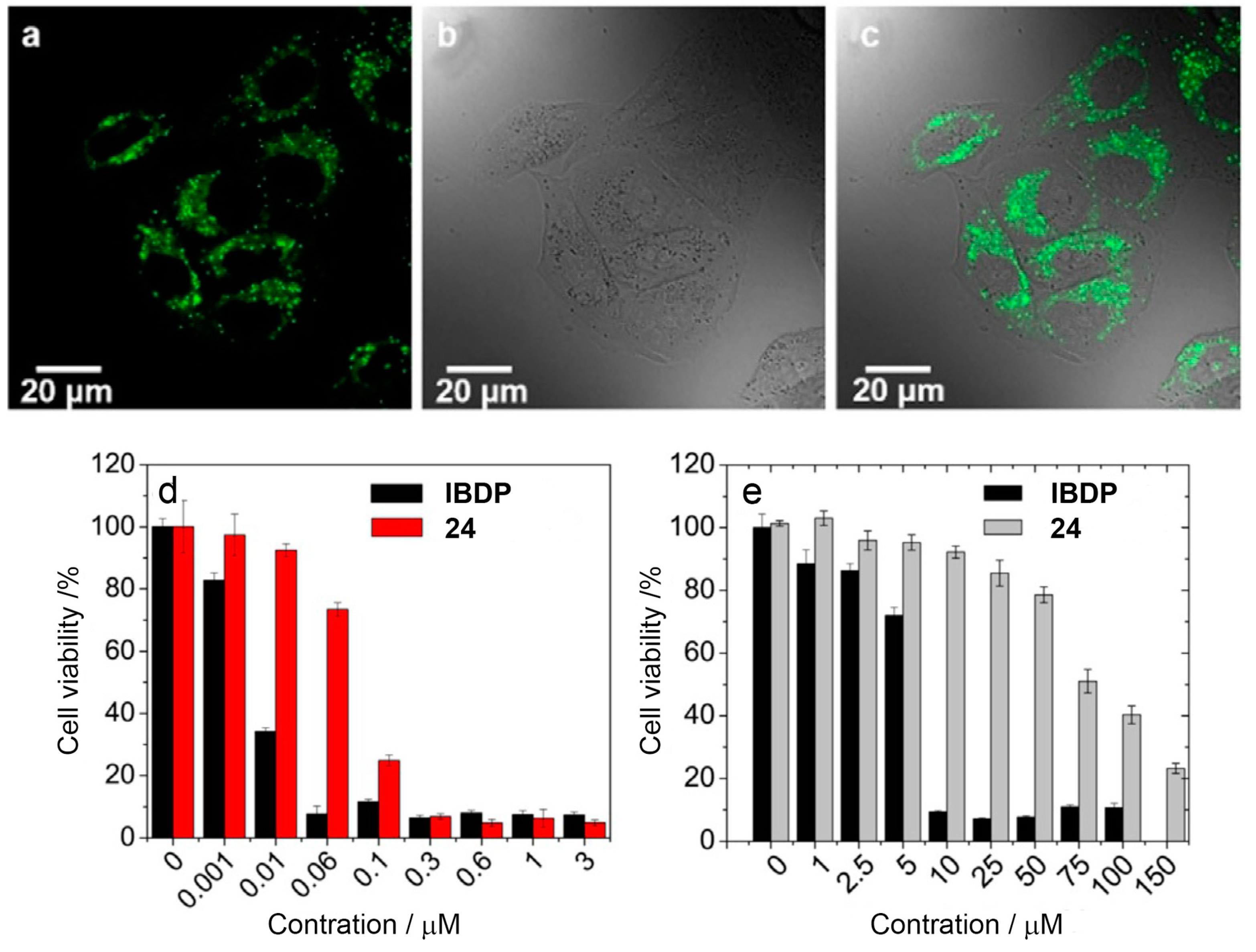
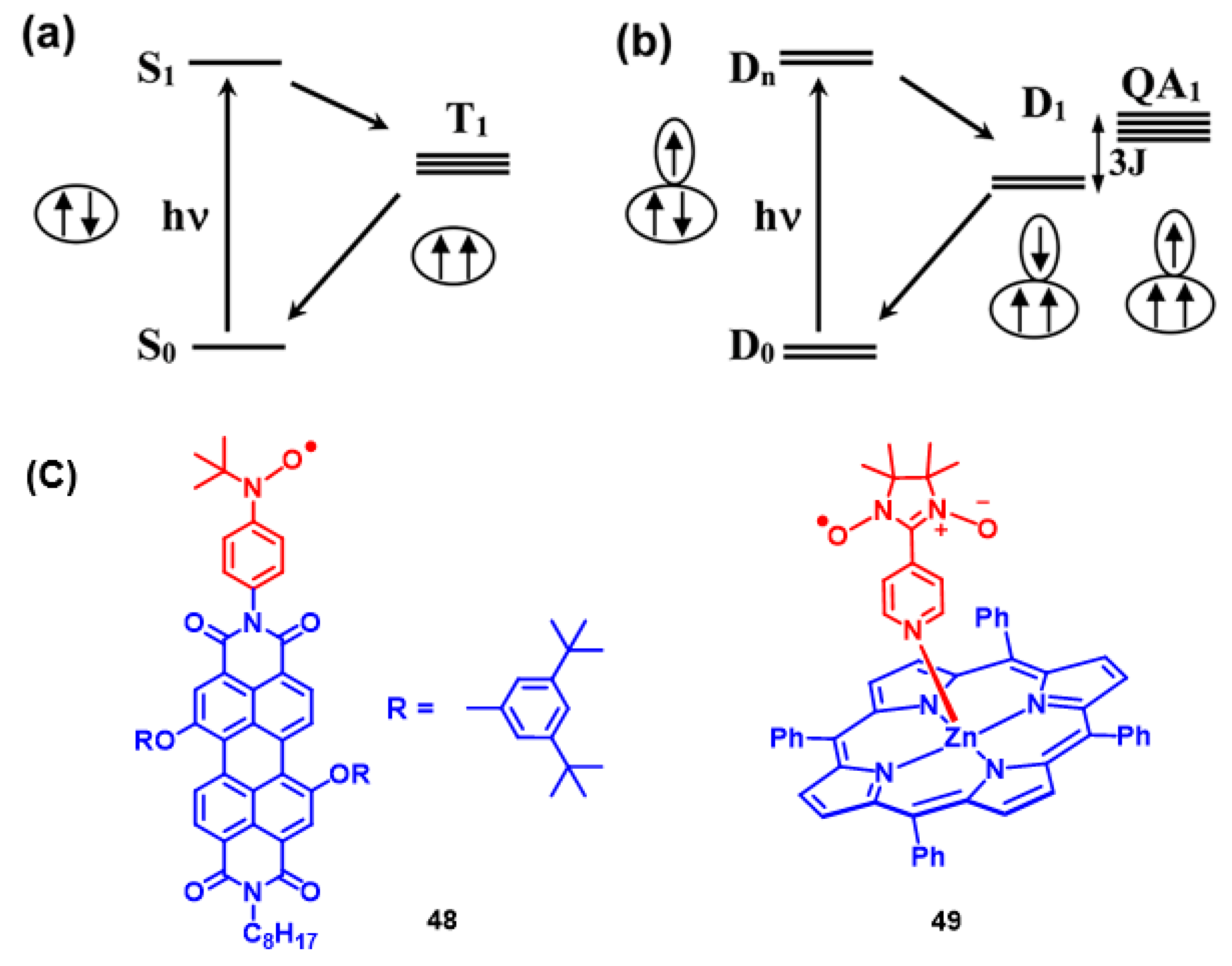
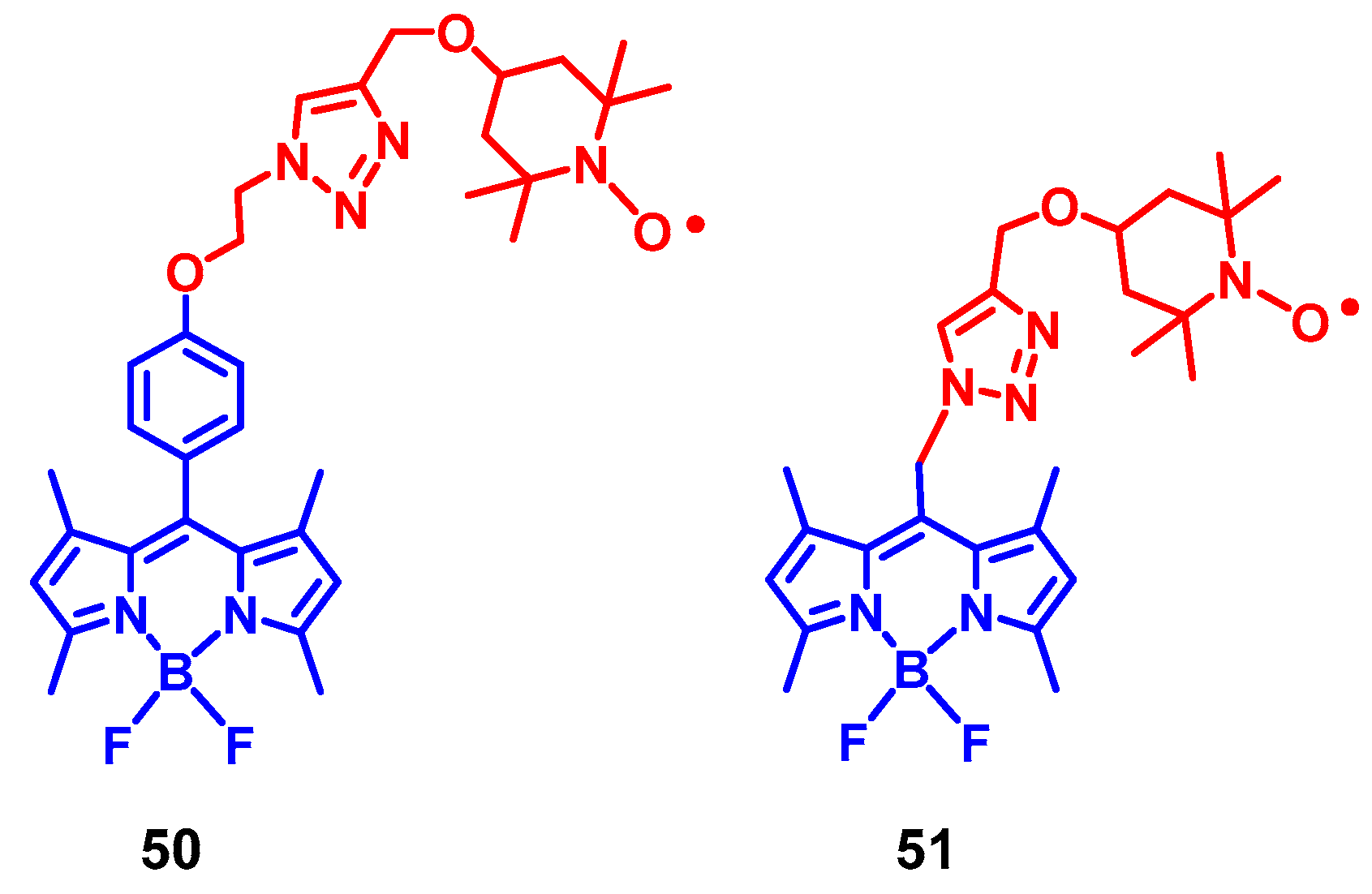
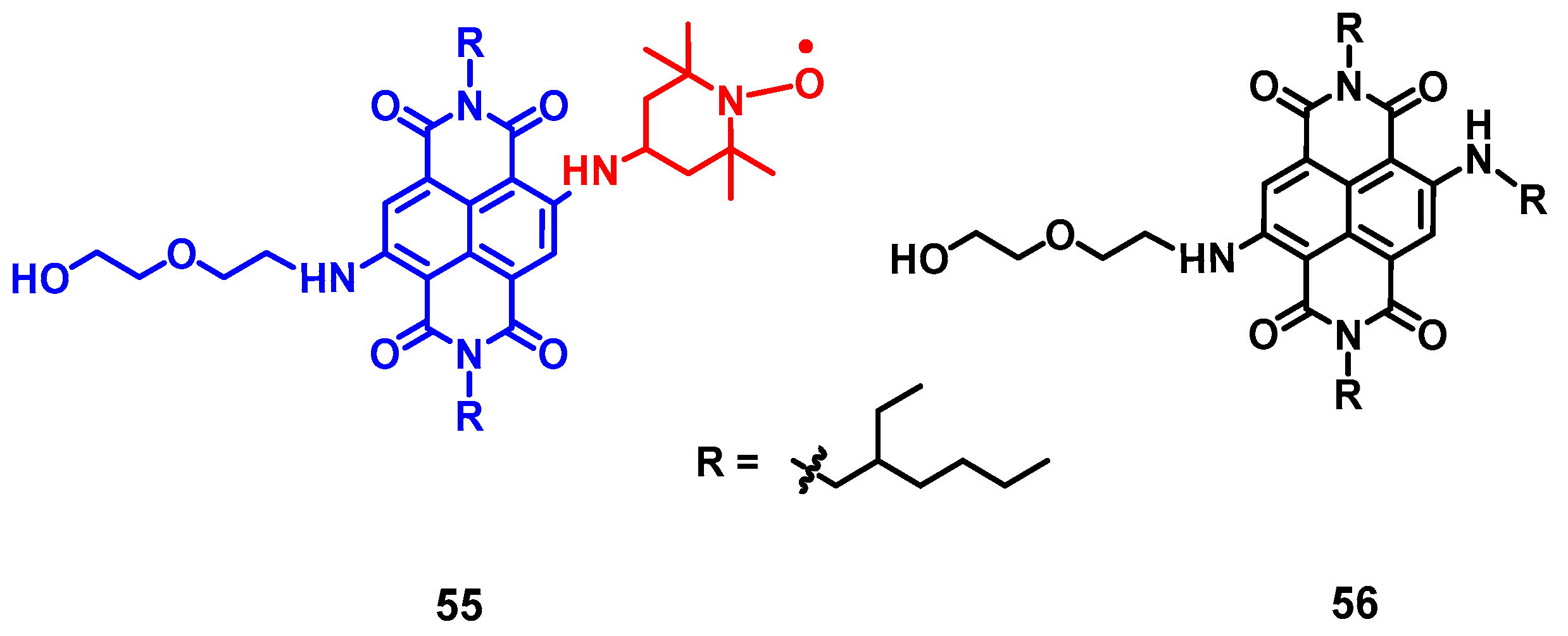
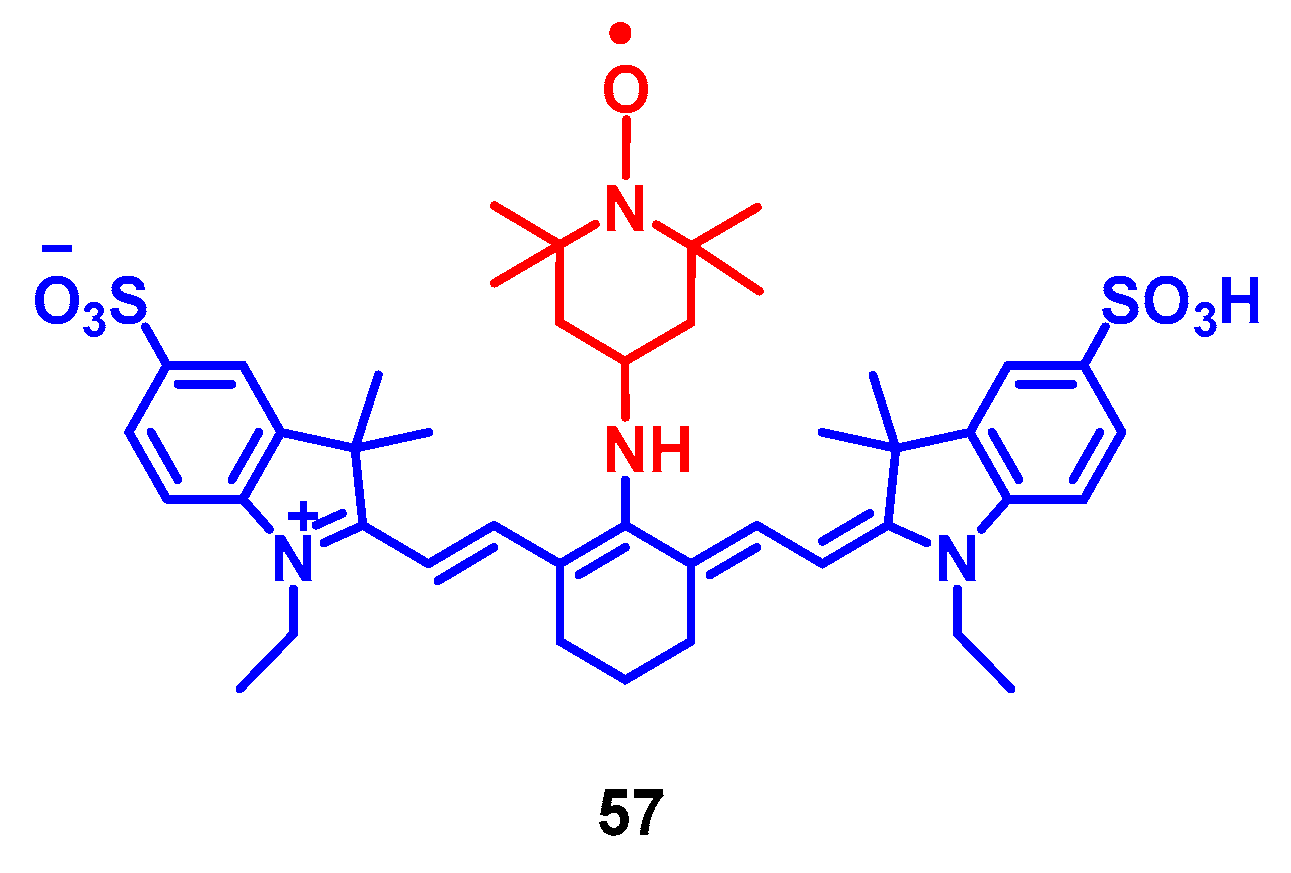
4. Radical Enhanced ISC: ISC by Electron Spin-Spin Interaction between a Persistent Radical and an Electronically Excited State of a Chromophore
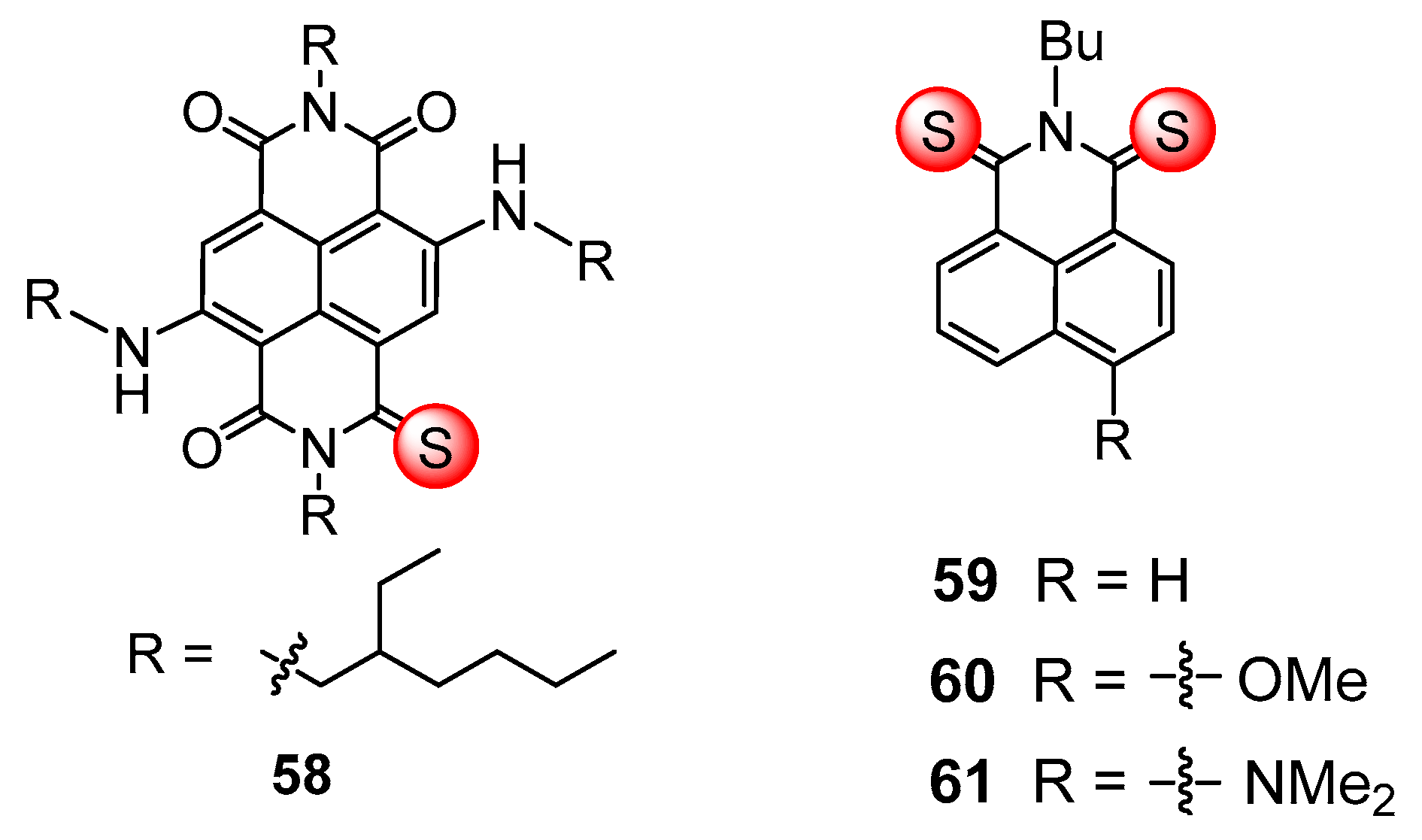
5. Triplet PSs with Thionated Carbonyl Groups
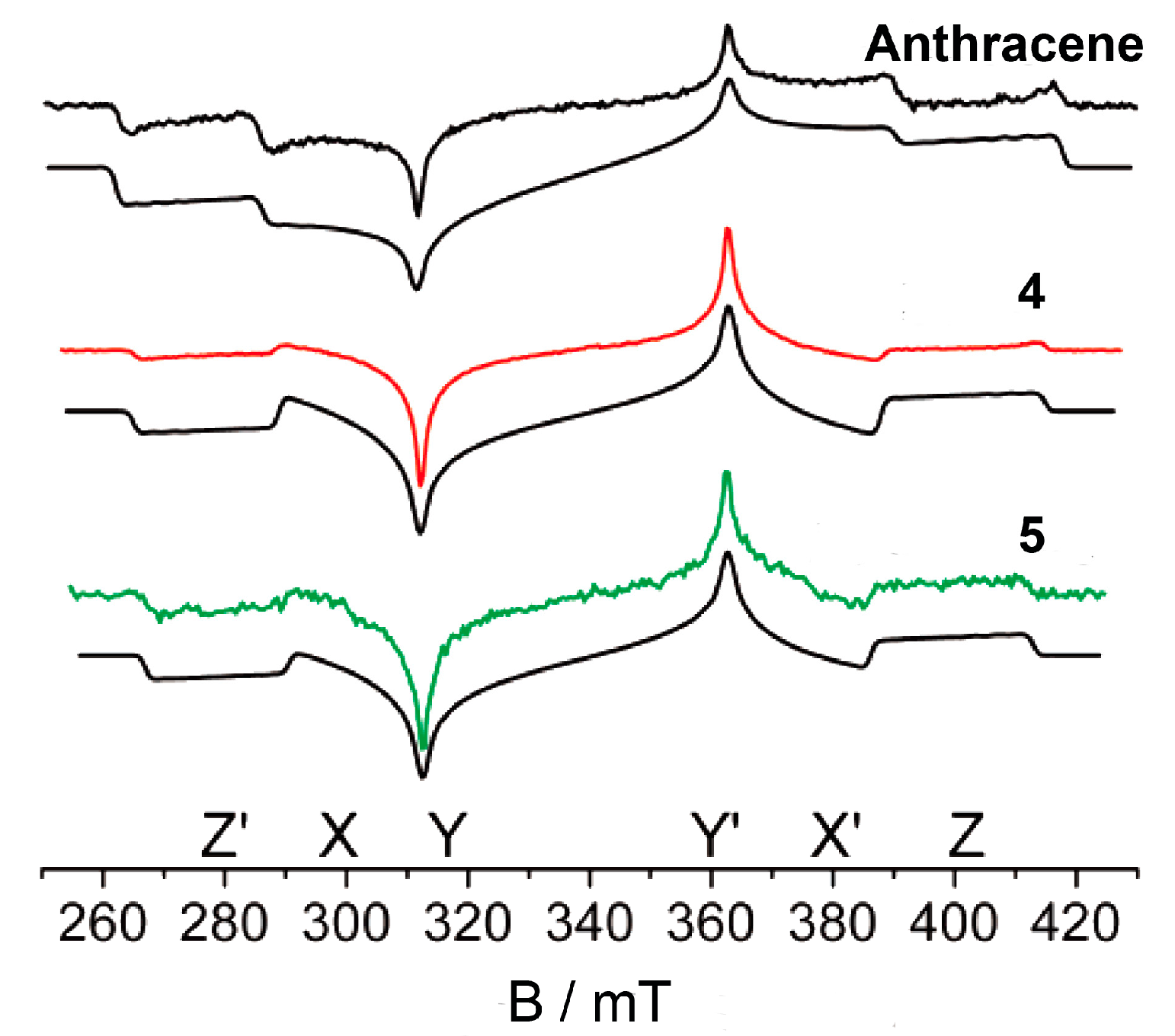
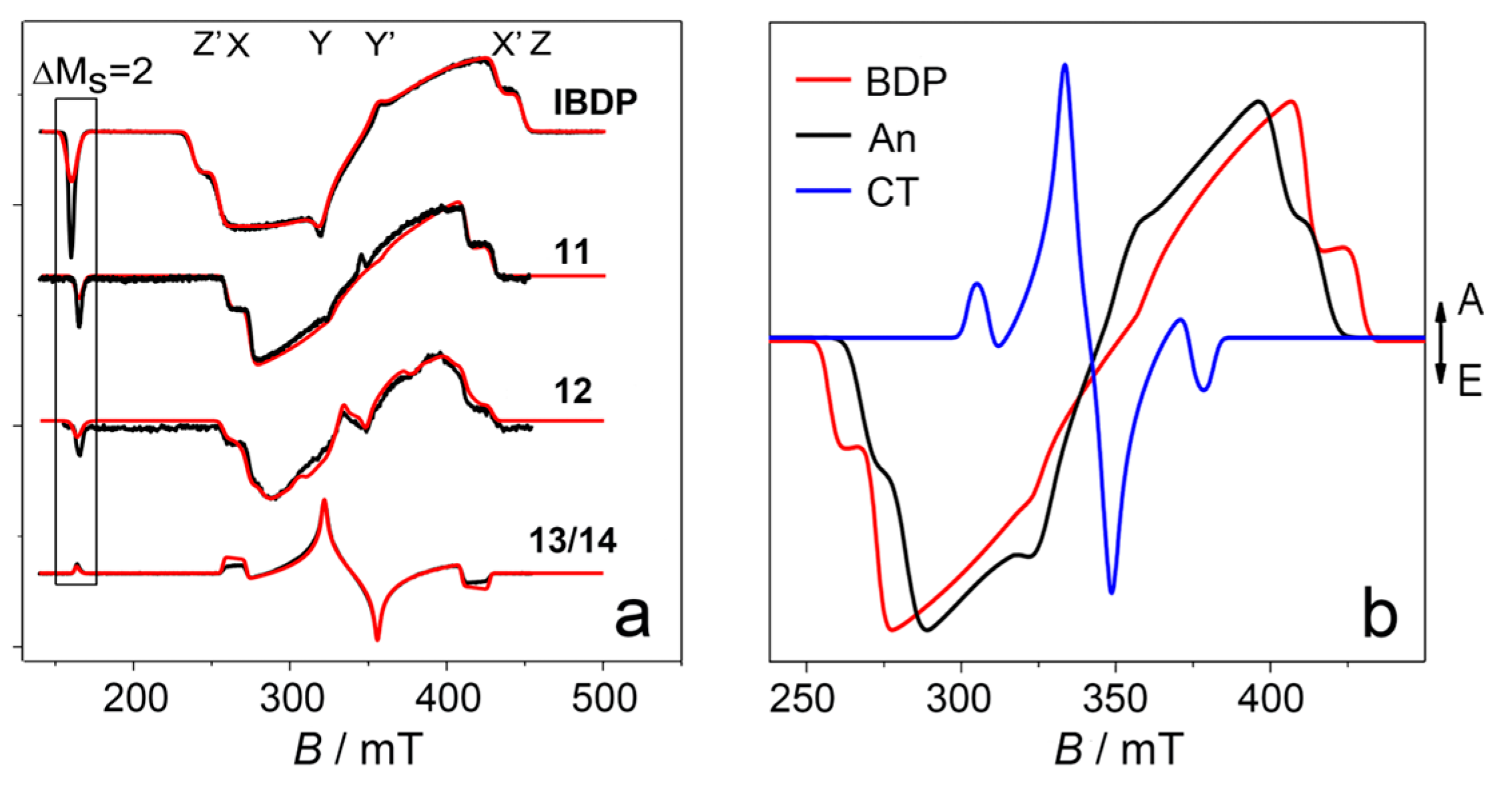
6. Population Rates of the Three Sublevels of the Triplet State: Time-Resolved EPR Spectra (TREPR)
7. Concluding Remarks and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeRosa, M.C.; Crutchley, R.J. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. Bodipy Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef]
- Majumdar, P.; Nomula, R.; Zhao, J. Activatable Triplet Photosensitizers: Magic Bullets for Targeted Photodynamic Therapy. J. Mater. Chem. C 2014, 2, 5982–5997. [Google Scholar] [CrossRef]
- Tian, J.; Zhou, J.; Shen, Z.; Ding, L.; Yu, J.-S.; Ju, H. A pH-Activatable and Aniline-Substituted Photosensitizer for Near-Infrared Cancer Theranostics. Chem. Sci. 2015, 6, 5969–5977. [Google Scholar] [CrossRef]
- Callaghan, S.; Senge, M.O. The Good, the Bad, and the Ugly—Controlling Singlet Oxygen through Design of Photosensitizers and Delivery Systems for Photodynamic Therapy. Photochem. Photobiol. Sci. 2018, 17, 1490–1514. [Google Scholar] [CrossRef]
- Tang, Q.; Xiao, W.; Li, J.; Chen, D.; Zhang, Y.; Shao, J.; Dong, X. A Fullerene-Rhodamine B Photosensitizer with pH-Activated Visible-Light Absorbance/Fluorescence/Photodynamic Therapy. J. Mater. Chem. B 2018, 6, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhong, Z.; Ma, Q.; Shao, J.; Huang, W.; Dong, X. Aza-Bodipy-Based Nanomedicines in Cancer Phototheranostics. ACS Appl. Mater. Interfaces 2020, 12, 26914–26925. [Google Scholar] [CrossRef]
- Bassan, E.; Gualandi, A.; Cozzi, P.G.; Ceroni, P. Design of Bodipy Dyes as Triplet Photosensitizers: Electronic Properties Tailored for Solar Energy Conversion, Photoredox Catalysis and Photodynamic Therapy. Chem. Sci. 2021, 12, 6607–6628. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.-N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Miao, J.; Huo, Y.; Yao, G.; Feng, Y.; Weng, J.; Zhao, W.; Guo, W. Heavy Atom-Free, Mitochondria-Targeted, and Activatable Photosensitizers for Photodynamic Therapy with Real-Time in-Situ Therapeutic Monitoring. Angew. Chem. Int. Ed. 2022, 61, e202201815. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Katsumiti, A.; Sola-Llano, R.; Agarrabeitia, A.R.; Cajaraville, M.P.; Ortiz, M.J.; Martinez-Martinez, V. Red Halobodipys as Theragnostic Agents: The Role of the Substitution at Meso Position. Dye. Pigm. 2022, 198, 110015. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, J.; Liu, W.; Ren, H.; Wang, S.; Wang, P. Novel Selenium-Containing Photosensitizers for Near-Infrared Fluorescence Imaging-Guided Photodynamic Therapy. J. Photochem. Photobiol. B 2022, 233, 112488. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, C.; Wu, Q.; Wen, H.; Sun, T.; Xie, Z. A Cationic Bodipy Photosensitizer Decorated with Quaternary Ammonium for High-Efficiency Photodynamic Inhibition of Bacterial Growth. J. Mater. Chem. B 2022, 10, 4967–4973. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, W.; Sun, J.; Guo, S. Triplet Photosensitizers: From Molecular Design to Applications. Chem. Soc. Rev. 2013, 42, 5323–5351. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Hou, Y.; Yan, Y.; Zhao, J.; Dick, B. Recent Development of Heavy-Atom-Free Triplet Photosensitizers: Molecular Structure Design, Photophysics and Application. J. Mater. Chem. C 2021, 9, 11944–11973. [Google Scholar] [CrossRef]
- Lincoln, R.; Kohler, L.; Monro, S.; Yin, H.; Stephenson, M.; Zong, R.; Chouai, A.; Dorsey, C.; Hennigar, R.; Thummel, R.P.; et al. Exploitation of Long-Lived 3IL Excited States for Metal-Organic Photodynamic Therapy: Verification in a Metastatic Melanoma Model. J. Am. Chem. Soc. 2013, 135, 17161–17175. [Google Scholar] [CrossRef]
- Wang, Z.; Ivanov, M.; Gao, Y.; Bussotti, L.; Foggi, P.; Zhang, H.; Russo, N.; Dick, B.; Zhao, J.; Di Donato, M.; et al. Spin–Orbit Charge-Transfer Intersystem Crossing (ISC) in Compact Electron Donor–Acceptor Dyads: ISC Mechanism and Application as Novel and Potent Photodynamic Therapy Reagents. Chem. Eur. J. 2020, 26, 1091–1102. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, Q.; Zhao, J. An Exceptionally Long-Lived Triplet State of Red Light-Absorbing Compact Phenothiazine-Styrylbodipy Electron Donor/Acceptor Dyads: A Better Alternative to the Heavy Atom-Effect? Chem. Commun. 2020, 56, 1721–1724. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, K.; Yang, W.; Wang, Z.; Zhong, F. The Triplet Excited State of Bodipy: Formation, Modulation and Application. Chem. Soc. Rev. 2015, 44, 8904–8939. [Google Scholar] [CrossRef]
- Teng, K.-X.; Chen, W.-K.; Niu, L.-Y.; Fang, W.-H.; Cui, G.; Yang, Q.-Z. Bodipy-Based Photodynamic Agents for Exclusively Generating Superoxide Radical over Singlet Oxygen. Angew. Chem. Int. Ed. 2021, 60, 19912–19920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, J.; Liu, A. NRNC-8, a Novel Mitochondria-Immobilization Superoxide Radical Generator for Photodynamic Therapy. Sens. Actuators B 2022, 369, 132322. [Google Scholar] [CrossRef]
- Guldi, D.M. Fullerenes: Three Dimensional Electron Acceptor Materials. Chem. Commun. 2000, 321–327. [Google Scholar] [CrossRef]
- Verhoeven, J.W.; van Ramesdonk, H.J.; Groeneveld, M.M.; Benniston, A.C.; Harriman, A. Long-Lived Charge-Transfer States in Compact Donor–Acceptor Dyads. ChemPhysChem 2005, 6, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, J.W. On the Role of Spin Correlation in the Formation, Decay, and Detection of Long-Lived, Intramolecular Charge-Transfer States. J. Photochem. Photobiol. C 2006, 7, 40–60. [Google Scholar] [CrossRef]
- Fukuzumi, S. New Development of Photoinduced Electron-Transfer Catalytic Systems. Pure Appl. Chem. 2007, 79, 981–991. [Google Scholar] [CrossRef]
- Vauthey, E. Photoinduced Symmetry-Breaking Charge Separation. ChemPhysChem 2012, 13, 2001–2011. [Google Scholar] [CrossRef]
- Kotani, H.; Ohkubo, K.; Fukuzumi, S. Formation of a Long-Lived Electron-Transfer State of a Naphthalene–Quinolinium Ion Dyad and the π-Dimer Radical Cation. Faraday Discuss. 2012, 155, 89–102. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, X.; Chen, K.; Liu, D.; Wang, Z.; Liu, Q.; Zhao, J.; Barbon, A. Charge Separation, Charge Recombination, Long-Lived Charge Transfer State Formation and Intersystem Crossing in Organic Electron Donor/Acceptor Dyads. J. Mater. Chem. C 2019, 7, 12048–12074. [Google Scholar] [CrossRef]
- Gibbons, D.J.; Farawar, A.; Mazzella, P.; Leroy-Lhez, S.; Williams, R.M. Making Triplets from Photo-Generated Charges: Observations, Mechanisms and Theory. Photochem. Photobiol. Sci. 2020, 19, 136–158. [Google Scholar] [CrossRef]
- Filatov, M.A. Heavy-Atom-Free Bodipy Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer. Org. Biomol. Chem. 2020, 18, 10–27. [Google Scholar] [CrossRef]
- Van Willigen, H.; Jones, G.; Farahat, M.S. Time-Resolved EPR Study of Photoexcited Triplet-State Formation in Electron-Donor-Substituted Acridinium Ions. J. Phys. Chem. 1996, 100, 3312–3316. [Google Scholar] [CrossRef]
- Dance, Z.E.X.; Mickley, S.M.; Wilson, T.M.; Ricks, A.B.; Scott, A.M.; Ratner, M.A.; Wasielewski, M.R. Intersystem Crossing Mediated by Photoinduced Intramolecular Charge Transfer: Julolidine−Anthracene Molecules with Perpendicular π Systems. J. Phys. Chem. A 2008, 112, 4194–4201. [Google Scholar] [CrossRef] [PubMed]
- Filatov, M.A.; Karuthedath, S.; Polestshuk, P.M.; Savoie, H.; Flanagan, K.J.; Sy, C.; Sitte, E.; Telitchko, M.; Laquai, F.; Boyle, R.W.; et al. Generation of Triplet Excited States via Photoinduced Electron Transfer in Meso-Anthra-Bodipy: Fluorogenic Response toward Singlet Oxygen in Solution and in Vitro. J. Am. Chem. Soc. 2017, 139, 6282–6285. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, S.; Filatov, M.A.; Savoie, H.; Boyle, R.W.; Senge, M.O. In Vitro Cytotoxicity of a Library of Bodipy-Anthracene and -Pyrene Dyads for Application in Photodynamic Therapy. Photochem. Photobiol. Sci. 2019, 18, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Biskup, T.; Rein, S.; Wang, Z.; Bussotti, L.; Russo, N.; Foggi, P.; Zhao, J.; Di Donato, M.; Mazzone, G.; et al. Spin–Orbit Charge Recombination Intersystem Crossing in Phenothiazine–Anthracene Compact Dyads: Effect of Molecular Conformation on Electronic Coupling, Electronic Transitions, and Electron Spin Polarizations of the Triplet States. J. Phys. Chem. C 2018, 122, 27850–27865. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J. Bodipy–Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet–Triplet Annihilation Upconversion. Org. Lett. 2017, 19, 4492–4495. [Google Scholar] [CrossRef]
- Wang, Z.; Sukhanov, A.A.; Toffoletti, A.; Sadiq, F.; Zhao, J.; Barbon, A.; Voronkova, V.K.; Dick, B. Insights into the Efficient Intersystem Crossing of Bodipy-Anthracene Compact Dyads with Steady-State and Time-Resolved Optical/Magnetic Spectroscopies and Observation of the Delayed Fluorescence. J. Phys. Chem. C 2019, 123, 265–274. [Google Scholar] [CrossRef]
- Lou, Z.; Hou, Y.; Chen, K.; Zhao, J.; Ji, S.; Zhong, F.; Dede, Y.; Dick, B. Different Quenching Effect of Intramolecular Rotation on the Singlet and Triplet Excited States of Bodipy. J. Phys. Chem. C 2018, 122, 185–193. [Google Scholar] [CrossRef]
- Singh-Rachford, T.N.; Castellano, F.N. Photon Upconversion Based on Sensitized Triplet–Triplet Annihilation. Coordin. Chem. Rev. 2010, 254, 2560–2573. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, S.; Guo, H. Triplet–Triplet Annihilation Based Upconversion: From Triplet Sensitizers and Triplet Acceptors to Upconversion Quantum Yields. RSC Adv. 2011, 1, 937–950. [Google Scholar] [CrossRef]
- Yanai, N.; Kimizuka, N. Stimuli-Responsive Molecular Photon Upconversion. Angew. Chem. Int. Ed. 2020, 59, 10252–10264. [Google Scholar] [CrossRef]
- Xiao, X.; Tian, W.; Imran, M.; Cao, H.; Zhao, J. Controlling the Triplet States and Their Application in External Stimuli-Responsive Triplet–Triplet-Annihilation Photon Upconversion: From the Perspective of Excited State Photochemistry. Chem. Soc. Rev. 2021, 50, 9686–9714. [Google Scholar] [CrossRef]
- Healy, C.; Hermanspahn, L.; Kruger, P.E. Photon Upconversion in Self-Assembled Materials. Coordin. Chem. Rev. 2021, 432, 213756. [Google Scholar] [CrossRef]
- Chen, K.; Yang, W.; Wang, Z.; Iagatti, A.; Bussotti, L.; Foggi, P.; Ji, W.; Zhao, J.; Di Donato, M. Triplet Excited State of Bodipy Accessed by Charge Recombination and Its Application in Triplet–Triplet Annihilation Upconversion. J. Phys. Chem. A 2017, 121, 7550–7564. [Google Scholar] [CrossRef]
- Wu, W.; Guo, H.; Wu, W.; Ji, S.; Zhao, J. Organic Triplet Sensitizer Library Derived from a Single Chromophore (Bodipy) with Long-Lived Triplet Excited State for Triplet–Triplet Annihilation Based Upconversion. J. Org. Chem. 2011, 76, 7056–7064. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, X.-F.; Lu, X.; Lan, S.; Tian, D.; Li, T.; Wang, L.; Zhao, S.; Feng, M.; Zhang, J. Modifying the Meso-Phenyl with Electron Donating Amino Groups Strongly Enhances Bodipy’s Ability as Good Singlet Oxygen Photosensitizer. Dyes Pigm. 2018, 149, 306–314. [Google Scholar] [CrossRef]
- Wang, C.; Qian, Y. A Water Soluble Carbazolyl-Bodipy Photosensitizer with an Orthogonal D–A Structure for Photodynamic Therapy in Living Cells and Zebrafish. Biomater. Sci. 2020, 8, 830–836. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.M.K.; Kim, G.; Kwon, N.; Kim, C.Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet Bodipy Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef]
- Amemori, S.; Sasaki, Y.; Yanai, N.; Kimizuka, N. Near-Infrared-to-Visible Photon Upconversion Sensitized by a Metal Complex with Spin-Forbidden yet Strong S0–T1 Absorption. J. Am. Chem. Soc. 2016, 138, 8702–8705. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Y.; Wang, Z.; Xu, K.; Zhao, J. Exploiting the Benefit of S0 → T1 Excitation in Triplet–Triplet Annihilation Upconversion to Attain Large Anti-Stokes Shifts: Tuning the Triplet State Lifetime of a Tris(2,2’-Bipyridine) Osmium(II) Complex. Dalton Trans. 2018, 47, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Gray, V.; Allardice, J.R.; Zhang, Z.; Pershin, A.; Beljonne, D.; Rao, A. Photon Upconversion from Near-Infrared to Blue Light with Tips-Anthracene as an Efficient Triplet–Triplet Annihilator. ACS Mater. Lett. 2019, 1, 660–664. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Zheng, M.; Zhou, X.; Zou, Y.; Yang, C. Simultaneously High Upconversion Efficiency and Large Anti-Stokes Shift by Using Os(II) Complex Dyad as Triplet Photosensitizer. Adv. Opt. Mater. 2020, 8, 1902157. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Di Donato, M.; Mazzone, G. Increasing the Anti-Stokes Shift in Tta Upconversion with Photosensitizers Showing Red-Shifted Spin-Allowed Charge Transfer Absorption but a Non-Compromised Triplet State Energy Level. Chem. Commun. 2019, 55, 1510–1513. [Google Scholar] [CrossRef]
- Hu, W.; Liu, M.; Zhang, X.-F.; Wang, Y.; Wang, Y.; Lan, H.; Zhao, H. Can Bodipy-Electron Acceptor Conjugates Act as Heavy Atom-Free Excited Triplet State and Singlet Oxygen Photosensitizers Via Photoinduced Charge Separation-Charge Recombination Mechanism? J. Phys. Chem. C 2019, 123, 15944–15955. [Google Scholar] [CrossRef]
- Imran, M.; Sukhanov, A.A.; Wang, Z.; Karatay, A.; Zhao, J.; Mahmood, Z.; Elmali, A.; Voronkova, V.K.; Hayvali, M.; Xing, Y.H.; et al. Electronic Coupling and Spin–Orbit Charge-Transfer Intersystem Crossing in Phenothiazine–Perylene Compact Electron Donor/Acceptor Dyads. J. Phys. Chem. C 2019, 123, 7010–7024. [Google Scholar] [CrossRef]
- Imran, M.; El-Zohry, A.M.; Matt, C.; Taddei, M.; Doria, S.; Bussotti, L.; Foggi, P.; Zhao, J.; Di Donato, M.; Mohammed, O.F.; et al. Intersystem Crossing via Charge Recombination in a Perylene–Naphthalimide Compact Electron Donor/Acceptor Dyad. J. Mater. Chem. C 2020, 8, 8305–8319. [Google Scholar] [CrossRef]
- Wang, L.; Qian, Y. Two Effective Strategies to Improve SOCT-ISC Type Photosensitizers: Triphenylamine Bodipy with A-D-A Configuration and Aie Effect and Its Application in A-549 cells and Zebrafish. Dye. Pigm. 2022, 198, 110018. [Google Scholar] [CrossRef]
- Cakmak, Y.; Kolemen, S.; Duman, S.; Dede, Y.; Dolen, Y.; Kilic, B.; Kostereli, Z.; Yildirim, L.T.; Dogan, A.L.; Guc, D.; et al. Designing Excited States: Theory-Guided Access to Efficient Photosensitizers for Photodynamic Action. Angew. Chem. Int. Ed. 2011, 50, 11937–11941. [Google Scholar] [CrossRef]
- Wu, W.; Cui, X.; Zhao, J. Hetero Bodipy-Dimers as Heavy Atom-Free Triplet Photosensitizers Showing a Long-Lived Triplet Excited State for Triplet–Triplet Annihilation Upconversion. Chem. Commun. 2013, 49, 9009–9011. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Wang, Z.; Yang, W.; Chen, K.; Zhao, J.; Gurzadyan, G.G. Precise Control of the Electronic Coupling Magnitude between the Electron Donor and Acceptor in Perylenebisimide Derivatives via Conformation Restriction and Its Effect on Photophysical Properties. J. Phys. Chem. C 2018, 122, 3756–3772. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, J.; Li, X.; Gurzadyan, G.G. Anthracene–Naphthalenediimide Compact Electron Donor/Acceptor Dyads: Electronic Coupling, Electron Transfer, and Intersystem Crossing. J. Phys. Chem. A 2019, 123, 2503–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Elmali, A.; Duan, R.; Liu, Q.; Ji, W.; Zhao, J.; Li, C.; Karatay, A. Charge Separation, Recombination and Intersystem Crossing of Directly Connected Perylenemonoimide–Carbazole Electron Donor/Acceptor Dyads. Phys. Chem. Chem. Phys. 2020, 22, 6376–6390. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Z.; Taddei, M.; Rehmat, N.; Bussotti, L.; Doria, S.; Guan, Q.; Ji, S.; Zhao, J.; Di Donato, M.; Huo, Y.; et al. Color-Tunable Delayed Fluorescence and Efficient Spin–Orbit Charge Transfer Intersystem Crossing in Compact Carbazole-Anthracene-Bodipy Triads Employing the Sequential Electron Transfer Approach. J. Phys. Chem. C 2020, 124, 5944–5957. [Google Scholar] [CrossRef]
- Marek-Urban, P.H.; Urban, M.; Wiklińska, M.; Paplińska, K.; Woźniak, K.; Blacha-Grzechnik, A.; Durka, K. Heavy-Atom Free spiro Organoboron Complexes as Triplet Excited States Photosensitizers for Singlet Oxygen Activation. J. Org. Chem. 2021, 86, 12714–12722. [Google Scholar] [CrossRef]
- Trinh, C.; Kirlikovali, K.; Das, S.; Ener, M.E.; Gray, H.B.; Djurovich, P.; Bradforth, S.E.; Thompson, M.E. Symmetry-Breaking Charge Transfer of Visible Light Absorbing Systems: Zinc Dipyrrins. J. Phys. Chem. C 2014, 118, 21834–21845. [Google Scholar] [CrossRef]
- Das, S.; Thornbury, W.G.; Bartynski, A.N.; Thompson, M.E.; Bradforth, S.E. Manipulating Triplet Yield through Control of Symmetry-Breaking Charge Transfer. J. Phys. Chem. Lett. 2018, 9, 3264–3270. [Google Scholar] [CrossRef]
- Turro, N.J.; Ramamurthy, V.; Scaiano, J.C. Principles of Molecular Photochemistry: An Introduction; University Science Books: Sausalito, CA, USA, 2009. [Google Scholar]
- Yushchenko, O.; Licari, G.; Mosquera-Vazquez, S.; Sakai, N.; Matile, S.; Vauthey, E. Ultrafast Intersystem-Crossing Dynamics and Breakdown of the Kasha–Vavilov’s Rule of Naphthalenediimides. J. Phys. Chem. Lett. 2015, 6, 2096–2100. [Google Scholar] [CrossRef]
- Ma, H.; Long, S.; Cao, J.; Xu, F.; Zhou, P.; Zeng, G.; Zhou, X.; Shi, C.; Sun, W.; Du, J.; et al. New Cy5 Photosensitizers for Cancer Phototherapy: A Low Singlet–Triplet Gap Provides High Quantum Yield of Singlet Oxygen. Chem. Sci. 2021, 12, 13809–13816. [Google Scholar] [CrossRef]
- Hao, B.; Wang, J.; Wang, C.; Xue, K.; Xiao, M.; Lv, S.; Zhu, C. Bridging D-A Type Photosensitizers with the Azo Group to Boost Intersystem Crossing for Efficient Photodynamic Therapy. Chem. Sci. 2022, 13, 4139–4149. [Google Scholar] [CrossRef]
- Mahmood, Z.; Rehmat, N.; Ji, S.; Zhao, J.; Sun, S.; Di Donato, M.; Li, M.; Teddei, M.; Huo, Y. Tuning the Triplet Excited State of Bis(Dipyrrin) Zinc(II) Complexes: Symmetry Breaking Charge Transfer Architecture with Exceptionally Long Lived Triplet State for Upconversion. Chem. Eur. J. 2020, 26, 14912–14918. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Elmali, A.; Zhao, J.; Dick, B.; Karatay, A. Long-Lived Triplet Excited State Accessed with Spin–Orbit Charge Transfer Intersystem Crossing in Red Light-Absorbing Phenoxazine-Styryl Bodipy Electron Donor/Acceptor Dyads. ChemPhysChem 2020, 21, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Deckers, J.; Cardeynaels, T.; Doria, S.; Tumanov, N.; Lapini, A.; Ethirajan, A.; Ameloot, M.; Wouters, J.; Di Donato, M.; Champagne, B.; et al. Balancing Fluorescence and Singlet Oxygen Formation in Push–Pull Type Near-Infrared Bodipy Photosensitizers. J. Mater. Chem. C 2022, 10, 9344–9355. [Google Scholar] [CrossRef]
- Xiao, Y.-F.; Chen, J.-X.; Chen, W.-C.; Zheng, X.; Cao, C.; Tan, J.; Cui, X.; Yuan, Z.; Ji, S.; Lu, G.; et al. Achieving High Singlet-Oxygen Generation by Applying the Heavy-Atom Effect to Thermally Activated Delayed Fluorescent Materials. Chem. Commun. 2021, 57, 4902–4905. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-Atom-Free Photosensitizers: From Molecular Design to Applications in the Photodynamic Therapy of Cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Sapir, M.; Donckt, E.V. Intersystem Crossing in the Helicenes. Chem. Phys. Lett. 1975, 36, 108–110. [Google Scholar] [CrossRef]
- Schmidt, K.; Brovelli, S.; Coropceanu, V.; Beljonne, D.; Cornil, J.; Bazzini, C.; Caronna, T.; Tubino, R.; Meinardi, F.; Shuai, Z.; et al. Intersystem Crossing Processes in Nonplanar Aromatic Heterocyclic Molecules. J. Phys. Chem. A 2007, 111, 10490–10499. [Google Scholar] [CrossRef]
- Wu, Y.; Zhen, Y.; Ma, Y.; Zheng, R.; Wang, Z.; Fu, H. Exceptional Intersystem Crossing in Di(Perylene Bisimide)s: A Structural Platform toward Photosensitizers for Singlet Oxygen Generation. J. Phys. Chem. Lett. 2010, 1, 2499–2502. [Google Scholar] [CrossRef]
- Menning, S.; Krämer, M.; Duckworth, A.; Rominger, F.; Beeby, A.; Dreuw, A.; Bunz, U.H.F. Bridged Tolanes: A Twisted Tale. J. Org. Chem. 2014, 79, 6571–6578. [Google Scholar] [CrossRef]
- Reger, D.; Haines, P.; Heinemann, F.W.; Guldi, D.M.; Jux, N. Oxa[7]Superhelicene: A π-Extended Helical Chromophore Based on Hexa-peri-Hexabenzocoronenes. Angew. Chem. Int. Ed. 2018, 57, 5938–5942. [Google Scholar] [CrossRef]
- Salla, C.A.M.; Farias, G.; Rouzières, M.; Dechambenoit, P.; Durola, F.; Bock, H.; de Souza, B.; Bechtold, I.H. Persistent Solid-State Phosphorescence and Delayed Fluorescence at Room Temperature by a Twisted Hydrocarbon. Angew. Chem. Int. Ed. 2019, 58, 6982–6986. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Mallia, A.R.; Muraleedharan, K.; Hariharan, M. Enhanced Intersystem Crossing in Core-Twisted Aromatics. Chem. Sci. 2017, 8, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Rachford, A.A.; Goeb, S.; Castellano, F.N. Accessing the Triplet Excited State in Perylenediimides. J. Am. Chem. Soc. 2008, 130, 2766–2767. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J. Visible Light-Harvesting Perylenebisimide–Fullerene (C60) Dyads with Bidirectional “Ping-Pong” Energy Transfer as Triplet Photosensitizers for Photooxidation of 1, 5-Dihydroxynaphthalene. Chem. Commun. 2012, 48, 3751–3753. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, J.; Sonn, C.; Escudero, D.; Karatay, A.; Yaglioglu, H.G.; Küçüköz, B.; Hayvali, M.; Li, C.; Jacquemin, D. Efficient Intersystem Crossing in Heavy-Atom-Free Perylenebisimide Derivatives. J. Phys. Chem. C 2016, 120, 10162–10175. [Google Scholar] [CrossRef]
- Rehmat, N.; Toffoletti, A.; Mahmood, Z.; Zhang, X.; Zhao, J.; Barbon, A. Carbazole-Perylenebisimide Electron Donor/Acceptor Dyads Showing Efficient Spin Orbit Charge Transfer Intersystem Crossing (SOCT-ISC) and Photo-Driven Intermolecular Electron Transfer. J. Mater. Chem. C 2020, 8, 4701–4712. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Q.; Wang, S.; Yu, C.; Li, J.; Hao, E.; Wei, Y.; Mu, X.; Jiao, L. Conformation-Restricted Partially and Fully Fused Bodipy Dimers as Highly Stable Near-Infrared Fluorescent Dyes. Org. Lett. 2015, 17, 5360–5363. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, J.; Gai, L.; Yuan, A.; Shen, Z. Naphtho[b]-Fused Bodipys: One Pot Suzuki–Miyaura–Knoevenagel Synthesis and Photophysical Properties. Chem. Commun. 2017, 53, 6621–6624. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Yan, Y.; El-Zohry, A.M.; Toffoletti, A.; Zhao, J.; Barbon, A.; Dick, B.; Mohammed, O.F.; Han, G. Elucidation of the Intersystem Crossing Mechanism in a Helical Bodipy for Low-Dose Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 16114–16121. [Google Scholar] [CrossRef]
- Dong, Y.; Dick, B.; Zhao, J. Twisted Bodipy Derivative as a Heavy-Atom-Free Triplet Photosensitizer Showing Strong Absorption of Yellow Light, Intersystem Crossing, and a High-Energy Long-Lived Triplet State. Org. Lett. 2020, 22, 5535–5539. [Google Scholar] [CrossRef]
- Dong, Y.; Kumar, P.; Maity, P.; Kurganskii, I.; Li, S.; Elmali, A.; Zhao, J.; Escudero, D.; Wu, H.; Karatay, A.; et al. Twisted Bodipy Derivative: Intersystem Crossing, Electron Spin Polarization and Application as a Novel Photodynamic Therapy Reagent. Phys. Chem. Chem. Phys. 2021, 23, 8641–8652. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Sakai, H.; Suzuki, Y.; Kawamata, J.; Hasobe, T. Systematic Control of Structural and Photophysical Properties of π-Extended Mono- and Bis-Bodipy Derivatives. Chem. Eur. J. 2020, 26, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zeng, W.; Huang, K.-W.; Wu, J. Benzene-Fused Bodipys: Synthesis and the Impact of Fusion Mode. Chem. Commun. 2013, 49, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sukhanov, A.A.; Bousquet, M.H.E.; Guan, Q.; Zhao, J.; Voronkova, V.K.; Escudero, D.; Barbon, A.; Xing, Y.; Gurzadyan, G.G.; et al. Does Twisted π-Conjugation Framework Always Induce Efficient Intersystem Crossing? A Case Study with Benzo[b]- and [a]Phenanthrene-Fused Bodipy Derivatives and Identification of a Dark State. J. Phys. Chem. B 2021, 125, 6280–6295. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Xuan, S.; Zhou, Z.; Fronczek, F.R.; Smith, K.M.; Vicente, G.H. Synthesis and Spectroscopic and Cellular Properties of Near-IR [a]Phenanthrene-Fused 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacenes. J. Org. Chem. 2017, 82, 9744–9750. [Google Scholar] [CrossRef]
- Kawai, A.; Shibuya, K. Electron Spin Dynamics in a Pair Interaction between Radical and Electronically-Excited Molecule as Studied by a Time-Resolved ESR Method. J. Photochem. Photobiol. C 2006, 7, 89–103. [Google Scholar] [CrossRef]
- Likhtenstein, G.I.; Ishii, K.; Nakatsuji, S.i. Dual Chromophore-Nitroxides: Novel Molecular Probes, Photochemical and Photophysical Models and Magnetic Materials. Photochem. Photobiol. 2007, 83, 871–881. [Google Scholar] [CrossRef]
- Dyar, S.M.; Margulies, E.A.; Horwitz, N.E.; Brown, K.E.; Krzyaniak, M.D.; Wasielewski, M.R. Photogenerated Quartet State Formation in a Compact Ring-Fused Perylene-Nitroxide. J. Phys. Chem. B 2015, 119, 13560–13569. [Google Scholar] [CrossRef]
- Teki, Y.; Miyamoto, S.; Nakatsuji, M.; Miura, Y. π-Topology and Spin Alignment Utilizing the Excited Molecular Field: Observation of the Excited High-Spin Quartet (S = 3/2) and Quintet (S = 2) States on Purely Organic π-Conjugated Spin Systems. J. Am. Chem. Soc. 2001, 123, 294–305. [Google Scholar] [CrossRef]
- Ishii, K.; Hirose, Y.; Fujitsuka, H.; Ito, O.; Kobayashi, N. Time-Resolved EPR, Fluorescence, and Transient Absorption Studies on Phthalocyaninatosilicon Covalently Linked to One or Two TEMPO Radicals. J. Am. Chem. Soc. 2001, 123, 702–708. [Google Scholar] [CrossRef]
- Giacobbe, E.M.; Mi, Q.; Colvin, M.T.; Cohen, B.; Ramanan, C.; Scott, A.M.; Yeganeh, S.; Marks, T.J.; Ratner, M.A.; Wasielewski, M.R. Ultrafast Intersystem Crossing and Spin Dynamics of Photoexcited Perylene-3,4:9,10-Bis(Dicarboximide) Covalently Linked to a Nitroxide Radical at Fixed Distances. J. Am. Chem. Soc. 2009, 131, 3700–3712. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.T.; Giacobbe, E.M.; Cohen, B.; Miura, T.; Scott, A.M.; Wasielewski, M.R. Competitive Electron Transfer and Enhanced Intersystem Crossing in Photoexcited Covalent TEMPO−Perylene-3,4:9,10-Bis(Dicarboximide) Dyads: Unusual Spin Polarization Resulting from the Radical−Triplet Interaction. J. Phys. Chem. A 2010, 114, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xie, T.; Duan, X.; Yu, F.; Wang, X.; Tang, B. A New Highly Selective and Sensitive Assay for Fluorescence Imaging of ⋅OH in Living Cells: Effectively Avoiding the Interference of Peroxynitrite. Chem. Eur. J. 2010, 16, 1834–1840. [Google Scholar] [CrossRef]
- Yapici, N.B.; Jockusch, S.; Moscatelli, A.; Mandalapu, S.R.; Itagaki, Y.; Bates, D.K.; Wiseman, S.; Gibson, K.M.; Turro, N.J.; Bi, L. New Rhodamine Nitroxide Based Fluorescent Probes for Intracellular Hydroxyl Radical Identification in Living Cells. Org. Lett. 2012, 14, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, J.-i.; Ishii, K.; Ohba, Y.; Yamauchi, S.; Fuhs, M.; Möbius, K. First Observation of the Excited Doublet State of a Radical−Triplet Pair in Solution: W-Band High-Field Time-Resolved Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. A 1999, 103, 213–216. [Google Scholar] [CrossRef]
- Ishii, K.; Fujisawa, J.-i.; Ohba, Y.; Yamauchi, S. A Time-Resolved Electron Paramagnetic Resonance Study on the Excited States of Tetraphenylporphinatozinc(II) Coordinated by p-Pyridyl Nitronyl Nitroxide. J. Am. Chem. Soc. 1996, 118, 13079–13080. [Google Scholar] [CrossRef]
- Teki, Y.; Miyamoto, S.; Iimura, K.; Nakatsuji, M.; Miura, Y. Intramolecular Spin Alignment Utilizing the Excited Molecular Field between the Triplet (S = 1) Excited State and the Dangling Stable Radicals (S = 1/2) as Studied by Time-Resolved Electron Spin Resonance: Observation of the Excited Quartet (S = 3/2) and Quintet (S = 2) States on the Purely Organic π-Conjugated Spin Systems. J. Am. Chem. Soc. 2000, 122, 984–985. [Google Scholar]
- Teki, Y.; Toichi, T.; Nakajima, S. π Topology and Spin Alignment in Unique Photoexcited Triplet and Quintet States Arising from Four Unpaired Electrons of an Organic Spin System. Chem. Eur. J. 2006, 12, 2329–2336. [Google Scholar] [CrossRef]
- Rane, V.; Das, R. Distance Dependence of Electron Spin Polarization During Photophysical Quenching of Excited Naphthalene by TEMPO Radical. J. Phys. Chem. A 2015, 119, 5515–5523. [Google Scholar] [CrossRef]
- Chernick, E.T.; Casillas, R.; Zirzlmeier, J.; Gardner, D.M.; Gruber, M.; Kropp, H.; Meyer, K.; Wasielewski, M.R.; Guldi, D.M.; Tykwinski, R.R. Pentacene Appended to a TEMPO Stable Free Radical: The Effect of Magnetic Exchange Coupling on Photoexcited Pentacene. J. Am. Chem. Soc. 2015, 137, 857–863. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, J.; Barbon, A.; Toffoletti, A.; Liu, Y.; An, Y.; Xu, L.; Karatay, A.; Yaglioglu, H.G.; Yildiz, E.A.; et al. Radical-Enhanced Intersystem Crossing in New Bodipy Derivatives and Application for Efficient Triplet–Triplet Annihilation Upconversion. J. Am. Chem. Soc. 2017, 139, 7831–7842. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Sukhanov, A.A.; Zhao, Y.; Zhao, J.; Ji, W.; Peng, X.; Escudero, D.; Jacquemin, D.; Voronkova, V.K. Unexpected Nucleophilic Substitution Reaction of Bodipy: Preparation of the Bodipy–TEMPO Triad Showing Radical-Enhanced Intersystem Crossing. Eur. J. Org. Chem. 2018, 2018, 885–895. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Jin, S.; Li, C.; Warncke, K.; Evangelista, F.A.; Lian, T.; Egap, E. Conjugated Oligomers with Stable Radical Substituents: Synthesis, Single Crystal Structures, Electronic Structure, and Excited State Dynamics. Chem. Mater. 2018, 30, 7840–7851. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Y.; Hussain, M.; Kundu, S.; Rane, V.; Hayvali, M.; Yildiz, E.A.; Zhao, J.; Yaglioglu, H.G.; Das, R.; et al. Efficient Radical-Enhanced Intersystem Crossing in an NDI-TEMPO Dyad: Photophysics, Electron Spin Polarization, and Application in Photodynamic Therapy. Chem. Eur. J. 2018, 24, 18663–18675. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Song, F.; Cui, J.; Peng, X. A Near-Infrared Heptamethine Aminocyanine Dye with a Long-Lived Excited Triplet State for Photodynamic Therapy. Chem. Commun. 2018, 54, 9198–9201. [Google Scholar] [CrossRef]
- Webster, S.; Peceli, D.; Hu, H.; Padilha, L.A.; Przhonska, O.V.; Masunov, A.E.; Gerasov, A.O.; Kachkovski, A.D.; Slominsky, Y.L.; Tolmachev, A.I.; et al. Near-Unity Quantum Yields for Intersystem Crossing and Singlet Oxygen Generation in Polymethine-Like Molecules: Design and Experimental Realization. J. Phys. Chem. Lett. 2010, 1, 2354–2360. [Google Scholar] [CrossRef]
- Hussain, M.; Zhao, J.; Yang, W.; Zhong, F.; Karatay, A.; Yaglioglu, H.G.; Yildiz, E.A.; Hayvali, M. Intersystem Crossing and Triplet Excited State Properties of Thionated Naphthalenediimide Derivatives. J. Luminsc. 2017, 192, 211–217. [Google Scholar] [CrossRef]
- Nguyen, V.-N.; Qi, S.; Kim, S.; Kwon, N.; Kim, G.; Yim, Y.; Park, S.; Yoon, J. An Emerging Molecular Design Approach to Heavy-Atom-Free Photosensitizers for Enhanced Photodynamic Therapy under Hypoxia. J. Am. Chem. Soc. 2019, 141, 16243–16248. [Google Scholar] [CrossRef]
- Pham, T.C.; Hoang, T.T.; Choi, Y.; Lee, S.; Joo, S.-W.; Kim, G.; Kim, D.; Jung, O.-S.; Lee, S. Dual Molecular Design toward a Lysosome-Tagged Aiegen and Heavy-Atom-Free Photosensitizers for Hypoxic Cancer Photodynamic Therapy. Biosensors 2022, 12, 420. [Google Scholar] [CrossRef]
- Levanon, H.; Norris, J.R. The Photoexcited Triplet State and Photosynthesis. Chem. Rev. 1978, 78, 185–198. [Google Scholar] [CrossRef]
- Weber, S. Transient EPR. eMagRes 2017, 6, 255–270. [Google Scholar]
- Richert, S.; Tait, C.E.; Timmel, C.R. Delocalisation of Photoexcited Triplet States Probed by Transient EPR and Hyperfine Spectroscopy. J. Magn. Reson. 2017, 280, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Hintze, C.; Steiner, U.E.; Drescher, M. Photoexcited Triplet State Kinetics Studied by Electron Paramagnetic Resonance Spectroscopy. ChemPhysChem 2017, 18, 6–16. [Google Scholar] [CrossRef]
- Hirota, N.; Yamauchi, S. Short-Lived Excited Triplet States Studied by Time-Resolved EPR Spectroscopy. J. Photochem. Photobiol. C 2003, 4, 109–124. [Google Scholar] [CrossRef]
- Dong, Y.; Sukhanov, A.A.; Zhao, J.; Elmali, A.; Li, X.; Dick, B.; Karatay, A.; Voronkova, V.K. Spin–Orbit Charge-Transfer Intersystem Crossing (SOCT-ISC) in Bodipy-Phenoxazine Dyads: Effect of Chromophore Orientation and Conformation Restriction on the Photophysical Properties. J. Phys. Chem. C 2019, 123, 22793–22811. [Google Scholar] [CrossRef]
- Hou, Y.; Kurganskii, I.; Elmali, A.; Zhang, H.; Gao, Y.; Lv, L.; Zhao, J.; Karatay, A.; Luo, L.; Fedin, M. Electronic Coupling and Spin–Orbit Charge Transfer Intersystem Crossing (SOCT-ISC) in Compact BDP–Carbazole Dyads with Different Mutual Orientations of the Electron Donor and Acceptor. J. Chem. Phys. 2020, 152, 114701. [Google Scholar] [CrossRef] [PubMed]
- Dance, Z.E.X.; Mi, Q.; McCamant, D.W.; Ahrens, M.J.; Ratner, M.A.; Wasielewski, M.R. Time-Resolved EPR Studies of Photogenerated Radical Ion Pairs Separated by p-Phenylene Oligomers and of Triplet States Resulting from Charge Recombination. J. Phys. Chem. B 2006, 110, 25163–25173. [Google Scholar] [CrossRef]
- Colvin, M.T.; Carmieli, R.; Miura, T.; Richert, S.; Gardner, D.M.; Smeigh, A.L.; Dyar, S.M.; Conron, S.M.; Ratner, M.A.; Wasielewski, M.R. Electron Spin Polarization Transfer from Photogenerated Spin-Correlated Radical Pairs to a Stable Radical Observer Spin. J. Phys. Chem. A 2013, 117, 5314–5325. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Kotani, H.; Ohkubo, K.; Ogo, S.; Tkachenko, N.V.; Lemmetyinen, H. Electron-Transfer State of 9-Mesityl-10-Methylacridinium Ion with a Much Longer Lifetime and Higher Energy than that of the Natural Photosynthetic Reaction Center. J. Am. Chem. Soc. 2004, 126, 1600–1601. [Google Scholar] [CrossRef]
- Karimata, A.; Kawauchi, H.; Suzuki, S.; Kozaki, M.; Ikeda, N.; Keyaki, K.; Nozaki, K.; Akiyama, K.; Okada, K. Photoinduced Charge Separation of 10-Phenyl-10H-Phenothiazine–2-Phenylanthraquinone Dyad Bridged by Bicyclo[2.2.2]octane. Chem. Lett. 2013, 42, 794–796. [Google Scholar] [CrossRef]
- Tang, G.; Sukhanov, A.A.; Zhao, J.; Yang, W.; Wang, Z.; Liu, Q.; Voronkova, V.K.; Di Donato, M.; Escudero, D.; Jacquemin, D. Red Thermally Activated Delayed Fluorescence and the Intersystem Crossing Mechanisms in Compact Naphthalimide–Phenothiazine Electron Donor/Acceptor Dyads. J. Phys. Chem. C 2019, 123, 30171–30186. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, K.; Yildiz, E.A.; Li, S.; Hou, Y.; Zhang, X.; Wang, Z.; Zhao, J.; Barbon, A.; Yaglioglu, H.G.; et al. Efficient Intersystem Crossing in the Tröger’s Base Derived from 4-Amino-1,8-Naphthalimide and Application as a Potent Photodynamic Therapy Reagent. Chem. Eur. J. 2020, 26, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Zhao, J. Electron Spin Dynamics of the Intersystem Crossing of Triplet Photosensitizers That Show Strong Absorption of Visible Light and Long-Lived Triplet States. J. Phys. Chem. C 2021, 125, 19097–19109. [Google Scholar] [CrossRef]
- Imran, M.; Zhang, X.; Wang, Z.; Chen, X.; Zhao, J.; Barbon, A.; Voronkova, V.K. Electron Spin Dynamics in Excited State Photochemistry: Recent Development in the Study of Intersystem Crossing and Charge Transfer in Organic Compounds. Phys. Chem. Chem. Phys. 2021, 23, 15835–15868. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, X.; Ye, K.; Imran, M.; Zhao, J. Recent Development of Heavy Atom-Free Triplet Photosensitizers for Photodynamic Therapy. Appl. Sci. 2022, 12, 9933. https://doi.org/10.3390/app12199933
Xiao X, Ye K, Imran M, Zhao J. Recent Development of Heavy Atom-Free Triplet Photosensitizers for Photodynamic Therapy. Applied Sciences. 2022; 12(19):9933. https://doi.org/10.3390/app12199933
Chicago/Turabian StyleXiao, Xiao, Kaiyue Ye, Muhammad Imran, and Jianzhang Zhao. 2022. "Recent Development of Heavy Atom-Free Triplet Photosensitizers for Photodynamic Therapy" Applied Sciences 12, no. 19: 9933. https://doi.org/10.3390/app12199933
APA StyleXiao, X., Ye, K., Imran, M., & Zhao, J. (2022). Recent Development of Heavy Atom-Free Triplet Photosensitizers for Photodynamic Therapy. Applied Sciences, 12(19), 9933. https://doi.org/10.3390/app12199933







