Novel Application of Infrared Radiation Therapy: Spirit Identity Powder-Generated Far Infrared Radiation Inhibits the Proliferation of Human Male Liver Cancer Cells by Activating the Transmembrane Attack Complex
Abstract
1. Introduction
2. Materials and Methods
2.1. Spirit Identity (SI) Fabrication
2.2. Cell Culture
2.3. Cell Viability
2.4. Adenine Tri-Phosphatase (ATP) Assay
2.5. Affymetrix Whole Transcript Expression Arrays
2.6. Quantitative Polymerase Chain Reaction (qPCR)
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
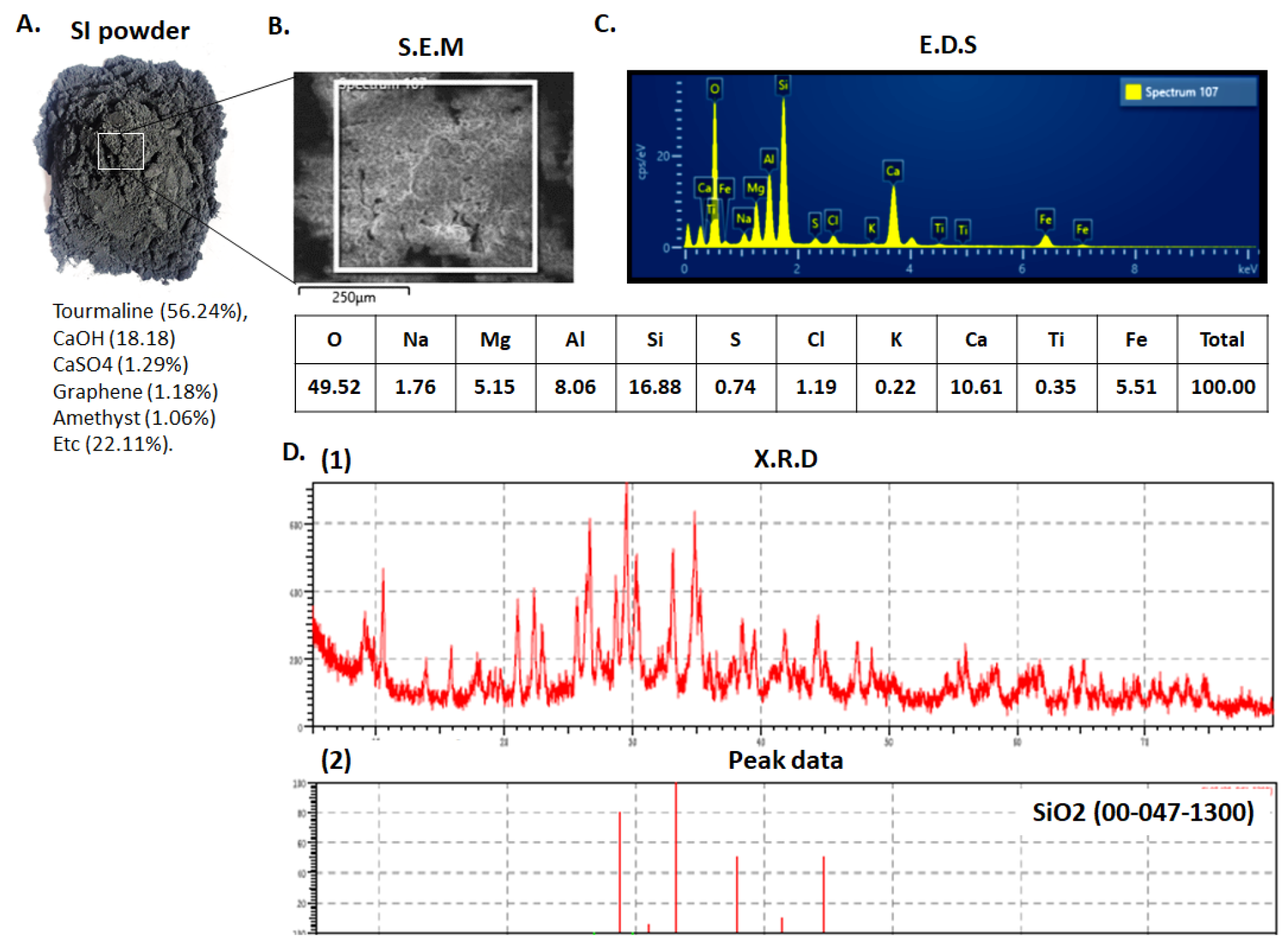
3.1. Characterization of the SI Powder
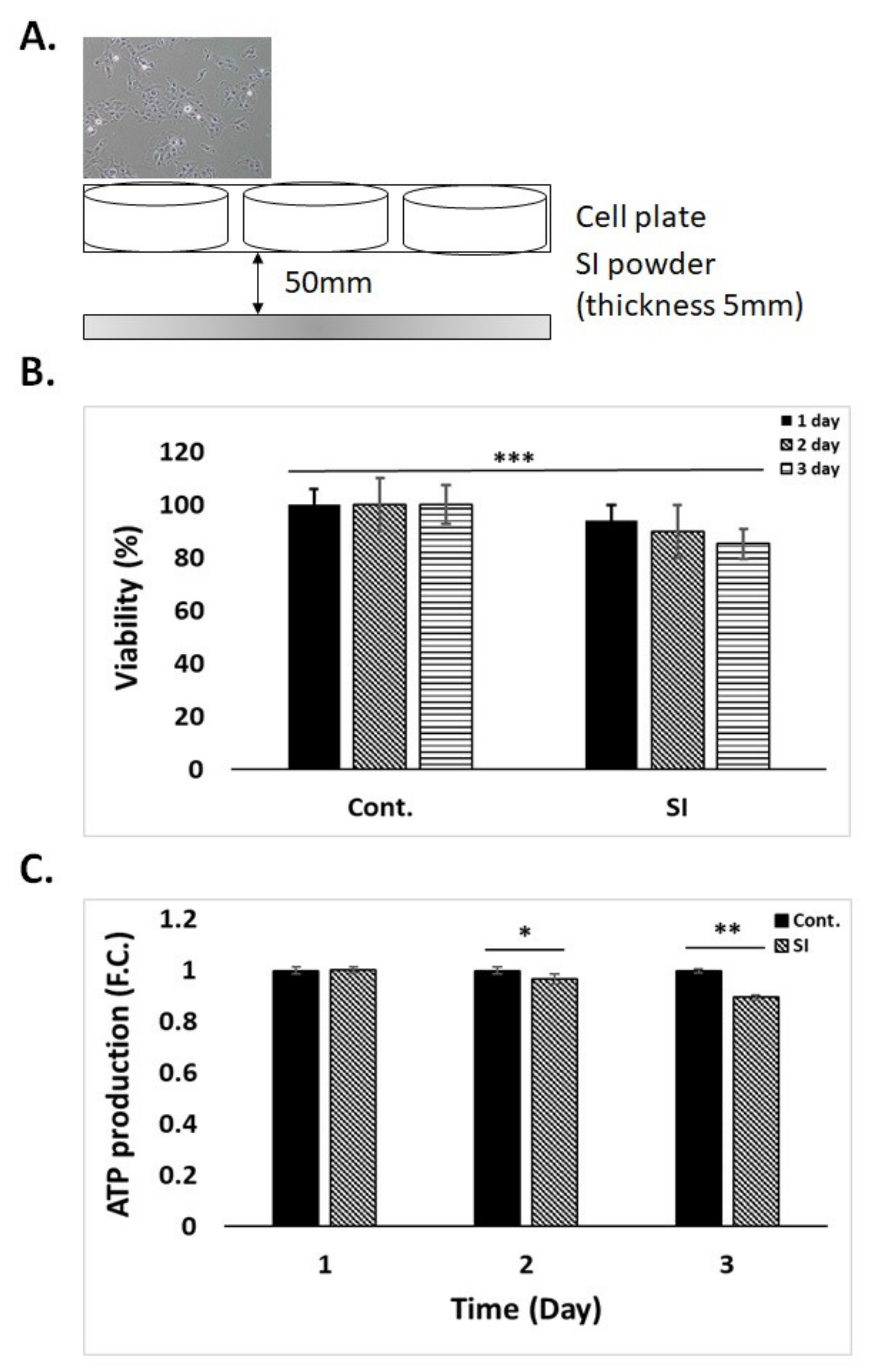
3.2. Cell Viability and ATP Production by SI Powder
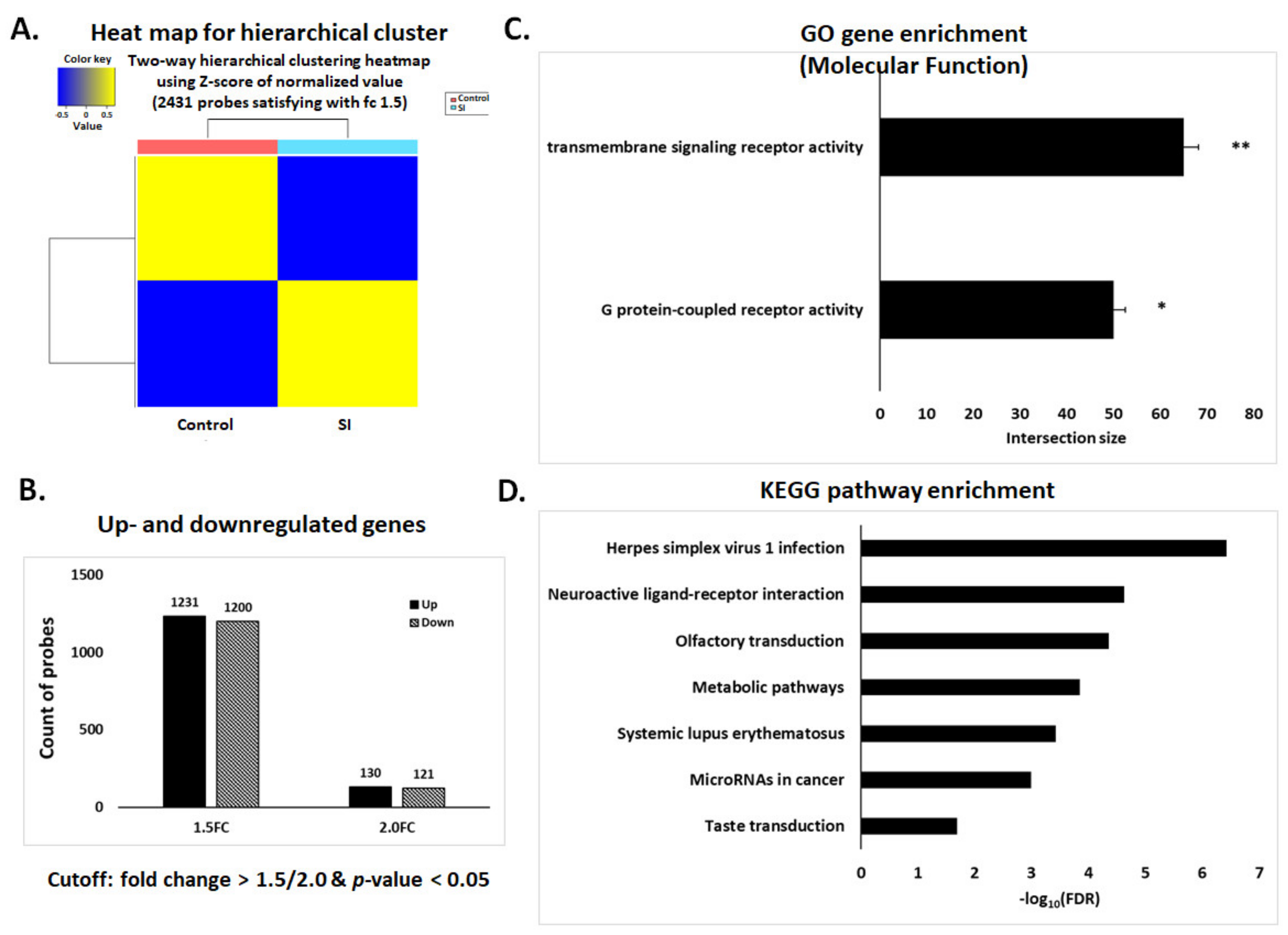
3.3. Affymetrix Gene Chip Analysis
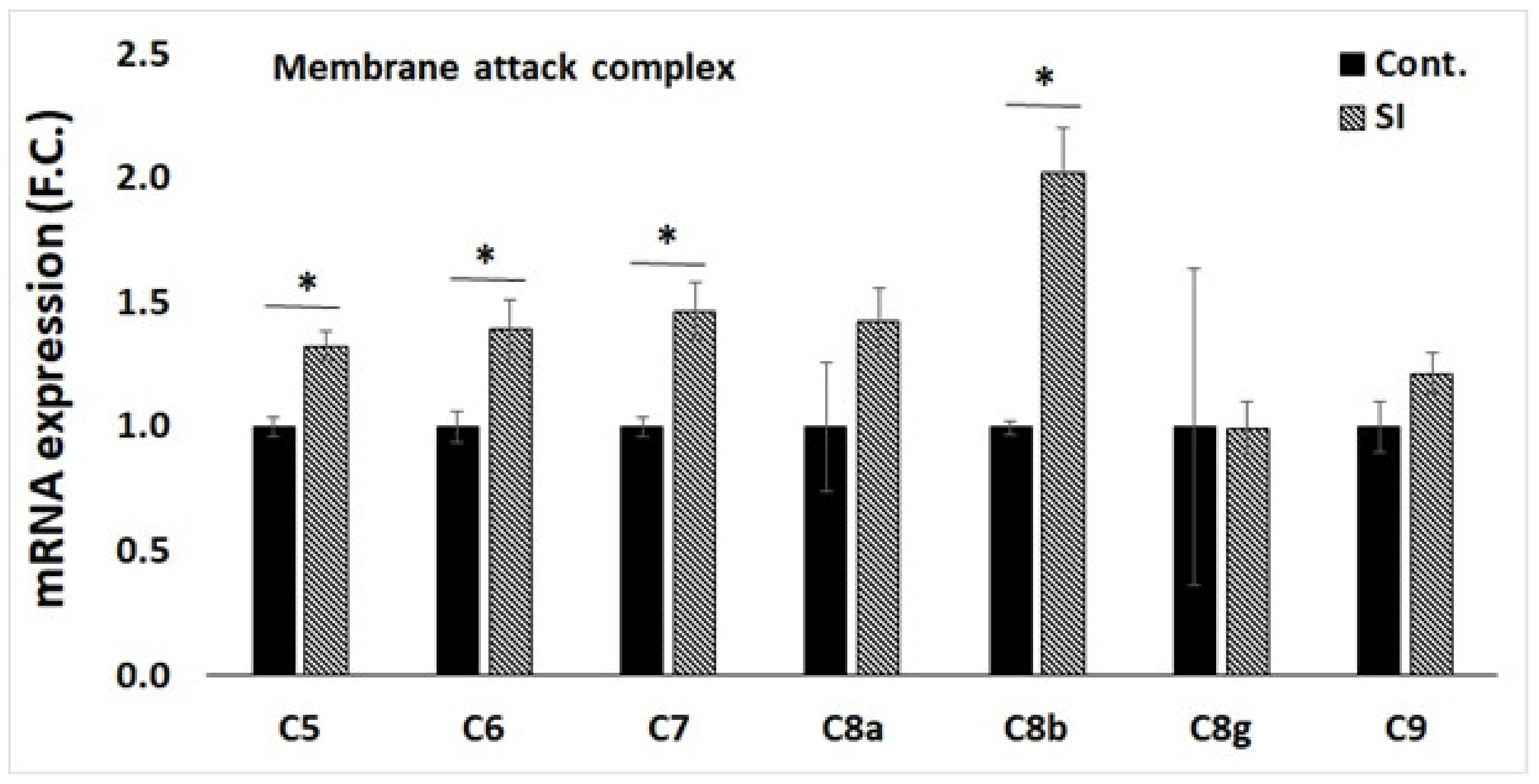
3.4. qPCR Validation of Transcriptome Data for Selected Genes
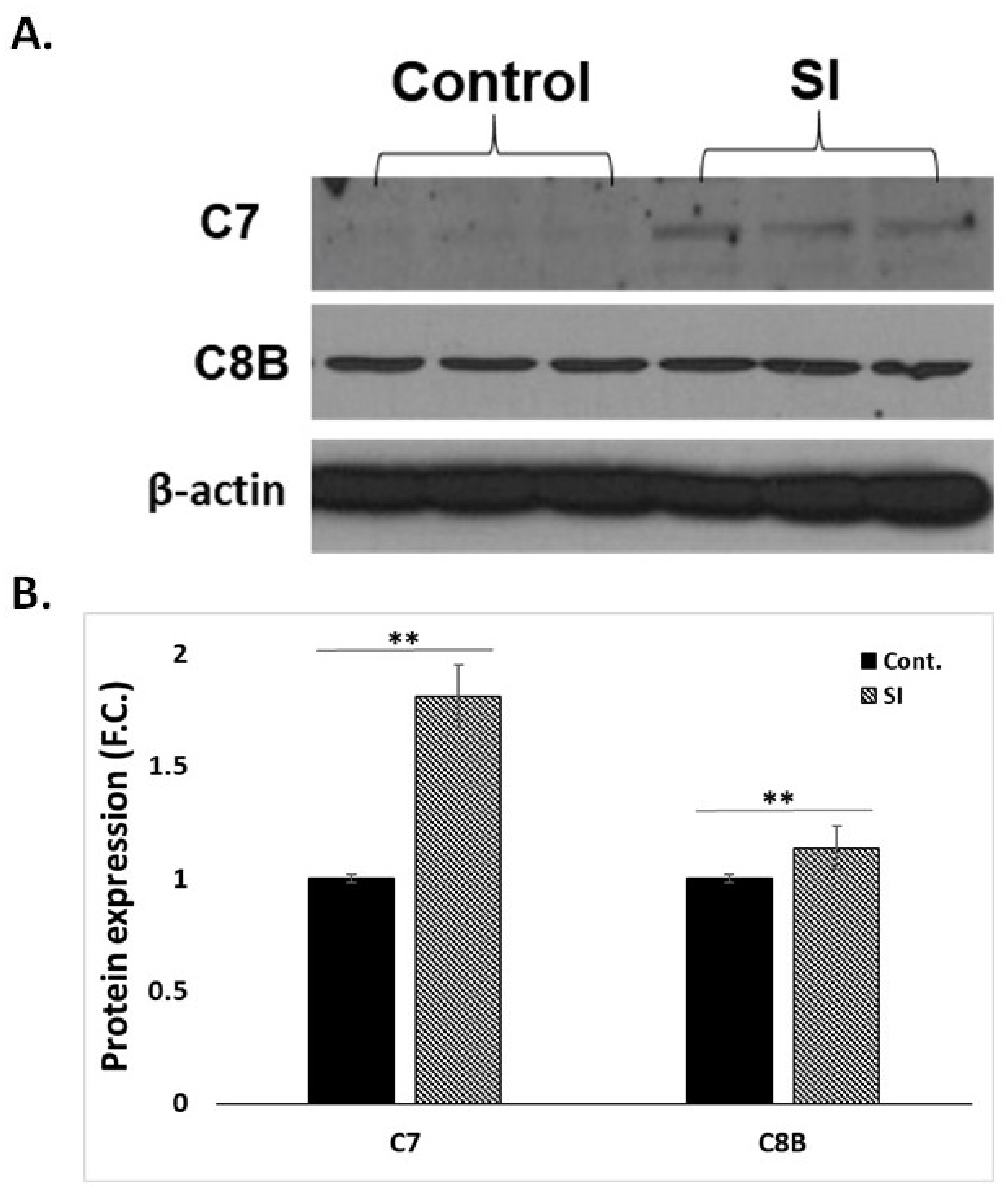
3.5. Western Blot Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vatansever, F.; Hamblin, M.R. Far infrared radiation (FIR): Its biological effects and medical applications. Photonics Lasers Med. 2012, 4, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Miyachi, Y.; Ishimoto, K.; Kanoh, T. Why are natural plant medicinal products effective in some patients and not in others with the same disease? Planta Med. 1991, 57, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Plaghki, L.; Decruynaere, C.; Van Dooren, P.; Le Bars, D. The fine tuning of pain thresholds: A sophisticated double alarm system. PLoS ONE 2010, 5, e10269. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.R.; Swicord, M.L.; Balzano, Q. Quantitative evaluations of mechanisms of radiofrequency interactions with biological molecules and processes. Health Phys. 2008, 95, 365–396. [Google Scholar] [CrossRef]
- Huang, P.H.; Chen, J.W.; Lin, C.P.; Chen, Y.H.; Wang, C.H.; Leu, H.B.; Lin, S.J. Far infra-red therapy promotes ischemia-induced angiogenesis in diabetic mice and restores high glucose-suppressed endothelial progenitor cell functions. Cardiovasc. Diabetol. 2012, 11, 99. [Google Scholar] [CrossRef]
- Akasaki, Y.; Miyata, M.; Eto, H.; Shirasawa, T.; Hamada, N.; Ikeda, Y.; Biro, S.; Otsuji, Y.; Tei, C. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ. J. 2006, 70, 463–470. [Google Scholar] [CrossRef]
- Imamura, M.; Biro, S.; Kihara, T.; Yoshifuku, S.; Takasaki, K.; Otsuji, Y.; Minagoe, S.; Toyama, Y.; Tei, C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J. Am. Coll. Cardiol. 2001, 38, 1083–1088. [Google Scholar] [CrossRef]
- Kihara, T.; Biro, S.; Ikeda, Y.; Fukudome, T.; Shinsato, T.; Masuda, A.; Miyata, M.; Hamasaki, S.; Otsuji, Y.; Minagoe, S.; et al. Effects of repeated sauna treatment on ventricular arrhythmias in patients with chronic heart failure. Circ. J. 2004, 68, 1146–1151. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, X.M.; Peyton, K.; Wang, H.; Yang, W.C.; Lin, S.J.; Durante, W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 739–745. [Google Scholar] [CrossRef]
- Lee, D.; Seo, Y.; Kim, Y.W.; Kim, S.; Bae, H.; Choi, J.; Lim, I.; Bang, H.; Kim, J.H.; Ko, J.H. Far-infrared radiation stimulates platelet-derived growth factor mediated skeletal muscle cell migration through extracellular matrix-integrin signaling. Korean J. Physiol. Pharmacol. 2019, 23, 141–150. [Google Scholar] [CrossRef]
- Lin, T.C.; Lin, C.S.; Tsai, T.N.; Cheng, S.M.; Lin, W.S.; Cheng, C.C.; Wu, C.H.; Hsu, C.H. Stimulatory influences of far infrared therapy on the transcriptome and genetic networks of endothelial progenitor cells receiving high glucose treatment. Acta Cardiol. Sin. 2015, 31, 414–428. [Google Scholar]
- Chen, C.H.; Chen, T.H.; Wu, M.Y.; Chou, T.C.; Chen, J.R.; Wei, M.J.; Lee, S.L.; Hong, L.Y.; Zheng, C.M.; Chiu, I.J.; et al. Far-infrared protects vascular endothelial cells from advanced glycation end products-induced injury via PLZF-mediated autophagy in diabetic mice. Sci. Rep. 2017, 7, 40442. [Google Scholar] [CrossRef]
- Tu, Y.P.; Chen, S.C.; Liu, Y.H.; Chen, C.F.; Hour, T.C. Postconditioning with far-infrared irradiation increases heme oxygenase-1 expression and protects against ischemia/reperfusion injury in rat testis. Life Sci. 2013, 92, 35–41. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, Y.C.; Chen, T.H.; Sue, Y.M.; Cheng, T.H.; Chen, J.R.; Chen, C.H. Far-infrared therapy induces the nuclear translocation of PLZF which inhibits VEGF-induced proliferation in human umbilical vein endothelial cells. PLoS ONE 2012, 7, e30674. [Google Scholar] [CrossRef]
- Ishibashi, J.; Yamashita, K.; Ishikawa, T.; Hosokawa, H.; Sumida, K.; Nagayama, M.; Kitamura, S. The effects inhibiting the proliferation of cancer cells by far-infrared radiation (FIR) are controlled by the basal expression level of heat shock protein (HSP) 70A. Med. Oncol. 2008, 25, 229–237. [Google Scholar] [CrossRef]
- Leung, T.K.; Chan, C.F.; Lai, P.S.; Yang, C.H.; Hsu, C.Y.; Lin, Y.-S. Inhibitory effects of far-infrared irradiation generated by ceramic material on murine melanoma cell growth. Int. J. Photoenergy 2012, 2012, 646845. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, D.H.; Lee, I.K.; Park, Y.M.; Jo, I. Far-infrared radiation inhibits proliferation, migration, and angiogenesis of human umbilical vein endothelial cells by suppressing secretory clusterin levels. Cancer Lett. 2014, 346, 74–83. [Google Scholar] [CrossRef]
- Cho, D.H.; Lee, H.J.; Lee, J.Y.; Park, J.H.; Jo, I. Far-infrared irradiation inhibits breast cancer cell proliferation independently of DNA damage through increased nuclear Ca2+/calmodulin binding modulated-activation of checkpoint kinase 2. J. Photochem. Photobiol. B 2021, 219, 112188. [Google Scholar] [CrossRef]
- Yamashita, K.; Dalkhsuren, S.O.; Ishikawa, T.; Sumida, K.; Ishibashi, J.; Hosokawa, H.; Ueno, A.; Nasu, F.; Kitamura, S. Far infrared ray radiation inhibits the proliferation of A549, HSC3 and Sa3 cancer cells through enhancing the expression of ATF3 gene. J. Electromagn. Anal. Appl. 2010, 2, 382–394. [Google Scholar] [CrossRef]
- Heitbrink, D.; Sigurdson, H.; Bolwien, C.; Brzezinski, P.; Heberle, J. Transient binding of CO to Cu(B) in cytochrome c oxidase is dynamically linked to structural changes around a carboxyl group: A time-resolved step-scan Fourier transform infrared investigation. Biophys. J. 2002, 82, 1–10. [Google Scholar] [CrossRef]
- Rich, P.R.; Breton, J. Attenuated total reflection Fourier transform infrared studies of redox changes in bovine cytochrome c oxidase: Resolution of the redox Fourier transform infrared difference spectrum of heme a(3). Biochemistry 2002, 41, 967–973. [Google Scholar] [CrossRef]
- Maréchal, A.; Rich, P.R. Water molecule reorganization in cytochrome c oxidase revealed by FTIR spectroscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 8634–8638. [Google Scholar] [CrossRef]
- Lane, N. Cell Biology: Cell biology: Power games. Nature 2006, 443, 901–903. [Google Scholar] [CrossRef]
- Chen, A.C.H.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef]
- Calì, T.; Ottolini, D.; Brini, M. Mitochondrial Ca(2+) as a key regulator of mitochondrial activities. Adv. Exp. Med. Biol. 2012, 942, 53–73. [Google Scholar]
- Xie, C.B.; Jane-Wit, D.; Pober, J.S. Complement membrane attack complex: New roles, mechanisms of action, and therapeutic targets. Am. J. Pathol. 2020, 190, 1138–1150. [Google Scholar] [CrossRef]
- Nauta, A.J.; Daha, M.R.; Tijsma, O.; van de Water, B.; Tedesco, F.; Roos, A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur. J. Immunol. 2002, 32, 783. [Google Scholar] [CrossRef]
- Bajerski, F.; Stock, J.; Hanf, B.; Darienko, T.; Heine-Dobbernack, E.; Lorenz, M.; Naujox, L.; Keller, E.R.J.; Schumacher, H.M.; Friedl, T.; et al. ATP content and cell viability as indicators for cryostress across the diversity of life. Front. Physiol. 2018, 9, 921. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Apoptosis and necrosis: Intracellular ATP level as a determinant for cell death modes. Cell Death Differ. 1997, 4, 429–434. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cristóbal-García, M.; García-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.H.; Johnson, R.J. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp. Nephrol. 2012, 121, e71–e78. [Google Scholar] [CrossRef]
- Rus, H.; Cudrici, C.; Niculescu, F. The role of the complement system in innate immunity. Immunol. Res. 2005, 33, 103–112. [Google Scholar] [CrossRef]
- Bayly-Jones, C.; Bubeck, D.; Dunstone, M.A. The mystery behind membrane insertion: A review of the complement membrane attack complex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160221. [Google Scholar] [CrossRef] [PubMed]
- Savill, J. Apoptosis and the kidney. J. Am. Soc. Nephrol. 1994, 5, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sandau, K.; Pfeilschifter, J.; Brüne, B. Nitric oxide and superoxide induced p53 and Bax accumulation during mesangial cell apoptosis. Kidney Int. 1997, 52, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Nangaku, M.; Alpers, C.E.; Shankland, S.J.; Couser, W.G.; Johnson, R.J. C5b-9 membrane attack complex mediates endothelial cell apoptosis in experimental glomerulonephritis. Am. J. Physiol. Renal Physiol. 2000, 278, F747–F757. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, D.; Zhao, K.; Wu, Y.; Zhang, P.; Liu, J.; Yang, G.; Gong, P.; Liu, Z. Donor-acceptor structured photothermal COFs for enhanced starvation therapy. Chem. Eng. J. 2022, 442, 135963. [Google Scholar] [CrossRef]
- Serna, M.; Giles, J.L.; Morgan, B.P.; Bubeck, D. Structural basis of complement membrane attack complex formation. Nat. Commun. 2016, 7, 10587. [Google Scholar] [CrossRef]
- Lovelace, L.L.; Cooper, C.L.; Sodetz, J.M.; Lebioda, L. Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J. Biol. Chem. 2011, 286, 17585–17592. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-B.; Park, J.-H. Novel Application of Infrared Radiation Therapy: Spirit Identity Powder-Generated Far Infrared Radiation Inhibits the Proliferation of Human Male Liver Cancer Cells by Activating the Transmembrane Attack Complex. Appl. Sci. 2022, 12, 9416. https://doi.org/10.3390/app12199416
Kim H-B, Park J-H. Novel Application of Infrared Radiation Therapy: Spirit Identity Powder-Generated Far Infrared Radiation Inhibits the Proliferation of Human Male Liver Cancer Cells by Activating the Transmembrane Attack Complex. Applied Sciences. 2022; 12(19):9416. https://doi.org/10.3390/app12199416
Chicago/Turabian StyleKim, Hong-Bae, and Jae-Heung Park. 2022. "Novel Application of Infrared Radiation Therapy: Spirit Identity Powder-Generated Far Infrared Radiation Inhibits the Proliferation of Human Male Liver Cancer Cells by Activating the Transmembrane Attack Complex" Applied Sciences 12, no. 19: 9416. https://doi.org/10.3390/app12199416
APA StyleKim, H.-B., & Park, J.-H. (2022). Novel Application of Infrared Radiation Therapy: Spirit Identity Powder-Generated Far Infrared Radiation Inhibits the Proliferation of Human Male Liver Cancer Cells by Activating the Transmembrane Attack Complex. Applied Sciences, 12(19), 9416. https://doi.org/10.3390/app12199416





