Employing Discrete Solid Phases to Represent C-S-H Solid Solutions in the Cemdata07 Thermodynamic Database to Model Cement Hydration Using the PHREEQC Geochemical Software
Abstract
1. Introduction
2. Derivation of Discrete Solid Phases (DSPs) from Cemdata07 Solid Solution End-Members
3. Thermodynamic Modelling of Cement Hydration
3.1. OPC Clinker Rate Equations
3.2. Dissolution of Oxides Dissolved in OPC Clinker
3.3. Accessory Clinker Phases
3.4. Oversaturation
3.5. Alkalis Binding to the C-S-H
4. Full Hydration Analysis Using Cemdata07
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, N.; Tyrer, M.; West, R.P.; Lowe, A.; Kelliher, D. Using PHREEQC to model cement hydration. Constr. Build. Mater. 2022, 319, 126–129. [Google Scholar] [CrossRef]
- Parkhurst, D.J.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport and Inverse Geochemical Calculations; The U.S. Department of the Interior and the U.S. Geological Survey: Baltimore, MD, USA, 2013.
- Elakneswaran, Y.; Ishida, T. Development and Verification of an Integrated Physicochemical and Geochemical Modelling Framework for Performance Assessment of Cement-Based Materials. J. Adv. Concr. Technol. 2014, 12, 111–126. [Google Scholar] [CrossRef]
- Maekawa, K.; Ishida, T.; Kishi, T. Multiscale Modeling of Structural Concrete; Taylor & Francis: Abingdon, UK, 2009. [Google Scholar]
- Elakneswaran, Y.; Owaki, E.; Miyahara, S.; Ogino, M.; Maruya, T.; Nawa, T. Hydration study of slag-blended cement based on thermodynamic considerations. Constr. Build. Mater. 2016, 124, 615–625. [Google Scholar] [CrossRef]
- Charlton, S.R.; Parkhurst, D.L. Modules based on the geochemical model PHREEQC for use in scripting and programming languages. Comput. Geosci. 2011, 37, 1653–1663. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Glasser, G.; Möschner, F. Thermodynamic modelling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Owaki, E.; Nawa, T. Modelling Long-Term Durability Performance of Cementitious Materials under Sodium Sulphate Interaction. Appl. Sci. 2018, 8, 2597. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Li, C.; Kajio, T.; Owaki, E.; Ogino, M.; Nawa, T. Durability of slag-blended cement due to U-phase instability in sulphate environment. Mater. Struct. 2020, 53, 146. [Google Scholar] [CrossRef]
- Krishnya, S.; Yoda, Y.; Elakneswaran, Y. A two-stage model for the prediction of mechanical properties of cement paste. Cem. Concr. Compos. 2021, 115, 103853. [Google Scholar] [CrossRef]
- Walker, C.; Savage, D.; Tyrer, M.; Ragnarsdottir, K. Non-ideal solid solution aqueous solution modeling of synthetic calcium silicate hydrate. Cem. Concr. Res. 2007, 37, 502–511. [Google Scholar] [CrossRef]
- Curti, E. Coprecipitation of radionuclides with calcite: Estimation of partition coefficients based on a review of laboratory investigations and geochemical data. Appl. Geochem. 1999, 14, 433–445. [Google Scholar] [CrossRef]
- Atkinson, A.; Hearne, J.A.; Knights, C.F. Aqueous chemistry and thermodynamic modelling of CaO-SiO2-H2O gels. J. Chem. Soc. Dalton Trans. 1989, 2371–2379. [Google Scholar]
- Aimoz, L.; Kulik, D.A.; Wieland, E.; Curti, E.; Lothenbach, B.; Mader, U. Thermodynamics of AFm-(I2,SO4) solid solution and of its end-members in aqueous media. Appl. Geochem. 2012, 27, 2117–2129. [Google Scholar] [CrossRef]
- Dilnesa, B.Z.; Lothenbach, B.; le Saout, G.; Renaudin, G.; Mesbah, A.; Filinchuk, Y.; Wichser, A.; Wieland, E. Iron in carbonate containing AFm phases. Cem. Concr. Res. 2011, 41, 311–323. [Google Scholar] [CrossRef]
- Hobbs, M.Y. Solubilities and ion exchange properties of solid solutions between the OH, Cl and CO3 end members of the monocalcium aluminate hydrates. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2001; p. 226. [Google Scholar]
- Möschner, G.; Lothenbach, B.; Winnefeld, F.; Ulrich, A.; Figi, R.; Kretzschmar, R. Solid solution between Al-ettringite and Fe-ettringite (Ca6[Al1−xFex(OH)6]2(SO4)3·26H2O). Cem. Concr. Res. 2009, 39, 482–489. [Google Scholar] [CrossRef]
- Dilnesa, B.Z.; Lothenbach, B.; Renaudin, G.; Wichser, A.; Kulik, D.A. Synthesis and characterization of hydrogarnet Ca3(AlxFe1-x)2(SiO4)y(OH)4(3-y). Cem. Concr. Res. 2014, 59, 96–111. [Google Scholar] [CrossRef]
- Walker, C.; Sutou, S.; Oda, C.; Mihara, M.; Honda, A. Calcium silicate hydrate (C-S-H) gel solubility data and a discrete solid phase model at 25 °C based on two binary non-ideal solid solutions. Cem. Concr. Res. 2016, 79, 1–30. [Google Scholar] [CrossRef]
- Svenum, I.-H.; Ringdalen, I.G.; Bleken, F.L.; Friis, J.; Höche, D.; Swang, O. Structure, hydration, and chloride ingress in C-S-H: Insight from DFT calculations. Cem. Concr. Res. 2020, 129, 105965. [Google Scholar] [CrossRef]
- BWong, M.; Lacina, D.; Nielsen, I.M.B.; Graetz, J.; Allendorf, M.D. Thermochemistry of Alane Complexes for Hydrogen Storage: A Theoretical and Experimental Investigation. J. Phys. Chem. C 2011, 115, 7778–7786. [Google Scholar] [CrossRef]
- Lothenbach, B.; le Saout, G.; Gallucci, E.; Scrivener, K. Influence of limestone on the hydration of Portland cements. Cem. Concr. Res. 2008, 38, 848–860. [Google Scholar] [CrossRef]
- Lothenbach, B.; Kulik, D.A.; Matschei, T.; Balonis, M.; Baquerizo, L.; Dilnes, B.; Miron, G.D.; Myers, R.J. Cemdata18: A chemical thermodynamic database for hydrated Portland cements and alkali-activated materials. Cem. Concr. Res. 2019, 115, 472–506. [Google Scholar] [CrossRef]
- Killoh, L.J.; Parrot, D.C. Prediction of cement hydration. Br. Ceram. Proc. 1984, 35, 41–53. [Google Scholar]
- Winnefeld, F.; Lothenbach, B. Thermodynamic modelling of the hydration of Portland Cement. Cem. Concr. Res. 2006, 36, 209–226. [Google Scholar]
- Hong, S.Y.; Glasser, F.P. Alkali binding in cement pastes: Part I. The C-S-H phase. Cem. Concr. Res. 1999, 29, 1893–1903. [Google Scholar] [CrossRef]
- Taylor, H.F.W. Cement Chemistry; Thomas Telford Publishing: London, UK, 1997. [Google Scholar]
- Savage, D.; Soler, J.M.; Yamaguchi, K.; Walker, C.; Honda, A.; Inagaki, M.; Watson, C.; Wilson, J.; Benbow, S.; Gaus, I.; et al. A comparative study of the modelling of cement hydration and cement–rock laboratory experiments. Appl. Geochem. 2011, 26, 1138–1152. [Google Scholar] [CrossRef]
- de Weerdt, K.; Haha, M.B.; le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Lothenbach, B.; Damidot, D.; Marchand, T.; Matschei, T. Thermodynamic modelling: State of knowledge and challenges. Adv. Cem. Res. 2010, 22, 211–223. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Atkins, M.; Bennett, D.G.; Dawes, A.C.; Glasser, F.P.; Kindness, A.; Read, D. A thermodynamic model for blended cements. Cem. Concr. Res. 1992, 22, 497–502. [Google Scholar] [CrossRef]
- Bennett, D.G.; Read, D.; Atkins, M.; Glasser, F.P. A thermodynamic model for blended cements. II: Cement hydrate phases; thermodynamic values and modelling studies. J. Nucl. Mater. 1992, 190, 315–325. [Google Scholar] [CrossRef]
- Damidot, D.; Glasser, F.P. Thermodynamic investigation of the CaO Al2O3 CaSO4 H2O system at 50°C and 85 °C. Cem. Concr. Res. 1992, 22, 1179–1191. [Google Scholar] [CrossRef]
- Damidot, D.; Glasser, F.P. Thermodynamic investigation of the CaO Al2O3 CaSO4 K2O H2O system at 25 °C. Cem. Concr. Res. 1993, 23, 1195–1204. [Google Scholar] [CrossRef]
- Damidot, D.; Glasser, F.P. Thermodynamic investigation of the CaO Al2O3 CaSO4 H2O system at 25 °C and the influence of Na2O. Cem. Concr. Res. 1993, 23, 221–238. [Google Scholar] [CrossRef]
- Damidot, D.; Stronach, S.; Kindness, A.; Atkins, M.; Glasser, F.P. Thermodynamic investigation of the CaO_Al2O3_CaCO3-H2O closed system at 25 °C and the influence of Na2O. Cem. Concr. Res. 1994, 24, 563–572. [Google Scholar] [CrossRef]
- Atkins, M.; Glasser, F.P.; Kindness, A. Cement hydrate phases: Solubility at 25 °C. Cem. Concr. Res. 1992, 22, 241–246. [Google Scholar] [CrossRef]
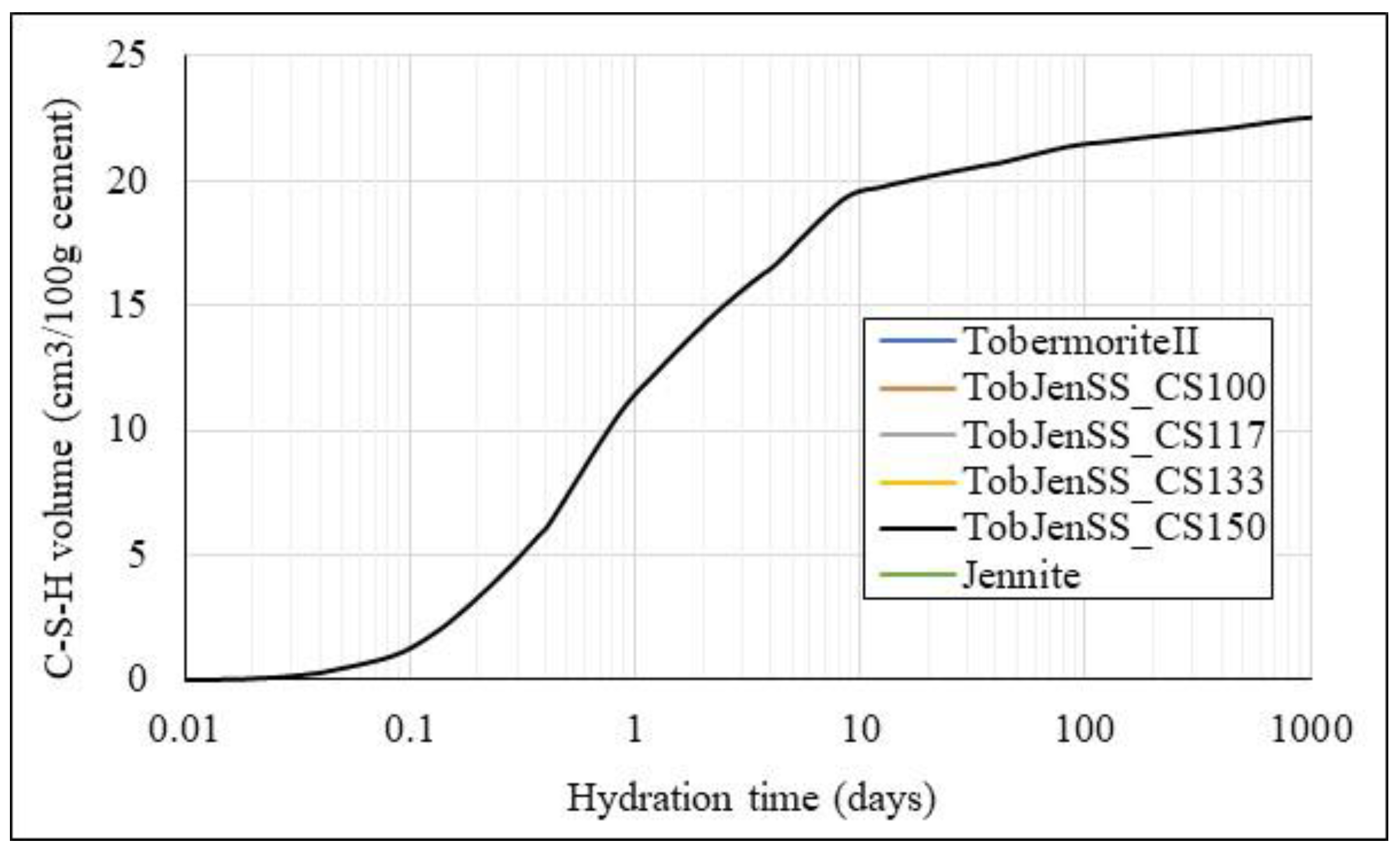

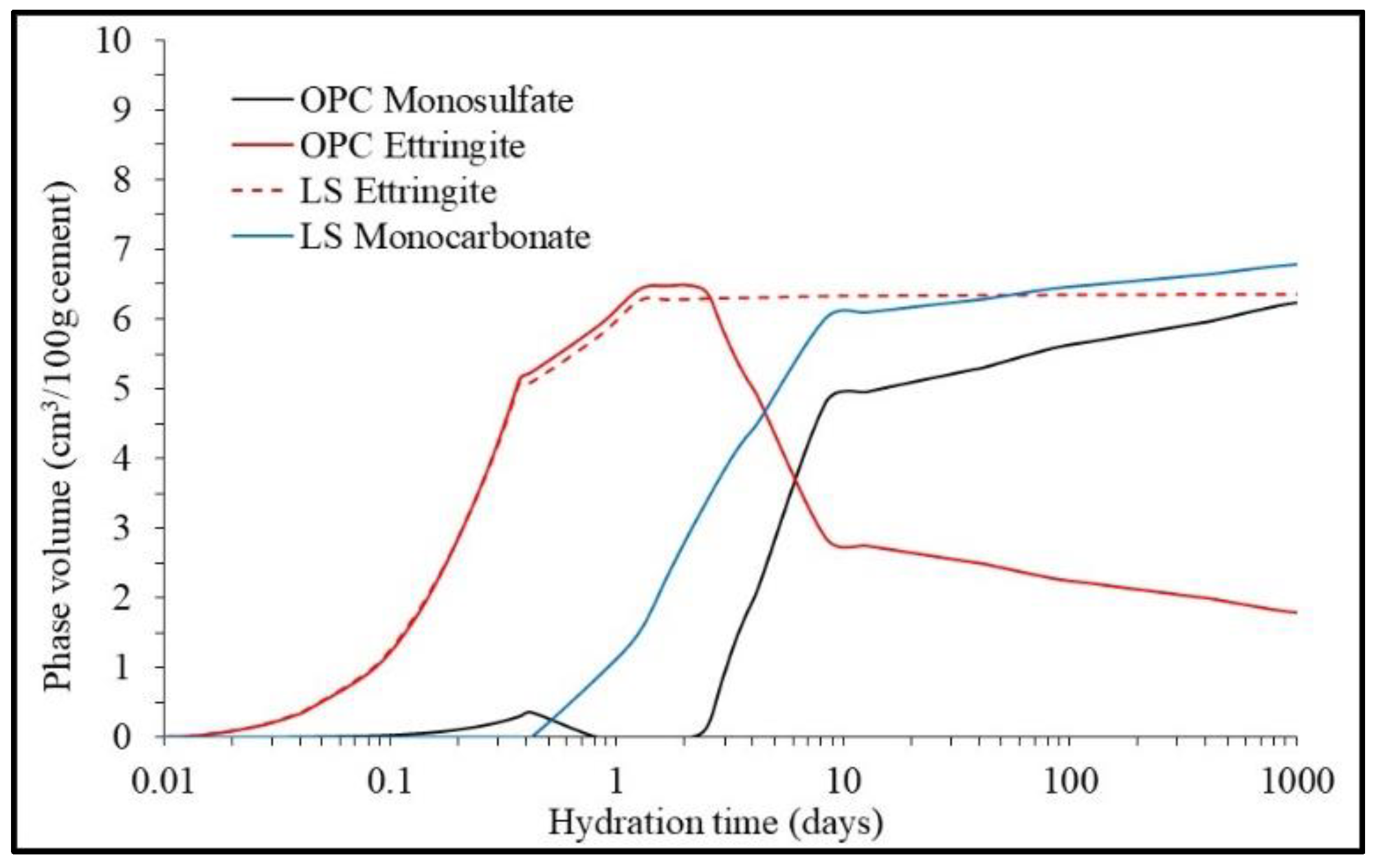

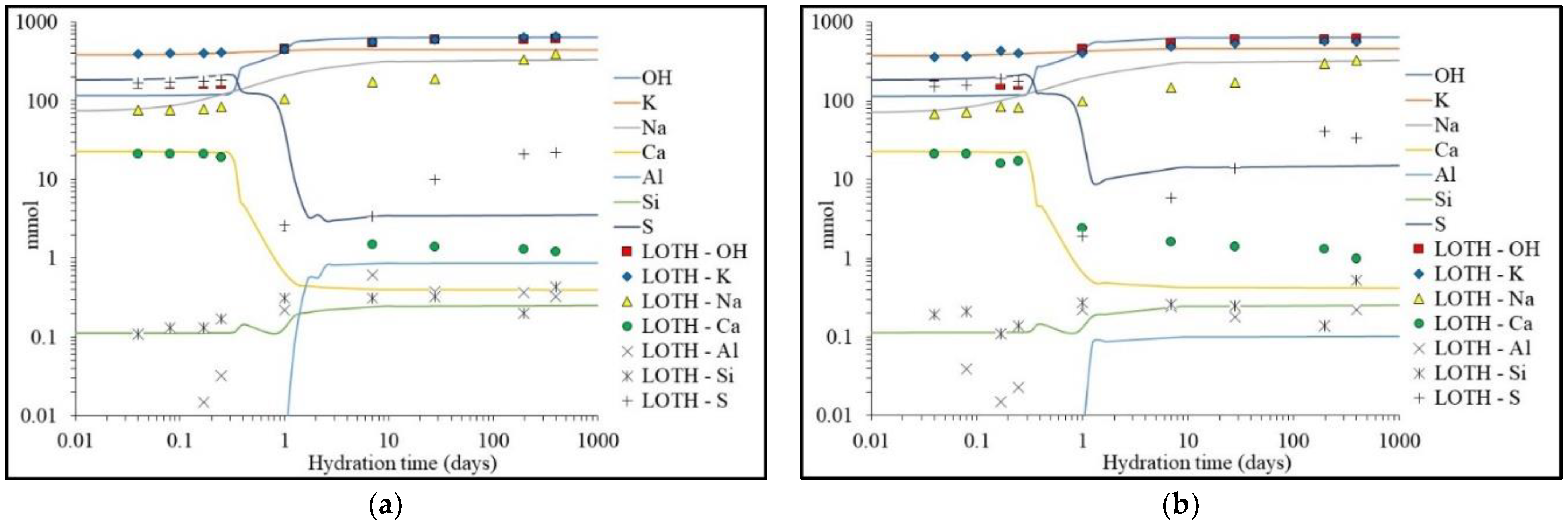
| Component | G i | H i | S ii | Cp ii |
|---|---|---|---|---|
| Tobermorite-II | −1,744,356 | −1,915,813.3 | 80 | 132.372 |
| Jennite | −2,480,808 | −2,723,484.3 | 140 | 210.805 |
| Ca+2 | −552,790 | −543,069 | −56.483997 | −30.922515 |
| SiO2(aq) | −833,411 | −887,856.17 | 41.338001 | 44.465416 |
| H2O | −237,183 | −285,881 | 69.923 | 75.3605 |
| H+ | 0 | 0 | 0 | 0 |
| Phase | Mole Fraction | DSP Dissolution Reactions | (25 °C) | Analytical Expression Parameters (Equation (1)) | Vol (cm3/mol) | |||
|---|---|---|---|---|---|---|---|---|
| Xi | Xj | a | C | d | ||||
| Tobermorite II | 1 | 0 | (CaO)0.8333(SiO2)1.0000(H2O)1.3330 + 1.6666 H+ = 0.8333 Ca+2 + 2.1663 H2O + 1.0000 SiO2 | 11.1373 | −13.91062 | 3.064440 × 103 | 5.962965 | 59 |
| TobJenSS CS100 | 0.8 | 0.2 | (CaO)1.0000(SiO2)1.0000(H2O)1.4864 + 2.0000 H+ = 1.0000 Ca+2 + 2.4864 H2O + 1.0000 SiO2 | 14.5527 | −15.22546 | 4.186636 × 103 | 6.357638 | 62.8 |
| TobJenSS CS117 | 0.6 | 0.4 | (CaO)1.1667(SiO2)1.0000(H2O)1.6398 + 2.3334 H+ = 1.1667 Ca+2 + 2.8065 H2O + 1.0000 SiO2 | 18.1104 | −16.39794 | 5.308832 × 103 | 6.752311 | 66.6 |
| TobJenSS CS133 | 0.4 | 0.6 | (CaO)1.3333(SiO2)1.0000(H2O)1.7932 + 2.6666 H+ = 1.3333 Ca+2 + 3.1265 H2O + 1.0000 SiO2 | 21.7431 | −17.49397 | 6.426629 × 103 | 7.146450 | 70.4 |
| TobJenSS CS150 | 0.2 | 0.8 | (CaO)1.5000(SiO2)1.0000(H2O)1.9466 + 3.0000 H+ = 1.5000 Ca+2 + 3.4466 H2O + 1.0000 SiO2 | 25.4508 | −18.51652 | 7.548825 × 103 | 7.541123 | 74.2 |
| Jennite | 0 | 1 | (CaO)1.6667(SiO2)1.0000(H2O)2.1000 + 3.3334 H+ = 1.6667 Ca+2 + 3.7667 H2O + 1.0000 SiO2 | 29.3008 | −19.39671 | 8.671021 × 103 | 7.935796 | 78 |
| Oxide Proportions (g/100 g Cement) | Phase Compositions (g/100 g Cement) | ||||||
|---|---|---|---|---|---|---|---|
| OPC | Limestone | OPC | Limestone | ||||
| SiO2 | 20.2 | SiO | 0.8 | C3S | 66.5 | C3S | 64.60 |
| Al2O3 | 4.9 | Al2O3 | 0.3 | C2S | 10.30 | C2S | 9.30 |
| Fe2O3 | 3.2 | Fe2O3 | 0.3 | C3A | 7.50 | C3A | 7.40 |
| CaO | 63.9 | CaO | 55 | C4AF | 8.50 | C4AF | 7.80 |
| MgO | 1.8 | MgO | 1.8 | CaO_free | 0.93 | CaO-free | 0.89 |
| Na2O | 0.42 | Na2O | <0.01 | Calcite | 0.60 | Calcite | 4.60 |
| K2O | 0.78 | K2O | <0.01 | Gypsum | 3.10 | Gypsum | 3.00 |
| CaO-free | 0.93 | CaO-free | <0.01 | Periclase | 0.90 | Periclase | 0.90 |
| CO2 | 0.26 | CO2 | 42.5 | K2SO4 | 1.30 | K2SO4 | 1.30 |
| SO3 | 2.29 | SO3 | 0.05 | Na2SO4 | 0.21 | Na2SO4 | 0.20 |
| Periclase | 0.9 | Periclase | 0.9 | Alkali | |||
| Blaine fineness (m2/kg) | K2O | 0.05 | K2O | 0.05 | |||
| 413 | 429 | Na2O | 0.33 | Na2O | 0.31 | ||
| Ignition loss | MgO | 0.94 | MgO | 0.87 | |||
| 0.37 | 43.4 | SO3 | 0.11 | SO3 | 0.11 | ||
| Clinker Phase | Oxide | |||||||
|---|---|---|---|---|---|---|---|---|
| K2O | Na2O | MgO | SO3 | K2O (wt.%) | Na2O (wt.%) | MgO (wt.%) | SO3 (wt.%) | |
| C3S | 0.1 | 0.1 | 1.1 | 0.1 | 5.26 | 7.69 | 18.33 | 33.33 |
| C2S | 0.9 | 0.1 | 0.5 | 0.2 | 47.37 | 7.69 | 8.33 | 67.67 |
| C3A | 0.7 | 1.0 | 1.4 | 0 | 36.84 | 76.92 | 23.33 | 0 |
| C4AF | 0.2 | 0.1 | 3.0 | 0 | 10.53 | 7.69 | 50.0 | 0 |
| Total | 1.9 | 1.3 | 6.0 | 0.3 | 100 | 100 | 100 | 100 |
| Clinker Phase | Model Approach |
|---|---|
| C3S | Kinetic |
| C2S | Kinetic |
| C3A | Kinetic |
| C4AF | Kinetic |
| K2O | Kinetic (dissolved in C3S, C2S, C3A and C4AF) |
| Na2O | Kinetic (dissolved in C3S, C2S, C3A and C4AF) |
| MgO | Kinetic (dissolved in C3S, C2S, C3A and C4AF) |
| SO3 | Kinetic (dissolved in C3S and C2S) |
| Na2SO4 | Equilibrium—immediately dissolved during step one |
| K2SO4 | Equilibrium—immediately dissolved during step one |
| Lime | Equilibrium—immediately dissolved during step one |
| Calcite | Equilibrium—reacting instantaneous equilibrium |
| Gypsum | Equilibrium—reacting instantaneous equilibrium |
| Periclase | Equilibrium—immediately dissolved during step one |
| Phase | n | S.I. < 12 h |
|---|---|---|
| Brucite | 3 | 0.45 |
| TobJenSS_CS150 | 4.50 | 0.68 |
| Ettringite | 15 | 2.25 |
| Gypsum | 2 | 0.30 |
| Portlandite | 3 | 0.45 |
| Syngenite | 5 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, N.; Tyrer, M.; Kelliher, D. Employing Discrete Solid Phases to Represent C-S-H Solid Solutions in the Cemdata07 Thermodynamic Database to Model Cement Hydration Using the PHREEQC Geochemical Software. Appl. Sci. 2022, 12, 10039. https://doi.org/10.3390/app121910039
Holmes N, Tyrer M, Kelliher D. Employing Discrete Solid Phases to Represent C-S-H Solid Solutions in the Cemdata07 Thermodynamic Database to Model Cement Hydration Using the PHREEQC Geochemical Software. Applied Sciences. 2022; 12(19):10039. https://doi.org/10.3390/app121910039
Chicago/Turabian StyleHolmes, Niall, Mark Tyrer, and Denis Kelliher. 2022. "Employing Discrete Solid Phases to Represent C-S-H Solid Solutions in the Cemdata07 Thermodynamic Database to Model Cement Hydration Using the PHREEQC Geochemical Software" Applied Sciences 12, no. 19: 10039. https://doi.org/10.3390/app121910039
APA StyleHolmes, N., Tyrer, M., & Kelliher, D. (2022). Employing Discrete Solid Phases to Represent C-S-H Solid Solutions in the Cemdata07 Thermodynamic Database to Model Cement Hydration Using the PHREEQC Geochemical Software. Applied Sciences, 12(19), 10039. https://doi.org/10.3390/app121910039








