Featured Application
The current investigation was conducted to characterize the Sidr honey through melissopalynological analysis, its physicochemical, and biochemical properties, antimicrobial, antioxidant activities as well as total phenolic and total flavonoid contents. For this purpose, Sidr honey samples collected from the Saudi market imported from 12 different countries were analyzed.
Abstract
The current investigation was conducted to assess the melissopalynological, physicochemical, and biochemical properties, antimicrobial and antioxidant activities as well as total phenolic and total flavonoid contents of 794 Sidr honey samples collected from the Saudi market that had been imported from 12 different countries. Testing Sidr honey from different countries showed different levels of growth suppression observed against five drug resistant bacterial strains. The pathogenic strains were Staphylococcus aureus, Streptococcus mutans, Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. The antimicrobial activity showed growth suppression levels which varied according to the origin of the honey. The comparative study of Sidr honeys revealed a strong correlation between total polyphenol and flavonoid contents and significant radical scavenging activities in particular Egyptian and Saudi Arabian honeys. The melissopalynological and physicochemical properties of different Sidr honeys complied with the recommendations of the WHO Codex Alimentarius, the European Union standards for honey quality, and the Gulf Technical Regulation on honey (GSO 147:2008-Standards Store-GCC Standardization Organization). It was concluded that Sidr honey from different geographical areas has the capacity to suppress the growth of pathogenic bacteria and perform significant radical scavenging activities.
1. Introduction
Apis mellifera worker bees produce honey from collected from plants [1,2]. Honey can be either monofloral or multifloral depending on the pollen plant source [3]. Honey composition is influenced by many factors such as plant species, climate, environmental conditions, harvesting time, beekeeper’s handling, processing and storage conditions [4]. Honey quality depends on the chemical composition of the source plants, and on the climatic conditions and soil mineral composition [4]. The main components in honey are fructose and glucose (monosaccharides) (65%), and water (18−20%), and the minor components include free amino acids, aroma compounds, vitamins, organic acids, minerals, and phenolic acids and flavonoids [5]. The beehive products (honey, propolis, royal jelly, bee venom, and beeswax) contain several [6,7] important medicinal compounds. Major honey bioactive compounds include phenolics, methylglyoxal, royal jelly proteins (MRJPs), and oligosaccharides that have anti-inflammatory, antimicrobial and antioxidant activities [7]. The presence of novel antimicrobial compounds in natural authentic honey is well documented [7,8,9]. Adulteration of honey either by adding cheap sugar syrup or by feeding the bees on sugar solutions is a major worldwide problem that leads to the loss of many biological properties of honey including its effective antimicrobial activity [10].
Therefore, the potential use of specific types of fresh authentic honey as candidate antibacterial agents is based on their physicochemical characteristics. Although monofloral honey is appreciated and it is also more expensive [11], Devi & Jangir (2018) [12] report that volatile compounds in honey come from its diverse floral origin and could be the source of its biological activity and medical importance.
Sidr (Ziziphus spp.) honey is produced from Ziziphus trees [13]. In Saudi Arabia, this is the most valuable honey, and customers believe that this type of honey is superior to other honey imported from other countries around the world or produced locally [13].
Several Sidr honeys are produced in different parts of the world. However, the available information on their physical and chemical properties is limited [14]. This honey is often subjected to adulteration due to limited availability and its high price [15]. Honey color and composition are dependent greatly on the geographical and botanical origins [10,11,12,13,14,15,16]. In addition, geographical and botanical origins are the two main criteria for general honey authenticity according to different national standards on honey authenticity and the Codex Alimentarius Standard [17]. Therefore, the current investigation was conducted to assess the melissopalynological, physicochemical, and biochemical properties, antimicrobial and antioxidant activities as well as total phenolic and total flavonoid contents of Sidr sider honey samples collected from the Saudi market that had been imported from 12 zones in different countries.
2. Materials and Methods
2.1. Materials
All used reagents and chemicals were of analytical grade and were purchased from Sigma (St. Louis, MO, USA).
2.2. Honey Samples
A total of 794 fresh Sidr honey samples (1 kg each) were collected from the Saudi Arabia markets during 2021. They were imported from 12 geographical areas in different countries. Each honey sample was collected in a sterile universal glass container and kept at 2−8 °C until tested. Melissopalynological analysis was used to corroborate the samples’ level of authenticity as Sidr honey, which means that the honey must have at least 55% of pollen from a specific floral source [18]. The collected honey samples are shown in Table 1.

Table 1.
Geographical origin of Sidr honey samples.
2.3. Melissopalynological and Physicochemical Analysis
Melissopalynological and physicochemical analyses were performed [19]. The pollen content was identified by the sedimentation technique as described by [18,20]. Other parameters determined were color, water content [21], insoluble solids [22], pH, acidity, optical rotation, and electrical conductivity [23]. The assessments for sugar content, inverted sugars, glucose, fructose, fructose/glucose ratio, fructose + glucose %, glucose/moisture ratio, and sucrose were performed by HPLC-DAD according to standard methods [24]. Additionally, diastase enzyme activity [16], and hydroxymethylfurfural (HMF) were analyzed [25].
2.4. Detection of Total Phenolic Content (TPC)
TPC was detected using Folin–Ciocalteu reagent [26] following [27,28]. The honey solution (0.5 mL) was mixed with 2.5 mL Folin–Ciocalteu reagent (2N) and incubated for 5 min. Subsequently, 2 mL sodium carbonate solution (75 gr/L) was added and incubated for 2 h at 25 °C. The absorbance of the solution was measured at 765 nm after incubation using a UV-Visible spectrophotometer (Perkin-Elmer Lambda 25, Waltham, MA, USA). For the calibration curve preparation, gallic acid (0–1000 mg/L) was used as a standard. The mean values of triplicate assays of TPC are reported, expressed as milligrams of gallic acid equivalent (GAE) per gram of honey [29].
2.5. Determination of Total Flavonoid Content (TFC)
TFC was determined using a 5 mL sample of diluted honey at 0.1 g/mL concentration. This solution was mixed with 5 mL of 2% aluminum chloride (AlCl3) for the determination of TFC. The mixture was then incubated for 10 min at 25 °C. The absorbance of the formed complex was measured at 415 nm using a UV-Visible spectrophotometer. Rutin was the standard chemical used for the calibration curve preparation, with a concentration 0–100 mg/L. The mean values of triplicate assays of TFC are reported, expressed as milligrams of rutin equivalent (RE) per gram of honey [28,29].
2.6. Antioxidant Assay to Determine the DPPH Scavenging Activity
An antioxidant assay was used to determine the DPPH scavenging activity of the different honey samples. This test is based on the change in the absorbance that results from reducing the purple DPPH radical using an oxidizing antioxidant. The scavenging effect of vitamin C and caffeic acid as well as the honey samples corresponded to the quenching intensity of 1,1-diphenyl-2-picrylhydrazyl (DPPH) as carried out by [30]. The absorbance resulting from reducing the purple DPPH radical by an oxidizing antioxidant was measured at 520 nm.
To determine the percent inhibition, the antioxidant ability of vitamin C samples was measured as a decrease in absorbance of DPPH solution (purple DPPH reduction) due to the addition of the sample solution. The absorbance value of the DPPH solution measurement results before and after the addition of the sample solution was calculated as percent inhibition. Using the percent inhibition obtained, a linear regression equation with the sample concentration (μg/mL) on the x axis and the inhibition value on the y axis was calculated. The antioxidant activity of the test material was assessed by calculating the inhibitory concentration 50% (IC50) using the following formula: 50 = ax + b. The IC50 value indicates the concentration of the test sample (µg/mL) which results in a 50% DPPH reduction (able to inhibit or reduce the oxidation process by 50%). The results of the calculation are entered into the obtained regulatory equation [31].
2.7. Bacterial Strains
The five antibiotic-resistant bacterial strains (Gram-positive and Gram-negative) included Staphylococcus aureus (ATCC 25923), Streptococcus mutans (1815T), Escherichia coli (ATCC 35218), Klebsiella pneumoniae (ATCC 27736), Escherichia coli (ATCC 35218) and Pseudomonas aeruginosa (ATCC 27853). These microorganisms were provided and maintained by the Department of Zoonotic Diseases, National Research Centre, Egypt. Each bacterial strain suspension was prepared by inoculating fresh stock culture into the broth tube containing 10 mL Muller Hinton Broth (Sigma Aldrich company). The inoculated tubes were incubated aerobically at 37 °C for 24 hr. The bacterial suspension was adjusted by comparison with 0.5 Mc Farland turbidity standards (5 × 107 cells/mL). It was then further diluted to obtain a final of 5 × 106 cells/mL. Physiological saline PBS pH 7.2 was used for all dilution steps under aseptic conditions. These bacterial strains were enriched on selective broth for bacterial propagation [32]. A separate tube containing 40 µL of 21.30% honey concentration was mixed with 0.20 µL/10 mL from the enriched broth of each propagated S. aureus, S. mutans, K. pneumoniae, E. coli, and P. aeruginosa [9,33,34]. These tubes were incubated at 37 °C for 24 h. The growths of the control bacterial strains and the inhibition of the bacterial growth due to mixing with honey were measured using the disc diffusion method. The mean values of inhibition were calculated from triplicate readings in each test. Evaluations of the antibacterial activity of different honey dilutions were performed according to Hegazi et al., 2017; 2020 and 2021) [9,33,34]. The results of antibacterial activity against different examined bacteria were recorded.
2.8. Minimum Inhibitory Concentration (MIC)
The MIC of different samples of Sidr honey were determined by a two-fold serial dilution method [6]. Serial dilution of 100 mg/mL for the rest of the samples were performed separately to achieve 50, 25, 12.50, 6.25, 3.12, 1.56, 0.78 mg/mL, and 390, 195, 97 µg/mL concentrations were used for the MIC determination. Briefly, 100 µL of varying sample concentrations were added separately to the test tubes containing 9 mL of the standardized suspension of the tested bacteria (108 CFU/mL). The test tubes were incubated at 37 °C for 24 h. Control tests with the test organisms were performed using distilled water instead of honey. The lowest concentration of these samples with no visible growth was taken as the MIC [6].
2.9. Statistical Analysis
The tests were conducted in triplicate and the statistical analysis then performed using SPSS Ver. 21 (IBM, New York, NY, USA) software. A one-way ANOVA was applied for comparisons between and within the tested groups. The mean ± standard error (SE) is presented for all data and p values less than 0.05 were considered significant.
3. Results
A total of 794 Sidr honey types that had been imported from different countries were collected from the Saudi market. Melissopalynological analysis of Sidr honey from different geographical origins proved that not only the expected pollen type based on the specific source of nectar but different pollen from some other sources were also present depending on the geographical origin (Table 2 and Figure 1). The Sidr honey included pollen from the following species: Ziziphus jujuba, Conocarpus erectus, and Eleusine coracana (Emirates); Ziziphus jujuba, and Brassica napus (China); Ziziphus jujuba, and Cichorium intybus (Iraq); Ziziphus spina-christi, Amaranthus blitum, Capsella bursa-pastoris, and Oryza meyeriana (Pakistan); Ziziphus spina-christi, Rhanterium epapposum, Sesamum indicum, and Oryza meyeriana (Bashawer); Ziziphus spina-christi, Amaranthus blitum, and Oryza meyeriana (Panjab); Ziziphus jujuba, Acacia asak, and Blepharis Ciliaris (Saudi Arabia); Ziziphus spina-christi, Capsella bursa-pastoris, Amaranthus blitum, and Chrysanthemum leucanthemum (Kashmir); Ziziphus jujuba, and Cynara auranitica (Libya); Ziziphus lotus, Oryza meyeriana, Zea mays, and Brassica tournefortii Gouan (Egypt); Ziziphus jujuba, Oryza meyeriana, and Acacia asak, (India); and Ziziphus jujuba, and Acacia asak (Yemen).

Table 2.
Melissopalynological analysis of Sidr honey from different origins.
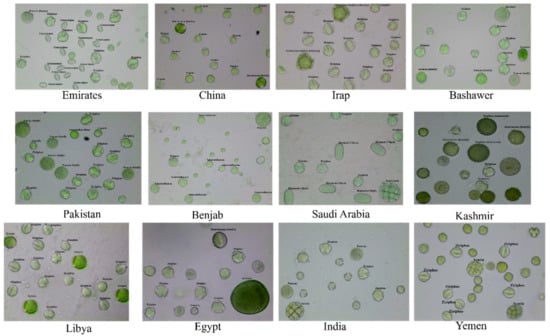
Figure 1.
Pollen grain images from microscope preparations of Sidr honey from different geographical origins.
The physicochemical properties as shown in Table 3 reveal that the Sidr honey samples were comparable in water content, which ranged from 14.2 ± 0.41(Libya) to 17.06 ± 0.47% (Iraq). The optical rotation ranged from −1.13° (Egypt) to −2.42° (China). The colors observed ranged from extra white (China, Kashmir and India) to deep amber (Yemen). The pH varied from 3.6 ± 0.1 (Egypt) to 7.4 ± 0.1 (Yemen). The acidity also varied from 7.7 ± 0.5 (Egypt) to 11.3 ± 1.4 meq/l (Libya). Electrical conductivity ranged from 0.55 ± 0.02 (China, and Libya) to 1.40 ± 0.39 mS/cm (Pakistan). The insoluble solids ranged from 0.05 ± 0.02 and 0.05 ± 0.03% (Saudi Arabia, and Libya, respectively) to 1.36 ± 0.02 (Pakistan).

Table 3.
Physicochemical parameters of Sidr honey samples from different origins.
The glucose, fructose, sucrose, diastase activity and HMF levels in different Sidr honey samples are shown in Table 4. Glucose was detected at the lowest level (24.77 ± 0.65%) in India Sidr honey, whereas the highest level (28.89 ± 0.11%) was observed in Iraq Sidr honey. The level of fructose ranged between 32.79 ± 0.64 (India) and 36.01 ± 1.05 (Iraq). The sucrose level ranged from 1.24 ± 0.37% (Pakistan) to 3.79 ± 0.27% (Egypt). Diastase activity had a range of 9.75 ± 1.78 D.U. (Yemen) to 17.4 ± 2.16 D.U. (Egypt). The lowest HMF (mg/kg) was observed in Libya Sidr honey (11.07 ± 4.38), whereas the highest level was detected in Yemen Sidr honey (25.11 ± 6.63).

Table 4.
Glucose, fructose, sucrose, diastase activity and HMF levels in different Sidr honey samples.
The TPC (mg GAE/100 g honey), TFC (mg RE/100 g honey) and DPPH (mg AAE/100 g honey) content is shown in Table 5. The highest levels for the three parameters were found in Egypt Sidr honey 159.3 ± 15.32, 83.1 ± 18.33 and 177.8 ± 10.51, respectively, whereas the lowest level for total phenolics was detected in Yemen Sidr honey. On the other hand, the lowest levels of TFC and DPPH were detected in China Sidr Honey (35.1 ± 7.10 and 75.1 ± 7.57, respectively).

Table 5.
TPC, TFC and DPPH of the Sidr honeys.
The antibacterial activity of the Sidr honey from twelve geographical origins was evaluated according to the zone of inhibition. The antibacterial potency of honey was investigated against various Gram-negative and Gram-positive pathogenic bacteria. All geographical honey types showed high antibacterial activity against most of the tested bacterial strains. Staphylococcus aureus showed the highest zones of inhibition 25.00 ± 0.58, 23.00 ± 0.58 mm in Egypt and Saudi Arabia honey respectively, followed by Emirates (21.33 ± 0.88 mm) and Punjab (21.00 ± 0.11 mm) honeys, while Egyptian (29.33 ± 0.64 mm), Saudi (23.00 ± 0.22 mm), and Iraq (22.00 ± 0.58 mm) honeys revealed the highest zones of inhibition against Streptococcus mutans. The highest zones of inhibition against Klebsiella pneumoniae were observed in the Saudi (25.00 ± 0.61 mm), Egyptian (24.00 ± 0.34 mm), Emirates (22.00 ± 0.68 mm), and Libya (21.00 ± 0.31 mm) honey samples. Additionally, Egyptian (29.16 ± 0.60 mm), Saudi (27.00 ± 0.61 mm) and Libya (20.33 ± 0.88 mm) honey were highly effective against Escherichia coli. Significantly higher zones of inhibition against Pseudomonas aeruginosa were shown for Saudi (40.67 ± 0.67 mm) and Egyptian (29.00 ± 0.58 mm) honeys (Table 6).

Table 6.
The inhibition zone of Sidr honey against various pathogenic microorganisms by well diffusion method.
The MIC of the Sidr honey from different geographical origins against the pathogenic bacteria Staphylococcus aureus, Streptococcus mutans, Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa is shown in Table 7. The results of the antibacterial activity assay showed significant differences in MIC values when antibiotic-resistant strains were tested. Analyzing MICs, the strongest antibacterial potential against all tested bacteria was observed for Saudi Arabian and Egyptian (MIC = 0.4 g/mL) honeys. Iraq, Panjab, Kashmir, Libya, and India honeys showed the strongest antibacterial potential against Staphylococcus aureus, Streptococcus mutans, and Klebsiella pneumoniae (MIC = 0.4 g/mL). In contrast, the weakest overall antibacterial activity was shown in honey samples from Emirates, China, Pakistan, and Bashawer. Yemen honey showed the lowest antibacterial potential against Escherichia coli (MIC = 0.05 g/mL).

Table 7.
Minimal inhibitory concentration (MIC) of Sidr honey against antibiotic resistant bacterial strains.
4. Discussion
This research revealed that monofloral honey obtained from the Ziziphus spp. nectar of different geographical origins contained more than 70% of a specific pollen. The most important physicochemical parameter of honey is the water content. Low water content increases honey shelf life while high levels promote its fermentation during storage. The water content of the investigated honey samples was within the accepted range which, in general, should not exceed 20% [12,35]. During honey production by bees, the water content is affected by the relative humidity and temperature. Water content results for honey samples of different geographical origins have been documented previously for their water content [34,36], being 18.32 ± 0.67 g/100 g for Egyptian, 16.28 ± 0.22 g/100 g for Yemeni, 15.64 ± 0.30 g/100 g for Saudi and 14.73 ± 0.3 g/100 g for Kashmiri honey.
The sugar content of honey is widely used to assess the authenticity and the overall quality of honey [37]. Honey adulteration by mixing the honey with other cheaper sugar syrups is a frequent problem in the worldwide market [38]. For this reason, the sugar analysis of honey is an indicator of whether the honeybees were fed naturally with flower nectar or were fed with sugar solution. The use of artificial feeding is evident when the glucose content of honey is much higher than its fructose content [39].
The results of this investigation revealed that the sum of glucose and fructose (content of reducing sugars in honey) was within the accepted range and is consistent with the standardization and authenticity of honey as observed by Aljohar et al., (2018) [40]. It has been demonstrated that the most dominant sugar in honey is fructose [39] and Szczesna et al., (2021), in their study of the winter feeding of honeybees, observed the fructose to glucose ratio (F/G) was higher than 1.00 indicating the natural feeding of honeybees [41]. In all the honey samples in this study, sucrose content did not exceed 5%, which is the accepted level for honey to be considered authentic as observed by Kazeminia et al., (2021) [42]. A higher content of sucrose may indicate the artificial feeding of bees with some types of sugar syrup or adulteration of the honey [38]. Two important parameters used to prove the freshness of honey are hydroxymethylfurfural content and diastase activity [43,44]. There are many factors affecting diastase activity, including the physiological period of the colony, age of the bees, the quantity of nectar and its sugar content as well as the nectar collection period [45]. The WHO Codex Alimentarius, the European Union and the Gulf Technical Regulation on honey (GSO 147:2008-Standards Store-GCC Standardization Organization) [46] recommend that the maximum level for HMF content in honey does not exceed 40 mg/kg but in countries with tropical temperatures, the HMF content of honey should not exceed 80 mg/kg [34,43,44]. In the present study, all the samples were within the allowed range for HMF content and diastase number These parameters indicate the freshness of the samples which was maintained by preventing their exposure to heat and shortening the storage time before the experiment. In addition, the acidity of all the honey samples was found to be within the accepted range. The acidity in honey is due to the presence of organic acids, in particular, gluconic acid, which has been found to affect honey flavor, texture, shelf life, and stability [47].
The TPC ranged from 118.9 to 159.3 mg GAE/100 g honey, which is higher than the results found in previous studies in honey from India [48] (47–98 mg GAE/100 g honey), Poland [49] (71.7 to 202.6 μg/g honey), Argentina [29] (18.730–107.213 mg GAE/100 g honey), Burkina Faso [50] (32.59–114.75 mg GAE/100 g honey) Portugal [51] (30.87 to 87.27 mg GAE/100 g), or from Romania [52] (2–125 mg GAE/100 g honey).
Variable levels of TPC were reported in different honey types as observed by [53] who observed that the TPC of forest honey (806.10 mg GAE/kg honey) was significantly higher (p < 0.05) in comparison with acacia and polyfloral samples (68.48 and 87.46 mg GAE/kg honey, respectively). In addition, Roby et al., (2020) [36] determined the phenolic compounds of Egyptian honeys, with TPC amounting to 338.5 and 536.4 mg GAE kg−1 in clover and citrus honeys, respectively. This variability was associated with the botanic origin of the honey, and multi-floral honey was found to have higher phenolic contents than monofloral honey [54]. The antioxidant, antiviral, antimicrobial, antifungal, and anti-inflammatory activities of honey are noteworthy due to phenolic compounds, especially flavonoids [55], with the quality of polyphenols being more important than their quantity [56].
The TPC and TFC of honey depend mainly on its botanical and geographical origins [57,58]. Considering the TFC results, it can be concluded that dark honey contains the highest concentration (p < 0.05). Flavonoids in Egyptian and Saudi Arabia Sidr honey (83.1 and 81.5 mg RE/100 g honey, respectively) are present in high amounts compared with other tested origins. Similar results were observed by analysis of three types of monofloral honey from Portugal which showed that dark honey was richer in phenolics content [59].
A DPPH radical scavenging method was used to determine the antioxidant activity of the honey samples. The Sidr honey from Egypt (177.8), Panjab (135.3), and Saudi Arabia (131.3) showed the highest level of DPPH radical scavenging activities (p < 0.05), compared with antioxidant activities of other tested honey. These results are in accordance with the results reported by van den Berg et al., (2008) about Buckwheat honey [60], Bueno-Costa et al., (2016) in Brazilian honeys [61] and Boussaid et al., (2018) in Tunisian honeys [62]. Alves et al., (2014) [63] demonstrated a positive relationship between phenolic concentration, antioxidant capacity, and the color of honey.
In our study, the antibacterial activity of all honeys may be attributed to the narrow ranges of their TFC, TPC, and sugar contents. There is a positive correlation between TPC and the antibacterial activity of honey [64], which is attributed to the inhibition of virulence factors in the pathogen [65].
The antibacterial activity of honey also results from the low pH and high osmolarity along with the hydrogen peroxide activity produced by the glucose oxidase enzyme [8,9,34,66,67]. The antibacterial activity of honey may also be attributed to the presence of lysozyme, methylglyoxal, and bee peptides as well as its high sugar content [9,34,68,69]. The presented results were in accordance with the research of Mandal & Mandal (2011) [70], Szweda (2017) [71], Libonatti et al., (2014) [72], Irish (2011) et.al., [73], Morroni et al., (2018) [74], and Al Masaudi (2021) [69], which also confirmed that Gram-positive bacteria were more sensitive to the honey samples than Gram-negative ones [75,76]. Vancomycin and meropenem were used as reference antibiotics, with MIC values much lower than those obtained for honey, ranging from 0.1–64 µg/mL [77].
The use of honey to treat microbial infections has been investigated previously [34,51,67,76]. The honey antimicrobial properties are due to a high sugar osmolarity or the presence of other biologically active compounds [33,77,78,79,80]. The osmotic stress of honey is due to the high content of various sugars in combination with its low moisture content, which prevents the spoilage of honey by microorganisms. Hydrogen peroxide (H2O2) is one of the antibacterial compounds of honey [81], whose presence and origin were confirmed by White et al., (1963) [82]. In the hydrogen peroxide formation, a glucose oxidase enzyme is added by the worker bee during the collection of the nectar, allowing the conversion of glucose to gluconic acid, whereby hydrogen peroxide is produced as a side product [82]. The antimicrobial activity of honey is directly related to its botanical origin [63] and is associated with the different content of phenolic compounds, flavonoids, and phenolic acids [68,83].
5. Conclusions
The physicochemical characteristics of honey determine its biological activity and at the same time they serve as tools for authentication. This research examines the authenticity of monofloral Sidr honey (Ziziphus spp.) from 12 different countries based on melissopalynological, physicochemical and bioactive compounds analyses. Additionally, the antimicrobial activity of each honey was determined, providing relevant information about its efficacy and clarifying its mechanisms of biological activity. Geographical differences were evident in the pollen profile of the samples. The physicochemical parameters were assessed according to the criteria from the different honey quality standards, and the biological activity revealed the Saudi and Egyptian Sidr honeys have the highest antibacterial activity as well as total phenolics and flavonoids contents. Our results identify Sidr honey as a promising natural product that can be potentially used as an alternative to synthetic antibiotics; however, further studies are also needed to identify and standardize protocols for the use of Sidr honey either in the protection against microbial infections or their treatments.
Author Contributions
Conceptualization, A.G.H.; methodology, M.F.A.R.; validation, M.F.A.R. and S.S.; formal analysis, M.F.A.R.; investigation, A.G.H., M.F.A.R. and A.F.M.A.G.; resources, F.M.A.G.; data curation, A.F.M.A.G. and A.M.C.; writing—original draft preparation, A.G.H. and A.M.C.; writing—review and editing, A.G.H., A.M.C. and S.S.; visualization, A.G.H. and A.M.C.; supervision, A.G.H. and S.S.; project administration, F.M.A.G.; funding acquisition, F.M.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Center of Egypt under registration number 12/5/1 and the Al Guthami Foundation (Saudi Arabia).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Silva, L.R.; Videira, R.; Monteiro, A.P.; Valentão, P.; Andrade, P.B. Honey from Luso region (Portugal): Physicochemical characteristics and mineral contents. Microchem. J. 2009, 93, 73–77. [Google Scholar] [CrossRef]
- Habib, H.M.; Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Physicochemical and biochemical properties of honeys from arid regions. Food Chem. 2014, 153, 35–43. [Google Scholar] [CrossRef]
- Mustafa, G.; Iqbal, A.; Javid, A.; Hussain, A.; Bukhari, S.M.; Ali, W.; Saleem, M.; Azam, S.M.; Sughra, F.; Ali, A.; et al. Variations in nutritional profile of honey produced by various species of genus Apis. Braz. J. Biol. Rev. Bras. Biol. 2021, 83, e246651. [Google Scholar] [CrossRef]
- Yeli, A.; Fabiola, M.-T.; Lobato-Rosales, G.; Landa, M.; Giovanni, Z.; Luis, L.-C.; García-Santamaría, E.; Fernández-Lambert, G. Influence variables for honey production using bees Apis mellifera in the Misantla region. Rev. Mex. Cienc. Agrícolas 2019, 10, 1353–1365. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for Nutrition and Health: A Review. J. Am. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Gobin, I.; Crnković, G.; Magdalenić, M.; Begić, G.; Babić, A.; Lušić, D.; Vučković, D. Antibacterial potential of Croatian honey against antibiotic resistant pathogenic bacteria. Med. Glas. 2018, 15, 139–144. [Google Scholar] [CrossRef]
- Fyfe, L.; Okoro, P.; Paterson, E.; Coyle, S.; McDougall, G.J. Compositional analysis of Scottish honeys with antimicrobial activity against antibiotic-resistant bacteria reveals novel antimicrobial components. LWT—Food Sci. Technol. 2017, 79, 52–59. [Google Scholar] [CrossRef]
- Hegazi, A.G. Antimicrobial activity of different Egyptian honeys as comparison of Saudi Arabia honey. Res. J. Microbiol. 2011, 6, 488–495. [Google Scholar] [CrossRef][Green Version]
- Hegazi, A.; Guthami, F.; Al Ghetami, A.; Abd Allah, F.M.; Saleh, A.A.; Fouad, E.A. Potential antibacterial activity of some Saudi Arabia honey. Vet. World 2017, 10, 233. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Mărghitaş, L.A.; Chirilă, F.; Copaciu, F.; Simonca, V.; Bobiş, O.; Erler, S. Influence of geographic origin, plant source and polyphenolic substances on antimicrobial properties of propolis against human and honeybee pathogens. J. Apic. Res. 2017, 56, 588–597. [Google Scholar] [CrossRef]
- di Rosa, A.R.; Marino, A.M.F.; Leone, F.; Corpina, G.G.; Giunta, R.P.; Chiofalo, V. Characterization of Sicilian Honeys Pollen Profiles Using a Commercial E-Tongue and Melissopalynological Analysis for Rapid Screening: A Pilot Study. Sensors 2018, 18, 4065. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Jangir, J. Chemical characterization complemented with chemometrics for the botanical origin identification of unifloral and multifloral honeys from India. Food Res. Int. 2018, 107, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.K.A.; Al-Kahtani, S.; Taha, R. Comparison of the physicochemical characteristics of sidr (Ziziphus spp.) honey produced by Apis florea F. and Apis mellifera L. J. Apic. Res. 2021, 60, 470–477. [Google Scholar] [CrossRef]
- Azonwade, F.E.; Paraïso, A.; Agbangnan Dossa, C.P.; Dougnon, V.T.; N’Tcha, C.; Mousse, W.; Baba-Moussa, L. Physicochemical Characteristics and Microbiological Quality of Honey Produced in Benin. J. Food Qual. 2018, 2018. [Google Scholar] [CrossRef]
- Crăciun, M.E.; Pârvulescu, O.C.; Donise, A.C.; Dobre, T.; Stanciu, D.R. Characterization and classification of Romanian acacia honey based on its physicochemical parameters and chemometrics. Sci. Rep. 2020, 10, 20690. [Google Scholar] [CrossRef]
- Miłek, M.; Bocian, A.; Kleczyńska, E.; Sowa, P.; Dżugan, M. The Comparison of Physicochemical Parameters, Antioxidant Activity and Proteins for the Raw Local Polish Honeys and Imported Honey Blends. Molecules 2021, 26, 2423. [Google Scholar] [CrossRef]
- Adamchuk, L.; Sukhenko, V.; Akulonok, O.; Bilotserkivets, T.; Vyshniak, V.; Lisohurska, D.; Lisohurska, O.; Slobodyanyuk, N.; Shanina, O.; Galyasnyj, I. Methods for determining the botanical origin of honey. Potravin. Slovak J. Food Sci. 2020, 14, 483–493. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 2015, 59, 139–157. [Google Scholar] [CrossRef]
- Kirs, E.; Pall, R.; Martverk, K.; Laos, K. Physicochemical and melissopalynological characterization of Estonian summer honeys. Procedia Food Sci. 2011, 1, 616–624. [Google Scholar] [CrossRef]
- Chukwuemeka, R.; Simeonov, V. Evaluation of Pollen and Chemical Composition of Honey Samples Sourced from Open Markets in Anambra State, Nigeria to Ascertain their Authenticity. J. Appl. Life Sci. Int. 2019, 22, 1–12. [Google Scholar] [CrossRef]
- Chataway, H.D. The determination of moisture in honey. Can. J. Res. 2011, 6, 532–547. [Google Scholar] [CrossRef]
- Nyau, V.; Mwanza, E.; Moonga, H. Physico- chemical qualities of honey harvested from different beehive types in Zambia. Afr. J. Food 2013 Agric. Nutr. Dev. 2013, 13, 7415. [Google Scholar] [CrossRef]
- Yadata, D. Detection of the Electrical Conductivity and Acidity of Honey from Different Areas of Tepi. Food Sci. Technol. 2014, 2, 59–63. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, L.; Li, Y.; Chen, L.; Wu, L.; Zhao, J. Floral classification of honey using liquid chromatography–diode array detection–tandem mass spectrometry and chemometric analysis. Food Chem. 2014, 145, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and color of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Chua, L.S.; Rahaman, N.L.A.; Adnan, N.A.; Eddie Tan, T.T. Antioxidant activity of three honey samples in relation with their biochemical components. J. Anal. Methods Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Isla, M.I.; Craig, A.; Ordoñez, R.; Zampini, C.; Sayago, J.; Bedascarrasbure, E.; Alvarez, A.; Salomón, V.; Maldonado, L. Physico chemical and bioactive properties of honeys from Northwestern Argentina. LWT—Food Sci. Technol. 2011, 44, 1922–1930. [Google Scholar] [CrossRef]
- Ruiz-Ciau, D.; Cuevas-Glory, L.; Quijano, L.; Sauri-Duch, E. Chemical Composition and Antioxidant DPPH Activity of the Floral and Leaves Essential Oils of Montanoa speciosa DC. Am. J. Plant Sci. 2017, 8, 745–753. [Google Scholar] [CrossRef]
- Daulay, A.S.; Syahputra, R.A.; Nafitri, A. Antioxidant Activity Test of Chayote (Sechium edule (Jacq.) Swartz) Ethanol Extract using DPPH Method. J. Phys. Conf. Ser. 2021, 1819, 12035. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gan, S.H.; Khalil, M.I. Neurological effects of honey: Current and future prospects. In Evidence-Based Complementary and Alternative Medicine; Oxford University Press: Oxford, UK, 2014. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Guthami, F.M.A.; Gethami, A.F.M.A.; Fouad, E.A. Antibacterial and Antioxidant Activities of Some Saudi Arabia Honey Products. Iran. J. Med. Microbiol. 2020, 14, 490–500. [Google Scholar] [CrossRef]
- Hegazi, A.G.; Guthami, F.M.; Al Gethami, A.F.M.; Fouad, E.A.; Abdou, A.M.; Hegazi, S.; Al Guthami, F.M.; Abdou, A.M. Antibacterial activity and characterisation of some Egyptian honey of different floral origin. Bulg. J. Vet. Med. 2021, 24, 278–290. [Google Scholar] [CrossRef]
- Wei Se, K.; Abdul Wahab, R.; Nuratiqah Syed Yaacob, S.; Krishna Ghoshal, S. Study review Detection techniques for adulterants in honey: Challenges and recent trends. J. Food Compos. Anal. 2019, 80, 16–32. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Abdelaliem, Y.F.; Esmail, A.H.M.; Mohdaly, A.A.A.; Ramadan, M.F. Evaluation of Egyptian honeys and their floral origins: Phenolic compounds, antioxidant activities, and antimicrobial characteristics. Environ. Sci. Pollut. Res. 2020, 27, 20748–20756. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Costinel, D.; Ionete, R.E. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control 2020, 109, 106919. [Google Scholar] [CrossRef]
- Salvador, L.; Guijarro, M.; Rubio, D.; Aucatoma, B.; Guillén, T.; Jentzsch, P.V.; Ciobotă, V.; Stolker, L.; Ulic, S.; Vásquez, L.; et al. Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador. Foods 2019, 8, 105. [Google Scholar] [CrossRef]
- El Sohaimy, S.A.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Ann. Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef]
- Aljohar, H.I.; Maher, H.M.; Albaqami, J.; Al-Mehaizie, M.; Orfali, R.; Alrubia, S. Physical and chemical screening of honey samples available in the Saudi market: An important aspect in the authentication process and quality assessment. Saudi Pharm. J. 2018, 26, 932–942. [Google Scholar] [CrossRef]
- Szczesna, T.; Waś, E.; Semkiw, P.; Skubida, P.; Jaśkiewicz, K.; Witek, M. Changes in the Physicochemical Properties of Starch Syrups after Processing by Honeybees. Agriculture 2021, 11, 335. [Google Scholar] [CrossRef]
- Kazeminia, M.; Mahmoudi, R.; Aali, E.; Ghajarbygi, P. Evaluation of Authenticity in Honey Samples from Qazvin, Iran. J. Chem. Health Risks 2021. [Google Scholar] [CrossRef]
- Bentabol Manzanares, A.; Hernández García, Z.; Rodríguez Galdón, B.; Rodríguez, E.; Díaz Romero, C. Physicochemical characteristics of minor monofloral honeys from Tenerife, Spain. LWT—Food Sci. Technol. 2014, 55, 572–578. [Google Scholar] [CrossRef]
- Pasias, I.N.; Kiriakou, I.K.; Proestos, C. HMF and diastase activity in honeys: A fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017, 229, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.; Haki, G.D.; Birringer, M.; Borck, H.; Lee, Y.C.; Kim, K.T.; Baye, K.; Melaku, S. Enzyme activity, amino acid profiles and hydroxymethylfurfural content in Ethiopian monofloral honey. J. Food Sci. Technol. 2017, 54, 2769. [Google Scholar] [CrossRef]
- GSO 147:2008-Standards Store-GCC Standardization Organization. Gulf Technical Regulation. Available online: https://www.gso.org.sa/store/standards/GSO:518550?lang=en (accessed on 17 February 2022).
- Warui, M.W.; Hansted, L.; Gikungu, M.; Mburu, J.; Kironchi, G.; Bosselmann, A.S. Characterization of Kenyan Honeys Based on Their Physicochemical Properties, Botanical and Geographical Origin. Int. J. Food Sci. 2019, 2019. [Google Scholar] [CrossRef]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Maruska, A.; Kornysova, O.; Ligor, M.; Kaškonienė, V.; Maruška, A.; Kornyšova, O.; Charczun, N.; Ligor, M.; Buszewski, B. Quantitative and qualitative determination of phenolic compounds in honey Plantarum project View project Impact of clear cuttings on transformation of biodiversity in forest ecosystems View project Quantitative and qualitative determination of phenolic compounds in honey. Cheminé Technol. 2009, 3, 74–80. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Gonçalves, J.; Ribeiro, I.; Marçalo, J.; Rijo, P.; Faustino, C.; Pinheiro, L. Physicochemical, Antioxidant and Antimicrobial Properties of selected Portuguese Commercial Monofloral Honeys. J. Food Nutr. Res. 2018, 6, 645–654. [Google Scholar] [CrossRef]
- Al, M.L.; Daniel, D.; Moise, A.; Bobis, O.; Laslo, L.; Bogdanov, S. Physico-chemical and bioactive properties of different floral origin honeys from Romania. Food Chem. 2009, 112, 863–867. [Google Scholar] [CrossRef]
- Srećković, N.Z.; Mihailović, V.B.; Katanić Stanković, J.S. Physico-chemical, antioxidant and antimicrobial properties of three different types of honey from central Serbia. Kragujev. J. Sci. 2019, 41, 53–68. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Tulipani, S.; Díaz, D.; Estevez, Y.; Romandini, S.; Giampieri, F.; Damiani, E.; Astolfi, P.; Bompadre, S.; Battino, M. Antioxidant and antimicrobial capacity of several monofloral Cuban honeys and their correlation with color, polyphenol content and other chemical compounds. Food Chem. Toxicol. 2010, 48, 2490–2499. [Google Scholar] [CrossRef]
- Silici, S.; Sagdic, O.; Ekici, L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010, 121, 238–243. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- van den Berg, A.J.; van den Worm, E.; van Ufford, H.C.Q.; Halkes, S.B.; Hoekstra, M.J.; Beukelman, C.J. An in vitro examination of the antioxidant and anti-inflammatory properties of buckwheat honey. J. Wound Care 2008, 17, 172–178. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; Silva, W.P.; da Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT—Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef]
- Pontis, J.A.; da Costa, L.A.M.A.; da Silva, S.J.R.; Flach, A. Color, phenolic and flavonoid content, and antioxidant activity of honey from Roraima, Brazil. Food Sci. Technol. 2014, 34, 69–73. [Google Scholar] [CrossRef]
- Nayaka, N.M.D.M.W.; Fidrianny, I.; Sukrasno Hartati, R.; Singgih, M. Antioxidant and antibacterial activities of multiflora honey extracts from the Indonesian Apis cerana bee. J. Taibah Univ. Med. Sci. 2020, 15, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Stagos, D.; Soulitsiotis, N.; Tsadila, C.; Papaeconomou, S.; Arvanitis, C.; Ntontos, A.; Karkanta, F.; Adamou-Androulaki, S.; Petrotos, K.; Spandidos, D.A.; et al. Antibacterial and antioxidant activity of different types of honey derived from Mount Olympus in Greece. Int. J. Mol. Med. 2018, 42, 726. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, A.G.; Abd Allah, F.M. Antimicrobial Activity of Different Saudi Arabia Honeys. Global Veterinaria 2012, 9, 53–59. [Google Scholar]
- Al Masaudi, S. The antibacterial activities of honey. Saudi J. Biol. Sci. 2021, 28, 2188–2196. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154. [Google Scholar] [CrossRef]
- Israili, Z.H. Antimicrobial properties of honey. Am. J. Ther. 2014, 21, 304–323. [Google Scholar] [CrossRef]
- Libonatti, C.; Varela, S.; Basualdo, M. Antibacterial activity of honey: A review of honey around the world. J. Microbiol. Antimicrob. 2014, 6, 51–56. [Google Scholar] [CrossRef]
- Szweda, P. Antimicrobial Activity of Honey. Honey Anal. 2017, 3, 15. [Google Scholar] [CrossRef]
- Morroni, G.; Alvarez-Suarez, J.M.; Brenciani, A.; Simoni, S.; Fioriti, S.; Pugnaloni, A.; Giampieri, F.; Mazzoni, L.; Gasparrini, M.; Marini, E.; et al. Comparison of the antimicrobial activities of four honeys from three countries (New Zealand, Cuba, and Kenya). Front. Microbiol. 2018, 9, 1378. [Google Scholar] [CrossRef]
- Irish, J.; Blair, S.; Carter, D.A. The antibacterial activity of honey derived from Australian flora. PLoS ONE 2011, 6, e18229. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.P.; Thakur, V.; Brar, S.K. Antibacterial Efficacy of Raw and Processed Honey. Biotechnol. Res. Int. 2011, 2011, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Matzen, R.D.; Leth-Espensen, J.Z.; Jansson, T.; Nielsen, D.S.; Lund, M.N.; Matzen, S. The Antibacterial Effect in Vitro of Honey Derived from Various Danish Flora. Dermatol. Res. Pract. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Molan, P.C. The antibacterial activity of honey 2. Variation in the potency of the antibacterial activity. Bee World 1992, 73, 59–76. [Google Scholar] [CrossRef]
- Lee, H.; Churey, J.J.; Worobo, R.W. Antimicrobial activity of bacterial isolates from different floral sources of honey. Int. J. Food Microbiol. 2008, 126, 240–244. [Google Scholar] [CrossRef]
- Anthimidou, E.; Mossialos, D. Antibacterial activity of Greek and Cypriot honeys against staphylococcus aureus and pseudomonas aeruginosa in comparison to manuka honey. J. Med. Food 2013, 16, 42–47. [Google Scholar] [CrossRef]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Borras-Linares, I.; Cadiz-Gurrea, M.d.l.L.; Mahmoodi-Khaledi, E. Potential antimicrobial activity of honey phenolic compounds against Gram positive and Gram-negative bacteria. LWT 2019, 101, 236–245. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- White, J.W.; Subers, M.H.; Schepartz, A.I. The identification of inhibine, the antibacterial factor in honey, as hydrogen peroxide and its origin in a honey glucose-oxidase system. Biochim. Biophys. Acta (BBA)—Spec. Sect. Enzymol. Subj. 1963, 73, 57–70. [Google Scholar] [CrossRef]
- Bhat, R.R.; Pai, M.v.; Ram, H.S.; Reddy, S.; Rai, S.; Thejeswi, P.; Prabhu, S. Comparison of Sugar and Honey Dressings in Healing of Chronic Wounds. IOSR J. Dent. Med. Sci. 2014, 13, 82–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).