Network Pharmacology-Based Study on the Efficacy and Mechanism of Lonicera japonica Thunberg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compound and Target Gene Collection
2.2. Network Construction
2.3. Functional Enrichment Analysis
3. Results
3.1. Network Analysis of LJ
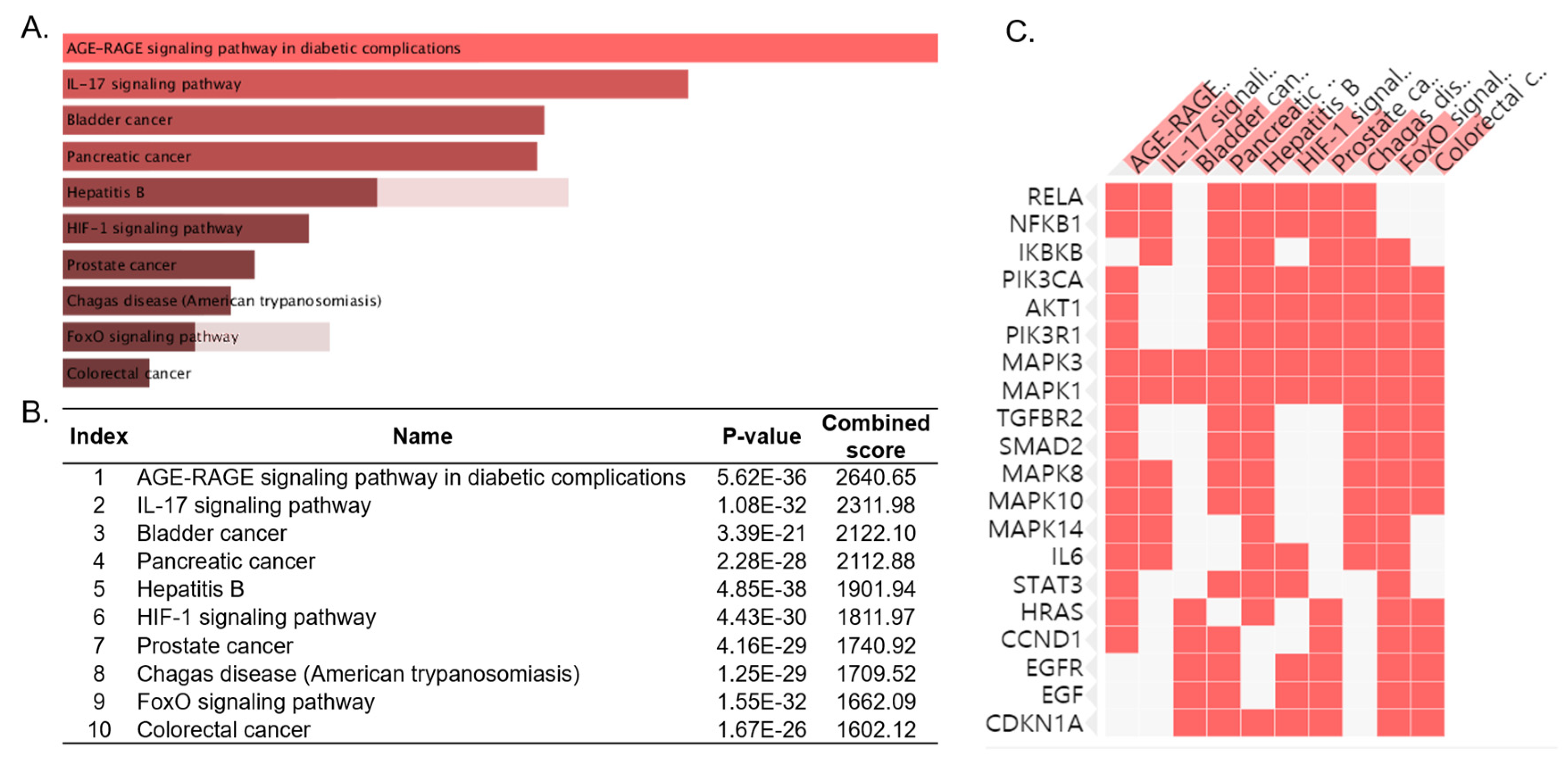
3.2. Functional Enrichment Analysis of the LJ Network
3.3. Specific Mechanisms of LJ in the AGE-RAGE Signaling Pathway in Diabetic Complications
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venkataramanan, R.; Komoroski, B.; Strom, S. In vitro and in vivo assessment of herb drug interactions. Life Sci. 2006, 78, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Park, S.-Y.; Lee, H.-J.; Kim, C.-E. A Systems-Level Analysis of Mechanisms of Platycodon grandiflorum Based on A Network Pharmacological Approach. Molecules 2018, 23, 2841. [Google Scholar] [CrossRef]
- Yuan, Y.; Zheng, Q.; Si, Z.; Liu, J.; Li, Z.; Xiong, L.; Liu, P.; Li, X.; He, C.; Liang, J. Efficacy of Chinese Herbal Injections for Elderly Patients With pneumonia—A Bayesian Network Meta-analysis of Randomized Control Trials. Front. Pharmacol. 2021, 12, 610745. [Google Scholar] [CrossRef]
- Xia, H.; Liu, J.; Yang, W.; Liu, M.; Luo, Y.; Yang, Z.; Xie, J.; Zeng, H.; Xu, R.; Ling, H.; et al. Integrated Strategy of Network Pharmacological Prediction and Experimental Validation Elucidate Possible Mechanism of Bu-Yang Herbs in Treating Postmenopausal Osteoporosis via ESR1. Front. Pharmacol. 2021, 12, 654714. [Google Scholar] [CrossRef]
- Raafat, K. Identification of phytochemicals from North African plants for treating Alzheimer’s diseases and of their molecular targets by in silico network pharmacology approach. J. Tradit. Complement. Med. 2020, 11, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xie, D.; Yu, Y.; Liu, H.; Shi, Y.; Shi, T.; Wen, C. TCMID 2.0: A comprehensive resource for TCM. Nucleic Acids Res. 2017, 46, D1117–D1120. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Kim, S.-K.; Nam, S.; Jang, H.; Kim, A.; Lee, J.-J. TM-MC: A database of medicinal materials and chemical compounds in Northeast Asian traditional medicine. BMC Complement. Altern. Med. 2015, 15, 218. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Q.; Liu, M. A Network Pharmacology Approach to Explore the Potential Mechanisms of Huangqin-Baishao Herb Pair in Treatment of Cancer. Med. Sci. Monit. 2020, 26, e923199-1. [Google Scholar] [CrossRef]
- Pan, B.; Shi, X.; Ding, T.; Liu, L. Unraveling the action mechanism of polygonum cuspidatum by a network pharmacology approach. Am. J. Transl. Res. 2019, 11, 6790. [Google Scholar]
- Dan, W.; Liu, J.; Guo, X.; Zhang, B.; Qu, Y.; He, Q. Study on Medication Rules of Traditional Chinese Medicine against Antineoplastic Drug-Induced Cardiotoxicity Based on Network Pharmacology and Data Mining. Evid. Based Complement. Altern. Med. 2020, 2020, 7498525. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-W.; Lee, Y.-J.; Yang, D.-J.; Hsieh, M.-C.; Chen, C.-C.; Hsu, W.-L.; Chang, Y.-Y.; Liu, C.-W. Anti-inflammatory effects of Flos Lonicerae Japonicae Water Extract are regulated by the STAT/NF-κB pathway and HO-1 expression in Virus-infected RAW264.7 cells. Int. J. Med. Sci. 2021, 18, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, S.; Wang, W.; Hong, Y.; Tang, K.; Luo, Q. Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem. 2013, 138, 327–333. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, H.; Bai, X.; Liu, P.; Yang, Y.; Huang, J.; Zhou, L.; Min, X. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. Int. J. Biol. Macromol. 2019, 151, 1058–1066. [Google Scholar] [CrossRef]
- Park, H.-S.; Park, K.-I.; Lee, D.-H.; Kang, S.-R.; Nagappan, A.; Kim, J.-A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Hah, Y.-S.; et al. Polyphenolic extract isolated from Korean Lonicera japonica Thunb. induce G2/M cell cycle arrest and apoptosis in HepG2 Cells: Involvements of PI3K/Akt and MAPKs. Food Chem. Toxicol. 2012, 50, 2407–2416. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Hong, S.-I.; Jung, Y.-H.; Kim, M.-J.; Kim, S.-Y.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. Lonicera japonica THUNB. protects 6-hydroxydopamine-induced neurotoxicity by inhibiting activation of MAPKs, PI3K/Akt, and NF-κB in SH-SY5Y cells. Food Chem. Toxicol. 2012, 50, 797–807. [Google Scholar] [CrossRef]
- Li, R.; Kuang, X.; Wang, W.; Wan, C.; Li, W. Comparison of chemical constitution and bioactivity among different parts of Lonicera japonica Thunb. J. Sci. Food Agric. 2019, 100, 614–622. [Google Scholar] [CrossRef]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Su, G.; Morris, J.H.; Demchak, B.; Bader, G.D. Biological network exploration with Cytoscape 3. Curr. Protoc. Bioinform. 2014, 47, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webber, C. Functional Enrichment Analysis with Structural Variants: Pitfalls and Strategies. Cytogenet. Genome Res. 2011, 135, 277–285. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2020, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, W.S.; Nagappan, A.; Hong, S.H.; Jung, J.H.; Park, C.; Kim, H.J.; Kim, G.-Y.; Kim, G.; Jung, J.-M.; et al. Flavonoids Isolated from Flowers of Lonicera japonica Thunb. Inhibit Inflammatory Responses in BV2 Microglial Cells by Suppressing TNF-α and IL-β Through PI3K/Akt/NF-kb Signaling Pathways. Phytother. Res. 2016, 30, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Ma, S.-X.; Hong, S.-I.; Lee, S.-Y.; Jang, C.-G. Lonicera japonica THUNB. Extract Inhibits Lipopolysaccharide-Stimulated Inflammatory Responses by Suppressing NF-κB Signaling in BV-2 Microglial Cells. J. Med. Food 2015, 18, 762–775. [Google Scholar] [CrossRef]
- Han, J.M.; Kim, M.H.; Choi, Y.Y.; Lee, H.; Hong, J.; Yang, W.M. Effects of Lonicera japonica Thunb. on Type 2 Diabetes via PPAR-γ Activation in Rats. Phytother. Res. 2015, 29, 1616–1621. [Google Scholar] [CrossRef]
- Tzeng, T.-F.; Liou, S.-S.; Chang, C.J.; Liu, I.-M. The Ethanol Extract of Lonicera japonica (Japanese Honeysuckle) Attenuates Diabetic Nephropathy by Inhibiting p-38 MAPK Activity in Streptozotocin-Induced Diabetic Rats. Planta Med. 2014, 80, 121–129. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, X.; Liu, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from flower buds of Lonicera japonica in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2017, 102, 396–404. [Google Scholar] [CrossRef]



| Compound Name | Molecule Formula | PubChem CID |
|---|---|---|
| Centauroside | C34H46O19 | 102183195 |
| Demethylsecologanol | C16H24O10 | 11617742 |
| 5-O-caffeoylquinic acid | C16H18O9 | 12310830 |
| Ketologanin | C16H22O10 | 12311348 |
| 1,4-di-O-caffeoylquinic acid | C25H24O12 | 12358846 |
| Sweroside | C16H22O9 | 161036 |
| Secologanin acid | C17H24O10 | 161276 |
| Secoxyloganin | C17H24O11 | 162868 |
| Chlorogenic acid | C16H18O9 | 1794427 |
| (E)-aldosecologanin | C34H46O19 | 45783101 |
| Quercetin | C15H10O7 | 5280343 |
| Coniferin | C16H22O8 | 5280372 |
| Luteolin | C15H10O6 | 5280445 |
| Neochlorogenic acid | C16H18O9 | 5280633 |
| Luteoloside | C21H20O11 | 5280637 |
| Isoquercitrin | C21H20O12 | 5280804 |
| Rutin | C27H30O16 | 5280805 |
| Hyperoside | C21H20O12 | 5281643 |
| 3,4-O-dicaffeoylquinic acid | C25H24O12 | 5281780 |
| Rhoifolin | C27H30O14 | 5282150 |
| Lonicerin | C27H30O15 | 5282152 |
| 4,5-O-dicaffeoylquinic acid | C25H24O12 | 6474309 |
| Isochlorogenic acid A | C25H24O12 | 6474310 |
| Caffeic acid | C9H8O4 | 689043 |
| Loganin | C17H26O10 | 87691 |
| Loganic acid | C16H24O10 | 89640 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J.; Kim, M.H.; Yang, W.M. Network Pharmacology-Based Study on the Efficacy and Mechanism of Lonicera japonica Thunberg. Appl. Sci. 2022, 12, 9122. https://doi.org/10.3390/app12189122
Park SJ, Kim MH, Yang WM. Network Pharmacology-Based Study on the Efficacy and Mechanism of Lonicera japonica Thunberg. Applied Sciences. 2022; 12(18):9122. https://doi.org/10.3390/app12189122
Chicago/Turabian StylePark, Sang Jun, Mi Hye Kim, and Woong Mo Yang. 2022. "Network Pharmacology-Based Study on the Efficacy and Mechanism of Lonicera japonica Thunberg" Applied Sciences 12, no. 18: 9122. https://doi.org/10.3390/app12189122
APA StylePark, S. J., Kim, M. H., & Yang, W. M. (2022). Network Pharmacology-Based Study on the Efficacy and Mechanism of Lonicera japonica Thunberg. Applied Sciences, 12(18), 9122. https://doi.org/10.3390/app12189122







