Geometric Characterization of Local Changes in Tungsten Microneedle Tips after In-Vivo Insertion into Peripheral Nerves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Data
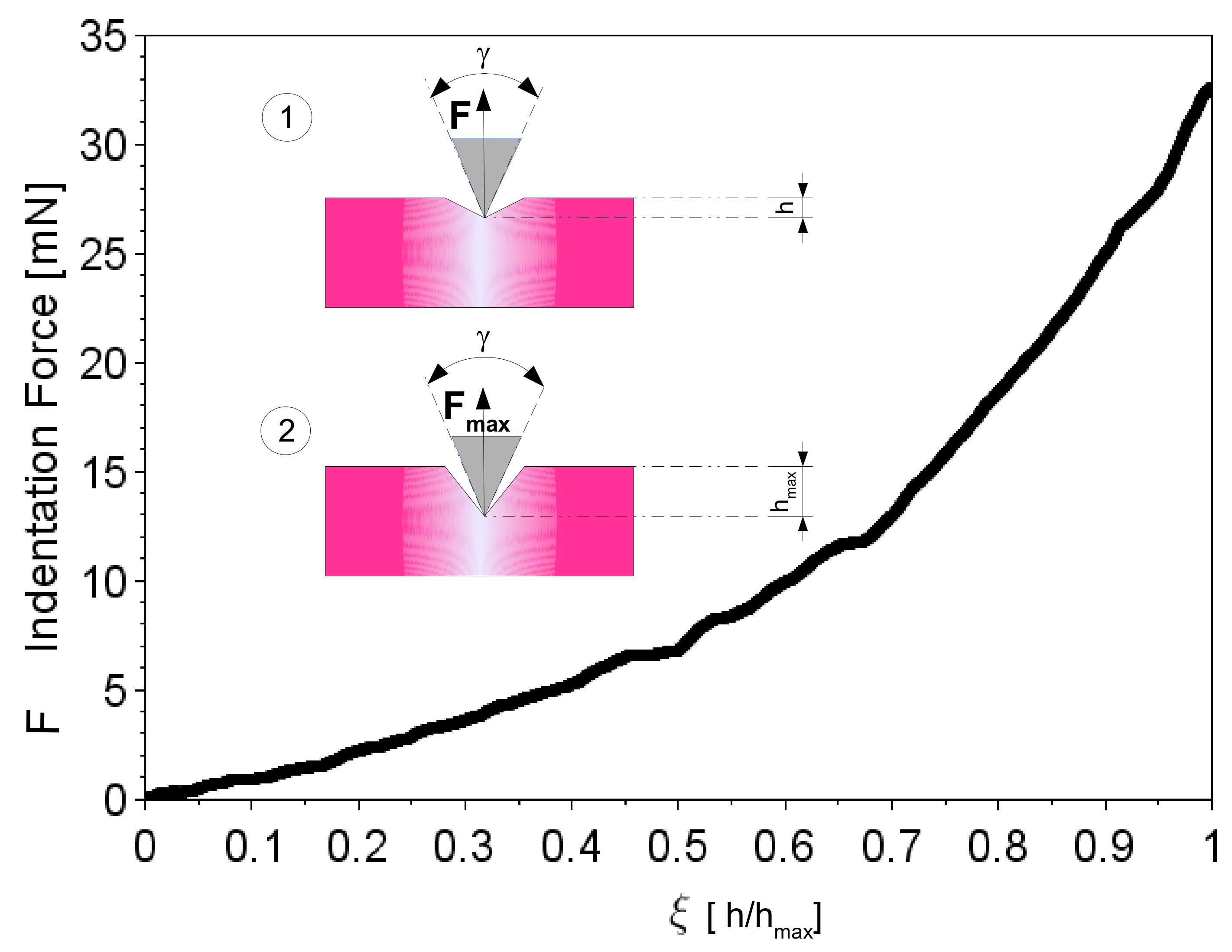
2.2. Modeling of the Interaction between W Microneedles and In Vivo Tissue
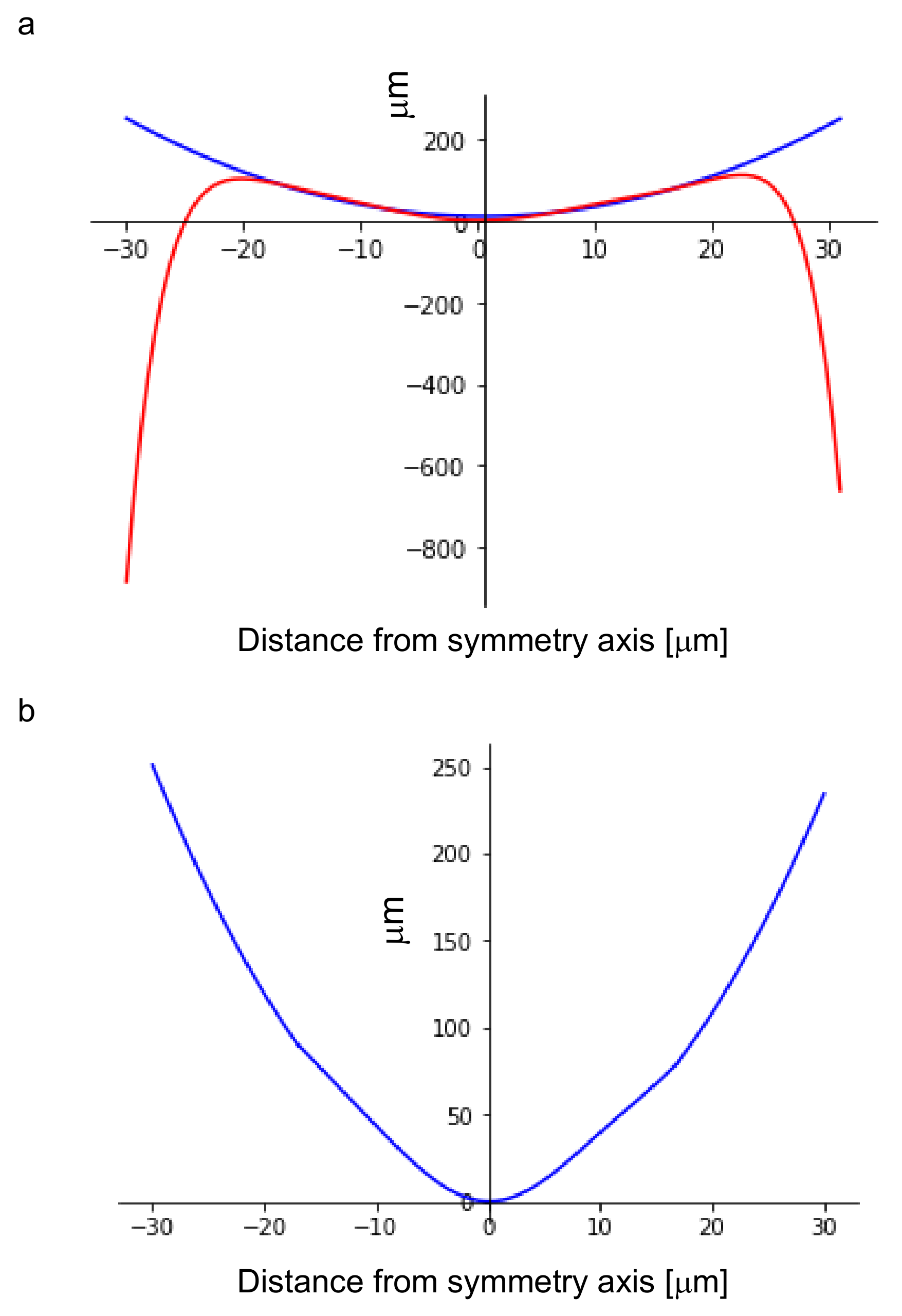
2.3. Modelling of the Undeformed Shape of W Microneedles
2.4. Quantification of Microneedle Tip Angle and Force-Angle Experimental Relationship
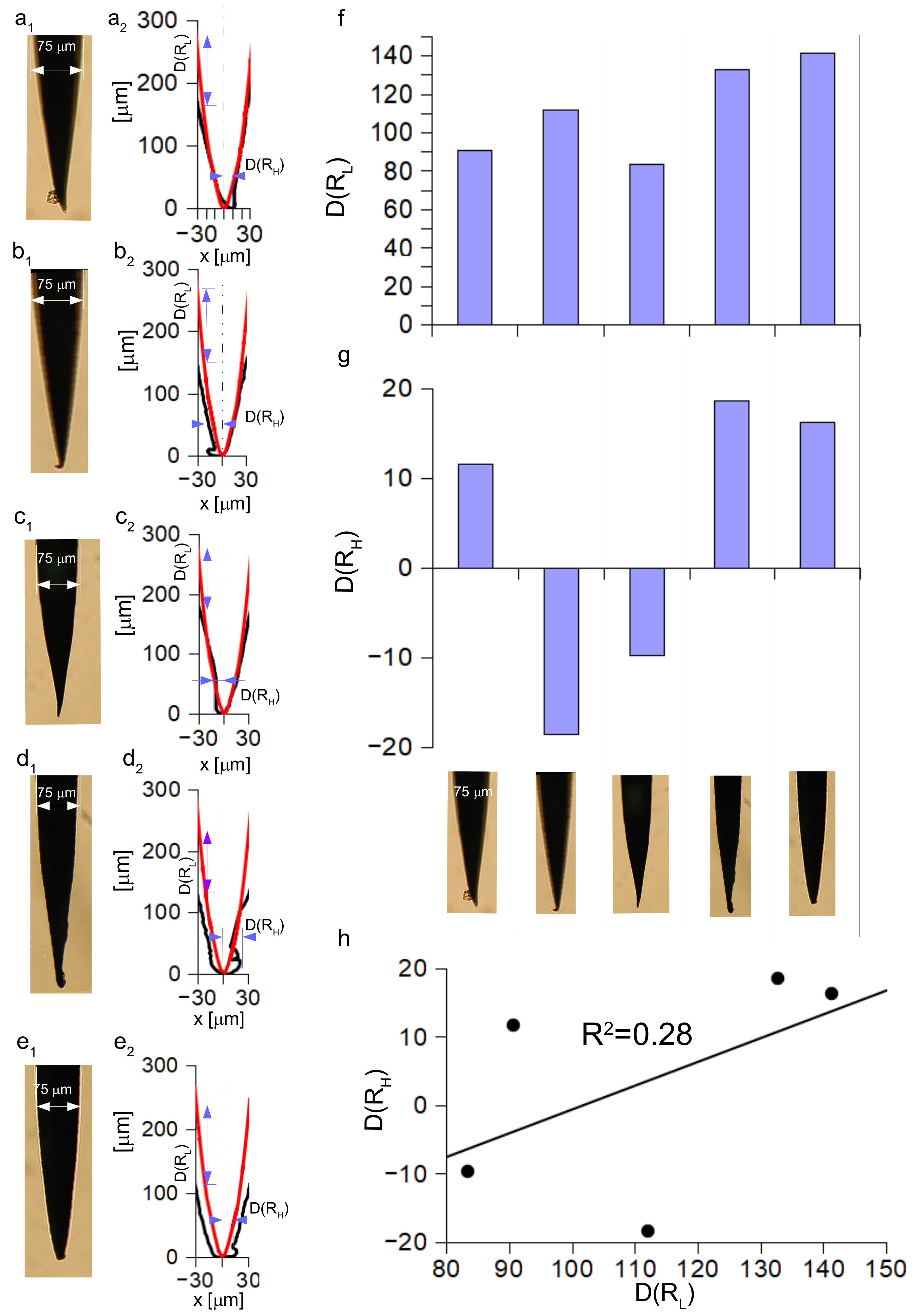
2.5. Investigation and Quantification of Post Insertion Shape Changes
3. Results
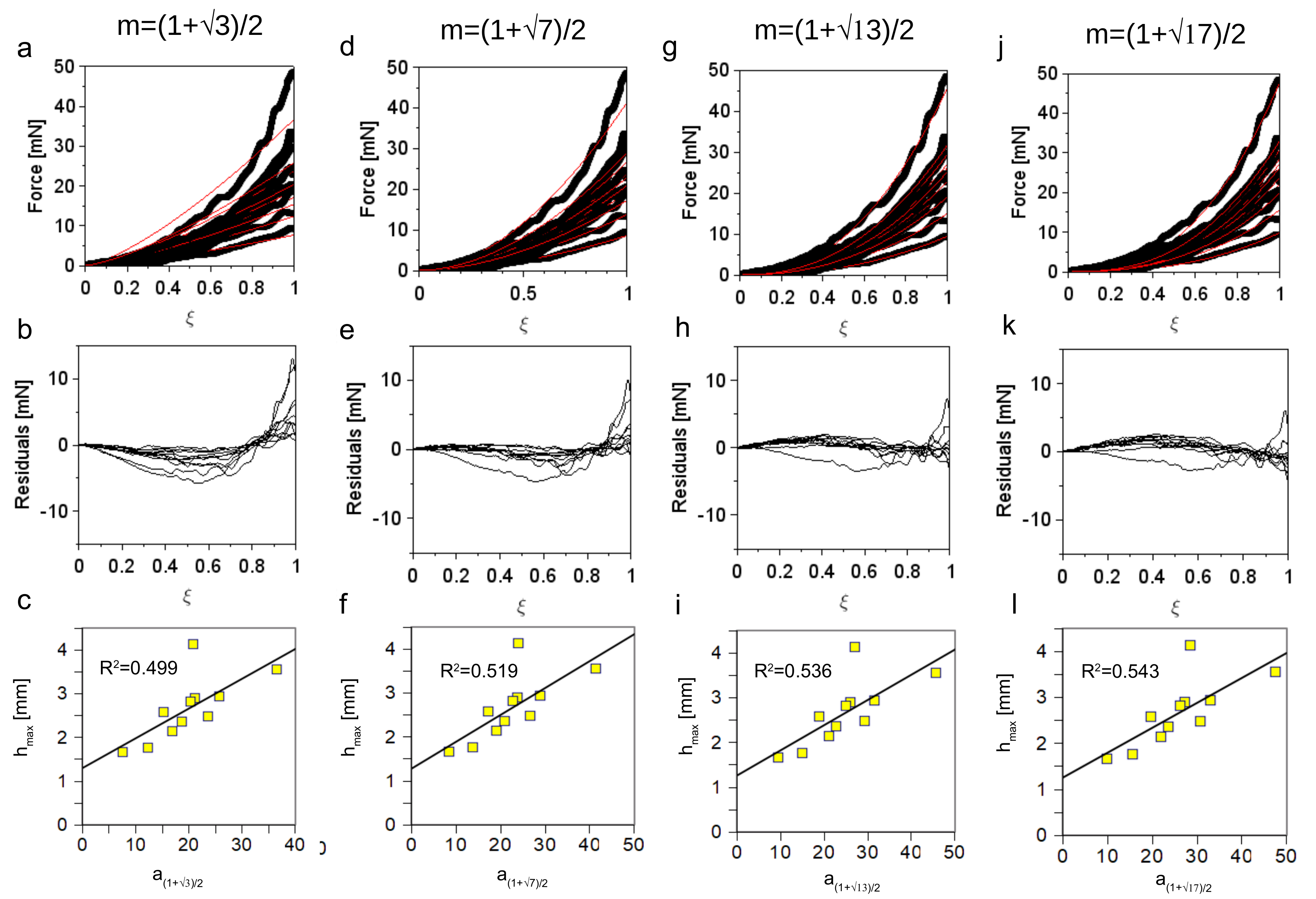
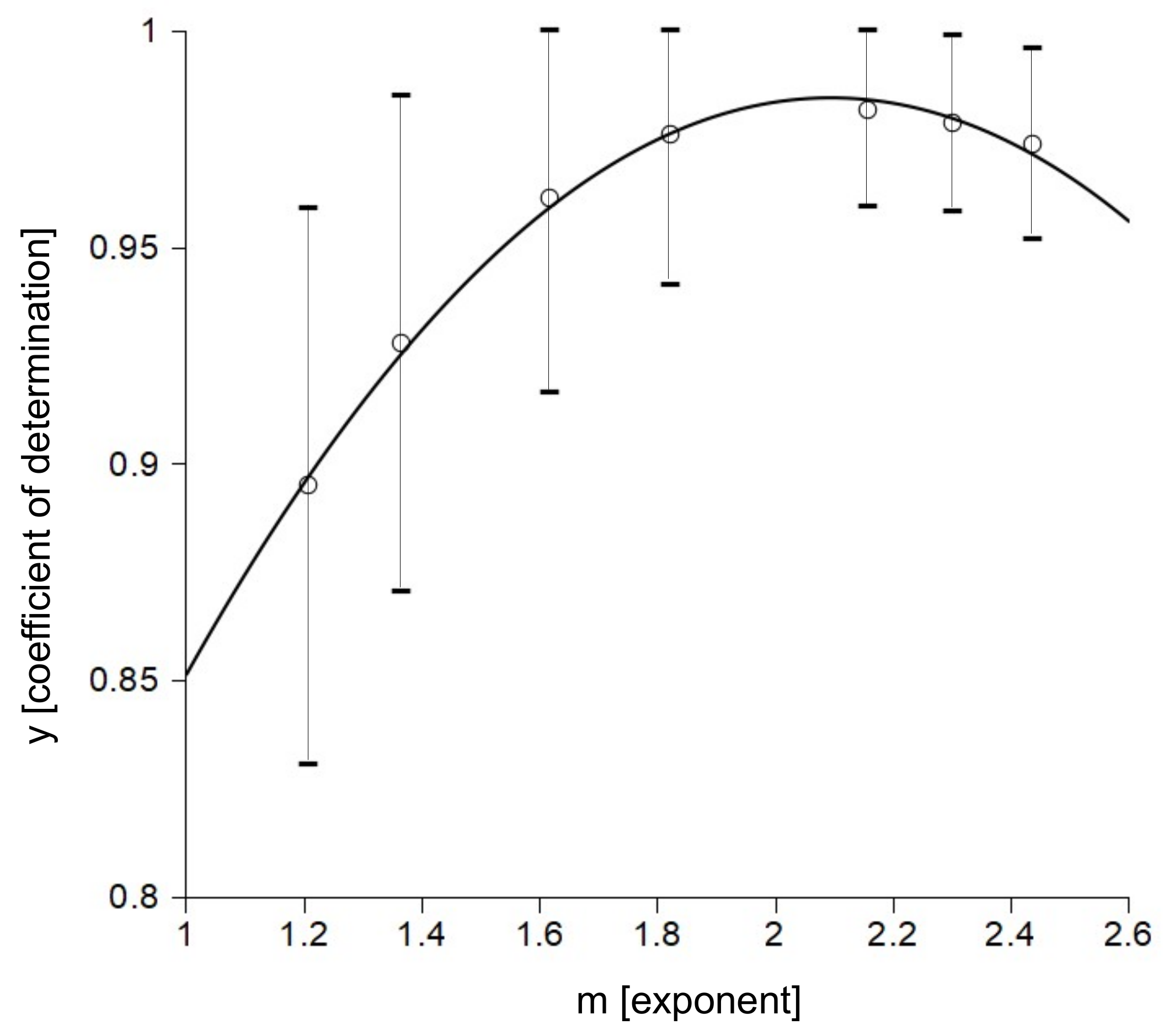
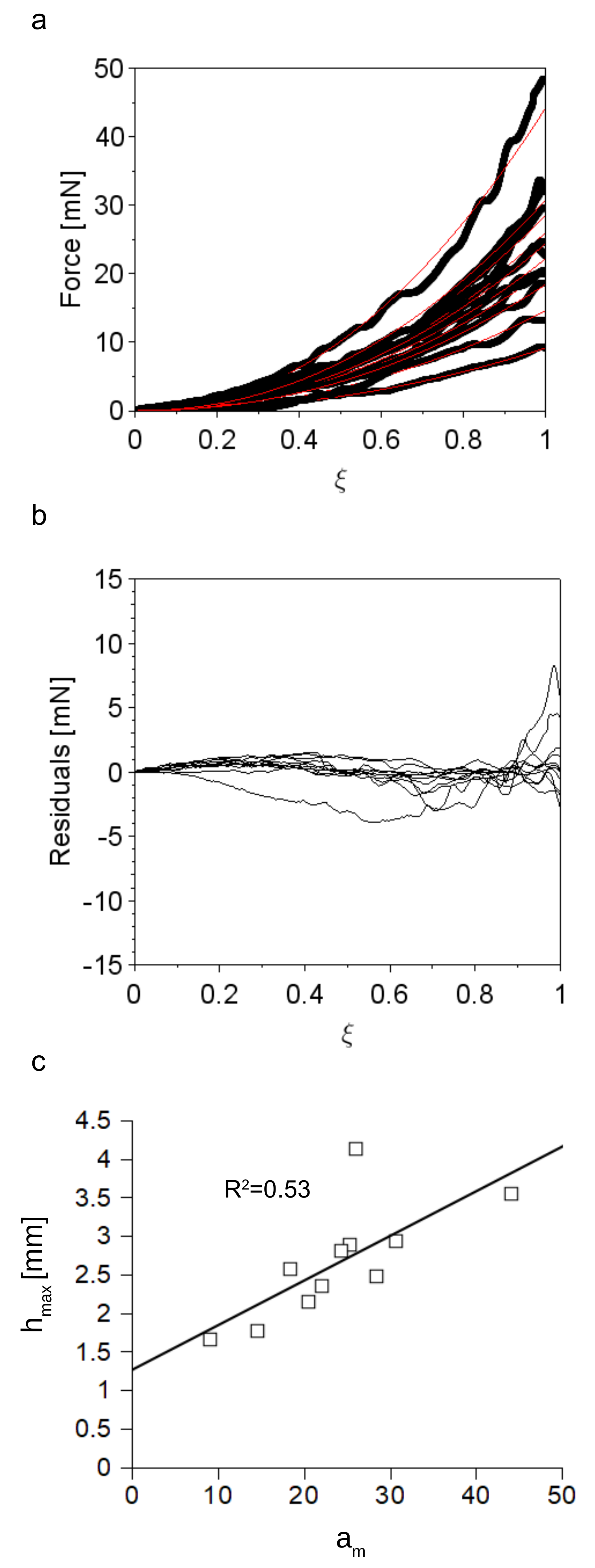
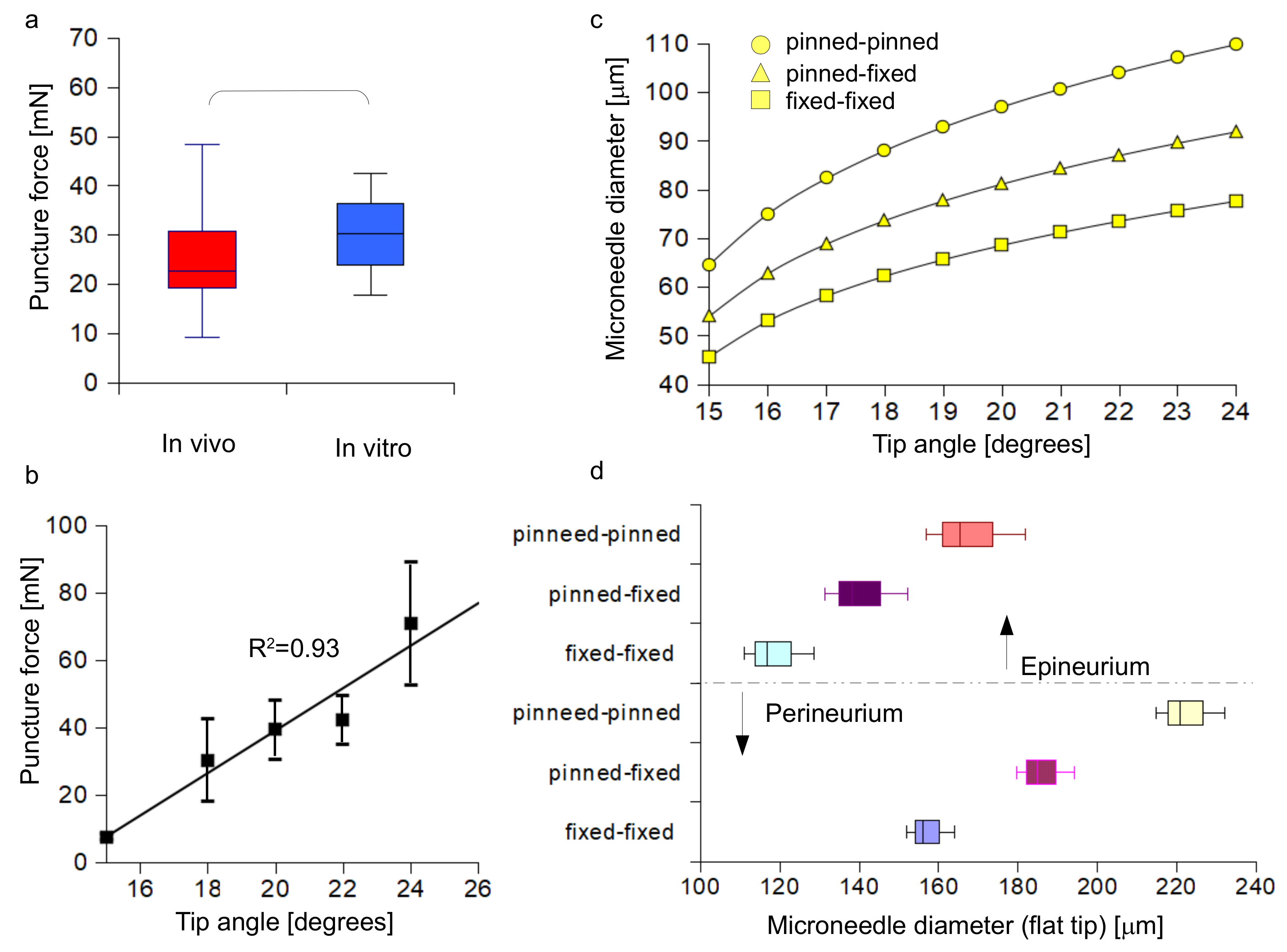
3.1. Accuracy of the Insertion Force Model of W Microneedles into In Vivo Peripheral Nerves
| Coefficient | Value | (95% Confidence Interval) |
|---|---|---|
| a | −1.024 × 10 | |
| b | ||
| c |
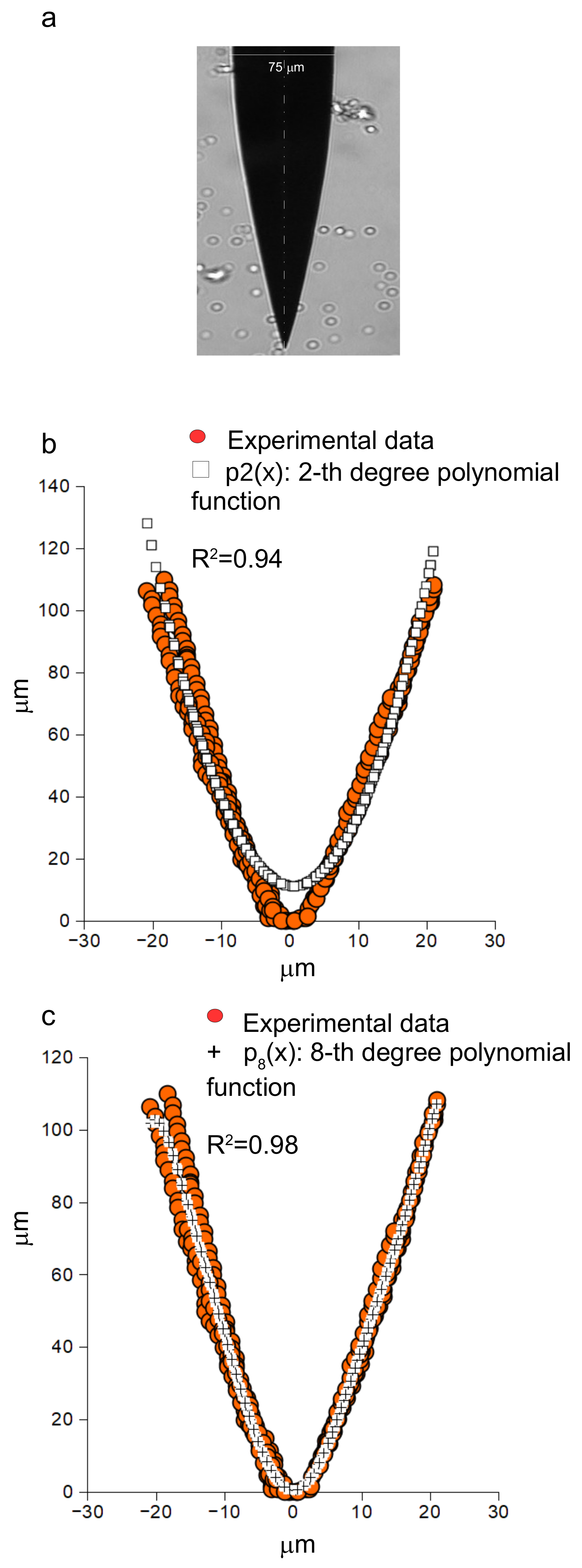
3.2. Tip Shape of Intact Microneedles
3.3. Quantification of the Microneedle Tip Angle and Its Relationship to the Insertion Force
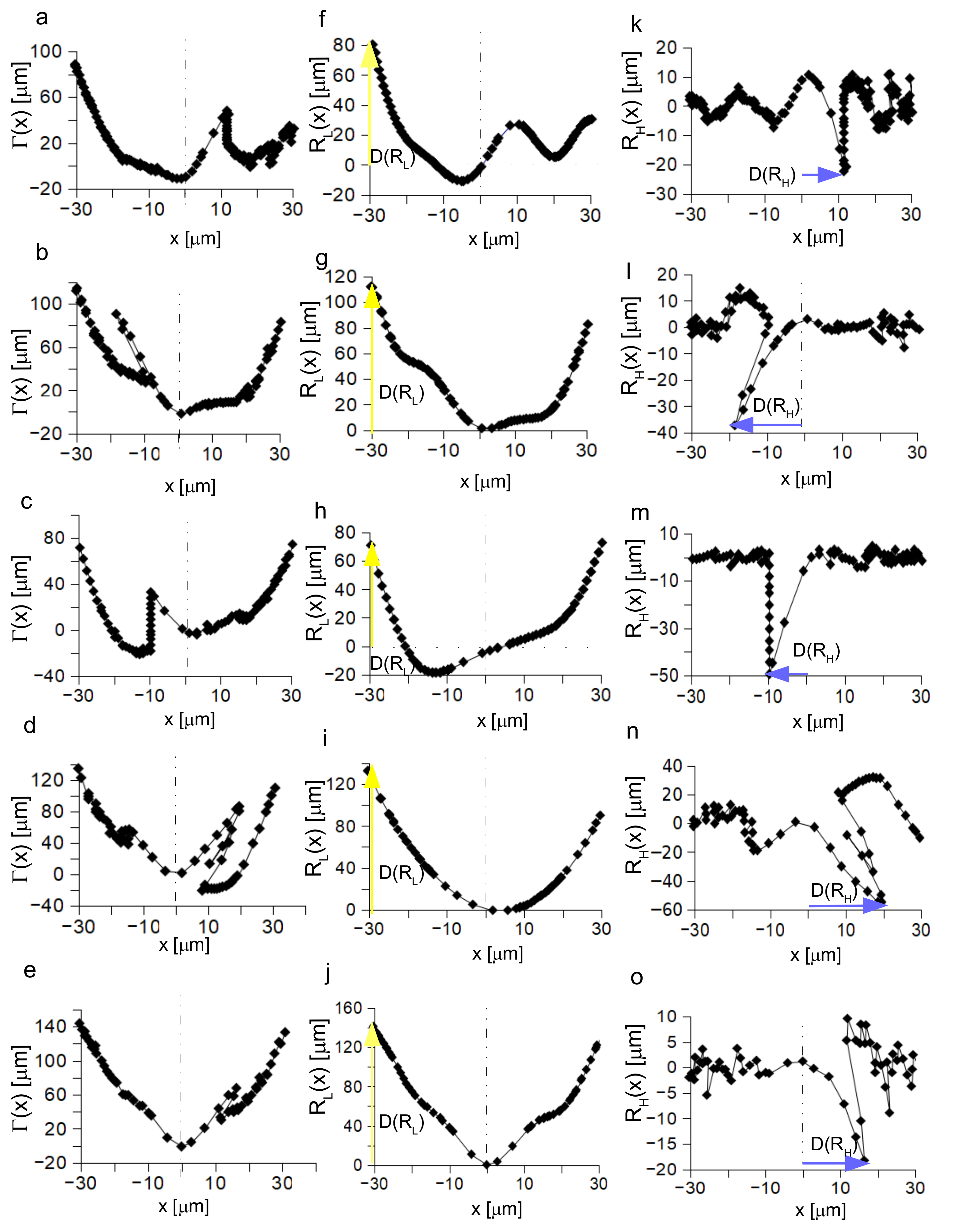
3.4. Analysis of the Post Insertion Shape
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Coefficients | Value | (95% Confidence Interval) |
|---|---|---|
| × 10 | ||
| Coefficients | Value | (95% Confidence Interval) |
|---|---|---|
| × 10 | ||
| × 10 | |
References
- Pervikov, A.; Krinitcyn, M.; Glazkova, E.; Rodkevich, N.; Lerner, M. Synthesis of tungsten carbide from bimodal tungsten powder produced by electrical explosion of wire. Int. J. Refract. Met. Hard Mater. 2022, 103, 105733. [Google Scholar] [CrossRef]
- Chayeuski, V.; Zhylinski, V.; Rudak, P.; Rusalsky, D.; Visniakov, N.; Cernasejus, O. Characteristics of ZrC/Ni-UDD coatings for a tungsten carbide cutting tool. Appl. Surf. Sci. 2018, 446, 18–26. [Google Scholar] [CrossRef]
- Li, X.; Yuan, D.; Jiang, X.; Wang, F. Damages and wear of tungsten carbide-tipped rippers of tunneling machines used to cutting large diameter reinforced concrete piles. Eng. Fail. Anal. 2021, 127, 105533. [Google Scholar] [CrossRef]
- Katiyar, P.K. A comprehensive review on synergy effect between corrosion and wear of cemented tungsten carbide tool bits: A mechanistic approach. Int. J. Refract. Met. Hard Mater. 2020, 92, 105315. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, G.; Liu, J.; Lv, Y.; Zhang, Y. Highly stable activity of cobalt based catalysts with tungsten carbide-activated carbon support for dry reforming of methane: Role of tungsten carbide. Fuel 2022, 311, 122512. [Google Scholar] [CrossRef]
- Salama, E.; Eissa, M.; Tageldin, A. Distinct properties of tungsten austenitic stainless alloy as a potential nuclear engineering material. Nucl. Eng. Technol. 2019, 51, 784–791. [Google Scholar] [CrossRef]
- Roth, J.; Tsitrone, E.; Loarte, A.; Loarer, T.; Counsell, G.; Neu, R.; Philipps, V.; Brezinsek, S.; Lehnen, M.; Coad, P.; et al. Recent analysis of key plasma wall interactions issues for ITER. J. Nucl. Mater. 2009, 390–391, 1–9. [Google Scholar] [CrossRef]
- Gilbert, M.; Dudarev, S.; Zheng, S.; Packer, L.; Sublet, J.C. An integrated model for materials in a fusion power plant: Transmutation, gas production, and helium embrittlement under neutron irradiation. Nucl. Fusion 2012, 52, 083019. [Google Scholar] [CrossRef]
- Chirayutthanasak, O.; Sarochawikasit, R.; Wisitsorasak, A.; Rujisamphan, N.; Frolov, T.; Oppelstrup, T.; Dangtip, S.; Rohrer, G.S.; Ratanaphan, S. Anisotropic grain boundary area and energy distributions in tungsten. Scr. Mater. 2022, 209, 114384. [Google Scholar] [CrossRef]
- Riesch, J.; Feichtmayer, A.; Coenen, J.W.; Curzadd, B.; Gietl, H.; Höschen, T.; Manhard, A.; Schwarz-Selinger, T.; Neu, R. Irradiation effects in tungsten—From surface effects to bulk mechanical properties. Nucl. Mater. Energy 2021, 30, 101093. [Google Scholar] [CrossRef]
- Debnath, S.; Masanta, M.; Bhattacharyya, B. Wire Electrochemical Machining employing newly developed tungsten micro wire with repeatedly similar cross sectional variations. J. Manuf. Process. 2022, 74, 535–543. [Google Scholar] [CrossRef]
- Burger, R.E.; Estavillo, J.; Osborne, J.; Stoll, P.J.; Wallace, W. Low-Impedance Tungsten Microelectrode for Recording from Sensory Ganglia. IEEE Trans. Biomed. Eng. 1973, BME-20, 378–380. [Google Scholar] [CrossRef]
- Williams, J.C.; Rennaker, R.L.; Kipke, D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 1999, 4, 303–313. [Google Scholar] [CrossRef]
- Patrick, E.; Orazem, M.E.; Sanchez, J.C.; Nishida, T. Corrosion of tungsten microelectrodes used in neural recording applications. J. Neurosci. Methods 2011, 198, 158–171. [Google Scholar] [CrossRef]
- Prasad, A.; Xue, Q.S.; Sankar, V.; Nishida, T.; Shaw, G.; Streit, W.J.; Sanchez, J.C. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural. Eng. 2012, 9, 056015. [Google Scholar] [CrossRef]
- Anderson, J.M.; Defife, K.; Mcnally, A.; Collier, T.; Jenney, C. Monocyte, macrophage and foreign body giant cell interactions with molecularly engineered surfaces. J. Mater. Sci. Mater. Med. 1999, 10, 579–588. [Google Scholar] [CrossRef]
- Luttikhuizen, D.T.; Harmsen, M.C.; Luyn, M.J.V. Cellular and Molecular Dynamics in the Foreign Body Reaction. Tissue Eng. 2006, 12, 1955–1970. [Google Scholar] [CrossRef]
- Sunderland, S. The intraneural topography of the radial, median and ulnar nerves. Brain 1945, 68, 243–299. [Google Scholar] [CrossRef]
- Sunderland, S. The connective tissues of peripheral nerves. Brain 1965, 88, 841–854. [Google Scholar] [CrossRef]
- Lundborg, G.; Hansson, H.A. Regeneration of peripheral nerve through a preformed tissue space. Preliminary observations on the reorganization of regenerating nerve fibres and perineurium. Brain Res. 1979, 178, 573–576. [Google Scholar] [CrossRef]
- Lundborg, G. Intraneural microcirculation. Orthop. Clin. N. Am. 1988, 19, 1–12. [Google Scholar]
- Zochodne, D.W.; Huang, Z.X.; Ward, K.K.; Low, P.A. Guanethidine-induced adrenergic sympathectomy augments endoneurial perfusion and lowers endoneurial microvascular resistance. Brain Res. 1990, 519, 112–117. [Google Scholar] [CrossRef]
- Stolinski, C. Structure and composition of the outer connective tissue sheaths of peripheral nerve. J. Anat. 1995, 186 Pt 1, 123–130. [Google Scholar] [PubMed]
- Millesi, H.; Zoch, G.; Reihsner, R. Mechanical properties of peripheral nerves. Clin. Orthop. Relat. Res. 1995, 314, 76–83. [Google Scholar]
- Topp, K.S.; Boyd, B.S. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006, 86, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Grill, W.M.; Norman, S.E.; Bellamkonda, R.V. Implanted Neural Interfaces: Biochallenges and Engineered Solutions. Annu. Rev. Biomed. Eng. 2009, 11, 1–24. [Google Scholar] [CrossRef]
- Carpaneto, J.; Cutrone, A.; Bossi, S.; Sergi, P.; Citi, L.; Rigosa, J.; Rossini, P.M.; Micera, S. Activities on PNS neural interfaces for the control of hand prostheses. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 4637–4640. [Google Scholar]
- Cutrone, A.; Sergi, P.N.; Bossi, S.; Micera, S. Modelization of a self-opening peripheral neural interface: A feasibility study. Med Eng. Phys. 2011, 33, 1254–1261. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Innate and Adaptive Immune Responses in Tissue Engineering. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural. Eng. 2009, 6, 056003. [Google Scholar] [CrossRef]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef]
- Sankar, V.; Patrick, E.; Dieme, R.; Sanchez, J.C.; Prasad, A.; Nishida, T. Electrode impedance analysis of chronic tungsten microwire neural implants: Understanding abiotic vs. biotic contributions. Front. Neuroeng. 2014, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, Foreign Body Giant Cells and Their Response to Implantable Biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef]
- Gori, M.; Vadala, G.; Giannitelli, S.M.; Denaro, V.; Di Pino, G. Biomedical and Tissue Engineering Strategies to Control Foreign Body Reaction to Invasive Neural Electrodes. Front. Bioeng. Biotechnol. 2021, 9, 411. [Google Scholar] [CrossRef]
- Lacour, S.P.; Courtine, G.; Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 2016, 1, 16063. [Google Scholar] [CrossRef]
- Tringides, C.M.; Vachicouras, N.; de Lazaro, I.; Wang, H.; Trouillet, A.; Seo, B.R.; Elosegui-Artola, A.; Fallegger, F.; Shin, Y.; Casiraghi, C.; et al. Viscoelastic surface electrode arrays to interface with viscoelastic tissues. Nat. Nanotechnol. 2021, 16, 1019–1029. [Google Scholar] [CrossRef]
- Nath, S.; Chen, Z.; Yue, N.; Trumpore, S.; Peschel, R. Dosimetric effects of needle divergence in prostate seed implant using and radioactive seeds. Med. Phys. 2000, 27, 1058–1066. [Google Scholar] [CrossRef]
- Youk, J.H.; Kim, E.K.; Kim, M.J.; Lee, J.Y.; Oh, K.K. Missed Breast Cancers at US-guided Core Needle Biopsy: How to Reduce Them. RadioGraphics 2007, 27, 79–94. [Google Scholar] [CrossRef]
- Gupta, S.; Madoff, D.C. Image-Guided Percutaneous Needle Biopsy in Cancer Diagnosis and Staging. Interventional Oncology—Part 2: Non-Hepatic Interventions and Biopsy Techniques. Tech. Vasc. Interv. Radiol. 2007, 10, 88–101. [Google Scholar] [CrossRef]
- Abolhassani, N.; Patel, R.; Moallem, M. Needle insertion into soft tissue: A survey. Med. Eng. Phys. 2007, 29, 413–431. [Google Scholar] [CrossRef]
- Gandevia, S.; Hales, J. The methodology and scope of human microneurography. J. Neurosci. Methods 1997, 74, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Vallbo, R.B.; Hagbarth, K.E.; Wallin, B.G. Microneurography: How the technique developed and its role in the investigation of the sympathetic nervous system. J. Appl. Physiol. 2004, 96, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, M.L.; Green, J.B.; Kaufman, J.; Blackwelder, P.L.; Freytag, D.L.; Frank, K.; Alfertshofer, M.; Cotofana, S. Deformation of Needle Tips During Facial Soft Tissue Filler Injections: An Electron-Microscopic Study. Aesthetic Surg. J. 2021, 41, NP2011–NP2019. [Google Scholar] [CrossRef] [PubMed]
- Skapetis, T.; Doan-Tran, P.D.; Hossain, N.M. Evaluation of bevelled needle tip deformation with Dental Inferior Alveolar Nerve blocks. Aust. Endod. J. 2019, 45, 325–330. [Google Scholar] [CrossRef]
- Amely, H.; Tina, L.; Luhrenberg, P.; Monika, D. Needle tip deformation in local dental anesthesia: A technical note. J. Mech. Behav. Biomed. Mater. 2022, 126, 105034. [Google Scholar] [CrossRef]
- Sergi, P.N.; Carrozza, M.C.; Dario, P.; Micera, S. Biomechanical characterization of needle piercing into peripheral nervous tissue. IEEE Trans. Biomed. Eng. 2006, 53, 2373–2386. [Google Scholar] [CrossRef]
- Yoshida, K.; Lewinsky, I.; Nielsen, M.; Hylleberg, M. Implantation mechanics of tungsten microneedles into peripheral nerve trunks. Med. Biol. Eng. Comput. 2007, 45, 413–420. [Google Scholar] [CrossRef]
- Sergi, P.N.; Jensen, W.; Micera, S.; Yoshida, K. In vivo interactions between tungsten microneedles and peripheral nerves. Med. Eng. Phys. 2012, 34, 747–755. [Google Scholar] [CrossRef]
- Sergi, P.N.; Jensen, W.; Yoshida, K. Interactions among biotic and abiotic factors affect the reliability of tungsten microneedles puncturing in vitro and in vivo peripheral nerves: A hybrid computational approach. Mater. Sci. Eng. C 2016, 59, 1089–1099. [Google Scholar] [CrossRef]
- Bora, F.W.; Richardson, S.; Black, J. The biomechanical responses to tension in a peripheral nerve. J. Hand Surg. 1980, 5, 21–25. [Google Scholar]
- Layton, B.E.; Sastry, A.M. A Mechanical Model for Collagen Fibril Load Sharing in Peripheral Nerve of Diabetic and Nondiabetic Rats. J. Biomech. Eng. 2005, 126, 803–814. [Google Scholar] [CrossRef]
- Layton, B.E.; Sastry, A.M. Equal and local-load-sharing micromechanical models for collagens: Quantitative comparisons in response of non-diabetic and diabetic rat tissue. Acta Biomater. 2006, 2, 595–607. [Google Scholar] [CrossRef]
- Main, E.K.; Goetz, J.E.; Rudert, M.J.; Goreham-Voss, C.M.; Brown, T.D. Apparent transverse compressive material properties of the digital flexor tendons and the median nerve in the carpal tunnel. J. Biomech. 2011, 44, 863–868. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Hu, S.; Tan, J.S.; Myer, C.; Njus, N.M.; Xia, Z. In vitro and in vivo mechanical properties of human ulnar and median nerves. J. Biomed. Mater. Res. A 2013, 101, 2718–2725. [Google Scholar] [CrossRef]
- Giannessi, E.; Stornelli, M.R.; Sergi, P.N. A unified approach to model peripheral nerves across different animal species. PeerJ 2017, 5, e4005. [Google Scholar] [CrossRef]
- Giannessi, E.; Stornelli, M.R.; Sergi, P.N. Fast in silico assessment of physical stress for peripheral nerves. Med. Biol. Eng. Comput. 2018, 56, 1541–1551. [Google Scholar] [CrossRef]
- Giannessi, E.; Stornelli, M.R.; Coli, A.; Sergi, P.N. A Quantitative Investigation on the Peripheral Nerve Response within the Small Strain Range. Appl. Sci. 2019, 9, 1115. [Google Scholar] [CrossRef]
- Giannessi, E.; Stornelli, M.R.; Sergi, P.N. Strain stiffening of peripheral nerves subjected to longitudinal extensions in vitro. Med. Eng. Phys. 2020, 76, 47–55. [Google Scholar] [CrossRef]
- Sergi, P.N. Deterministic and Explicit: A Quantitative Characterization of the Matrix and Collagen Influence on the Stiffening of Peripheral Nerves Under Stretch. Appl. Sci. 2020, 10, 6372. [Google Scholar] [CrossRef]
- Sneddon, I.N. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int. J. Eng. Sci. 1965, 3, 47–57. [Google Scholar] [CrossRef]
- Anik, M. Anodic Behavior of Tungsten in H3PO4-K2SO4-H2SO4/KOH Solutions. Turk. J. Chem. 2002, 26, 915–924. [Google Scholar]
- Okamura, A.M.; Simone, C.; O’Leary, M.D. Force modeling for needle insertion into soft tissue. IEEE Trans. Biomed. Eng. 2004, 51, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Malamou, A. Hertz model or Oliver and Pharr analysis? Tutorial regarding AFM nanoindentation experiments on biological samples. Mater. Res. Express 2020, 7, 033001. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Koppaka, S.; Hess-Dunning, A.; Tyler, D.J. Biomechanical characterization of isolated epineurial and perineurial membranes of rabbit sciatic nerve. J. Biomech. 2022, 136, 111058. [Google Scholar] [CrossRef]
- Jiang, X.J.; Gao, F.; Martin, H.; Williamson, J.; Li, D. Chapter 10—On-Machine Metrology for Hybrid Machining. In Hybrid Machining; Luo, X., Qin, Y., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 239–268. [Google Scholar] [CrossRef]
- Ciofani, G.; Sergi, P.N.; Carpaneto, J.; Micera, S. A hybrid approach for the control of axonal outgrowth: Preliminary simulation results. Med. Biol. Eng. Comput. 2011, 49, 163–170. [Google Scholar] [CrossRef]
- Sergi, P.N.; Cavalcanti-Adam, E.A. Biomaterials and computation: A strategic alliance to investigate emergent responses of neural cells. Biomater. Sci. 2017, 5, 648–657. [Google Scholar] [CrossRef]
- Sergi, P.N.; Morana Roccasalvo, I.; Tonazzini, I.; Cecchini, M.; Micera, S. Cell Guidance on Nanogratings: A Computational Model of the Interplay between PC12 Growth Cones and Nanostructures. PLoS ONE 2013, 8, e70304. [Google Scholar] [CrossRef]
- Sergi, P.N.; Marino, A.; Ciofani, G. Deterministic control of mean alignment and elongation of neuron-like cells by grating geometry: A computational approach. Integr. Biol. 2015, 7, 1242–1252. [Google Scholar] [CrossRef]
- Roccasalvo, I.M.; Micera, S.; Sergi, P.N. A hybrid computational model to predict chemotactic guidance of growth cones. Sci. Rep. 2015, 5, 11340. [Google Scholar] [CrossRef]
- Navarro, X.; Krueger, T.B.; Lago, N.; Micera, S.; Stieglitz, T.; Dario, P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 2005, 10, 229–258. [Google Scholar] [CrossRef]
- Sergi, P.N.; Valle, J.D.; Oliva, N.D.L.; Micera, S.; Navarro, X. A data-driven polynomial approach to reproduce the scar tissue outgrowth around neural implants. J. Mater. Sci. Mater. Med. 2020, 31, 59. [Google Scholar] [CrossRef]
- Sergi, P.N.; De la Oliva, N.; del Valle, J.; Navarro, X.; Micera, S. Physically Consistent Scar Tissue Dynamics from Scattered Set of Data: A Novel Computational Approach to Avoid the Onset of the Runge Phenomenon. Appl. Sci. 2021, 11, 8568. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Nikita, K.S.; Malamou, A.; Stylianopoulos, T. A simplified approach for the determination of fitting constants in Oliver-Pharr method regarding biological samples. Phys. Biol. 2019, 16, 056003. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, P.N.; Jensen, W.; Yoshida, K. Geometric Characterization of Local Changes in Tungsten Microneedle Tips after In-Vivo Insertion into Peripheral Nerves. Appl. Sci. 2022, 12, 8938. https://doi.org/10.3390/app12188938
Sergi PN, Jensen W, Yoshida K. Geometric Characterization of Local Changes in Tungsten Microneedle Tips after In-Vivo Insertion into Peripheral Nerves. Applied Sciences. 2022; 12(18):8938. https://doi.org/10.3390/app12188938
Chicago/Turabian StyleSergi, Pier Nicola, Winnie Jensen, and Ken Yoshida. 2022. "Geometric Characterization of Local Changes in Tungsten Microneedle Tips after In-Vivo Insertion into Peripheral Nerves" Applied Sciences 12, no. 18: 8938. https://doi.org/10.3390/app12188938
APA StyleSergi, P. N., Jensen, W., & Yoshida, K. (2022). Geometric Characterization of Local Changes in Tungsten Microneedle Tips after In-Vivo Insertion into Peripheral Nerves. Applied Sciences, 12(18), 8938. https://doi.org/10.3390/app12188938







