Effect of Ozone Treatment on the Contents of Selected Bioactive Phytochemicals in Leaves of Alligator Plant Kalanchoe daigremontiana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Determination of Antioxidant Activity
2.3. Total Phenolic Content Assay
2.4. Total Ascorbic Acid Assay
2.5. ROS
2.6. SOD and CAT
2.7. Mechanical Properties
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity and Total Phenolic Content
3.2. Ascorbic Acid Content
3.3. Reactive Oxygen Species Accumulation
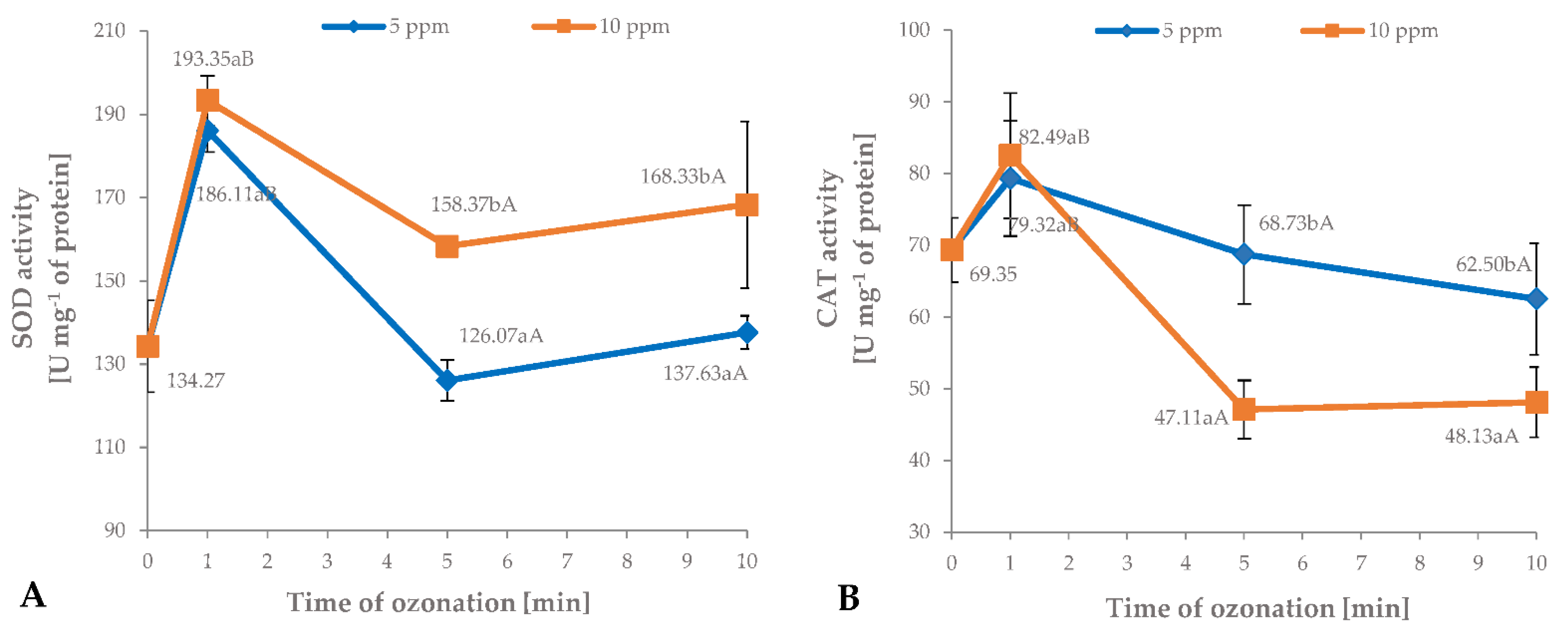
3.4. Antioxidant Enzyme Activity
3.5. Mechanical Properties
4. Conclusions
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Abdellaoui, S.; Destandau, E.; Toribio, A.; Elfakir, C.; Lafosse, M.; Renimel, I.; André, P.; Cancellieri, P.; Landemarre, L. Bioactive molecules in Kalanchoe pinnata leaves: Extraction, purification, and identification. Anal. Bioanal. Chem. 2010, 398, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Biswas, S.K.; Das, J.; Karmakar, U.K.; Shill, M.C.; Dutta, N. Investigation of cytotoxicity and antifungal activities of petroleum ether and aqueous extracts of leaves and stems of Kalanchoe pinnata L. (Crassulaceae). Asian. J. Plant Sci. 2011, 10, 274–277. [Google Scholar]
- Pattewar, S.V. Kalanchoe pinnata: Phytochemical and pharmacological profle. Int. J. Phytoph. 2012, 2, 1–8. [Google Scholar]
- Rajsekhar, P.B.; Bharani, A.R.S.; Ramachandran, M. The “wonder plant” Kalanchoe pinnata (Linn.) Pers.: A review. J. Appl. Pharm. Sci. 2016, 6, 151–158. [Google Scholar]
- Eggli, U. Illustrated Handbook of Succulent Plants; Crassulaceae; Springer: Berlin/Heidelberg, Germany, 2003; pp. 143–153. [Google Scholar]
- Moniuszko-Szajwaj, B.; Pecio, Ł.; Kowalczyk, M.; Stochmal, A. New bufadienolides isolated from the roots of Kalanchoe daigremontiana (Crassulaceae). Molecules 2016, 21, 1–13. [Google Scholar]
- Bogucka-Kocka, A.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoe species. Sau. J. Biol. Sci. 2018, 25, 622–630. [Google Scholar]
- Parkoła, M.; Matławska, I. Znaczenie gatunków z rodzaju Kalanchoe. Herba Pol. 2007, 53, 106–107. [Google Scholar]
- Ürményi, F.G.; Saraiva, G.D.; Casanova, L.M.; Matos, A.D.S.; Camargo, L.M.d.; Romanos, M.T.V.; Costa, S.S. Anti-HSV-1 and HSV-2 flavonoids and a new kaempferol triglycoside from the medicinal plant Kalanchoe daigremontiana. Chem. Biodivers. 2016, 13, 1707–1714. [Google Scholar]
- Hermawan, W.; Maharani, R.; Fajriah, S.; Hardiawan, R.; Supratman, U. Insecticidal bufadienolides from the leaves of Kalanchoe daigremontiana (Crassulaceae). J. Ilmu Dasar 2010, 11, 115–119. [Google Scholar]
- Studzińska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-inflammatory Activity and Phytochemical Profile of Galinsoga Parviflora Cav. Molecules 2018, 23, 2133. [Google Scholar] [CrossRef]
- Chibli, L.A.; Rodrigues, K.C.; Gasparetto, C.M.; Pinto, N.C.; Fabri, R.L.; Scio, E.; Alves, M.S.; Del-Vechio-Vieira, G.; Sousa, O.V. Anti-inflammatory effects of Bryophyllum pinnatum (Lam.) Oken ethanol extract in acute and chronic cutaneous inflammation. J. Ethnopharmacol. 2014, 11, 330–338. [Google Scholar] [CrossRef]
- Singab, A.N.B.; El-Ahmady, S.H.; Labib, R.M.; Fekry, S.S. Phenolics from Kalanchoe marmorata Baker, Family Crassulaceae. Bul. Fac. Pharm. Cairo Univ. 2011, 49, 1–5. [Google Scholar]
- Cruz, E.; Reuter, S.; Martin, H.; Dehzad, N.; Muzitano, M.; Costa, S.; Rossi-Bergmann, B.; Buhl, R.; Stassen, M.; Taube, C. Kalanchoe pinnata inhibits mast cell activation and prevents allergic airway disease. Phytomed 2012, 19, 115–121. [Google Scholar]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of ozonation process on the microbiological and antioxidant status of raspberry (Rubus ideaeus L.) fruit during storage at room temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitor effects on reactive oxygen species in liquid cultures of Penicillium chrysogenum. Biotechnol. Lett. 2004, 18, 282–289. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Rahman, A.; Inafuku, M.; Oku, H.; Fujita, M. Coordinated Actions of Glyoxalase and Antioxidant defense systems in conferring abiotic stress tolerance in plants. Int. J. Mol. Sci. 2017, 18, 200–228. [Google Scholar]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar]
- Dąbrowska, G.; Aleksandra, K.; Goc, A.; Szechyńska-Hebda, M.; Skrzypek, E. Characteristics od the plant ascorbate peroxidase family. Acta. Biol. Cracov. Bot. Ser. Bot. 2007, 49, 7–17. [Google Scholar]
- Zapałowska, A.; Matłok, N.; Zardzewiały, M.; Piechowiak, T.; Balawejder, M. Effect of Ozone Treatment on the Quality of Sea Buckthorn (Hippophae rhamnoides L.). Plants 2021, 10, 847. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of Organic and Mineral Fertilization on Yield and Selected Quality Parameters for Dried Herbs of Two Varieties of Oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Piechowiak, T.; Balawejder, M. Impact of Ozonation Process on the Level of Selected Oxidative Stress Markers in Raspberries Stored at Room Temperature. Food Chem. 2019, 298, 125093. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of Antioxidant Compounds from Blueberry Fruit Waste and Evaluation of Their In Vitro Biological Activity in Human Keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method for Protein Quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar]
- Gorzelany, J.; Migut, D.; Matlok, N.; Antos, P.; Kuzniar, P.; Balawejder, M. Impact of Pre-Ozonation on Mechanical Properties of Selected Genotypes of Cucumber Fruits During the Souring Process. Ozone Sci. Eng. 2016, 39, 188–195. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencingvitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Onopiuk, A.; Półtorak, A.; Moczkowska, M.; Szpicer, A.; Wierzbicka, A. The impact of ozone on health-promoting, microbi-ological, and colour properties of Rubus ideaus raspberries. J. Food 2017, 15, 563–573. [Google Scholar]
- Huang, S.; Aken, O.; Schwarzlander, M.; Belt, K.; Millar, A. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- van Breusegem, F.; Bailey-serres, J.; Mittler, R. Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol. 2008, 147, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Uehara, N.; Sasaki, H.; Kobayashi, K.; Yamakawa, T. Impacts of acute ozone stress on superoxide dismutase (SOD) expression and reactive oxygen species (ROS) formation in rice leaves. Plant Physiol. Biochem. 2013, 70, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Bhumika, Y.; Chandrakar, V.; Keshavkant, S. Responses of plants to fluoride: An overview of oxidative stress and defense mechanisms. Fluoride 2016, 49, 293. [Google Scholar]
- Sharma, Y.K.; Davis, K.R. The effects of ozone on antioxidant responses in plants. Free Radic. Biol. Med. 1997, 23, 480–488. [Google Scholar] [CrossRef]
- Rybus-Zając, M.; Kubiś, J. Effect of Uv-B Radiation On Antioxidative Enzyme Activity In Cucumber Cotyledons. Acta. Biol. Crac. Ser. Bot. 2010, 52, 97–102. [Google Scholar] [CrossRef]
- Piechowiak, T.; Sowa, P.; Balawejder, M. Effect of Ozonation Process on the Energy Metabolism in Raspberry Fruit During Storage at Room Temperature. Food Bio. Technol. 2021, 14, 483–491. [Google Scholar] [CrossRef]
- Pellinen, R.; Palva, T.; Kangasjärvi, J. Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 1999, 20, 349–356. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 1990, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Camp, W.; Willekens, H.; Bowler, C.; van Montagu, M.; Inzé, D.; Reupold-Popp, P.; Sandermann, H., Jr.; Langebartels, C. Elevated Levels of Superoxide Dismutase Protect Transgenic Plants Against Ozone Damage. Nat. Biotechnol. 1994, 12, 165–168. [Google Scholar] [CrossRef]
- Morita, S.; Nakatani, S.; Koshiba, T.; Masumura, T.; Ogihara, Y.; Tanaka, K. Differential expression of two cytosolic ascorbate peroxidases and two superoxide dismutase genes in response to abiotic stress in rice. Rice Sci. 2011, 18, 157–166. [Google Scholar] [CrossRef]
- Samuel, M.A.; Miles, G.P.; Ellis, B.E. Ozone treatment rapidly activates MAP kinase signalling in plants. Plant J. 2001, 22, 367–376. [Google Scholar] [CrossRef]
- Stephenson, K.; Harwood, C.R. Influence of a cell-wall-associated protease on production of alpha-amylase by Bacillus subtilis. Appl. Env. Microbiol. 1998, 64, 2875–2881. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gallegos, J.F.; Jurado-Alameda, E.; Carrasquilla-Carmona, J.L.; Jiménez-Pérez, J.; Romero-Pareja, P.M. Characterization of the ozone effect over an α-amylase from Bacillus licheniformis. Biochem. Eng. J. 2014, 85, 119–124. [Google Scholar] [CrossRef]
- Piechowiak, T.; Józefczyk, R.; Balawejder, M. Impact of ozonation process of wheat flour on the activity of selected enzymes. J. Cereal Sci. 2018, 84, 30–37. [Google Scholar] [CrossRef]
- Zardzewiały, M.; Matlok, N.; Piechowiak, T.; Gorzelany, J.; Balawejder, M. Ozone Treatment as a Process of Quality Improvement Method of Rhubarb (Rheum rhaponticum L.) Petioles during Storage. Appl. Sci. 2020, 10, 8282. [Google Scholar] [CrossRef]
- Antos, P.; Piechowicz, B.; Gorzelany, J.; Matłok, N.; Migut, D.; Józefczyk, R.; Balawejder, M. Effect of Ozone on Fruit Quality and Fungicide Residue Degradation in Apples during Cold Storage. Ozone Sci. Eng. 2018, 40, 1–5. [Google Scholar] [CrossRef]
- Available online: http://herbapolonica.pl/magazines-files/336747-Conference%20Proceedings.pdf#page=78 (accessed on 2 August 2022).
- Prabha, V.; Deb Barma, R.; Singh, R.; Madan, A. Ozone Technology in Food Processing: A Review. Trends Biosci. 2015, 8, 4031–4047. [Google Scholar]




| Ozone Dose | Fmax (N) | dL at Fmax (mm) | Emod (MPa) | W to Fmax (Nmm) |
|---|---|---|---|---|
| 0 ppm 0 min | 3.06 ± 0.28 a | 0.76 ± 0.16 a | 0.0009 ± 0.0002 a | 1.13 ± 0.27 a |
| 5 ppm 1 min | 2.99 ± 0.36 a | 0.74 ± 0.32 a | 0.0010 ± 0.0003 a | 1.02 ± 0.48 a |
| 5 ppm 5 min | 2.91 ± 0.27 a | 0.68 ± 0.20 a | 0.0010 ± 0.0003 a | 0.95 ± 0.24 a |
| 5 ppm 10 min | 2.94 ± 0.33 a | 0.69 ± 0.16 a | 0.0010 ± 0.0002 a | 1.01 ± 0.23 a |
| 10 ppm 1 min | 2.83 ± 0.44 a | 0.62 ± 0.12 a | 0.0011 ± 0.0002 a | 0.87 ± 0.21 a |
| 10 ppm 5 min | 3.04 ± 0.46 a | 0.78 ± 0.19 a | 0.0009 ± 0.0002 a | 1.09 ± 0.25 a |
| 10 ppm 10 min | 3.04 ± 0.32 a | 0.75 ± 0.27 a | 0.0011 ± 0.0003 a | 1.03 ± 0.25 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Balawejder, M. Effect of Ozone Treatment on the Contents of Selected Bioactive Phytochemicals in Leaves of Alligator Plant Kalanchoe daigremontiana. Appl. Sci. 2022, 12, 8934. https://doi.org/10.3390/app12188934
Matłok N, Piechowiak T, Zardzewiały M, Balawejder M. Effect of Ozone Treatment on the Contents of Selected Bioactive Phytochemicals in Leaves of Alligator Plant Kalanchoe daigremontiana. Applied Sciences. 2022; 12(18):8934. https://doi.org/10.3390/app12188934
Chicago/Turabian StyleMatłok, Natalia, Tomasz Piechowiak, Miłosz Zardzewiały, and Maciej Balawejder. 2022. "Effect of Ozone Treatment on the Contents of Selected Bioactive Phytochemicals in Leaves of Alligator Plant Kalanchoe daigremontiana" Applied Sciences 12, no. 18: 8934. https://doi.org/10.3390/app12188934
APA StyleMatłok, N., Piechowiak, T., Zardzewiały, M., & Balawejder, M. (2022). Effect of Ozone Treatment on the Contents of Selected Bioactive Phytochemicals in Leaves of Alligator Plant Kalanchoe daigremontiana. Applied Sciences, 12(18), 8934. https://doi.org/10.3390/app12188934








