Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles
Abstract
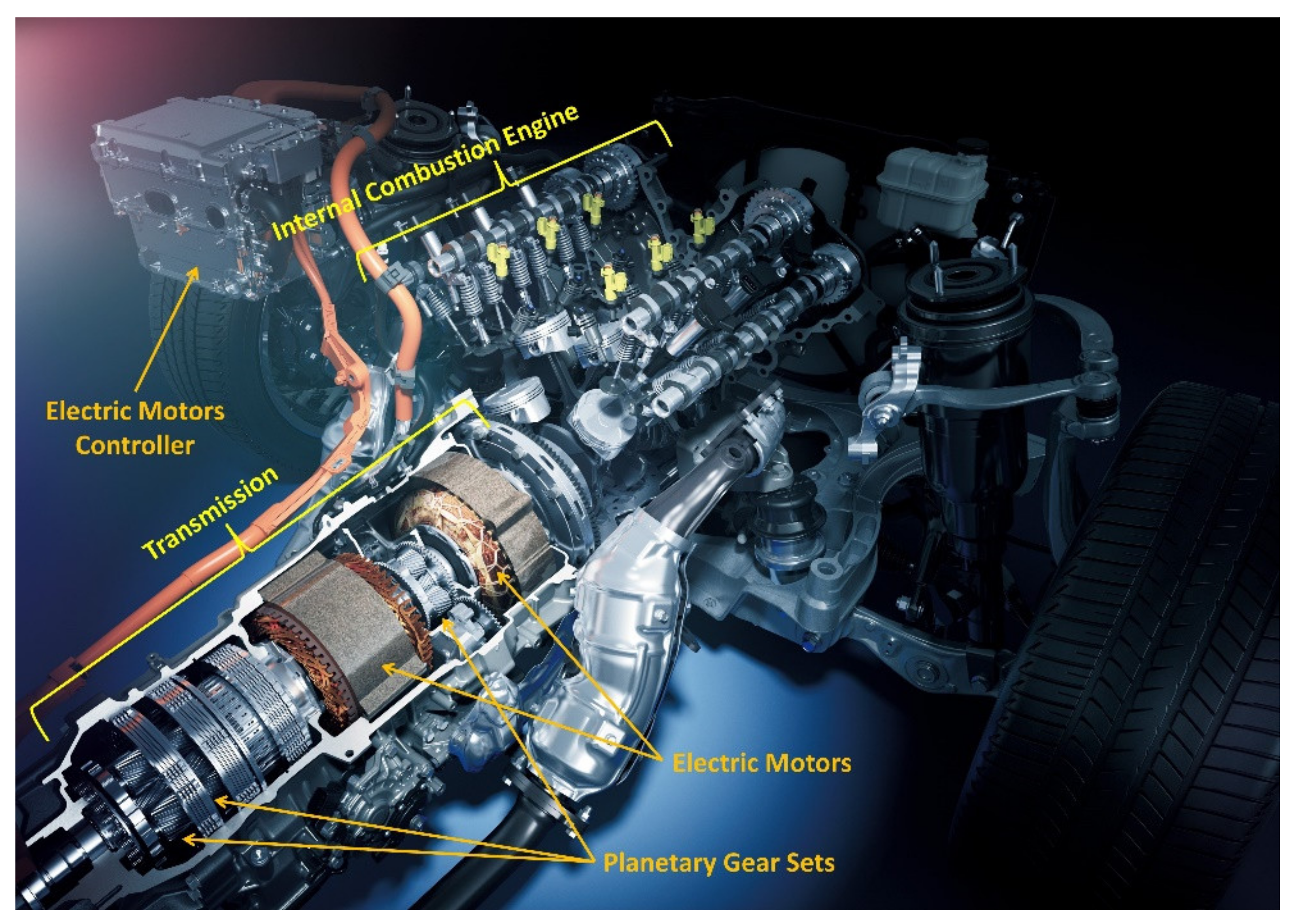
:1. Introduction
2. Materials and Methods
2.1. Automatic Transmission Fluids (ATFs)
2.2. Ageing Process
2.3. Thermophysical Properties Measurements
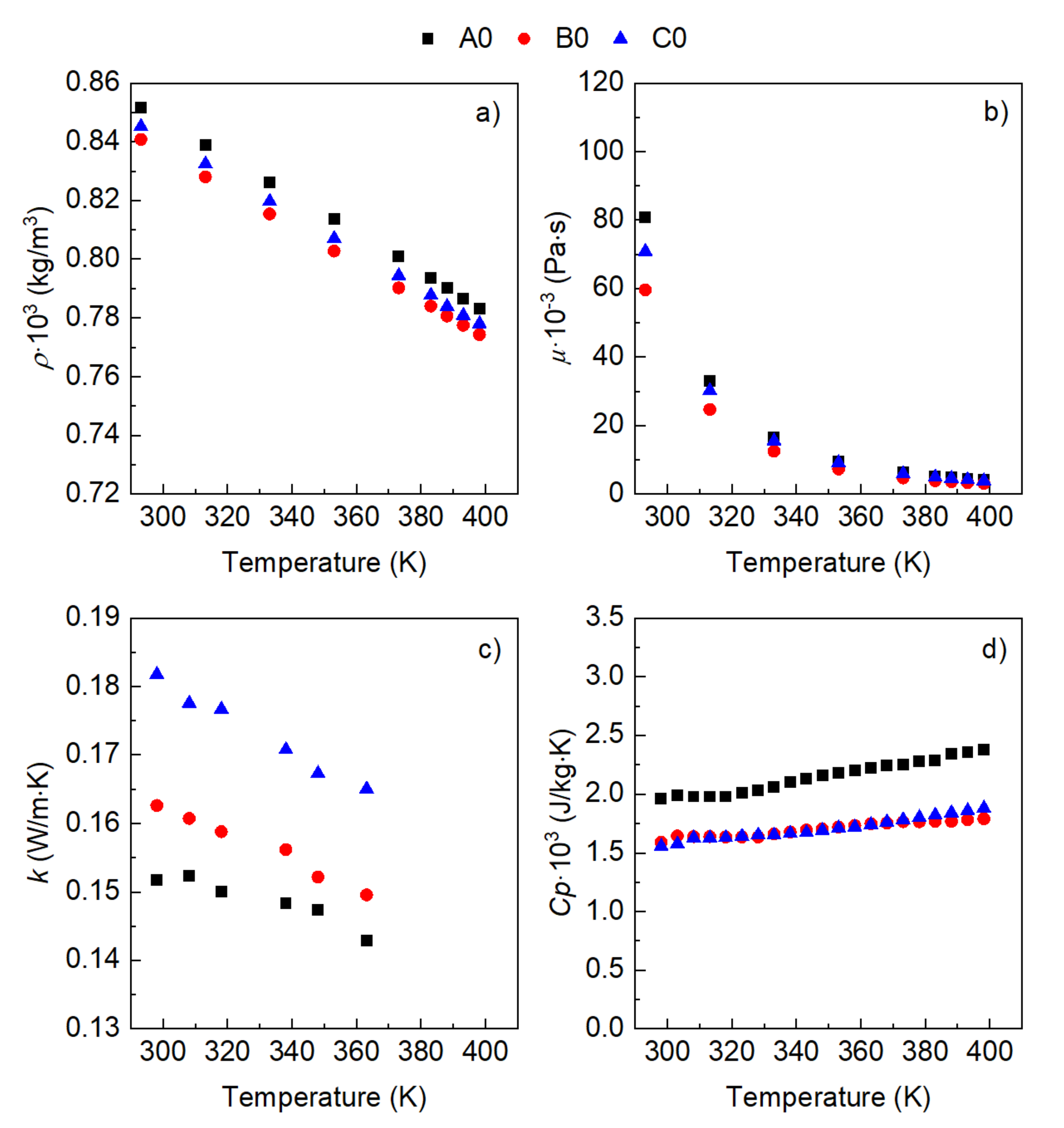
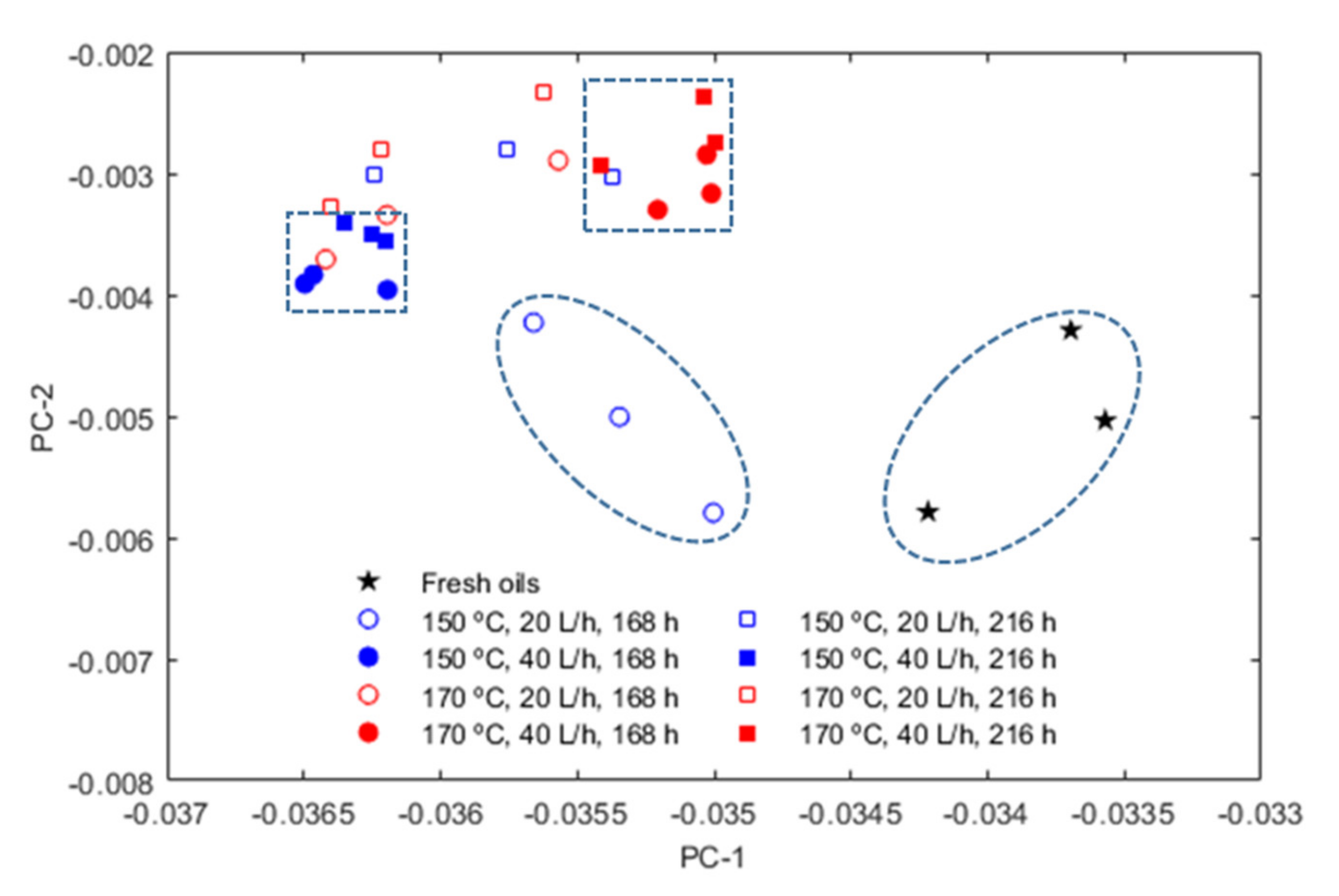
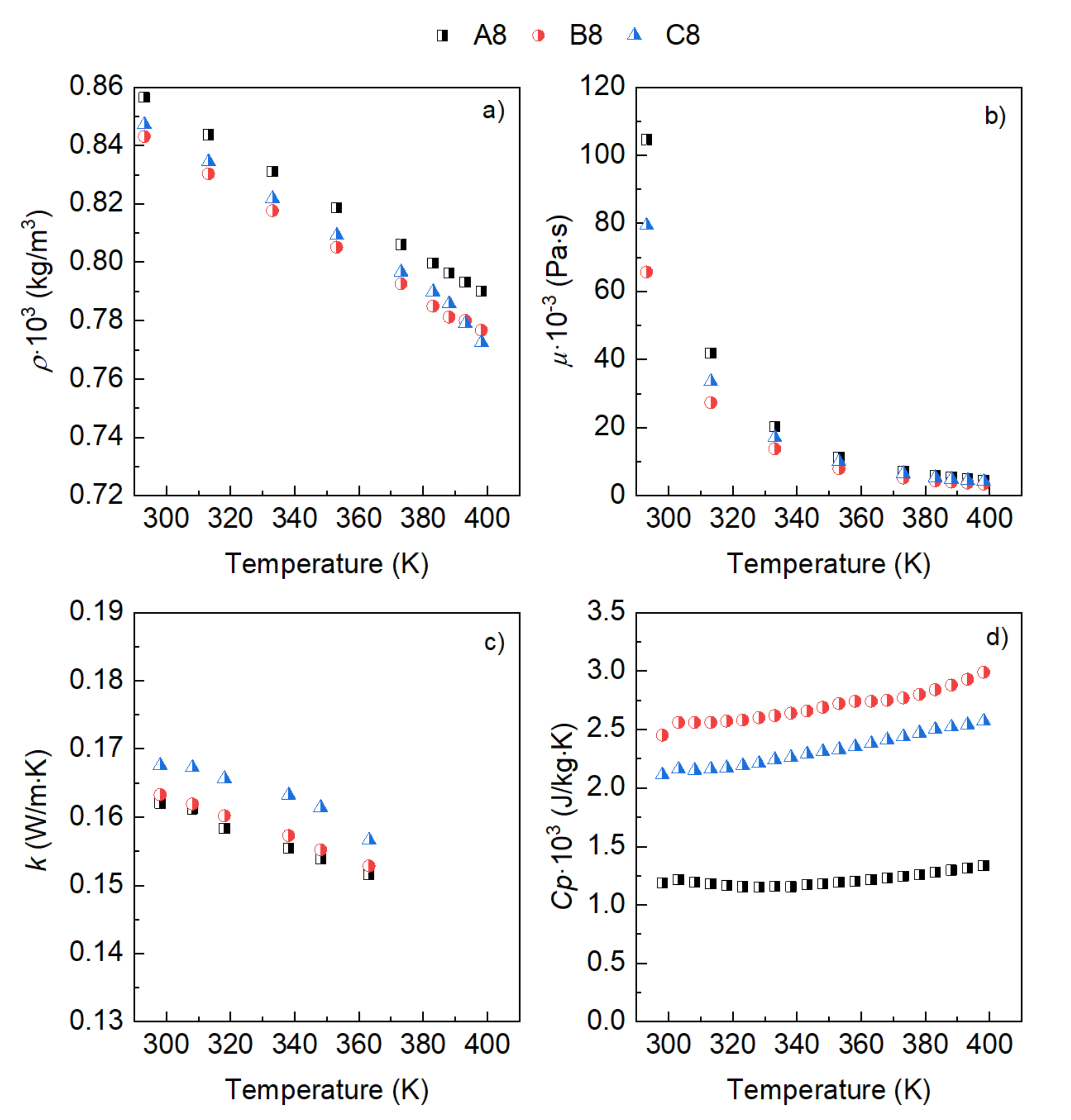
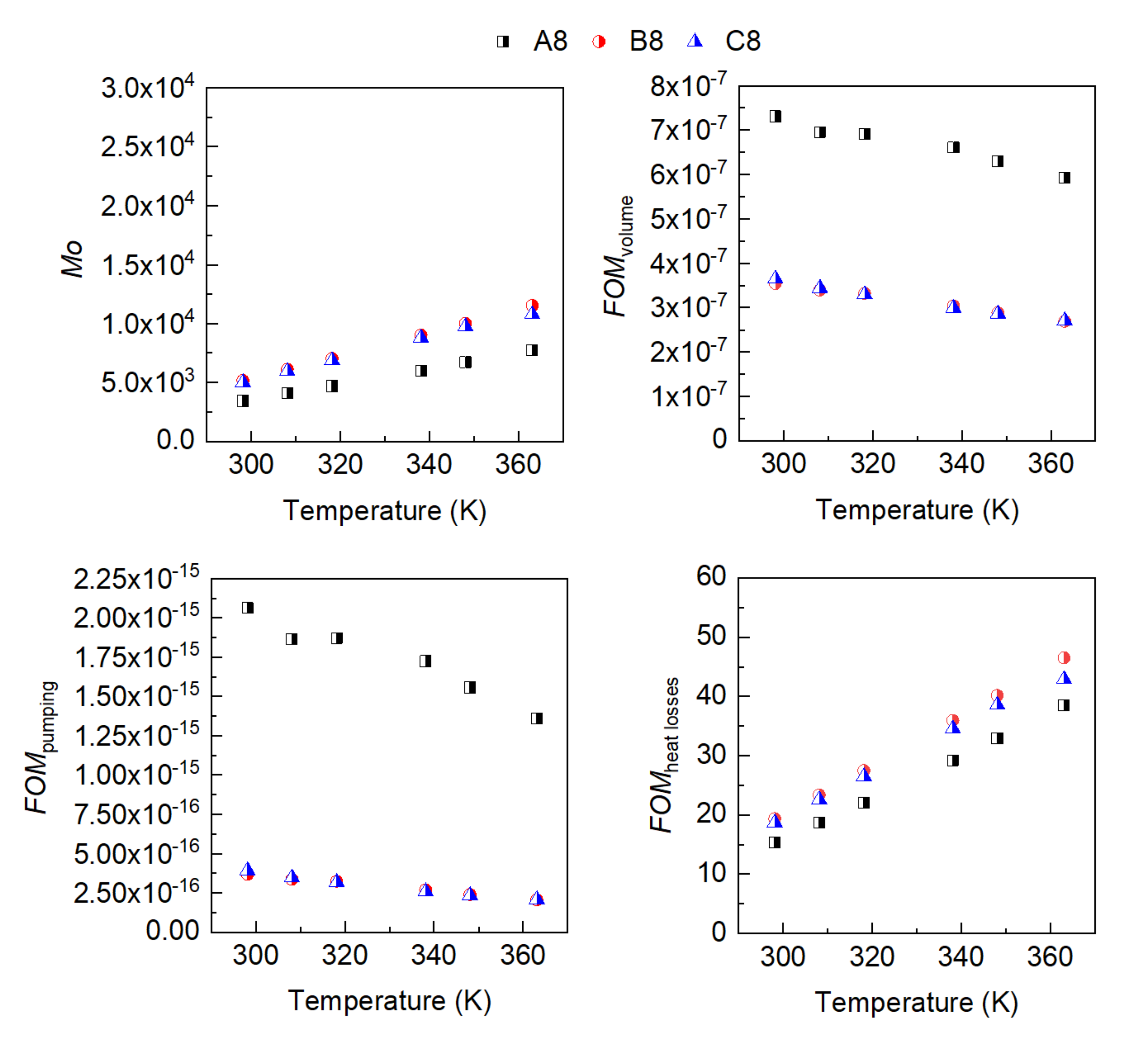
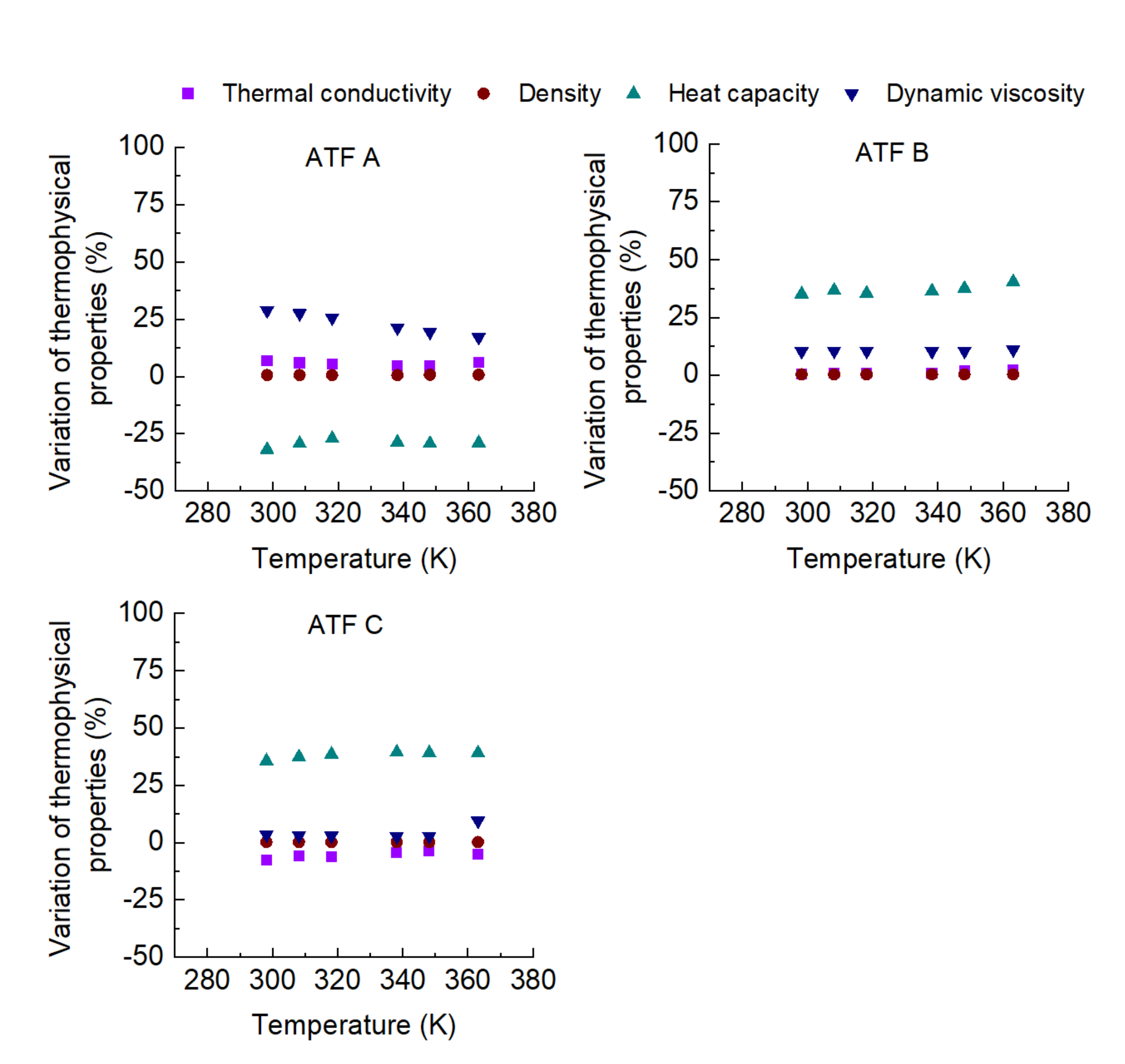
3. Results
4. Conclusions
- The influence of the molecular structure on thermal conductivity and heat capacity is stronger than on density and viscosity, but the differences in these properties of the three conventional ATFs in fresh conditions did not differentiate their cooling performance as expressed through some figures-of-merit (FOMs).
- The oxidation of the ATFs changed thermal conductivity and heat capacity in a different manner depending on the API Group of the base oils. ATFs B and C, formulated with base oils from API Group III, demonstrated better cooling performance than ATF A, which was formulated with base oils from API Group I
- The sensitivity to temperature of the variation with oxidation of the studied properties, including the FOMs, was almost null, except for ATF A.
- The FOMs should be used to compare cooling performance of ATFs for electric drivetrains instead of a single property such as thermal conductivity.
- Testing of the cooling performance of ATFs at higher oxidation levels should be addressed in future research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Cp | heat capacity |
| FOM | figure of merit |
| k | thermal conductivity |
| Mo | Mouromtseff number |
| Greek symbols | |
| μ | dynamic viscosity |
| ρ | density |
References
- Beyer, M.; Brown, G.; Gahagan, M.; Higuchi, T.; Hunt, G.; Huston, M.; Jayne, D.; McFadden, C.; Newcomb, T.; Patterson, S.; et al. Lubricant Concepts for Electrified Vehicle Transmissions and Axles. Tribol. Online 2019, 14, 428–437. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I. Tribology of Electric Vehicles: A Review of Critical Components, Current State and Future Improvement Trends. Tribol. Int. 2019, 138, 473–486. [Google Scholar] [CrossRef]
- Berly, M. Next Generation Driveline Lubricants for Electrified Vehicles. Tribol. Lubr. Technol. 2021, 77, 38–40. [Google Scholar]
- Eckenfels, T.; Kaksa, A.; Marek, C. 48 V Hybridization. CTI Symp. 2018, 2020, 110–119. [Google Scholar] [CrossRef]
- Dilzer, M. P2 Hybridization—Tailored Solutions. CTI Symp. 2018, 2020, 80–86. [Google Scholar] [CrossRef]
- Toyota Multi-Stage Hybrid System. Available online: https://global.toyota/en/download/34288283/ (accessed on 18 August 2022).
- Ha, T.; Han, N.G.; Kim, M.S.; Rho, K.H.; Kim, D.K. Experimental Study on Behavior of Coolants, Particularly the Oil-Cooling Method, in Electric Vehicle Motors Using Hairpin Winding. Energies 2021, 14, 956. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Gerada, D.; Huang, K.; Stone, I.; Worrall, S.; Yan, Y. A Critical Review on Thermal Management Technologies for Motors in Electric Cars. Appl. Therm. Eng. 2022, 201, 117758. [Google Scholar] [CrossRef]
- Huang, Z.; Nategh, S.; Lassila, V.; Alaküla, M.; Yuan, J. Direct Oil Cooling of Traction Motors in Hybrid Drives. In Proceedings of the 2012 IEEE International Electric Vehicle Conference, IEVC, Greenville, CA, USA, 4–8 March 2012. [Google Scholar] [CrossRef]
- Huang, Z.; Marquez, F.; Alakula, M.; Yuan, J. Characterization and Application of Forced Cooling Channels for Traction Motors in HEVs. In Proceedings of the 20th International Conference on Electrical Machines, ICEM, Marseille, France, 2–5 September 2012; pp. 1212–1218. [Google Scholar] [CrossRef]
- Alexandrova, Y.; Semken, R.S.; Pyrhönen, J. Permanent Magnet Synchronous Generator Design Solution for Large Direct-Drive Wind Turbines: Thermal Behavior of the LC DD-PMSG. Appl. Therm. Eng. 2014, 65, 554–563. [Google Scholar] [CrossRef]
- Lim, D.H.; Lee, M.Y.; Lee, H.S.; Kim, S.C. Performance Evaluation of an in-Wheel Motor Cooling System in an Electric Vehicle/Hybrid Electric Vehicle. Energies 2014, 7, 961–971. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.S.; Sabharwall, P.; Anderson, N. Development of Figure of Merits (FOMs) for Intermediate Coolant Characterization and Selection. In Proceedings of the American Nuclear Society Annual Meeting, Hollywood, FL, USA, 25–30 June 2011. [Google Scholar]
- Yu, L.; Liu, D. Study of the Thermal Effectiveness of Laminar Forced Convection of Nanofluids for Liquid Cooling Applications. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 3, 1693–1704. [Google Scholar] [CrossRef]
- Mouromtseff, I.E. Water and Forced-Air Cooling of Vacuum Tubes: Nonelectronic Problems in Electronic Tubes. Proc. IRE 1942, 30, 190–205. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I.; Gallardo-Hernández, E.A.; Vite-Torres, M.; Godínez-Salcedo, J.G. Influence of Oxidation of Automatic Transmission Fluids (ATFs) and Sliding Distance on Friction Coefficients of a Wet Clutch in the Running-in Stage. Friction 2021, 9, 401–414. [Google Scholar] [CrossRef]
- Fatima, N.; Holmgren, A.; Marklund, P.; Minami, I.; Larsson, R. Degradation Mechanism of Automatic Transmission Fluid by Water as a Contaminant. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2015, 229, 74–85. [Google Scholar] [CrossRef]
- McFadden, C.; Hughes, K.; Raser, L.; Newcomb, T. Electrical Conductivity of New and Used Automatic Transmission Fluids. SAE Int. J. Fuels Lubr. 2016, 9, 519–526. [Google Scholar] [CrossRef]
- Kwak, Y.; Cleveland, C.; Adhvaryu, A.; Fang, X.; Hurley, S.; Adachi, T. Understanding Base Oils and Lubricants for Electric Drivetrain Applications. SAE Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Rodríguez, E.; Rivera, N.; Fernández-González, A.; Pérez, T.; González, R.; Battez, A.H. Electrical Compatibility of Transmission Fluids in Electric Vehicles. Tribol. Int. 2022, 171, 107544. [Google Scholar] [CrossRef]
- Prado, J.I.; Calviño, U.; Lugo, L. Experimental Methodology to Determine Thermal Conductivity of Nanofluids by Using a Commercial Transient Hot-Wire Device. Appl. Sci. 2021, 12, 329. [Google Scholar] [CrossRef]
- Narita, K.; Takekawa, D. Lubricants Technology Applied to Transmissions in Hybrid Electric Vehicles and Electric Vehicles. SAE Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Rihani, D.N.; Doraiswamy, L.K. Estimation of Heat Capacity of Organic Compounds from Group Contributions. Ind. Eng. Chem. Fundam. 1965, 4, 17–21. [Google Scholar] [CrossRef]
- Besser, C.; Agocs, A.; Ronai, B.; Ristic, A.; Repka, M.; Jankes, E.; McAleese, C.; Dörr, N. Generation of Engine Oils with Defined Degree of Degradation by Means of a Large Scale Artificial Alteration Method. Tribol. Int. 2019, 132, 39–49. [Google Scholar] [CrossRef]
- El-Naggar, A.Y.; El-Adly, R.A.; Altalhi, T.A.; Alhadhrami, A.; Modather, F.; Ebiad, M.A.; Salem, A. Oxidation Stability of Lubricating Base Oils. Pet. Sci. Technol. 2018, 36, 179–185. [Google Scholar] [CrossRef]
- Perez, J.M. Oxidative Properties of Lubricants Using Thermal Analysis. Thermochim. Acta 2000, 357, 47–56. [Google Scholar] [CrossRef]


| Properties | ATF A | ATF B | ATF C |
|---|---|---|---|
| Base oils (wt.%) | 88.3 | 89.0 | 83.0 |
| Additive package (wt.%) | 11.7 | 11 | 17 |
| Kinematic viscosity (mm2/s) at 313.15 K (40 °C) | 39.4 | 29.8 | 36.2 |
| Kinematic viscosity (mm2/s) at 373.15 K (100 °C) | 7.7 | 5.8 | 7.4 |
| Viscosity Index | 170 | 144 | 176 |
| Samples | Temp. (K) | Air Flow (L/h) | Time (h) |
|---|---|---|---|
| A0, B0, C0 | Fresh oil | Fresh oil | Fresh oil |
| A1, B1, C1 | 423.15 | 20 | 168 |
| A2, B2, C2 | 423.15 | 40 | 168 |
| A3, B3, C3 | 443.15 | 20 | 168 |
| A4, B4, C4 | 443.15 | 40 | 168 |
| A5, B5, C5 | 423.15 | 20 | 216 |
| A6, B6, C6 | 423.15 | 40 | 216 |
| A7, B7, C7 | 443.15 | 20 | 216 |
| A8, B8, C8 | 443.15 | 40 | 216 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, N.; Viesca, J.L.; García, A.; Prado, J.I.; Lugo, L.; Battez, A.H. Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles. Appl. Sci. 2022, 12, 8911. https://doi.org/10.3390/app12178911
Rivera N, Viesca JL, García A, Prado JI, Lugo L, Battez AH. Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles. Applied Sciences. 2022; 12(17):8911. https://doi.org/10.3390/app12178911
Chicago/Turabian StyleRivera, Noelia, José Luis Viesca, Alberto García, Jose I. Prado, Luis Lugo, and Antolin Hernández Battez. 2022. "Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles" Applied Sciences 12, no. 17: 8911. https://doi.org/10.3390/app12178911
APA StyleRivera, N., Viesca, J. L., García, A., Prado, J. I., Lugo, L., & Battez, A. H. (2022). Cooling Performance of Fresh and Aged Automatic Transmission Fluids for Hybrid Electric Vehicles. Applied Sciences, 12(17), 8911. https://doi.org/10.3390/app12178911









