Gas Bubble Photonics: Manipulating Sonoluminescence Light with Fluorescent and Plasmonic Nanoparticles
Abstract
:1. Introduction
2. Discussion
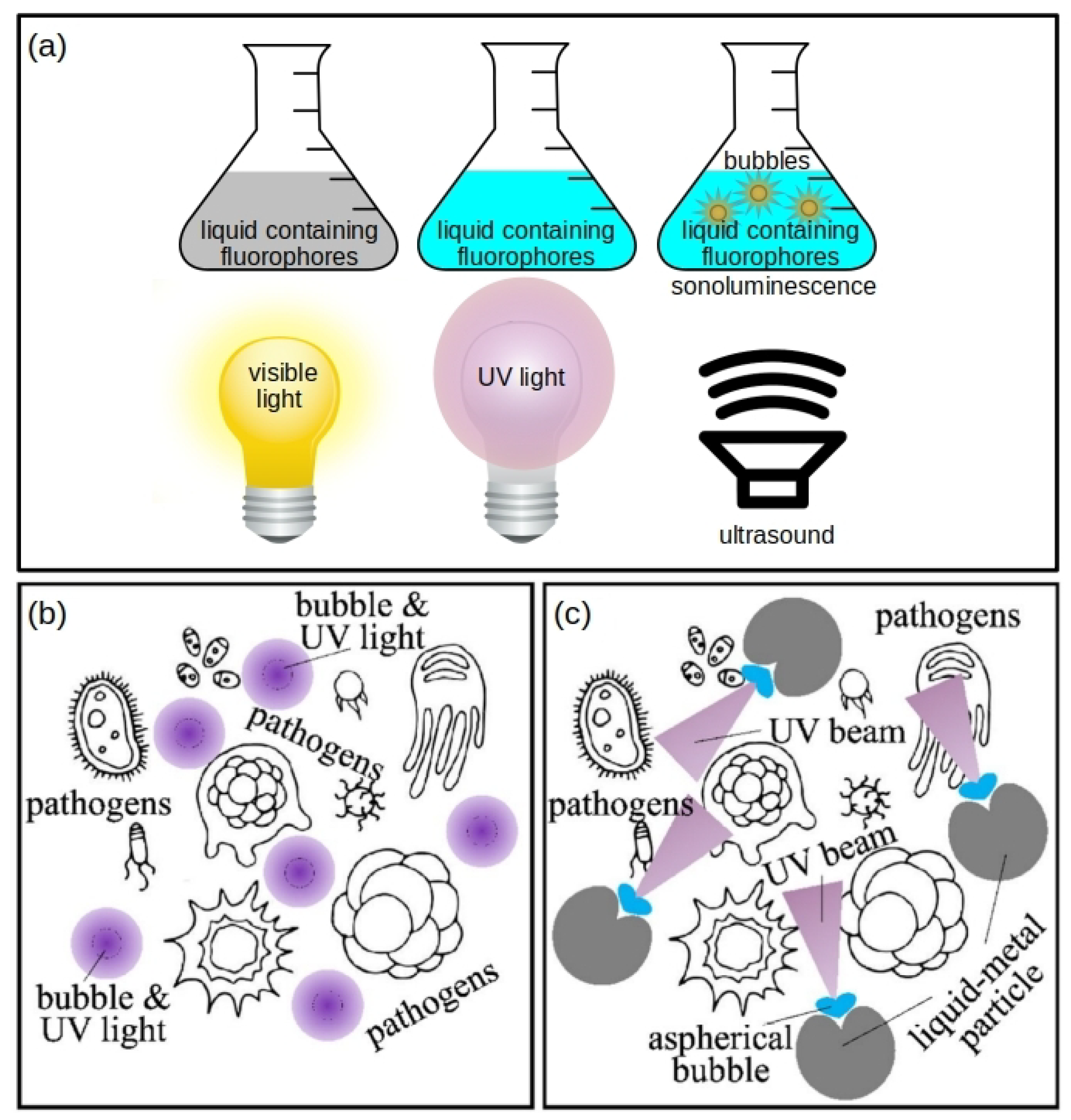
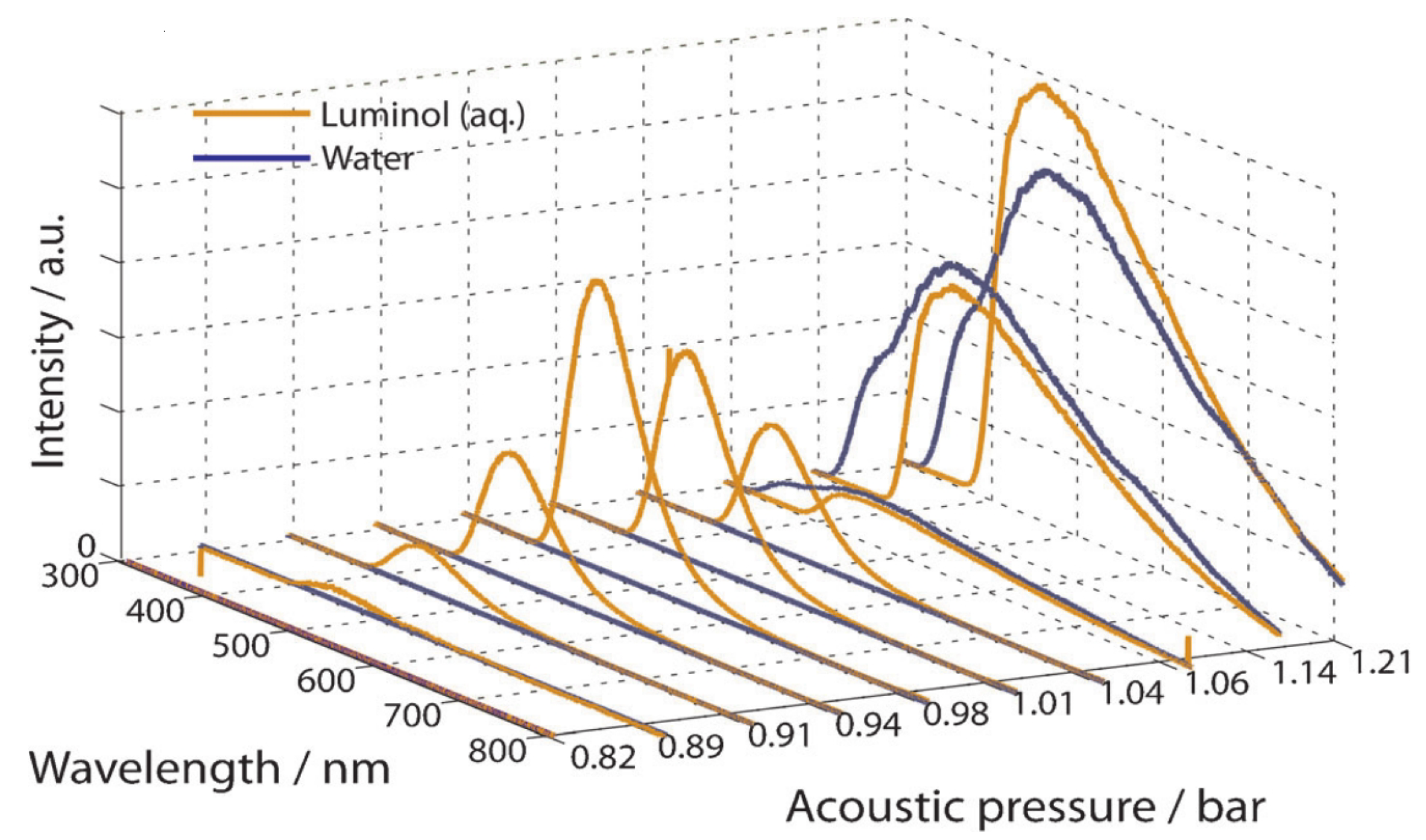
2.1. Sonochemiluminescence
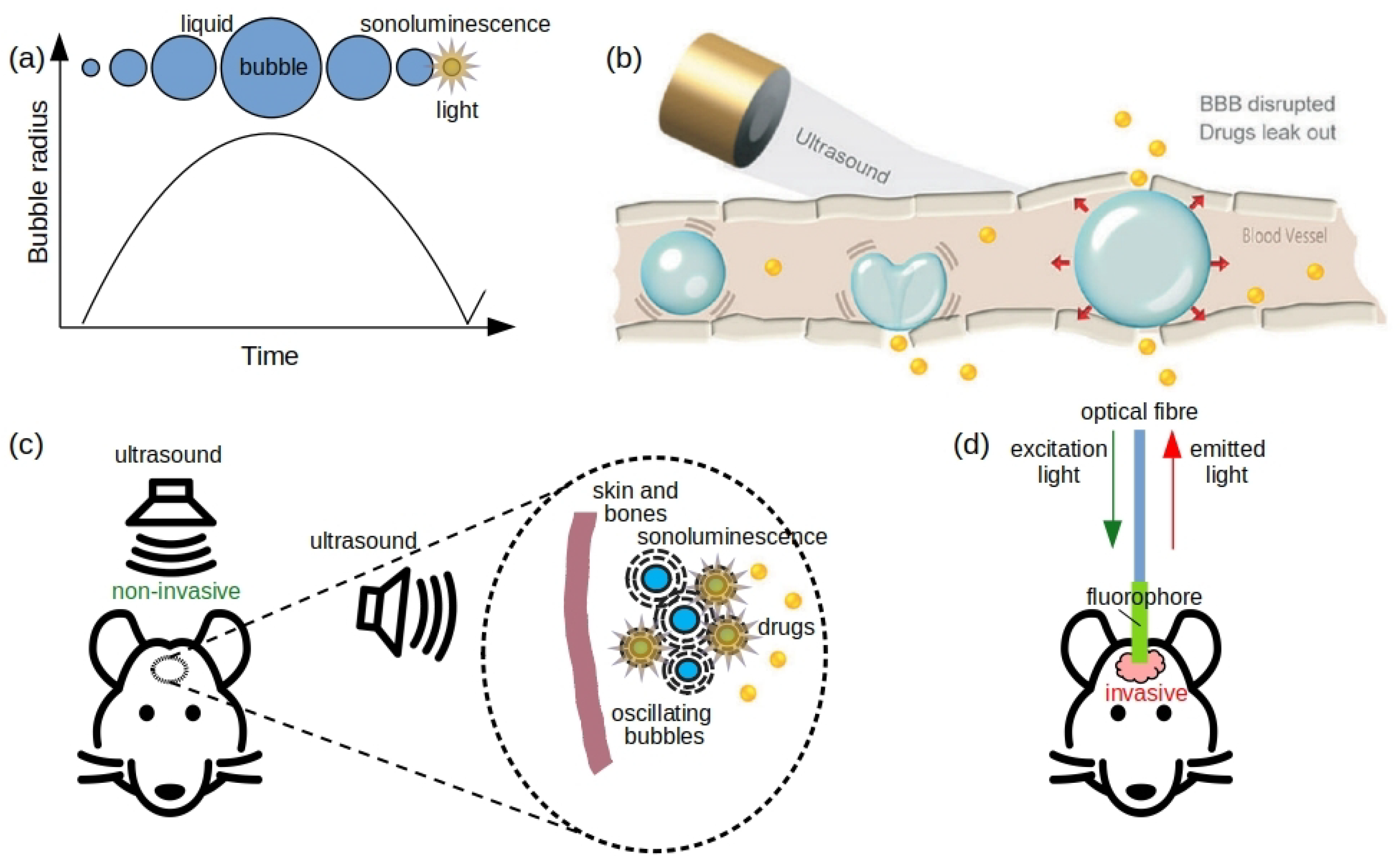
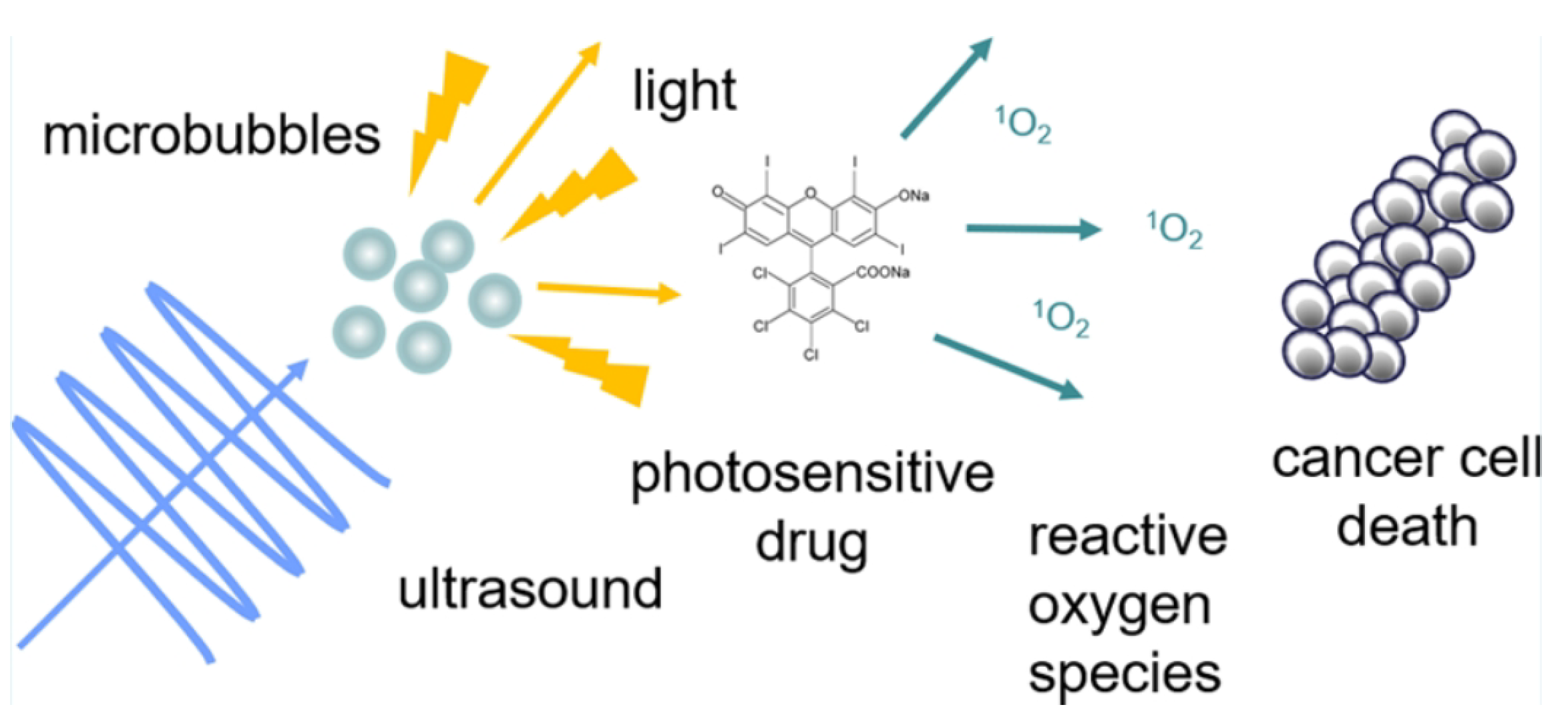
2.2. Sonoluminescence Using Therapeutic Ultrasound
2.3. Photonically Enhanced Sonoluminescence
2.4. Sonoluminescence Light Manipulation with Carbon Nanodots
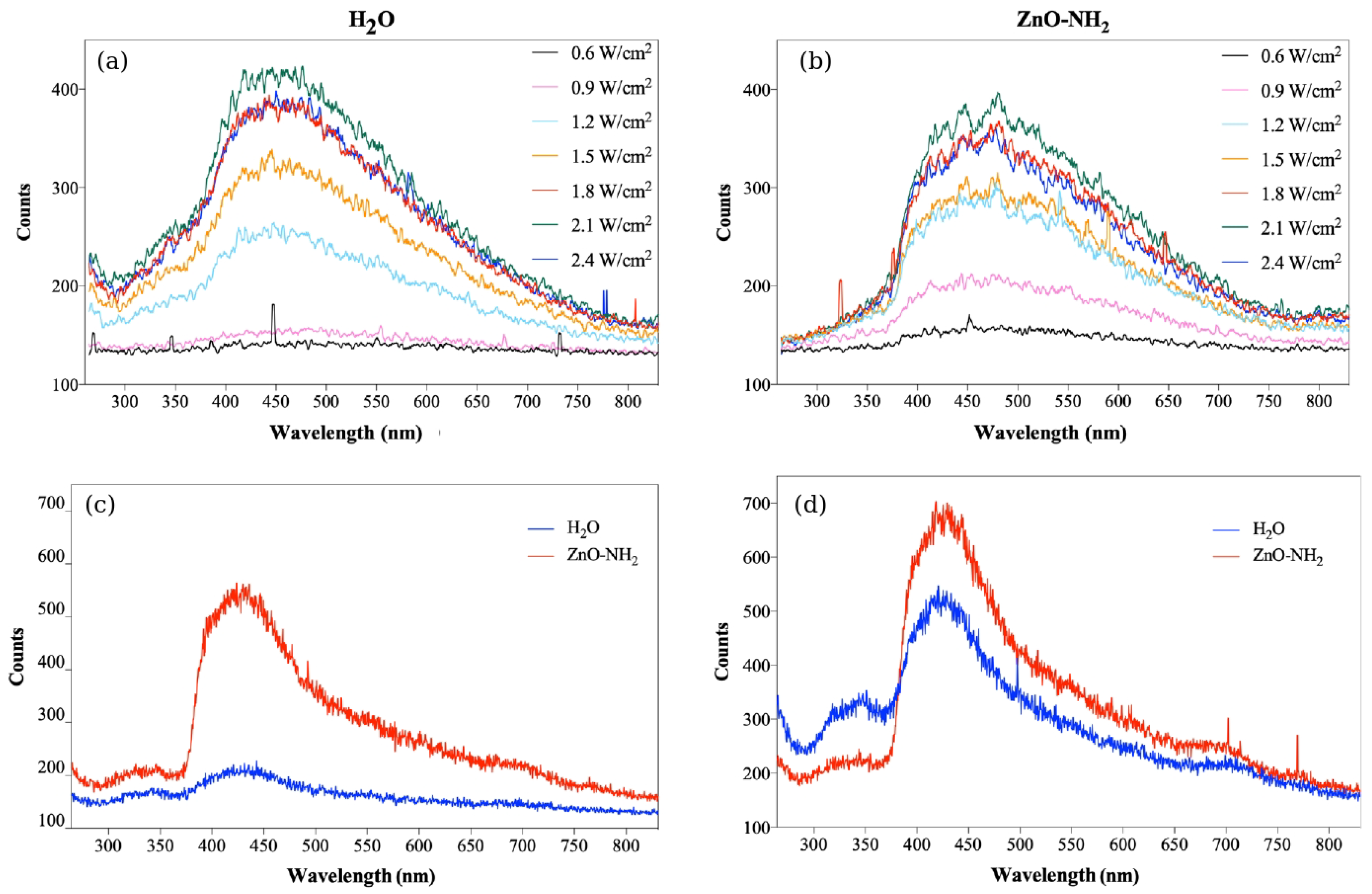
2.5. Liquid Metal-Alloy-Nanoparticle-Enhanced Sonoluminescence
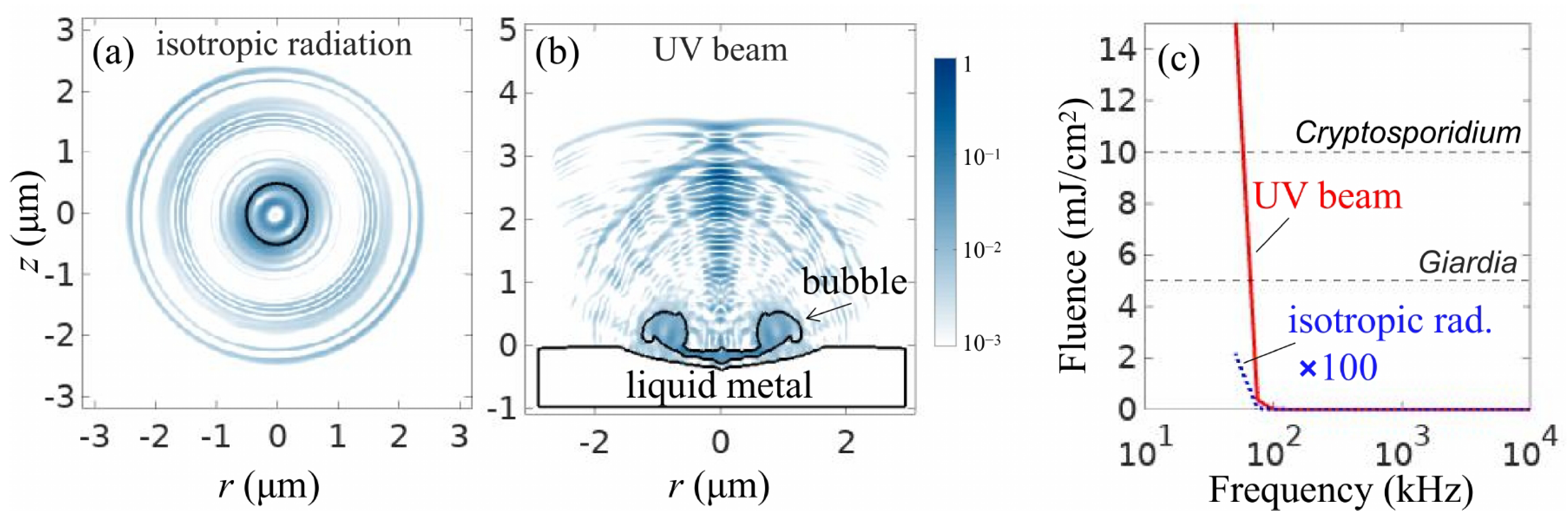
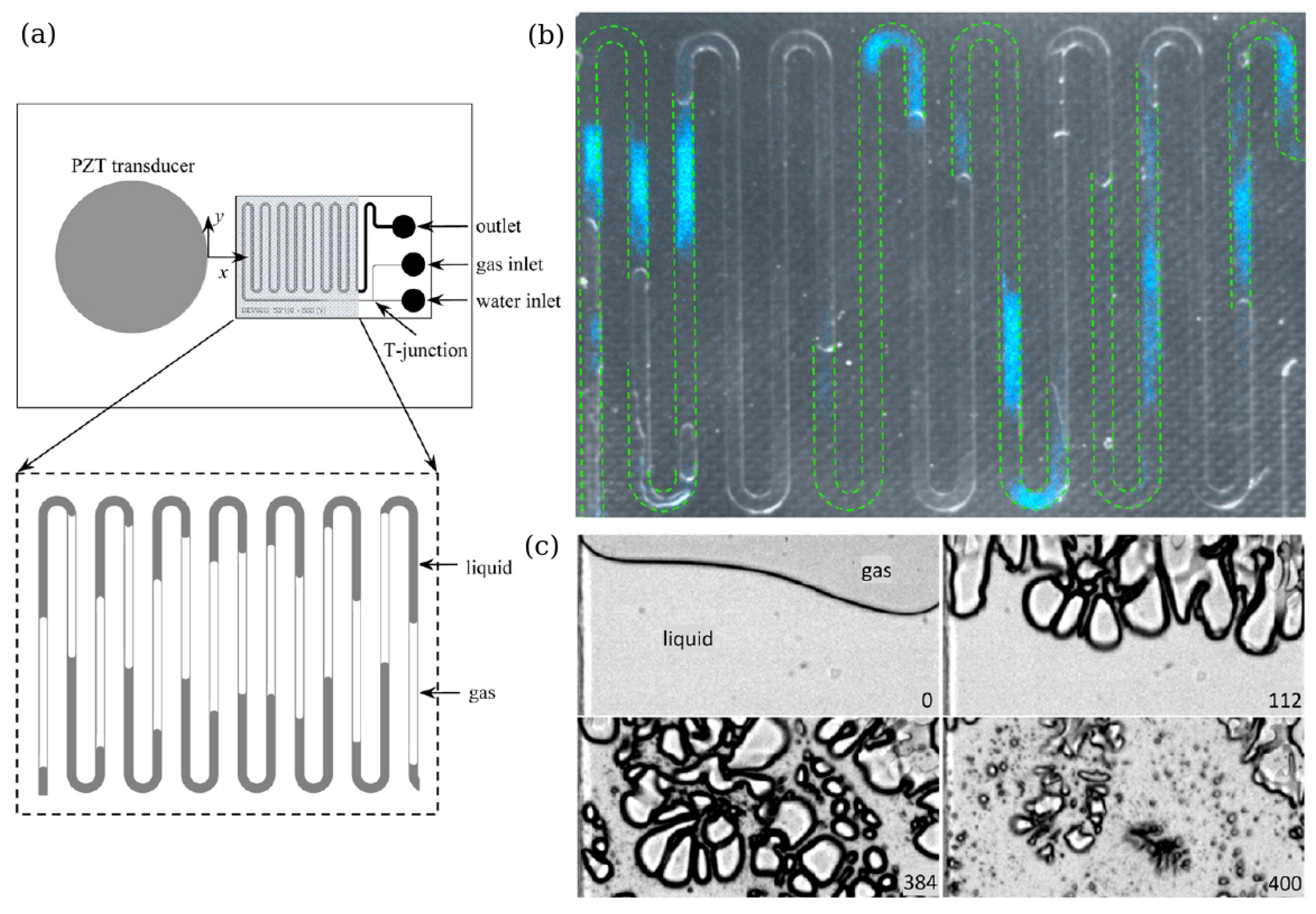
3. Sonoluminescence in Microfluidics-Based Systems
4. Conclusions and Open Questions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| blood–brain barrier | BBB |
| Food and Drug Administration | FDA |
| in vitro fertilisation | IVF |
| photomultiplier tube | PMT |
| photodynamic therapy | PTD |
| piezoelectric transducer | PZT |
| reactive oxygen species | ROS |
| sonodynamic therapy | SDT |
| ultraviolet | UV |
References
- Brennen, C.E. Cavitation and Bubble Dynamics; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Lauterborn, W.; Kurz, T. Physics of bubble oscillations. Rep. Prog. Phys. 2010, 73, 106501. [Google Scholar] [CrossRef]
- Raciti, D.; Brocca, P.; Raudino, A.; Corti, M. Interferometric detection of hydrodynamic bubble-bubble interactions. J. Fluid Mech. 2022, 942, R1. [Google Scholar] [CrossRef]
- Miller, S.; Ding, Y.; Jiang, L.; Tu, X.; Pau, S. Observation of elliptically polarized light from total internal reflection in bubbles. Sci. Rep. 2020, 10, 8725. [Google Scholar] [CrossRef] [PubMed]
- Shifrin, K.S. Scattering of Light in a Turbid Medium; National Aeronautics and Space Administration: Washington, DC, USA, 1959. [Google Scholar]
- Hansen, G.M. Mie scattering as a technique for the sizing of air bubbles. Appl. Opt. 1985, 24, 3214–3220. [Google Scholar] [CrossRef]
- Xu, H.; Wang, G.; Ma, J.; Jin, L.; Oh, K.; Guan, B. Bubble-on-fiber (BoF): A built-in tunable broadband acousto-optic sensor for liquid-immersible in situ measurements. Opt. Express 2018, 26, 11976–11983. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Greentree, A.D. Coupling light and sound: Giant nonlinearities from oscillating bubbles and droplets. Nanophotonics 2019, 8, 367–390. [Google Scholar] [CrossRef]
- Tsuge, H. Micro- and Nanobubbles: Fundamental and Applications; Pan Stanford: Singapore, 2014. [Google Scholar]
- Burgess, A.; Shah, K.; Hough, O.; Hynynen, K. Focused ultrasound-mediated drug delivery through the blood-brain barrier. Expert. Rev. Neurother. 2015, 15, 477–491. [Google Scholar] [CrossRef]
- Kang, S.T.; Yeh, C.K. Ultrasound microbubble contrast agents for diagnostic and therapeutic applications: Current status and future Design. Chang Gung Med. J. 2012, 35, 125–139. [Google Scholar]
- Deng, W.; Chen, W.; Clement, S.; Guller, A.; Zhao, Z.; Engel, A.; Goldys, E.M. Controlled gene and drug release from a liposomal delivery platform triggered by X-ray radiation. Nat. Commun. 2018, 9, 2713. [Google Scholar] [CrossRef]
- Lindner, J.R. Microbubbles in medical imaging: Current applications and future directions. Nat. Rev. Drug Discov. 2004, 3, 527–532. [Google Scholar] [CrossRef]
- Postema, M.; Gilja, O.H. Contrast-enhanced and targeted ultrasound. World J. Gastroenterol. 2011, 17, 28–41. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Khumri, T.M.; Main, M.L. Safety and risk-benefit profile of microbubble contrast agents in echocardiography. US Cardiol. 2009, 6, 16–19. [Google Scholar] [CrossRef]
- Marston, P.L.; Trinh, E.H.; Depew, J.; Asaki, J. Response of bubbles to ultrasonic radiation pressure: Dynamics in low gravity and shape oscillations. In Bubble Dynamics and Interface Phenomena; Blake, J.R., Boulton-Stone, J.M., Thomas, N.H., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1994; pp. 343–353. [Google Scholar]
- Ohl, S.W.; Klaseboer, E.; Khoo, B.C. Bubbles with shock waves and ultrasound: A review. Interface Focus 2015, 5, 20150019. [Google Scholar] [CrossRef]
- Manasseh, R. Acoustic Bubbles, Acoustic Streaming, and Cavitation Microstreaming. In Handbook of Ultrasonics and Sonochemistry; Springer: Singapore, 2016; pp. 33–68. [Google Scholar] [CrossRef]
- Dutta, A.; Chengara, A.; Nikolov, A.D.; Wasan, D.T.; Chen, K.; Campbell, B. Destabilization of aerated food products: Effects of Ostwald ripening and gas diffusion. J. Food Eng. 2004, 62, 177–184. [Google Scholar] [CrossRef]
- May, E.F.; Lim, V.W.; Metaxas, P.J.; Du, J.; Stanwix, P.L.; Rowland, D.; Johns, M.L.; Haandrikman, G.; Crosby, D.; Aman, Z.M. Gas Hydrate Formation Probability Distributions: The Effect of Shear and Comparisons with Nucleation Theory. Langmuir 2018, 34, 3186–3196. [Google Scholar] [CrossRef]
- Khan, S.A.; Duraiswamy, S. Controlling bubbles using bubbles-microfluidic synthesis of ultra-small gold nanocrystals with gas-evolving reducing agents. Lab Chip 2012, 12, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.; Yu, G.; Reilly-Collette, M.; Heiman, G.; Xu, J. Oscillating bubbles: A versatile tool for lab on a chip applications. Lab Chip 2012, 12, 4216. [Google Scholar] [CrossRef]
- Gogate, P. Treatment of wastewater streams containing phenolic compounds using hybrid techniques based on cavitation: A review of the current status and the way forward. Ultrason. Sonochem. 2008, 15, 1–15. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry and sonoprocessing: The link, the trends and (probably) the future. Ultrason. Sonochem. 2003, 10, 175–179. [Google Scholar] [CrossRef]
- Tandiono; Ohl, S.W.; Ow, D.S.W.; Klaseboer, E.; Wong, V.V.; Dumke, R.; Ohl, C.D. Sonochemistry and sonoluminescence in microfluidics. Proc. Natl. Acad. Sci. USA 2011, 108, 5996–5998. [Google Scholar] [CrossRef] [PubMed]
- Rubio, F.; Blandford, E.D.; Bond, L.J. Survey of advanced nuclear technologies for potential applications of sonoprocessing. Ultrasonics 2016, 71, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Preisig, J. The impact of bubbles on underwater acoustic communications in shallow water environments. J. Acoust. Soc. Am. 2003, 114, 2370. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Nguyen, B.Q.H.; Pototsky, A.; Suslov, S.A. Acoustic, phononic, Brillouin light scattering and Faraday wave-based frequency combs: Physical foundations and applications. Sensors 2022, 22, 3921. [Google Scholar] [CrossRef] [PubMed]
- Kalson, N.H.; Furman, D.; Zeiri, Y. Cavitation-induced synthesis of biogenic molecules on primordial Earth. ACS Cent. Sci. 2017, 3, 1041–1049. [Google Scholar] [CrossRef]
- Cernak, I. Understanding blast-induced neurotrauma: How far have we come? Concussion 2017, 2, CNC42. [Google Scholar] [CrossRef] [PubMed]
- Barney, C.W.; Dougan, C.E.; McLeod, K.R.; Kazemi-Moridani, A.; Zheng, Y.; Ye, Z.; Tiwari, S.; Sacligil, I.; Riggleman, R.A.; Cai, S.; et al. Cavitation in soft matter. Proc. Natl. Acad. Sci. USA 2020, 117, 9157–9165. [Google Scholar] [CrossRef]
- Cole, R.H. Underwater Explosions; Princeton University Press: New York, NY, USA, 1948. [Google Scholar]
- Putterman, S.J.; Weninger, K.R. Sonoluminescence: How bubbles turn sound into light. Annu. Rev. Fluid Mech. 2000, 32, 445–476. [Google Scholar] [CrossRef]
- Brenner, M.P.; Hilgenfeldt, S.; Lohse, D. Single-bubble sonoluminescence. Rev. Mod. Phys. 2002, 74, 425–484. [Google Scholar] [CrossRef]
- Farhat, M.; Chakravarty, A.; Field, J.E. Luminescence from hydrodynamic cavitation. Proc. R. Soc. A 2011, 467, 591–606. [Google Scholar] [CrossRef] [Green Version]
- Borisenok, V.A. Sonoluminescence: Experiments and models (review). Acoust. Phys. 2015, 61, 308–332. [Google Scholar] [CrossRef]
- Yasui, K. Multibubble sonoluminescence from a theoretical perspective. Molecules 2021, 26, 4624. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.; Suslov, S.A.; Becker, S.; Greentree, A.D.; Maksymov, I.S. Beamed UV sonoluminescence by aspherical air bubble collapse near liquid-metal microparticles. Sci. Rep. 2020, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Beguin, E.; Shrivastava, S.; Dezhkunov, N.V.; McHale, A.P.; Callan, J.F.; Stride, E. Direct evidence of multibubble sonoluminescence using therapeutic ultrasound and microbubbles. ACS Appl. Mater. Interfaces 2019, 11, 19913–19919. [Google Scholar] [CrossRef] [PubMed]
- Canaparo, R.; Foglietta, F.; Giuntini, F.; Francovich, A.; Serpe, L. The bright side of sound: Perspectives on the biomedical application of sonoluminescence. Photochem. Photobiol. Sci. 2020, 10, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond proof of principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed]
- Wegener, M.; Hansen, M.J.; Driessen, A.J.M.; Szymanski, W.; Feringa, B.L. Photocontrol of antibacterial activity: Shifting from UV to red light activation. J. Am. Chem. Soc. 2017, 139, 17979–17986. [Google Scholar] [CrossRef]
- Smith, A.M.; Mancini, M.C.; Nie, S. Second window for in vivo imaging. Nat. Nanotech. 2009, 4, 710–711. [Google Scholar] [CrossRef]
- Li, J.; Ebendorff-Heidepriem, H.; Gibson, B.C.; Greentree, A.D.; Hutchinson, M.R.; Jia, P.; Kostecki, R.; Liu, G.; Orth, A.; Ploschner, M.; et al. Perspective: Biomedical sensing and imaging with optical fibers–Innovation through convergence of science disciplines. APL Photon. 2018, 3, 100902. [Google Scholar] [CrossRef]
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J. Am. Chem. Soc. 2012, 134, 8848–8855. [Google Scholar] [CrossRef]
- Reineck, P.; Gibson, B.C. Near-infrared fluorescent nanomaterials for bioimaging and sensing. Adv. Opt. Mater. 2017, 5, 1600446. [Google Scholar] [CrossRef]
- Errico, C.; Osmanski, B.F.; Pezet, S.; Couture, O.; Lenkei, Z.; Tanter, M. Transcranial functional ultrasound imaging of the brain using microbubble-enhanced ultrasensitive Doppler. NeuroImage 2015, 124, 752–761. [Google Scholar] [CrossRef]
- Purdey, M.S.; Thompson, J.G.; Monro, T.M.; Abell, A.D.; Schartner, E.P. A dual sensor for pH and hydrogen peroxide using polymer-coated optical fibre tips. Sensors 2015, 15, 31904–31913. [Google Scholar] [CrossRef]
- Musolino, S.; Schartner, E.P.; Tsiminis, G.; Salem, A.; Monro, T.M.; Hutchinson, M.R. Portable optical fiber probe for in vivo brain temperature measurements. Biomed. Opt. Express 2016, 7, 3069–3078. [Google Scholar] [CrossRef]
- Zabolocki, M.; McCormack, K.; van den Hurk, M.; Milky, B.; Shoubridge, A.P.; Adams, R.; Tran, J.; Mahadevan-Jansen, A.; Reineck, P.; Thomas, J.; et al. BrainPhys neuronal medium optimized for imaging and optogenetics in vitro. Nat. Commun. 2020, 11, 5550. [Google Scholar] [CrossRef]
- Whitworth, M.; Bricker, L.; Mullan, C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD007058. [Google Scholar] [CrossRef]
- Yusefi, H.; Helfield, B. Ultrasound contrast imaging: Fundamentals and emerging technology. Front. Phys. 2022, 10, 791145. [Google Scholar] [CrossRef]
- Wang, B.; Su, J.L.; Karpiouk, A.B.; Sokolov, K.V.; Smalling, R.W.; Emelianov, S.Y. Intravascular photoacoustic imaging. IEEE J. Sel. Topics Quantum Electron. 2010, 16, 588–599. [Google Scholar] [CrossRef]
- Wilson, K.; Homan, K.; Emelianov, S. Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nat. Commun. 2011, 3, 618. [Google Scholar] [CrossRef]
- Dove, J.D.; Murray, T.W.; Borden, M.A. Enhanced photoacoustic response with plasmonic nanoparticle-templated microbubbles. Soft Matter 2013, 9, 7743–7750. [Google Scholar] [CrossRef]
- Doinikov, A.A. Bubble and Particle Dynamics in Acoustic Fields: Modern Trends and Applications; Research Signpost: Kerala, India, 2005. [Google Scholar]
- Vighetto, V.; Troia, A.; Laurenti, M.; Carofiglio, M.; Marcucci, N.; Canavese, G.; Cauda, V. Insight into sonoluminescence augmented by ZnO-functionalized nanoparticles. ACS Omega 2020, 7, 6591–6600. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Xu, W.; Luo, M.; You, K.; Tang, J.; Wen, H.; Cheng, X.; Luo, X.; Wang, Z. Turning single bubble sonoluminescence from blue in pure water to green by adding trace amount of carbon nanodots. Ultrason. Sonochem. 2021, 78, 105727. [Google Scholar] [CrossRef] [PubMed]
- Brotchie, A.; Schneider, J.; Pflieger, R.; Shchukin, D.; Möhwald, H. Sonochemiluminescence from a single cavitation bubble in water. Chem. Eur. J. 2012, 18, 11201–11204. [Google Scholar] [CrossRef] [PubMed]
- Maksymov, I.S. Magneto-plasmonic nanoantennas: Basics and applications. Rev. Phys. 2016, 1, 36–51. [Google Scholar] [CrossRef]
- Marinesco, N.; Trillat, J.J. Action des ultrasons sur les plaques photographiques. Proc. R. Acad. Sci. 1933, 196, 858–860. [Google Scholar]
- Frenzel, H.; Schultes, H. Lumineszenz im ultraschall-beschickten Wasser. Z. Phys. Chem. Abt. 1934, 27B, 421–424. [Google Scholar] [CrossRef]
- Suslick, K.S.; Flannigan, D.J. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Annu. Rev. Phys. Chem. 2008, 59, 659–683. [Google Scholar] [CrossRef]
- Gaitan, D.F.; Crum, L.A.; Church, C.C.; Roy, R.A. Sonoluminescence and bubble dynamics for a single, stable, cavitation bubble. J. Acoust. Soc. Am. 1992, 91, 3166–3183. [Google Scholar] [CrossRef]
- Yosioka, K.; Omura, A. The light emission from a single bubble driven by ultrasound and the spectra of acoustic oscillation. Proc. Annu. Meet. Acoust. Soc. Jpn. 1962, 125–126. [Google Scholar]
- Pickworth, M.J.W.; Dendy, P.P.; Leighton, T.G.; Walton, A.J. Studies of the cavitational effects of clinical ultrasound by conoluminescence: 2. Thresholds for sonoluminescence from a therapeutic ultrasound beam and the effect of temperature and duty cycle. Phys. Med. Biol. 1988, 33, 1249–1260. [Google Scholar] [CrossRef]
- Umemura, S.; Yumita, N.; Nishigaki, R.; Umemura, K. Mechanism of cell damage by ultrasound in combination with hematoporphyrin. Jpn. J. Cancer Res. 1990, 81, 962–966. [Google Scholar] [CrossRef]
- Srinivasan, D.; Holroyd, L.V. Optical spectrum of the sonoluminescence emitted by cavitated water. J. Appl. Phys. 1961, 32, 446–449. [Google Scholar] [CrossRef]
- Zhadnov, V.Z.; Mishanov, R.F.; Chernov, V.V. Sonoluminiscence of blood plasma in the differential diagnosis of tuberculosis, cancer and sarcoidosis of the lungs. Probl. Tuberk. 1994, 6, 42–43. [Google Scholar]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Q.; Guo, X.; Tu, J.; Zhang, D. Mechanisms underlying sonoporation: Interaction between microbubbles and cells. Ultrason. Sonochem. 2020, 67, 105096. [Google Scholar] [CrossRef]
- Bjerknes, V. Fields of Force; The Columbia University Press: New York, NY, USA, 1906. [Google Scholar]
- Crum, L.A. Bjerknes forces on bubbles in a stationary sound field. J. Acoust. Soc. Am. 1975, 57, 1363–1370. [Google Scholar] [CrossRef]
- Hatanaka, S.; Mitome, H.; Yasui, K.; Hayashi, S. Single-bubble sonochemiluminescence in aqueous luminol solutions. J. Am. Chem. Soc. 2002, 124, 10250–10251. [Google Scholar] [CrossRef]
- McMurray, H.N.; Wilson, B.P. Mechanistic and spatial study of ultrasonically induced luminol chemiluminescence. J. Phys. Chem. A 1999, 103, 3955–3962. [Google Scholar] [CrossRef]
- Ninomiya, K.; Noda, K.; Ogino, C.; Kuroda, S.I.; Shimizu, N. Enhanced OH radical generation by dual-frequency ultrasound with TiO2 nanoparticles: Its application to targeted sonodynamic therapy. Ultrason. Sonochem. 2014, 21, 289–294. [Google Scholar] [CrossRef]
- Osaki, T.; Yokoe, I.; Uto, Y.; Ishizuka, M.; Tanaka, T.; Yamanaka, N.; Kurahashi, T.; Azuma, K.; Murahata, Y.; Tsuka, T.; et al. Bleomycin enhances the efficacy of sonodynamic therapy using aluminum phthalocyanine disulfonate. Ultrason. Sonochem. 2016, 28, 161–168. [Google Scholar] [CrossRef]
- Wright, A.G. The Photomultiplier Handbook; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Miller, D.; Smith, N.; Bailey, M.; Czarnota, G.; Hynynen, K.; Makin, I.; American Institute of Ultrasound in Medicine Bioeffects Committee. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 1, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J. Zinc oxide nanomaterials for biomedical fluorescence detection. J. Nanosci. Nanotechnol. 2014, 14, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Ancona, A.; Troia, A.; Garino, N.; Dumontel, B.; Cauda, V.; Canavese, G. Leveraging re-chargeable nanobubbles on amine-functionalized ZnO nanocrystals for sustained ultrasound cavitation towards echographic imaging. J. Acoust. Soc. Am. 2011, 130, 3184–3208. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.T.; Seo, K.W.; Shim, I.W.; Kwak, H.Y. Syntheses of ZnO and ZnO-coated TiO2 nanoparticles in various alcohol solutions at multibubble sonoluminescence (MBSL) condition. Chem. Eng. J. 2008, 135, 168–173. [Google Scholar] [CrossRef]
- Goryacheva, I.Y.; Sapelkin, A.V.; Sukhorukov, G.B. Carbon nanodots: Mechanisms of photoluminescence and principles of application. TrAC–Trends Anal. Chem 2017, 90, 27–37. [Google Scholar] [CrossRef]
- Righetto, M.; Carraro, F.; Privitera, A.; Marafon, G.; Moretto, A.; Ferrante, C. The elusive nature of carbon nanodot fluorescence: An unconventional perspective. J. Phys. Chem. C 2020, 124, 22314–22320. [Google Scholar] [CrossRef]
- Rivas, D.F.; Ashokkumar, M.; Leong, T.; Yasui, K.; Tuziuti, T.; Kentish, S.; Lohse, D.; Gardeniers, H.J.G.E. Sonoluminescence and sonochemiluminescence from a microreactor. Ultrason. Sonochem. 2012, 19, 1252–1259. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.; Lin, Y.; Pacardo, D.B.; Wang, C.; Sun, W.; Ligler, F.S.; Dickey, M.D.; Gu, Z. Transformable liquid-metal nanomedicine. Nat. Commun. 2015, 6, 10066. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; Castro, I.A.D.; Esrafilzadeh, D.; Barrow, S.; Dickey, M.; Zadeh, K.K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef]
- Reineck, P.; Lin, Y.; Gibson, B.C.; Dickey, M.D.; Greentree, A.D.; Maksymov, I.S. UV plasmonic properties of colloidal liquid-metal eutectic gallium-indium alloy nanoparticles. Sci. Rep. 2019, 9, 5345. [Google Scholar] [CrossRef]
- Raether, H. Surface Plasmons on Smooth and Rough Surfaces and on Gratings; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Enoch, S.; Bonod, N. Plasmonics: From Basic to Advanced Topics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Chen, J.; Albella, P.; Pirzadeh, Z.; Alonso-González, P.; Huth, F.; Bonetti, S.; Bonanni, V.; Åkerman, J.; Nogués, J.; Vavassori, P.; et al. Plasmonic nickel nanoantennas. Small 2011, 7, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, V.; Bonetti, S.; Pakizeh, T.; Pirzadeh, Z.; Chen, J.; Nogués, J.; Vavassori, P.; Hillenbrand, R.; Åkerman, J.; Dmitriev, A. Designer magnetoplasmonics with nickel nanoferromagnets. Nano Lett. 2011, 11, 5333–5338. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Greentree, A.D. Dynamically reconfigurable plasmon resonances enabled by capillary oscillations of liquid-metal nanodroplets. Phys. Rev. A 2017, 96, 043829. [Google Scholar] [CrossRef]
- Fong, S.W.; Klaseboer, E.; Turangan, C.K.; Khoo, B.C.; Hung, K.C. Numerical analysis of a gas bubble near bio-materials in an ultrasound field. Ultrasound Med. Biol. 2006, 32, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, G.A.; Leppinen, D.M.; Wang, Q.X.; Blake, J.R. Ultrasonic cavitation near a tissue layer. J. Fluid Mech. 2013, 730, 245–272. [Google Scholar] [CrossRef]
- Boyd, B.; Becker, S. Numerical modelling of an acoustically-driven bubble collapse near a solid boundary. Fluid Dyn. Res. 2018, 50, 065506. [Google Scholar] [CrossRef]
- Boyd, B.; Becker, S. Numerical modeling of the acoustically driven growth and collapse of a cavitation bubble near a wall. Phys. Fluids 2019, 31, 032102. [Google Scholar] [CrossRef]
- Bolton, J.; Colton, C. The Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, USA, 2008. [Google Scholar]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.; Leech, P.W.; Manasseh, R. Production of monodispersed micron-sized bubbles at high rates in a microfluidic device. Appl. Phys. Lett. 2009, 95, 144101. [Google Scholar] [CrossRef]
- Rabaud, D.; Thibault, P.; Mathieu, M.; Marmottant, P. Acoustically bound microfluidic bubble crystals. Phys. Rev. Lett. 2011, 106, 134501. [Google Scholar] [CrossRef]
- Zhou, Y.; Seshia, A.A.; Hall, E.A.H. Microfluidics-based acoustic microbubble biosensor. In Proceedings of the SENSORS, IEEE, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Greentree, A.D. Synthesis of discrete phase-coherent optical spectra from nonlinear ultrasound. Opt. Express 2017, 25, 7496–7506. [Google Scholar] [CrossRef] [Green Version]
- Toegel, R.; Luther, S.; Lohse, D. Viscosity destabilizes sonoluminescing bubbles. Phys. Rev. Lett. 2006, 96, 114301. [Google Scholar] [CrossRef]
- Lu, X.; Prosperetti, A.; Toegel, R.; Lohse, D. Harmonic enhancement of single-bubble sonoluminescence. Phys. Rev. E 2003, 67, 056310. [Google Scholar] [CrossRef]
- Nguyen, B.Q.H.; Maksymov, I.S.; Suslov, S.A. Acoustic frequency combs using gas bubble cluster oscillations in liquids: A proof of concept. Sci. Reps. 2021, 11, 38. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Besbes, M.; Hugonin, J.P.; Yang, J.; Beveratos, A.; Sagnes, I.; Robert-Philip, I.; Lalanne, P. Metal-coated nanocylinder cavity for broadband nonclassical light emission. Phys. Rev. Lett. 2010, 105, 180502. [Google Scholar] [CrossRef]
- Sauvan, C.; Hugonin, J.P.; Maksymov, I.S.; Lalanne, P. Theory of the spontaneous optical emission of nanosize photonic and plasmon resonators. Phys. Rev. Lett. 2013, 110, 237401. [Google Scholar] [CrossRef]
- Akselrod, G.M.; Argyropoulos, C.; Hoang, T.B.; Ciracì, C.; Fang, C.; Huang, J.; Smith, D.R.; Mikkelsen, M.H. Probing the mechanisms of large Purcell enhancement in plasmonic nanoantenna. Nat. Photon. 2014, 8, 835–840. [Google Scholar] [CrossRef]
- Krasnok, A.E.; Slobozhanyuk, A.P.; Simovski, C.R.; Tretyakov, S.A.; Poddubny, A.N.; Miroshnichenko, A.E.; Kivshar, Y.S.; Belov, P.A. An antenna model for the Purcell effect. Sci. Rep. 2015, 5, 12956. [Google Scholar] [CrossRef]
- Eberlein, C. Theory of quantum radiation observed as sonoluminescence. Phys. Rev. A 1996, 53, 2772–2787. [Google Scholar] [CrossRef]
- Eberlein, C. Sonoluminescence as quantum vacuum radiation. Phys. Rev. Lett. 1996, 76, 3842–3845. [Google Scholar] [CrossRef] [PubMed]
- Maksymov, I.S.; Greentree, A.D. Acoustically tunable optical transmission through a subwavelength hole with a bubble. Phys. Rev. A 2017, 95, 033811. [Google Scholar] [CrossRef] [Green Version]
- Landi, M.; Zhao, J.; Prather, W.E.; Wu, Y.; Zhang, L. Acoustic Purcell effect for enhanced emission. Phys. Rev. Lett. 2018, 120, 114301. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.K.; Helt, L.G.; Poulton, C.G.; Steel, M.J. Elastic Purcell effect. Phys. Rev. Lett. 2018, 121, 064301. [Google Scholar] [CrossRef]
- Schmidt, M.K.; Helt, L.G.; Poulton, C.G.; Steel, M.J. Harnessing simultaneous optical and acoustic Purcell effects. In Proceedings of the 3rd International Workshop on Optomechanics and Brillouin Scattering (WOMBAT 3), Tel-Aviv, Israel, 26–28 March 2019. [Google Scholar]
- Zheludev, N.I.; Kivshar, Y.S. From metamaterials to metadevices. Nat. Mater. 2012, 11, 917–924. [Google Scholar] [CrossRef]
- Poddubny, A.; Iorsh, I.; Belov, P.; Kivshar, Y. Hyperbolic metamaterials. Nat. Photon. 2013, 7, 948–957. [Google Scholar] [CrossRef]
- Trigo, M.; Bruchhausen, A.; Fainstein, A.; Jusserand, B.; Thierry-Mieg, V. Confinement of acoustical vibrations in a semiconductor planar phonon cavity. Phys. Rev. Lett. 2002, 89, 227402. [Google Scholar] [CrossRef]
- Smolyaninov, I.I.; Smolyaninova, V.N. Analogue quantum gravity in hyperbolic metamaterials. Universe 2022, 8, 242. [Google Scholar] [CrossRef]
- Smolyaninov, I.I.; Smolyaninova, V.N. Fine tuning and MOND in a metamaterial “multiverse”. Sci. Rep. 2017, 7, 8023. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksymov, I.S. Gas Bubble Photonics: Manipulating Sonoluminescence Light with Fluorescent and Plasmonic Nanoparticles. Appl. Sci. 2022, 12, 8790. https://doi.org/10.3390/app12178790
Maksymov IS. Gas Bubble Photonics: Manipulating Sonoluminescence Light with Fluorescent and Plasmonic Nanoparticles. Applied Sciences. 2022; 12(17):8790. https://doi.org/10.3390/app12178790
Chicago/Turabian StyleMaksymov, Ivan S. 2022. "Gas Bubble Photonics: Manipulating Sonoluminescence Light with Fluorescent and Plasmonic Nanoparticles" Applied Sciences 12, no. 17: 8790. https://doi.org/10.3390/app12178790
APA StyleMaksymov, I. S. (2022). Gas Bubble Photonics: Manipulating Sonoluminescence Light with Fluorescent and Plasmonic Nanoparticles. Applied Sciences, 12(17), 8790. https://doi.org/10.3390/app12178790





