Abstract
The choice of an appropriate implant suprastructure, which should be made according to the individual characteristics of each patient, is a leading factor in achieving good aesthetic results. The aim of this study was to assess the clinical behavior of implant suprastructures according to functional, biological, and aesthetic criteria. Methods: The study enrolled 23 patients with a total of 27 implants in different areas of the oral cavity. The following groups were studied for a period of 1 year: Group A, the control group (monolithic implant crowns made of zirconium dioxide on a titanium base); Group B (monolithic implant crowns made of lithium disilicate with individualized (custom) titanium alloy abutment); and Group C (implant crowns made of zirconium dioxide with individualized titanium alloy abutment). The functional criteria included suprastructure fracture, crown fracture or chipping, screw fracture or loosening, faceting or wearing of the occlusal surface of the crown, articulation relations, and suprastructure fracture. The biological indicators included the probing pocket depth (PPD) and Mombelli et al.’s plaque index and bleeding on probing. The bone loss level was measured radiographically. Digital measurements of the bone loss level were performed when definitive prosthetics were placed and 1 year after. The platform of the implant was taken as a reference point. Two parallel lines ran distally and medially to the implant and descended to the level of the first thread, which was in contact with the bone. The aesthetic evaluation was based on Jempt’s papilla index and considered differences in the color of the peri-implant mucosa. The suprastructures were examined at 2 weeks and 1 year after the initiation of prosthetic treatment. Results: Regarding the functional criteria, no deviations from the reference range were registered in any of the study groups for both measurements. Radiography showed no bone loss in any of the study groups. Group B showed the best behavior regarding biological complications, followed by Group C. There was no plaque accumulation in Group B, but statistically significant amounts were found in Groups A (p = 0.08) and C (p = 0.01). Group B had the lowest bleeding index, but the differences between the groups were found to be insignificant during the observations at the one-year mark. On the vestibular side of the papillae, p = 0.39, while on the oral side, p = 0.35. The PPD measurements showed that there were statistically significant differences between the three groups when they were compared after the second week and after one year (p = 0.00). Jempt’s papilla height index showed the highest values in Group B, which increased by two times throughout the study period. The increase was statistically significant in Groups B and C, while the growth in Group A remained statistically insignificant (p = 0.10). The aesthetic indicator “Gingiva color around the restoration” showed mild margo gingivalis graying in Groups B and C. Conclusions: Individualized implant abutments made of titanium alloy and monolithic restorations made of lithium disilicate or zirconium dioxide have stable biomechanical behavior and may be the optimal choice for the prosthetic treatment of partial edentulousness. Because this study took place within a limited period of time, clinical trials with a longer follow-up period need to be carried out.
1. Introduction
The complete aesthetic and functional rehabilitation of partial edentulousness is challenging, especially when it is dependent on single implant treatment. Restoring a missing tooth with an implant-supported prosthesis could be the optimal treatment choice; however, it requires good clinical planning and the sophisticated management of hard and soft tissues. Contemporary implant dentistry is constantly searching for solutions to improve biological and surgical outcomes [1].
The choice of an appropriate implant suprastructure, which should be made according to the individual characteristics of each patient, is a leading factor in achieving good aesthetic results [2]. Despite the availability of standardized prosthetic components, treatments often cannot meet the individual needs and personal requirements of each patient and cannot provide the most favorable position for future restoration and gingival architectonics [3,4]. However, individualized (custom) abutments can prevent many of the complications observed in prosthetic treatments using factory-manufactured abutments in cases in which there are anatomical limitations, such as lack of sufficient distance from the antagonist, large interproximal space, unparallel implant position, etc. [5]. New materials and production technologies allow for fast and accurate manufacturing [6,7,8]. Custom-made CAD/CAM abutments have a better survival rate compared to conventional suprastructures, but there are still only a few clinical trials that have been described in the literature.
Because of this, there is a lack of studies considering individualized CAD/CAM-milled abutments. Most of the existing studies did not include control groups or make comparisons with conventional implant abutments. The most commonly reported clinical outcomes that were measured are soft tissue volume and health, marginal bone level, aesthetic score, and success rate. These studies also demonstrated substantial heterogeneity regarding the manufacturing systems and materials of the abutments examined, the specific clinical outcomes observed, the design of the studies, and the measurement techniques employed. Studies represent a follow-up period of 1–3 years. For studies comparing CAD/CAM abutments with conventional abutments, no significant differences have been observed in most of the clinical outcomes at the 1-year follow-up [9].
There are no available studies that consider the pre-milled abutment titanium blanks included in the present research. These blanks are prefabricated with genuine connections to ensure accurate fit and superior performance with the implant connection.
Definite guidance regarding the use of different types of abutments over implant superstructures that considers their specific morphological and biomechanical characteristics, especially in the critical transmucosal area, is needed. These statements indicate the need to formulate recommendations for the optimal application of different implant superstructures according to their biomechanical and clinical behavior.
The working hypothesis of the present research was that the pre-milled abutment titanium blanks with genuine connections in combination with the ceramic material of the crown would have an advantage over the prefabricated Ti-base abutments and zirconium crowns due to the possibility of individual varieties of transmucosal profiles based on functional, biological, and aesthetic indicators, supporting their long-term clinical use.
2. Materials and Methods
The study enrolled 23 patients with a total of 27 TSV implants (Zimmer Biomet, Warsaw, IN, USA) located in different areas of the oral cavity (Table 1 and Table 2). Patients were selected based on several inclusion criteria, including good general health status, no systemic or chronic diseases, no parafunctions or inflammatory processes in the oral cavity, and non-smokers. All cases were selected at the stage of complete osteointegration and after the formation of the peri-implant mucosa by the provisional restorations. After a thorough extra- and intra-oral examination, segmental radiography, and a clinical evaluation of the transmucosal area, the definitive implant prosthetic restorations were made. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Scientific Research with trial registry approval 12/14.05.2020.

Table 1.
Distribution of patients by sex and age.

Table 2.
Zones of implantation.
The following groups were studied (Table 3):

Table 3.
The three study groups according to the criteria.
For each case, prosthetic treatment was performed according to an entirely digital workflow and using CAD/CAM technology (Figure 1, Figure 2 and Figure 3). The prosthetic field was scanned with an iTero Element intraoral scanner (Align Technology, San Jose, CA, USA), and the implant position was registered by a TSV Scan Body (Zimmer Biomet, Warsaw, IN, USA) that matched the diameter of the implant platform. The STL files were imported into ExoCad modeling software (GmbH, Darmstadt, Germany) when the custom abutments for Groups B and C and the monolithic crowns were used for all of the study groups were designed. The custom abutments and the zirconia crowns for Groups A and C were milled in an inLab MC X5 CAD/CAM machine (Dentsply, Sirona, Charlotte, NC, USA). The lithium disilicate crowns for Group B were milled in Lab MC XL (Dentsply, Sirona, Charlotte, NC, USA).
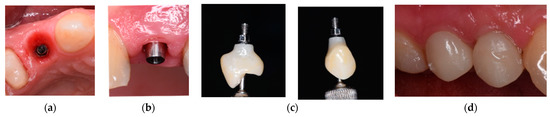
Figure 1.
Prosthetic restoration used for Group A.
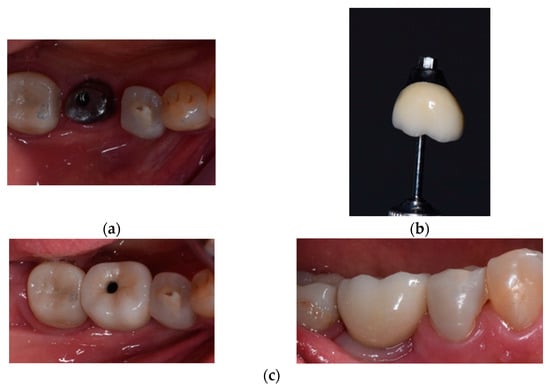
Figure 2.
Prosthetic restoration used for Group B.
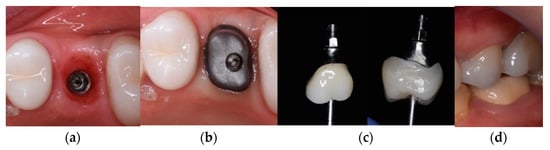
Figure 3.
Prosthetic restoration used in Group C.
The images in Figure 1 depict the following:
- (a)
- The emergence profile formed by the provisional prosthetic structure;
- (b)
- The standard titanium base fixed to the implant at Tooth 14;
- (c)
- The implant suprastructure of Tooth 14 cemented extraorally;
- (d)
- The implant suprastructure fixed on the implant.
The images in Figure 2 depict the following:
- (a)
- Individualized titanium suprastructure fixed to the implant at Tooth 36;
- (b)
- The implant suprastructure cemented extraorally at Tooth 36;
- (c)
- The implant suprastructure fixed on the implant—occlusal and vestibular view.
The images in Figure 3 depict the following:
- (a)
- The emergence profile formed by the provisional prosthetic structure;
- (b)
- The individualized titanium suprastructure fixed to the implant at Tooth 26;
- (c)
- The implant suprastructure cemented extraorally to Tooth 36;
- (d)
- The implant suprastructure fixed on the implant in the mouth—vestibular view.
All of the patients were invited to a follow-up examination at 2 weeks and 1 year after the definitive prosthesis Restoration was assessed according to the functional, biological [10,11,12,13], and aesthetic indicators [12] described in Table 4. The follow-up for all of the cases was carried out by an experienced clinician (Table 2). The statistical analysis was conducted using SPSS 22.0 software (SPSS, IBM, Armonk, NY, USA).

Table 4.
Assessment according to functional, biological, and aesthetic indicators.
The indicators detailed below were evaluated following the applied methods:
The examination was performed using magnifiers with a magnification of x3.6 (Zeiss, Jena, Germany) and with a straight probe.
The articulation relations were verified using articulation paper with a micro thickness of 40 µ (Bausch, Hainspitz, Germany).
Using the parallel technique (Updegrave 1951), segmental radiography was performed by an experienced X-ray technician for each patient. Digital measurements of the bone loss level were performed in the event of definitive prosthetics and at 1 year. The platform of the implant was taken as a reference point. Two lines parallel to the implant descended medially and distally to the level of the first thread, which was in contact with the bone (Figure 4).
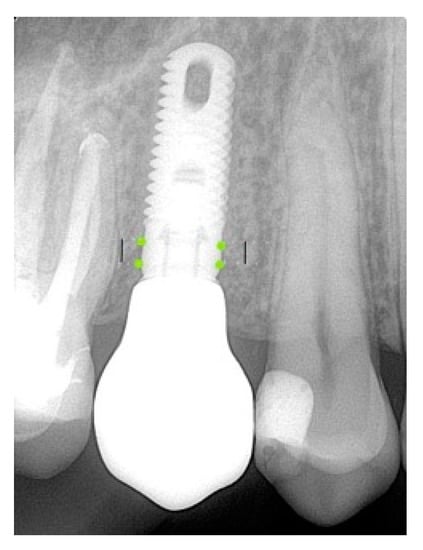
Figure 4.
Measurement of the bone loss level.
3. Results
Regarding the functional criteria, no deviations from the reference range were registered in any of the study groups for both measurements.
The measurement results for the biological criteria were as follows:
With regard to the plaque retentive index, it can be concluded that in the supra-implant restorations, a combination of an individualized titanium base and monolithic lithium disilicate crown (Group B) exhibits the best behavior (the absence of plaque around the restorations). This conclusion was valid for both observation periods.
The highest amount of plaque was observed in Group C.
In Groups A and C, the plaque increased during the observation period. This increase was more noticeable in Group C. The value of the index reached 0.56 in one year, and the increase was statistically significant (p = 0.01). In Group A, the observed increase from 0.17 to 0.22 was characterized by static insignificance (p = 0.08) (Table 5).

Table 5.
Analysis of the plaque retention index: temporal changes and differences between the groups.
Group A showed the worst performance in terms of bleeding on the oral side at the 1-year follow-up. The best general mean values of the bleeding indicator were observed in Group B, where initially, there was no bleeding in the second week, while at 1 year, the value remained relatively low at 0.22 on both the vestibular and oral sides. On both sides—vestibular and oral—there were statistically significant differences in the second week due to the increased values of the bleeding indicator in Group A. The differences became insignificant between the groups after one year of observations. On the vestibular side, p = 0.39, while on the oral side, p = 0.35 (Table 6).

Table 6.
Temporal analysis of bleeding index in patients with different types of implant suprastructures.
Group B had the lowest periodontal pocket depth (PPD) values throughout the study period, followed by Group C. The highest values were reported in the prosthetic restorations used in Group A, increasing by almost two times over a period of 1 year.
In Group C, there was a statistically insignificant decrease in the pocket depth value (p = 0.46). In Groups A and B, there were increases in the observed period, as shown in the table. In both cases, these were statistically significant, with p = 0.03 and p = 0.00 (Table 7).

Table 7.
Pocket depth analysis while probing: temporal changes and differences between groups.
Despite the observed differences in the dynamic development of the three groups, the patients in Group B maintained the lowest pocket depth values during probing compared to the other groups. It is also important to note that statistically significant differences were observed when the three groups were compared in the second week and after one year (p = 0.00). In both measurements, the indicator remained statistically significantly lower in Group B compared to the other two groups.
The radiography results showed no bone loss in any of the study groups.
A series of T-tests were performed in order to assess the significance of the variations in the mean values of bone loss for each group on the basis of the two time periods (Table 8).

Table 8.
Bone loss measurements—statistical findings (mm).
Jempt’s papillae height index had the highest values in Group B, increasing by two times over the course of the study period.
The results showed an increase in this index in all three patient groups, as measured by the means. The increase was statistically significant in Groups B and C, while the growth in Group A remained statistically insignificant (p = 0.10).
In the distal papillae, Group B once again showed the highest mean for Jempt’s papillary height index compared to the other two groups, reaching an average of 2.56. However, the observed increases were only statistically significant only Group C, where the largest increase in the indicator was observed. In Group B, the increase was statistically insignificant over the study period (p = 0.17). Similarly, Group A also recorded a statistically insignificant increase (p = 0.30) (Table 9).

Table 9.
Jempt’s papillae height analysis for each group.
With regard to the medial papilla, there were no statistically significant differences between the groups in the two measurements at both two weeks and one year.
The aesthetic indicator “Gingiva color around the restoration” showed a mild margo gingivalis graying of the otherwise clinically healthy PIM in Groups B and C, with index scores of 1–2 (pale pink to pink) compared to Group A (Figure 5).
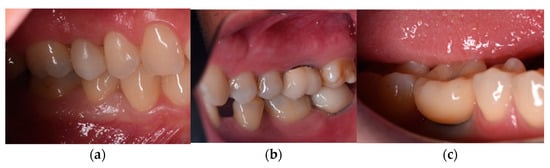
Figure 5.
Aesthetic indicator “Gingiva color around the restoration”—(a) Group A—15. (b) Group B—47. (c) Group C—46.
4. Discussion
No functional or technical complications were observed during the study period, which is consistent with the results reported by Bosch et al. [14]. This allows us to conclude that the standard and individualized abutments have equally favorable behavior in terms of mechanical stability.
In their study, Bompolaki et al. [15] found a greater number of functional complications in supra-implant restorations made with factory abutments. De Angelis et al. [16] reported isolated cases of chipping in the lithium disilicate crown group; however, the replacement was unnecessary. All of the authors of previously published studies indicated a low rate of functional complications between study groups, which was statistically insignificant.
Gulje et al. [17] followed up on certain zirconium implant restorations with individualized titanium abutments for a period of 1 year and achieved low values of the considered biological indicators, similar to our results. Schepke et al. [18] conducted a comparative assessment of factory-made and individualized abutments and reported that they did not observe any statistically significant differences in the biological factors, which is not consistent with our findings.
However, we found statistically significant values for some of the biological indicators, highlighting that the group with individualized titanium abutments with monolithic restorations made of lithium disilicate had the lowest values in all of the measured criteria.
In their study, Joda и Bragger [19] compared digitally fabricated implant suprastructures according to aesthetic criteria and noted that “graying” was visually observed in the marginal area of prosthetic restorations with individualized abutments. This opinion was confirmed by other authors [19,20,21] and coincided with our observations.
Our results confirmed the opinion of Borges et al. [22] that prosthetic restorations on individualized abutments manufactured with CAD/CAM technology improve aesthetics more than standard abutments due to the better design of the soft tissues of the peri-implant and the restoration of the interproximal papillae.
The presence of a statistically significant difference between the groups and the best values of Group B best shows the effectiveness of the material and design of permanent supra-implant prosthetics in achieving significantly higher Jempt scores for papillae growth than alternative methods. At present, there have been no other studies considering pre-milled abutments with titanium blanks.
The study that was conducted in this work enrolled 23 patients with a total of 27 implants in different areas of the mouth, and a clinical evaluation of the biomechanical parameters of the individualized titanium alloy abutments showed that individualized implant abutments made of titanium alloy and monolithic restorations made of lithium disilicate or zirconium dioxide have stable biomechanical behavior and may be the optimal choice for prosthetic treatment The working hypothesis of this study, which was that the pre-milled abutments with titanium blanks with genuine connections in the combination ceramic material of the crown would be advantageous due to the possibility of individual varieties of transmucosal profiles based on functional, biological, and aesthetic indicators, was proven. Definitive guidance regarding the use of pre-milled abutments with titanium blanks was presented in this study.
Our findings coincide with the opinions of a number of authors that this type of abutment recreates the emergence profile of the natural tooth as accurately as possible, eliminating the possibility of over contouring the entire suprastructure caused by the volumetric cylindrical cross-section of the standardized abutment and the required thickness of the definitive crown [13,23,24,25].
5. Conclusions
Individualized implant abutments made of titanium alloy and monolithic restorations made of lithium disilicate or zirconium dioxide have stable biomechanical behavior and may be the optimal choice for the prosthetic treatment of partial edentulousness. Because this study was carried out over a limited period of time, clinical trials with a longer follow-up period need to be carried out.
Author Contributions
Conceptualization, D.S. and D.F.; methodology, D.S.; software, G.I.; validation, G.I. and N.N.; formal analysis, D.S. and S.S.; investigation, D.S. and S.S.; resources, D.S.; data curation, D.F.; writing—original draft preparation, D.S.; writing—review and editing, G.I. and Z.P.; visualization, N.N. and Z.P.; supervision, G.I.; project administration, N.N.; funding acquisition, D.S. All authors have read and agreed to the published version of the manuscript.
Funding
The materials necessary to implement this scientific research were provided under GRANT No. 110/24.06.2020 project.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Scientific Research (Medical University Sofia, Bulgaria (part of project GRANT No. 110/24.06.2020)) under trial registry approval 12/14.05.2020.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
For further information, please contact the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bennardo, F.; Barone, S.; Vocaturo, C.; Nucci, L.; Antonelli, A.; Giudice, A. Usefulness of Magnetic Mallet in Oral Surgery and Implantology: A Systematic Review. J. Pers. Med. 2022, 12, 108. [Google Scholar] [CrossRef]
- Blatz, M.B.; Bergler, M.; Holst, S.; Block, M.S. Zirconia abutments for single-tooth implants-rationale and clinical guidelines. J. Oral Maxillofac. Surg. 2009, 67, 74–81. [Google Scholar] [CrossRef]
- Alikhasi, M.; Monzavi, A.; Bassir, S.; Naini, R.; Khosronedjad, N.; Keshavarz, S. Comparison of precision of fit, rotational freedom, and torque loss with copy-milled zirconia and prefabricated titanium abutments. Int. J. Oral Maxillofac. Surg. Res. 2013, 28, 996–1002. [Google Scholar] [CrossRef]
- Adatia, N.D.; Bayne, S.; Cooper, L.; Thomson, J. Fracture resistance of yttria-stabilized zirconia dental implant abutment. J. Prosthodont. 2009, 18, 17–22. [Google Scholar] [CrossRef]
- Marchack, B. A custom titanium abutment for the anterior single-tooth implant. J. Prosthet. Dent. 1996, 76, 288–291. [Google Scholar] [CrossRef]
- Bittner, N.; Lal, K.; Neurohr, J. Fabrication of custom abutment for a wide-diameter implant in a situation with limited interocclusal space. J. Prosthet. Dent. 2008, 100, 474–477. [Google Scholar] [CrossRef]
- Magne, P.; Magne, M.; Jovanovic, S. An esthetic solution for single-implant restorations—Type III porcelain veneer bonded to a screw-retained custom abutment: A clinical report. J. Prosthet. Dent. 2008, 99, 2–7. [Google Scholar] [CrossRef]
- Kim, H.; Peak, J. Customized locator abutment fabrication on inclined implants: A clinical report. J. Prosthet. Dent. 2017, 119, 522–525. [Google Scholar] [CrossRef]
- Long, L.; Alqarni, H.; Masri, R. Influence of implant abutment fabrication method on clinical outcomes: A systematic review. Eur. J. Oral Implantol. 2017, 10 (Suppl. S1), 67–77. [Google Scholar]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol. Scand 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Jemt, T. Regeneration of gingival papillae after single-implant treatment. Int. J. Periodontics Restor. Dent. 1997, 17, 326–333. [Google Scholar]
- Parpariola, A.; Norton, M.; Cecchinato, D.; Berssan, E.; Toia, M. Virtual Abutment Design: A concept for delivery of CAD/CAM customized abutments-report of a retrospective cohort. Int. J. Periodontics Restor. Dent. 2013, 33, 51–58. [Google Scholar] [CrossRef]
- Bosh, A.; Jung, R.; Sailer, I.; Hammerle, C.; Thoma, D. Single-tooth replacement using dental implants supporting all-ceramic and metal-based reconstructions: Results at 18 monts of loading. Int. J. Periodontics Restor. Dent. 2018, 38, 173–179. [Google Scholar] [CrossRef]
- Bompolaki, D.; Punj, A.; Fellows, C.; Truong, C.; Ferracane, J. Clinical Performance of CAD/CAM Monolithic Lithium Disilicate Implant-Supported Single Crowns Using Solid or Predrilled Blocks in a Fully Digital Workflow: A Retrospective Cohort Study with up to 33 Months of Follow up. J. Prosthodont. 2021, 31, 38–44. [Google Scholar] [CrossRef]
- De Angelis, P.; Passarelli, C.; Gasparini, G.; Boniello, R.; DÁmato, G.; De Angelis, S. Monolithic CAD-CAM lithium disilicate versus monolithic CAD-CAM zirconia for single implant-supported posterior crowns using a digital workflow: A 3-year cross-sectional retrospective study. J. Prosthet. Dent. 2020, 123, 252–256. [Google Scholar] [CrossRef]
- Gulje, F.; Raghoebar, G.; Vissink, A.; Meijer, H. Single Restorations in the Resorbed Posterior Mandible Supported by 6-mm Implants: A 1-Year Prospective Case Series Study. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S2), e465–e471. [Google Scholar] [CrossRef]
- Schepke, U.; Meijer, H.; Kerdijk, W.; Raghoebar, G.; Cune, M. Stock versus CAD/CAM customized zirconia implant abutments—clinical and patient-based outcomes in a randomized controlled clinical trial. Clin. Implant. Dent. Relat. Res. 2017, 19, 74–84. [Google Scholar] [CrossRef]
- Joda, T.; Bragger, U. Complete digital workflow for the production of implant-supported single-unit monolithic crowns. Clin. Oral Implant. Res. 2014, 25, 1304–1306. [Google Scholar] [CrossRef]
- Sailer, I.; Zembic, A.; Jung, R.; Siegenthaler, D.; Holderegger, C.; Hammerle, C. Randomized controlled clinical trial of customized zirconia and titanium implant abutments for canine and posterior single-tooth implant reconstructions: Preliminary results at 1 year of function. Clin. Oral Implant. Res. 2009, 20, 219–225. [Google Scholar] [CrossRef]
- Zembic, A.; Sailer, I.; Jung, R.E.; Hammerle, C. Randomized-controlled clinical trial of customized zirconia and titanium implant abutment for single-tooth implants in canine and posterior regions: 3 year results. Clin. Oral Implant. Res. 2009, 20, 802–808. [Google Scholar] [CrossRef]
- Borges, T.; Lima, T.; Carvalho, A.; Dourado, C.; Carvalho, V. The influence of customized abutments and custom metal abutments on the presence of the interproximal papilla at implants inserted in single-unit gaps: A 1-year prospective clinical study. Clin. Oral Implant. Res. 2014, 25, 1222–1227. [Google Scholar] [CrossRef]
- Tripodakis, A.P.D.; Strub, J.R.; Kappert, H.F.; Witkowski, S. Strength and mode of failure of single implant all-ceramic abutment restorations under static load. Int. J. Prosthodont. 1995, 8, 265–272. [Google Scholar]
- Nikolova, N. The Application of Provisional Implant Crowns as Prosthetic Instrument for Management of Peri-Implant Soft Tissue. Ph.D. Thesis, Medical University of Sofia, Sofia, Bulgaria, 2020. [Google Scholar]
- Happe, A.; von Glasser, G.; Neugebauer, J.; Strick, K.; Smeets, R.; Rutkowski, R. Clinical performance of zirconia implant abutments luted to a titanium base—A retrospective cross-sectional study. Int. J. Comput. Dent. 2022, 25, 37–45. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).