Abstract
In this work, the surface dielectric barrier discharge (SDBD) plasma treatment was used to sterilize the palm date fruits. N2SPS, N2FNS, and hydroxyl radical have emerged in the emission spectrum of the plasma from SDBD. The effects of SDBD plasma on A. niger that was extracted from palm date varieties were investigated. After 15 days of incubation, the reduction of A. niger at a 3 min exposure time was 4 log. The total phenolic content of the Ajwa variety after SDBD plasma treatment has been documented as the highest value among the other varieties; it was 1.65-fold of the untreated one. The treated Ajwa variety using SDBD plasma has recorded the highest increase in antioxidant activity; it was increased to 67.69% compared to the control one. After SDBD plasma treatment, the HMF was not detected in the Maghol variety. According to the PCA model, the first two PCs demonstrated strong positive correlations with most of the examined variables and demonstrated a strong positive correlation between these variables when assessed in both untreated and treated with SDBD plasma of palm date types in this stud.
1. Introduction
Consumers always look for safe foods that meet quality standards that traditional food preservation methods do not provide [1]. To enhance the safety of foodstuffs and extend their shelf life, thermal treatments have been used for many years and have been met with general satisfaction by consumers. However, the deficiency of nutrients and the low quality of organoleptic properties are considered the disadvantages of thermal treatments for food items [2]. Due to this, researchers have been trying to come up with new, low-cost, and environmentally friendly ways to kill microbes and extend the shelf life of foods without changing their nutrients or physicochemical properties [3].
Recently, non-thermal atmospheric pressure plasma technology has emerged to overcome the disadvantages of thermal treatments [4,5]. Non-thermal plasma technology has not only been used to decontaminate and extend the shelf life of food products, but it is also employed to improve the bioactive components of food products [6]. The reactive species generated during non-thermal plasma treatment may contribute to the increase in nutritious and bioactive compounds [7].
Recent experiments have demonstrated that the non-thermal plasma method can enhance bioactive compounds [5], inhibit microorganisms [8], inactivate endogenous spoilage enzymes, improve shelf life [9], and maintain the physicochemical properties of food items [10,11]. Previous research has shown that non-thermal plasma had little or no effect on phenolic content and antioxidant capacity as bioactive components [12]. The change of bioactive components is dependent on non-thermal plasma parameters and the food matrix [13]. Since it is performed at low temperatures, non-thermal plasma may maintain bioactive components, thus overcoming the effect of heat on food quality [12].
Non-thermal plasma technology has played a crucial role in reducing oxygen and increasing carbon dioxide concentrations in the gaseous environment when treating fresh apples, resulting in modifications to the respiratory pathway [14]. This alteration inactivates the indigenous enzymes of fruits and vegetables, resulting in a slowed maturation rate [15].
Palm date is one of the most consumed fruits in the Middle East, particularly in Saudi Arabia, due to its high nutritional content and delicious flavor. Palm dates have a variety of bioactive compounds, including antimutagenic, antioxidants, carotenoids, flavonoids, and phenolics [16]. Unfortunately, palm dates are susceptible to several funguses in the field, during transit, and during marketing. Moreover, palm dates are susceptible to several diseases and pests that degrade this strategic crop. In many instances, most dates are consumed without treatment to reduce microbial contamination. A. niger is the most common form of fungus that infects dates in Saudi Arabia [17]. The contamination of dates with A. niger is a major concern owing to the creation of poisonous chemicals that spread throughout the fruit’s flesh, creating severe health issues for humans. One of the most significant sources of mycotoxins is black fungus [18].
Different techniques, including ozone, UV, gamma radiation, and antifungal drugs, have been used to suppress fungus and limit the development of mycotoxins in products [17]. Although these procedures may avoid surface contamination, they have unfavorable side effects, such as lowering product quality and altering nutrients, which lowers customer demand for foods treated with these methods [18]. In addition, these techniques are considered impractical due to their high cost or slow speed [19].
In this study, surface dielectric barrier discharges (SDBD) were used to inhibit the growth of black fungi (A. niger). In addition, A. niger has been extracted from some palm date cultivars. The impact of SDBD plasma on the bioactive material, chemical content, and hydroxymethylfurfural (HMF) levels of some palm date cultivars has been evaluated. According to our knowledge, no previous studies have examined the impact of non-thermal plasma on the chemical composition of palm date fruits.
2. Experimental Setup
2.1. Plasma System
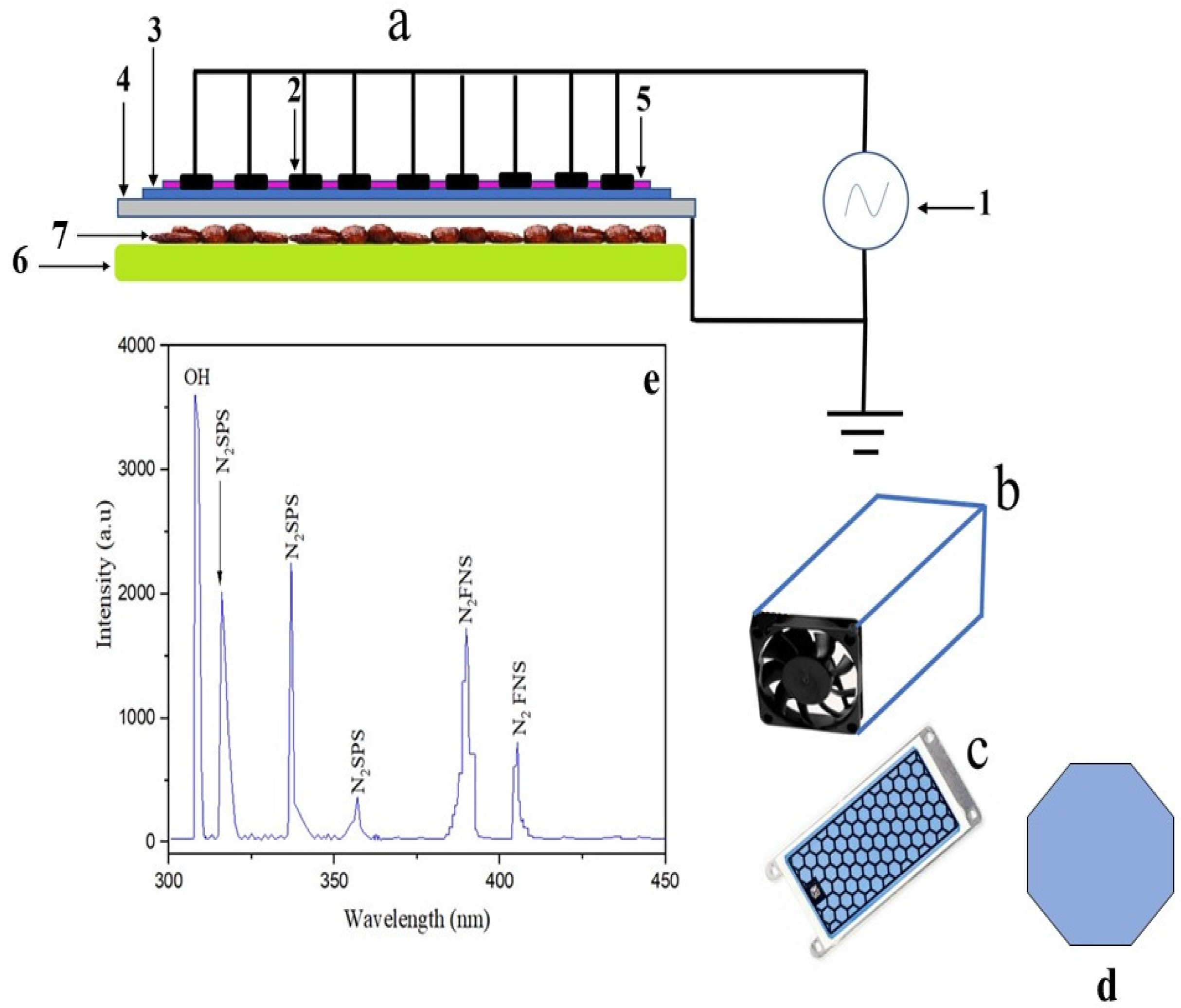
The electric circuit for surface dielectric barrier discharges (SDBD) (Figure 1a) was set in a Teflon box (24 × 12 × 8 cm) (Figure 1b). A cooling fan was added to the Teflon box to reduce the plasma temperature. The speed of the cooler’s fan ranged between 200 and 1200 rpm. After putting the specimen in the Teflon box, the opposite side of the cooling fan was sealed with a cork plug. The plasma treatment was performed by a commercially available (SDBD) with alumina dielectric and printed electrodes in a honeycomb configuration (Figure 1c). The honeycomb breadboard was comprised of 9 × 4 cm2 of alumina dielectric surface with a 1 mm thickness and a 7 mm diameter aluminum esagonal exposed electrode. The ground electrode was a metallic plain plate. Plasma was created via a high voltage (HV) power supply operating between 8 and 30 W [20] and a frequency of the order of 10 kHz. The applied HV was in the 5–10 kVpp range [21,22]. In addition, (HR4000CG-UV-NIR Ocean insight, Orlando, FL, USA) spectroscopy was used to identify the emission spectra of producing plasma across a wide wavelength range of 200–900 nm in the present experiment. In addition, the spectrometer has a 400-mm diameter lens coupled to a detector via fiber optic cable (QP400-2-SR, Ocean insight, Orlando, FL, USA).
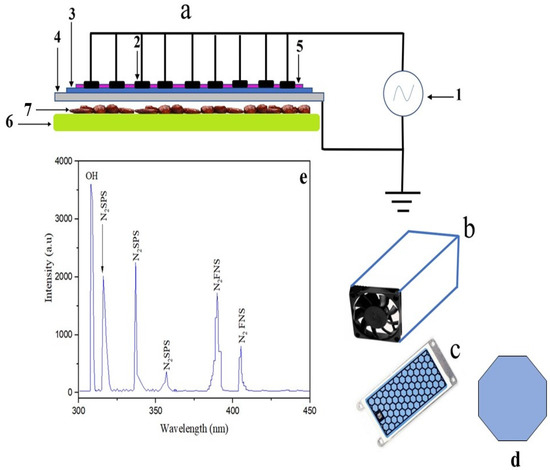
Figure 1.
SDBD electric circuit (a); (Power supply (1), HV electrode (2), Dielectric barrier (3), Ground electrode (4), Plasma (5), Sample holder (6) Palm date samples (7)), Teflon box contains the SDBD system (b), honeycomb geometry of SDBD (c), the cell geometry of honeycomb (d), and the emission spectrum of the plasma from SDBD in the range of (200–900 nm) (e).
2.2. Sample Collection for Fungi Inhibition Using SDBD
Twenty-five samples of dates (2 kg/sample-Tamr stage) were randomly chosen from seven date cultivars (Ajwa, Rashodia, Nabtet Ali, Magdol, Shalabi, Sukkari, and Khudari) in the Al-Ahsa region of Saudi Arabia. Special paper bags were used for sampling, and the samples were transferred aseptically to the Dates Quality and Safety Unit at the Palm and Dates Center within 15 min in an ice box container.
2.3. Isolation and Identification of Fungi Associated with Dates
One hundred grams of each sample was physically examined for fungi [23] in order to separate the obviously infected dates. In addition, 10 dates from each sample were surface-sterilized with a 2% sodium hypochlorite solution for 2 min, washed twice with sterile double-distilled water, and then cut into tiny pieces (1 cm2/piece) under aseptic conditions. Each fragment was individually cultivated on Potato Dextrose Agar (PDA) plates and incubated at 26 to 28 °C ± 1; RH 72–75 ± 1 for 5 days. Single hyphen tips of each different shape or color were sub-cultured under the same previous conditions until pure fungal growth was observed. Aspergillus niger, Fusarium, and Penicillium are the fungal species that have been isolated from all varieties of palm dates. The pure isolates were identified manually in accordance with [24,25] and then stored at 4 °C until use. A. niger represented the highest percentage of isolated fungus in our study. In this experiment, A. niger was chosen due to its high resistance to non-thermal plasma treatment [26]. A glass slide was sterilized with ethanol and then cleaned with a towel sprayed with 70% ethanol. In every run, a 5 mm A. niger disc was treated. The fungi disc has been put onto the glass slide. The SDBD plasma system was used to inhibit A. niger. The samples of fungi were placed on the sample holder at a distance of 0.5 cm from the electrode. In addition, the plasma dose supplied by the SDBD plasma system was 1, 1.5, 2, 2.5, and 3 min.
2.4. Sample Collection for Palm Dates Treatment Using SDBD
All experiments employed seven prevalent Saudi date cultivars at the Tamer stage (Ajwa, Rashodia, Nabtet Ali, Magdol, Shalabi, Sukkari, and Khudari). The experiments took place in Al-Ahsa, Saudi Arabia, at the Date Palm Research Center. After sorting the dates, the deteriorated fruits were removed. Healthy fruits were kept at 5 °C for less than 24 h. The palm dates were treated with the SDBD system for 3 min. In each run, 10–15 samples of one sort were spaced 0.5 cm apart from the electrode according to the electrode’s surface area and the volume of dates.
2.5. Physicochemical Measurements
Total carbohydrate, protein, lipid, and ash contents of date fruits samples were evaluated utilizing AOAC procedures [27]. The amount of ash was calculated using technique [28]. Before being weighed, samples were dried and cooled in a desiccator. Two grammes of the sample were measured and weighed in the crucible. The sample-containing crucible was inserted in the muffle furnace and ignited at 550 °C. The temperature remained constant for three hours. After allowing the muffle furnace to cool, the crucibles were removed, cooled, and weighed. The ash percentage was determined as follows:
where W2 = weight of crucible + ash, W1 = weight of empty crucible.
According to [29], the lipid content of the samples was measured using solvent extraction in a Soxhlet system. Two grammes of each sample was wrapped in filter paper and put in a Soxhlet reflux flask connected to a condenser and an oil extraction flask containing 200 mL of petroleum ether. When the ether reached its boiling point, the vapor condensed in the reflux flask, totally submerging the samples for extraction upon filling the reflux flask syphons and returning the oil extract to the boiling ether in the flask. Four hours were given for the boiling, condensation, and reflux processes before the defoamed samples were removed. The oil extract in the flux was oven-dried at 60 degrees Celsius for thirty minutes before being weighed.
where: W1 = weight of oven dried thimble, W2 = weight of sample used, W3 = weight of round bottom flask, and W4 = weight of round bottom flask with fat residue.
According to [28], the protein content of the samples was evaluated using the Kjedahl technique. Incorporating one gram of the sample into the digestion flask. An addition of Kjedahl catalyst (selenium tablets) was made to the sample. The sample was treated with 20 milliliters of strong sulfuric acid for eight hours, until a clear solution was produced. The digest was put into a 100 mL volumetric flask and topped up with distilled water. After boiling, the equipment for distillation was arranged and washed for 10 min. Twenty mils of boric acid at a concentration of 4% were pipetted into a conical flask. As an indicator, five drops of methyl red were added to the flask, and the sample was diluted with seventy-five milliliters of distilled water. Ten milliliters of the digest were alkalized with 20 milliliters of NaOH (20%) and then distilled. The distillery’s steam outlet was sealed, and the moment at which the boric acid solution became green was recorded. The combination underwent fifteen minutes of distillation. Afterwards, the filtrate was titrated against 0.1 N HCL.
The overall percentage was approximated:
% protein = % nitrogen × conversion factor (6.25)
The total sugar content in different varieties of control and treated date fruits was analyzed using a dinitrosalicylic acid reagent according to the standard procedure reported by [30]. Carbohydrate content of the Palm date samples was determined according to [29].
To determine the amount of amino acid of treated and control palm dates; 1 g of the sample was mixed with 5 mL H2O and 5 mL of HCl (Note: final conc. of HCl is 6 M) and then heated at 100 °C for 24 h and then filtered. Finally, 1 mL of the filtrate was injected into HPLC [31]. On the other hand, calcium and magnesium have been determined using the Versenate method [32], while the Ascorbic acid method was used to estimate phosphorus. However, the flame photometer was utilized to appreciate potassium and sodium [33]. Moreover, Inductively Coupled Plasma (ICP) Emission Spectroscopy has been used to determine Mn, Fe, Cu, and Zn in date samples [34]. On the other hand, extraction of HMF has been performed using solid phase microextraction (SPME) techniques [35]. Further, the contents of total phenolics in samples were analyzed by the Folin–Ciocalteu colorimetric method [36]. The 100 g of fresh weight of the edible part of fruits was weighed and homogenized with chilled 80% acetone (1:2, w/v) using a chilled Waring blender for 5 min. Following that, a Polytron homogenizer was used to homogenize the date samples for a further 3 min. Whatman paper (No. 2) has been used to filter the homogenates under vacuum in a Buchner funnel. At 45 °C, the acetone in the filtrate vaporized until 90% of the filtrate was vaporized. The filtrate was then recovered to a final volume of 50 mL using water. The soluble free phenolic extracts were stored until used at 40 °C. It has been found to contain both free aglycones and soluble conjugates (glycosylated forms). However, HPLC analysis was carried out to determine water soluble vitamins in treated and untreated palm dates [37]. Moreover, the radical scavenging activity (RSA) has been evaluated by DPPH according to the following procedure [38]. Ascorbic acid has been dissolved in methanol to produce a 50 mL stock solution with a concentration of 500 ppm to be used as a standard antioxidant for comparison in the activity. Fifty milliliter stock solutions for each palm date extract have been prepared by the same method. From each stock solution of palm date extract, a triplicate of 5 mL of solution each of 40, 60, 80, and 100 ppm concentrations was prepared in separate test tubes by using the formula of serial dilution C1V1 = C2V2. For each test tube, 1 mL of 2,2-diphenyl-2-picrylhydrazyl (DPPH) has been added. All the test tubes were incubated in the dark for 30 min at room temperature. Following that, the absorbance was measured using a spectrophotometer at 517 nm. The difference in the absorbance between the test solution and the control (DPPH in methanol) was calculated and expressed as percent scavenging or percent inhibition of the DPPH radical.
RSA was calculated as follows:
2.6. Statistical Analysis
The mean standard error (SE) is used to express data values. An analysis of variance (ANOVA) was performed to determine the effect of treatment using the SPSS software package (version 20). The test of significance of the means was by the Least Significant Difference (LSD) when the ANOVA suggested a significant difference at p ≤ 0.05. The principal component analysis was applied for a better understanding of the relationship among studied measures across palm date varieties that were both untreated and treated with SDBD plasma, using Origin Pro 2021 version b 9.5.0.193, Northampton, MA 01060, US computer software program.
3. Results and Discussion
3.1. Plasma Diagnostics
SDBD is based on the separation of metallic electrodes by a dielectric material, which is typically powered by an alternating current (AC) (0.05–500 kHz) high-voltage (HV) source [39]. The dielectric barrier limits current and collects surface charges to avoid sparks and arcs. Typically, SDBD micro-discharges are short-lived streamers that form either in volume or along the dielectric surface [40]. In dielectric barrier discharges, the production of a micro-discharge is invariably associated with charge accumulations on the dielectric surface and, subsequently, the generation of an electrical field in the opposite direction. This eventually causes a quick drop in current flow and the micro-discharge to self-terminate. The charge buildup causes a fresh micro-discharge in a new AC half-period; hence, the applied AC frequency must be sufficient to maintain the electrode surfaces with the charge collected because of the previous discharge event [41]. The generation of plasma at low temperatures and the high degree of diffusivity of SDBD are their primary benefits, which makes them effective surface treatments. The creation of plasma with low-temperature diffusivity is mostly dependent on the frequency and gas used to form the plasma [42]. Due to the plane-parallel geometry, huge effective plasma surface, and the ability to operate with just air as the supplying gas, we used air SDBD. When the discharge was ignited, transient microfilament streamers developed at random spots, and they transmitted a larger discharge current than the remainder of the discharge. However, because of the nature of an SDBD source, the total filament current was restricted, limiting the creation of hotspots. The design and application ease of this kind of plasma source was another factor in its selection. It does not need the addition of feeding gas, as a key aspect of future technology, and can be scaled up [43].
Optical emission spectroscopy (OES) was used to measure the SDBD plasma emission spectra in the 200–900 nm range. Figure 1e shows the emission spectra up to 450 nm. In the spectra of SDBD plasma, the nitrogen second positive system (N2 SPS), nitrogen first negative system (N2 FNS), and OH radical were seen. (N2 SPS) spectral lines have appeared at 316, 337, and 357 nm. Moreover, at 389 and 405 nm, (N2 FNS) lines have developed. Furthermore, the OH radical has occurred at a wavelength of 308.9 nm. In addition, OES of air plasma often reveals the presence of excited nitrogen species, atomic oxygen, and hydroxyl radicals [44]. The reactive species created by non-thermal plasma induce an oxidative stress response that leads to harmful oxidative cell deterioration [45], therefore inhibiting microorganisms [46]. Hydroxyl radical is among the most powerful oxidants that decompose and oxidize toxic substances [47]. Alternatively, hydroxyl radicals enhance the microbicidal action [48]. Hydroxyl radicals have a serious influence on the double layer of glycerophospholipids and proteins that form the cell membranes of microorganisms [49].
3.2. Inhibition of A. niger Using SDBD Plasma
It is generally recognized that microbial development is one of the main causes of palm date deterioration during storage. Therefore, deactivating or eliminating microorganisms is necessary for the safe storage of palm dates. Furthermore, non-thermal plasma is regarded as an effective method for surface decontamination of solid foods due to its inability to penetrate food samples deeper than a few micrometers [50]. Therefore, non-thermal plasma has no effect on the basic nutritious components of fresh fruits and vegetables [51].
Therefore, we examined the impact of plasma treatment with SDBD on A. niger extracted from several palm date varieties. The effects of different amounts of SDBD plasma on A. niger after 15 days of incubation are shown in Table 1. As the duration of treatment increases, so does the ability of SDBD plasma to suppress A. niger. According to these findings, as plasma processing time increases, the number of A. niger survivors (CFU/g) decreases. Moreover, the number of A. niger survivors in control samples was 4.33 ± 0.09 CFU/g. However, the number of A. niger cells was reduced from 2.50 ± 0.29 to 0.33 ± 0.03 CFU/g when the SDBD plasma duration was extended from 1 to 3 min. The reduction of A. niger at a 1 min exposure period was 1.83 logs, whereas the reduction at a 3 min exposure time was 4 logs. The results showed statistically significant (p < 0.05) differences in the percentages of A. niger survivors depending on how long they were treated with plasma.

Table 1.
The effect of SDBD plasma on A. niger spore log (CFU/g) at different treatment time; p value = 0.00.
After 16 and 17 days of incubation, the numbers of A. niger survivors were 4.47 ± 0.03 and 4.50 ± 0.00 CFU/g for control samples, respectively. In addition, when the exposure duration increases from 1 to 2.5 min, the number of A. niger survivors increases throughout the incubation period. These findings indicate that the number of A. Niger survivors did not vary with incubation time when samples were treated for three minutes. After 16 and 17 days of incubation, the growth rate of A. niger cells decreased gradually as the treatment period increased from 1 to 3 min, eventually reaching zero at 3 min. After 16 and 17 days of incubation, there was no statistically significant influence (p > 0.05) on the growth rate of A. niger. It was found that 3 min of exposure to SDBD plasma was the best way to stop A. niger from growing.
Findings indicated that double jet argon plasma treatment suppressed A. niger spores on date palm fruit slices [18]. In addition, they observed that non-thermal plasma reduced the ability of toxic fungi to produce mycotoxins in food and feed. The destruction of microorganisms by the use of non-thermal plasma is considered a complicated process due to the many factors involved [19].
Reactive species generated by non-thermal plasma may significantly impair the cell integrity of microorganisms [44]. The polysaccharide-rich wall and outermost layer of microbial cells are directly altered by the reactive species generated by the non-thermal plasma. The organic molecules composing microbe cells are subjected to non-thermal plasma etching; as a result, the bonds of hydrocarbon compounds that comprise these organic compounds are destroyed [52]. Due to the easily reactive species with the broken links, these compounds will be etched more rapidly [53]. As a result, microorganism cells will be completely distracted.
3.3. Total Phenolic
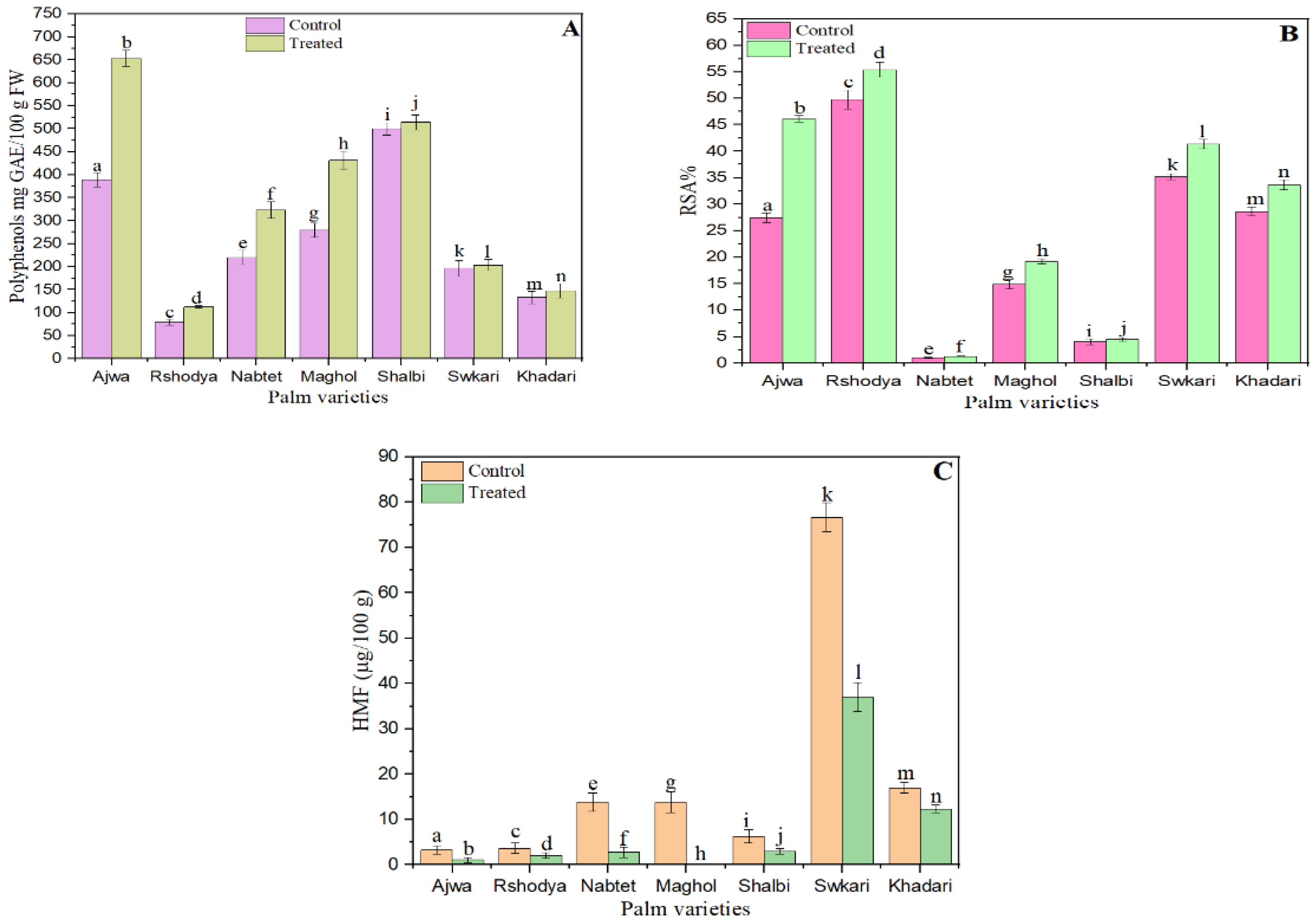
In terms of mg/g gallic acid equivalents (GAE), the influence of SDBD plasma on the total phenolic content of palm varieties was evaluated. As seen in Figure 2A, the total phenol content of all treated date cultivars increased when palm dates were treated with plasma generated from SDBD. The total phenolic content of untreated samples varied from 79.60 ± 6.36 to 500.33 ± 13.50, whereas treated samples ranged from 112.71 ± 3.16 to 653 ± 17.47. According to the findings, the total phenol content of treated and untreated samples differed significantly (p < 0.05). The plasma-treated Shalbi cultivars had the smallest rise in total phenolic, which was 1.02 times that of untreated ones. On the other hand, the treated Ajwa variety showed the greatest rise in total phenolic, with a 1.65-fold increase over the untreated type.
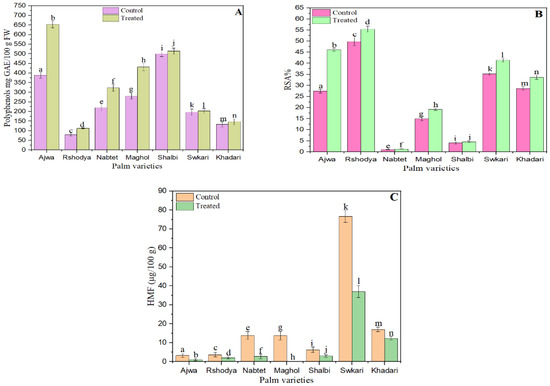
Figure 2.
The effect of SDBD plasma on (A) total phenol p value = 0.00, (B) antioxidant, p value = 0.00, and (C) HMF, p value = 0.00 of palm dates, lowercase letters a–n in the same column represent significance analysis; the different letters mean significant difference among various treatments at p < 0.05 level.
The impact of non-thermal plasma on mandarin peel increased dramatically, although the same conditions of non-thermal plasma had no effect on mandarin flesh [54]. Previous research has shown that non-thermal plasma treatment significantly increased the total phenolic content of strawberries [55], fresh cut pitaya fruits [56], bananas (Pour et al., 2022), and blueberries [57]. However, [58] have hypothesized that phenols accumulate because they function as antioxidants that prevent the damage due to free radicals. Non-thermal plasma treatment might damage the polysaccharide cell walls of plants, leading to an increase in polyphenol extraction [59,60]. After non-plasma treatment, the bond between phenolics and the cell membrane may break down, leading to the release of phenolics [57,61].
3.4. The Antioxidant
Non-thermal plasma has the ability to affect the antioxidant activity of fresh foods, resulting in the oxidation of antioxidants and a change in respiration rate [62]. Figure 2B depicts the effect of SDBD plasma on the antioxidant capacity of palm date varieties. This graph illustrates that the antioxidant capacity of untreated samples varied from 1.06 ± 0.09% to 49.77 ± 1.75%, while the antioxidant capacity of treated samples ranged from 1.28 ± 0.04% to 55.41 ± 1.45%. The antioxidant capacity of all palm date cultivars is enhanced after plasma treatment. There was a statistically significant difference (p < 0.05) between treated and untreated samples in their antioxidant capacity. The lowest rise in antioxidant capacity was documented for the plasma-treated Shalbi variety, which increased by 10.56 percent compared to the untreated type. In contrast, the treated Ajwa variety had the greatest increase in antioxidant capacity; it increased by 67.69% in comparison to the untreated variety. The increases in total phenolic content and antioxidant activity between untreated and treated palm dates followed a similar pattern, as seen by these data.
Previous research found no discernible differences in the antioxidant capacity of Abate Fetel pear [63], blueberries [64], and strawberries [65] after non-thermal plasma treatments. On the other hand, mandarin [54], strawberries [55], and bananas [66] are significantly increased after non-thermal plasma treatment. Due to an increase in total phenol and flavonoid content, the antioxidant activity values of non-plasma-treated samples increased. The increase in antioxidant activity of cloudy apple juice [67] and tomato pomace [68] after non-thermal plasma treatment has been attributed to the higher extraction of polyphenols. In addition, non-thermal plasma treatment induced the elevation of phenylpropanoids and ROS metabolism, as well as the augmentation of enzyme activities and the activation of related gene expression, hence increasing the antioxidant activity of fruit [56]. In addition, non-thermal plasma treatment increased phenolic accumulation and, therefore, antioxidant activity [55].
3.5. HMF
During food preparation, HMF is produced from the degradation of sugar via the Maillard reaction [69]. HMF and its derivatives have several negative impacts on public health, including DNA-damaging, genotoxic, organotoxic, mutagenic, carcinogenic, and enzyme-inhibiting properties [70].
The HMF of all palm date types was reduced after plasma treatment with SDBD (Figure 2C). The findings demonstrated a statistically significant difference (p < 0.05) between treated and untreated samples in terms of HMF. The HMF content of untreated samples varied from 3.25 to 76.67 μg/g, whereas treated samples contained between 1.05 and 55.41 μg/g. The HFM of Ajwa, Rashodya, Nbtet Ali, Shalbi, Swkary, and Khadari has decreased by 67.7%, 42.0%, 80.0%, 52.6%, 51.5%, and 27.5%, respectively, after SDBD plasma treatment. The HMF was not found in the Maghol sample following plasma treatment with SDBD. In addition, a recent study discovered that non-thermal ultrasound, nitrogen-GAD, and air-GAD plasma treatments decreased the HMF content of Turkish Blossom Honey by 19%, 32%, and 37%, respectively [71].
3.6. Chemical Compositions
The change in chemical composition of palm dates after SDBD plasma treatment is presented in Figure 3. Most ready-to-eat fruits and vegetables contain high levels of free water, which, when exposed to non-thermal plasma, can cause undesirable quality changes. However, because non-thermal plasma cannot penetrate the product, these changes occur only on the surface and do not affect the fresh produce’s intrinsic composition or nutritional properties [51].
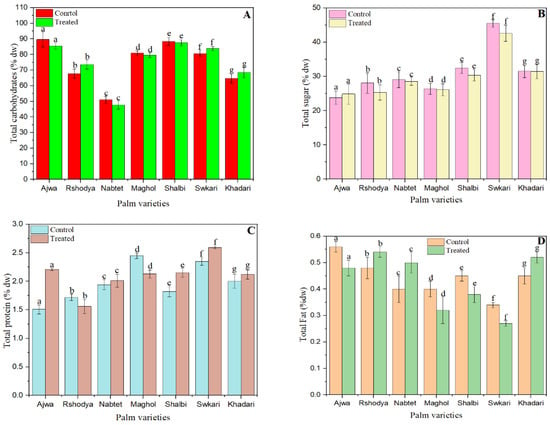
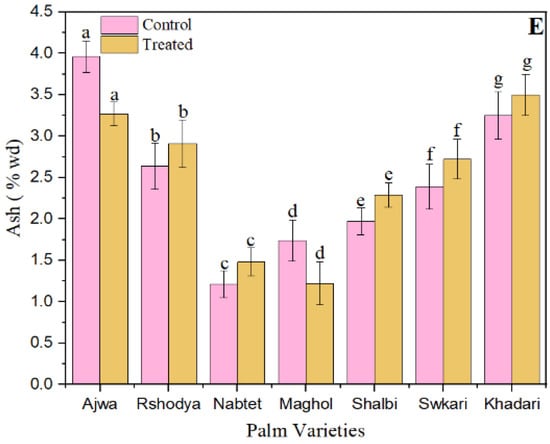
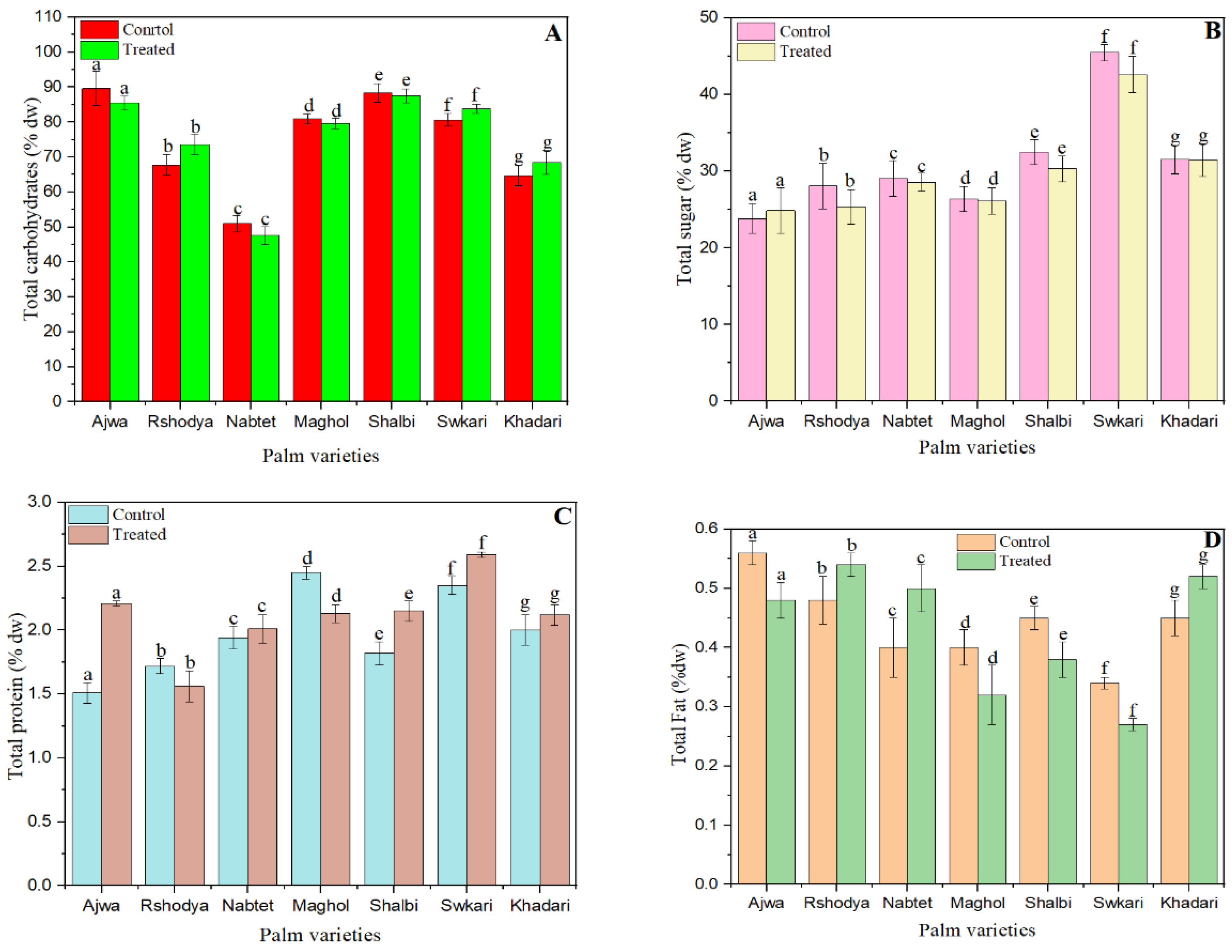
Figure 3.
The effect of SDBD plasma on composition of palm dates; (A) total carbohydrates, p value = 0.75, (B) total sugars p value = 0.34, (C) total protein p value = 0.06, (D) total fat p value = 0.48, and (E) ash p value = 0.25, lowercase letters a–g in the same column represent significance analysis; the different letters mean significant difference among various treatments at p < 0.05 level.
As seen in Figure 3A, the total carbohydrate content of untreated palm date types varied from 51 ± 2 to 90 ± 5, while the values for those treated with SDBD plasma for 3 min ranged from 47 ± 2 to 85 ± 2. The total carbohydrate levels in Rshodya plasma increased from 68 ± 3 to 74 ± 3, in Swkari plasma from 80 ± 2 to 84 ± 1, and in Khadari plasma from 65 ± 2 to 68 ± 3. Conversely, the overall carbohydrate content of the other palm date types has decreased. There were no significant changes (p > 0.05) in the total carbohydrates of treated and untreated palm dates.
Figure 3B demonstrates the influence of SDBD plasma on the total sugar content of palm date samples. The plasma-treated palm dates decreased in total sugar content for all date varieties except the Ajwa cultivar, where the total sugar content increased from 24 ± 2 to 25 ± 3. The range of total sugars in untreated samples is between 24 ± 2 and 45 ± 1, while the range for treated samples is between 25 ± 3 and 43 ± 1. Despite this, there are no significant changes (p > 0.05) in the total sugar values of treated and untreated samples. In a recent article, demonstrated that, no significant change in total sugar content between untreated and non-thermal plasma-treated bananas [72]. In addition, it observed that non-thermal plasma treatment had little effect on the composition and concentration of reducing sugar in radish paocai [73]. However, following non-thermal plasma treatment, the sugar content of blueberry fruits increased [74] and was reduced [55] in pitaya.
The impact of SDBD plasma on total protein is depicted in Figure 3C. The range of total protein concentration in untreated samples was 1.51 ± 0.08 to 2.45 ± 0.05, while the range for treated samples was 1.56 ± 0.12 to 2.59 ± 0.02. After plasma treatment, the total protein content of two types, Roshody (1.72 ± 0.06) and Maghol (2.45 ± 0.05), reduced to 1.56 ± 0.16 and 2.45 ± 0.07, respectively. Other treated palm date cultivars have increased in total protein content. Still, there were no statistically significant differences (p > 0.05) between the values of total protein in samples that were treated and those that were not.
As indicated in Figure 3D, the total fat values increased for Rshodya from 0.48 ± 0.02 to 0.54 ± 0.02, for Nabtet Ali from 0.40 ± 0.02 to 0.50 ± 0.02, and for Khadari from 0.45 ± 0.02 to 0.52 ± 0.02. Total fat content of the other palm date cultivars was decreased. The total fat content of untreated samples varied from 0.34 ± 0.03 to 0.56 ± 0.02, whereas treated samples had values between 0.27 ± 0.03 to 0.54 ± 0.02. However, there were no significant changes (p > 0.05) between the treated and control samples in terms of total fat content.
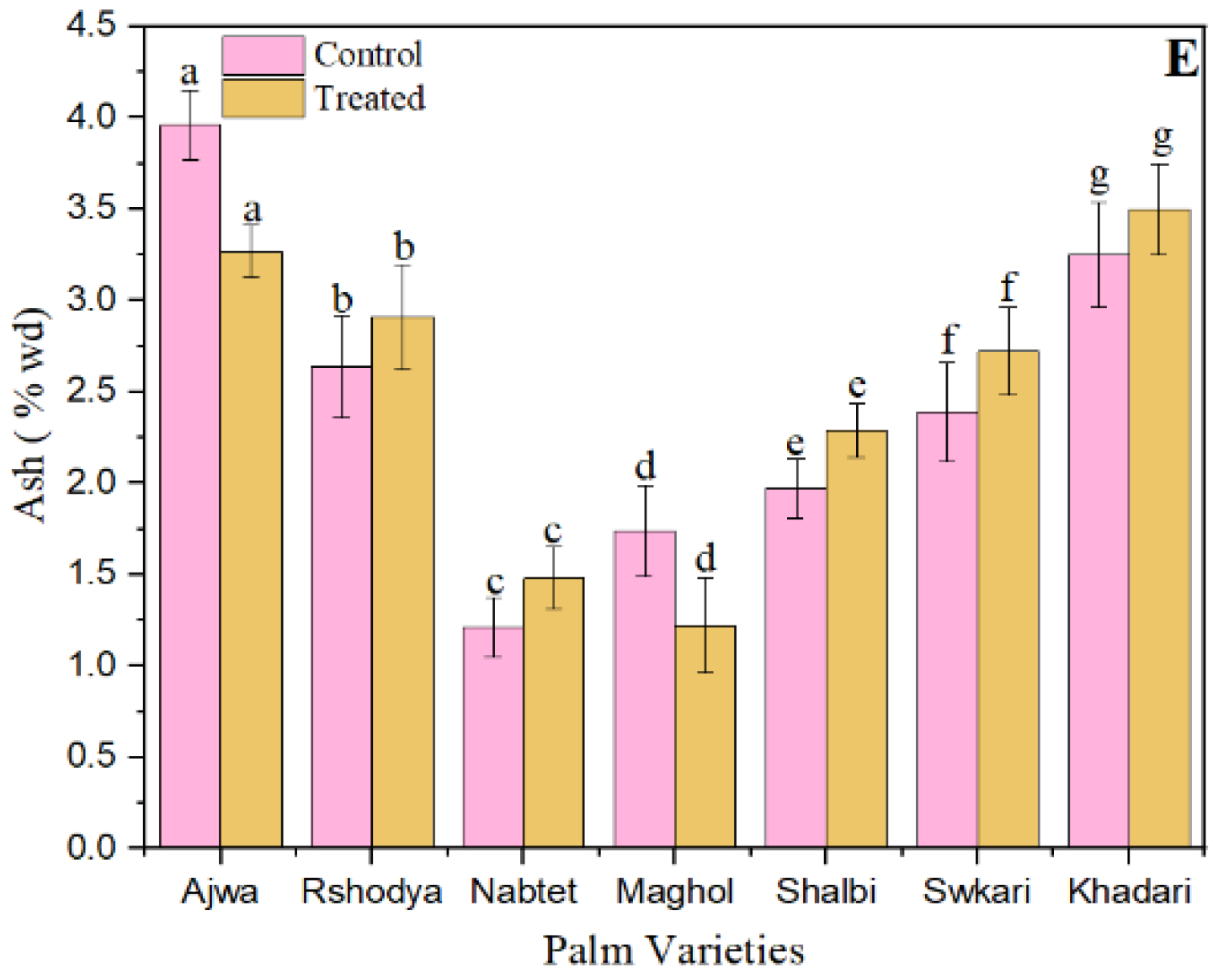
Figure 3E demonstrates that the SDBD treatment decreased the ash values of Ajwa and Maghol from 3.96 ± 0.19 to 3.27 ± 0.14 and from 1.74 ± 0.25 to 1.27 ± 0.25, respectively. In contrast, the ash values of the other palm date cultivars treated with SDBD plasma have increased. Total ash amounts in untreated samples range from 1.21 ± 0.16 to 3.96 ± 0.19, while total ash amounts in treated samples range from 1.22 ± 0.26 to 3.50 ± 0.25. Nevertheless, there were no significant changes (p > 0.05) between treated and untreated samples in terms of total ash concentration. Since SDBD plasma cannot easily penetrate the product and modify the chemical composition of palm dates, the optimal dose of SDBD plasma had no effect on the total carbohydrate, total protein, total sugars, total ash, and total fat content of palm date varieties. In agreement with [75], they discovered that non-thermal plasma had no effect on the majority of nutritious components in brown rice snack bars.
3.7. Water Soluble Vitamins
Table 2 depicts the effect of SDBD plasma treatment on water-soluble vitamins. The B1 vitamin values of untreated samples varied from 170.00 ± 2.89 to 1014.22 ± 4.22, while the values of treated samples ranged from 174.08 ± 4.61 to 987.76 ± 3.99. B1 vitamin values increased in the palm date varieties Rshodya and Nabtet Ali following SDBD plasma treatment, but they reduced in the other palm date varieties.

Table 2.
The influence of SDBD plasma on water-soluble vitamins of different varieties of palm dates.
The range of B2 vitamin levels for untreated samples was 85.95 ± 3.74 to 894.87 ± 3.00, while the range for treated samples was 74.08 ± 2.89 to 916.64 ± 3.32. Except for Rshodya, Maghol, and Khadari, the B2 vitamin content of all palm date cultivars was reduced following plasma treatment with SDBD.
The range of B6 vitamin levels for the untreated sample was 2301.66 ± 59.61 to 9588.19 ± 47.88, while the range for the treated sample was 2317.68 ± 39.02 to 9549.32 ± 28.88. With the exception of the Maghol and Shalbi varieties, all palm date samples treated with SDBD plasma showed a rise in B6 vitamin levels.
The range of B9 vitamin levels for untreated samples was 35.39 ± 2.21 to 240.63 ± 2.40, while the range for treated samples was 36.79 ± 0.98 to 238.5 ± 11.25. The B9 levels of all palm date samples increased following plasma treatment with SDBD, except for the Rshodya, Maghol, and Shalbi varieties, whose values decreased.
The range of B12 vitamin levels for the untreated sample was 173.08 ± 8.58 to 585.56 ± 8.02, whereas the range for the treated sample was 161.11 ± 5.88 to 553.46 ± 8.72. After plasma treatment with SDBD, B12 levels increased in the palm date varieties Nabtet Ali and Khadari but were reduced in the other treated palm date varieties.
However, there were no significant changes (p > 0.05) between treated and untreated samples for B1, B2, B6, B9, and B12 content. These data suggest that the appropriate amount of SDBD plasma treatment has no effect on the water-soluble vitamin content. It demonstrated that non-thermal plasma exposure processing had no effect on the vitamin C and beta-carotene content of L. barbarum samples [76].
3.8. Amino Acids
The amino acid composition was studied to determine the impact of SDBD plasma treatment on the composition of proteins. The impact of SDBD plasma on the amino acid content of palm dates is seen in Table 3a,b. After SDBD treatment, the contents of alanine, arginine, leucine, and phenylalanine decreased in Ajwa, Shalabi, and Swkari, but increased in the other palm date species. Regarding the levels of histidine, isoleucine, lysine, methionine, serine, and tyrosine, an increasing trend was seen for all palm date types except Shalabi and Swkari, whose values decreased following SDBD treatment, respectively. After being treated with SDBD, the proline and glu values went up in Ajwa and Swkari but went down in all palm date cultivars. After treatment with SDBD, the contents of valine and glycine in Maghol, Shalbi, and Khadari increased, but they decreased in the other palm date varieties. After SDBD treatment, the threonine levels of Ajwa, Rashodya, and Nabtet Ali palm dates increased, but they were reduced for all other palm date varieties. Except for the Ajwa and Shalbi varieties, the Asp values increased for all palm date varieties. Based on these results, it can be said that there are no significant differences (p > 0.05) in the concentration of amino acids between palm dates that have been treated and those that have not.

Table 3.
(a) The influence of SDBD plasma on amino acids of different varieties of palm dates. (b) The influence of SDBD plasma on amino acids of different varieties of palm dates.
3.9. Minerals
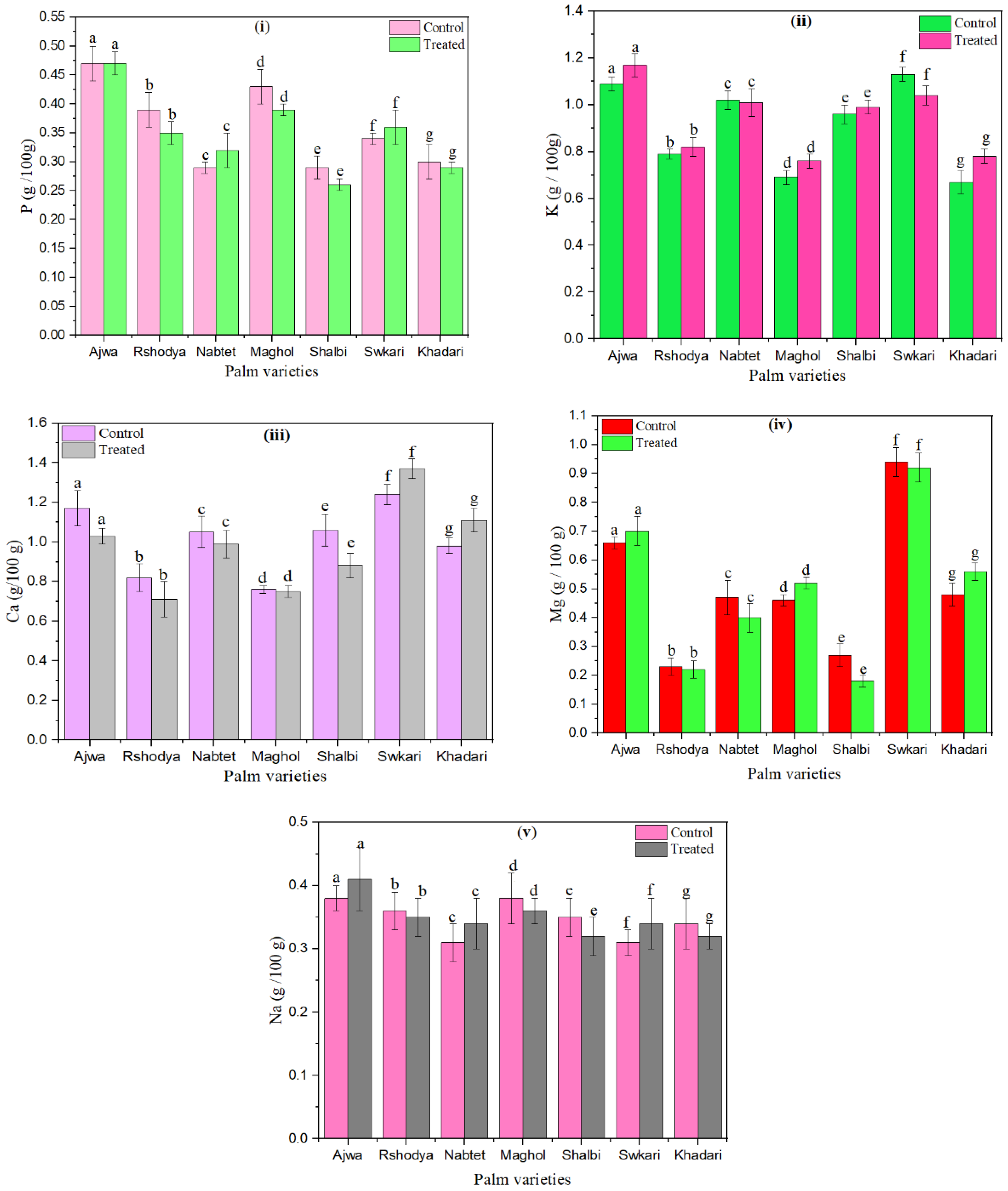
The chosen date fruit varieties were undergone for analysis of macro and microelements. The influence of SDBD plasma on macro (P, K, Ca, Mg, and Na) of palm dates are shown in Figure 4.
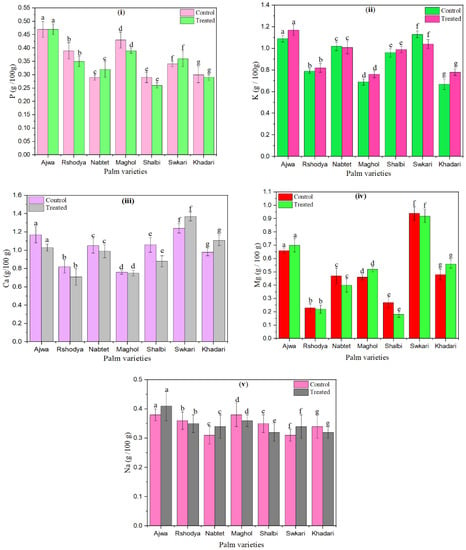
Figure 4.
The effect of SDBD plasma on macro elements (g/100 g) of palm dates, (i) P, p value = 0.99, (ii) K, p value = 0.06, (iii) Ca, p value = 0.4i, (iv) Mg, p value = 0.05, and (v) Na, p value = 0.35, lowercase letters a–g in the same column represent significance analysis; the different letters mean significant difference among various treatments at p < 0.05 level.
P values of control palm dates ranged from 0.29 ± 0.01 up to 0.47 ± 0.03. However, p values of treated palm dates using SDBD plasma for 3 min ranged from 0.26 ± 0.01 up to 0.47 ± 0.02, as shown in Figure 4i. p values of palm dates have increased after SDBD treatment for Nabtet Ali and Swkari varieties from 0.29 ± 0.01 to 0.32 ± 0.03 and from 0.34 ± 0.01 to 0.36 ± 0.03, respectively. On the other hand, the p value of Ajwa did not change after SDBD treatment. However, p values were decreased for the rest of the palm date treated varieties.
Figure 4ii shows that K values after SDBD plasma decreased for Nabtet Ali from 1.020.04 to 1.010.06 and for Swkari from 1.130.03 to 1.040.04. The K values of the other palm date varieties were increased. The K values of the untreated samples ranged from 0.67 ± 0.05 to 1.13 ± 0.03, while their values for treated samples ranged from 0.78 ± 0.03 to 1.17 ± 0.05.
Moreover, Ca values decreased for all palm date varieties except for Swkari and Khadari varieties. They increased from 1.24 ± 0.05 to1.37 ± 0.05 and from 0.98 ± 0.04 to 1.11 ± 0.06, respectively, as explained in Figure 4iii. Ca values of untreated ranged from 0.76 ± 0.02 up to 1.17 ± 0.05 while treated one it ranged from 0.71 ± 0.09 up to 1.11 ± 0.06.
The measured Mg values of palm dates have increased after SDBD treatment for Ajwa from 0.66 ± 0.02 to 0.70 ± 0.05, Maghol from 0.52 ± 0.02 to 7.49 ± 0.29, and Khadari from 0.48 ± 0.04 to 0.56 ± 0.03 but they have increased for the rest of the treated palm date types (Figure 4iv). Mg values varied from 0.23 ± 0.03 to 0.94 ± 0.05 for untreated samples and from 0.18 ± 0.04 to 0.70 ± 0.05 for treated ones.
Moreover, Na values have increased after SDBD treatment for Ajwa from 0.38 ± 0.02 to 0.41 ± 0.05, Nabtet Ali from 0.31 ± 0.03 to 0.34 ± 0.04 and Swkari from 0.31 ± 0.02 to 0.34 ± 0.04 while they have decreased for the rest of the treated palm date varieties, as illustrated in Figure 4v. Na values varied from 0.31 ± 0.02 to 0.38 ± 0.02 for untreated samples and from 0.32 ± 0.02 to 0.41 ± 0.05 for treated ones. In these figures, there are no significant differences (p > 0.05) in macro-elements content between palm date varieties treated using SDBD plasma and untreated ones.
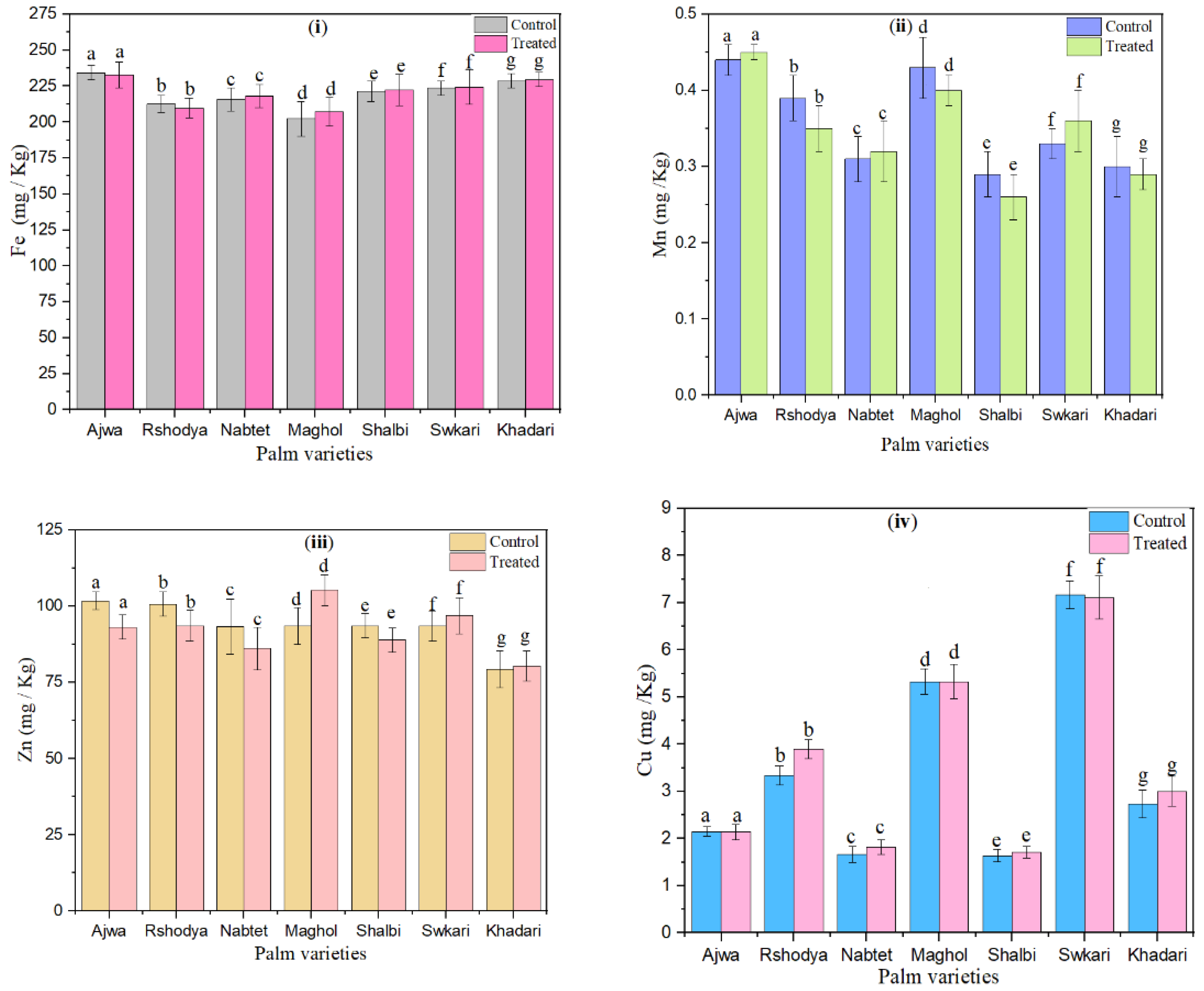
The influence of SDBD plasma on the microelement content (Fe, Mn, Zn, and Cu) of palm dates is shown in Figure 5. Except for Ajwa and Rashodya, Fe values increased with SDBD treatment for all treated palm dates, whereas they decreased from 234.33 ± 5.02 to 232.67 ± 9.01 and from 212.67 ± 6.03 to 209.67 ± 7.10, as shown in Figure 5i. Fe values varied from 202.33 ± 12.00 to 234.33 ± 5.08 for untreated samples and varied from 207.33 ± 10.10 to 232.67 ± 9.10 for treated ones.
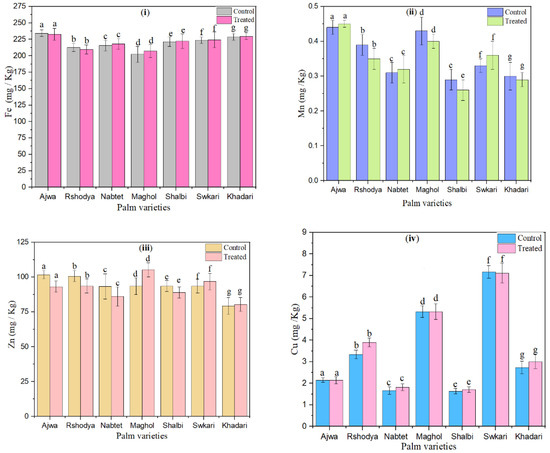
Figure 5.
The effect of SDBD plasma on microelements (mg/Kg) of palm dates, (i) Fe, p value = 0.23, (ii) Mn, p value = 0.99, (iii) Zn, p value = 0.18, and (iv) Cu, p value = 0.07, lowercase letters a–g in the same column represent significance analysis; the different letters mean significant difference among various treatments at p < 0.05 level.
Furthermore, the results of Figure 5ii, which depicts the measured Mn values of treated palm date varieties using SDBD for 3 min, show that Mn values increased after SDBD treatment for Ajwa from 0.44 ± 0.02 to 0.45 ± 0.02, Nabtet Ali from 0.31 ± 0.03 to 0.32 ± 0.04, and Swkari from 0.33 ± 0.02 to 0.36 ± 0.04, while they decreased for other treated palm date varieties. Mn values varied from 0.29 ± 0.03 to 0.45 ± 0.01 for untreated palm dates and from 0.26 ± 0.03 to 0.45 ± 0.01 for treated ones.
According to Figure 5iii, after SDBD treatment, Zn values increased for Maghol from 93.50 ± 6.10 to 105.30 ± 5.30, Swkari from 93.47 ± 5.06 to 96.88 ± 6.04, and Khadari from 79.33 ± 6.09 to 80.33 ± 5.60, but decreased for the remaining palm date varieties. Moreover, in the case of Zn, untreated samples ranged from 79.33 ± 6.09 to 101.80 ± 3.01; on the other hand, they ranged from 80.33 ± 5.60 to 105.30 ± 5.30 for treated ones.
Cu values of control palm dates ranged from 1.64 ± 0.13 up to7.17 ± 0.29. Figure 5iv shows that the Cu values of treated palm dates using SDBD plasma for 3 min ranged from 1.71 ± 0.12 to 7.11 ± 0.46. Cu values of palm dates has decreased after SDBD treatment for Ajwa, and Swkari varieties from 2.15 ± 0.01 to 2.14 ± 0.16 and from 7.17 ± 0.29 to 7.11 ± 0.46, respectively. Moreover, Cu values of the Maghole date cultivar did not change after SDBD treatment. However, Cu values were increased for the rest of the palm date treated varieties. Statistical analysis showed that there are no significant differences (p > 0.05) in macro and microelement content between palm date varieties treated using SDBD plasma and untreated ones.
These findings support the conclusion of Suhem et al. (2013) that non-thermal plasma has no effect on the trace elements of brown rice snack bars [75].
3.10. Principal Component Analysis
Across palm date types that were both untreated and treated with SDBD plasma, the relationships among the evaluated variables were evaluated using principal component analysis (PCA), as shown in Table 4. The six principal components exhibited eigenvalues higher than unity (Eigenvalue > 1) under untreated and treated with SDBD plasma of palm date types in this study. Since PC1 and PC2 have the greatest eigenvalues greater than unity and account for 54.29% and 53.08 of the total variance in all studied variables under untreated and treated palm dates with SDBD plasma, respectively, they were both retained for the final analysis. These findings confirm that the assessed variables were different between the two treatments and that the variability in treated varieties was higher than in untreated varieties by most PCs. As noticed in Table 2, the variance % by PC1 of the total variation of the studied variables in untreated types had more than in treated types, while the opposite is true for PC2. Based on the first two PCs, we can evaluate the association between the studied variables of the untreated and treated palm date types with SDBD plasma. PC1 and PC2 explained 31.00% and 21.00%, 60.27% and 17.11%, as well as 77.90% and 11.40% of the total variation of date palm fruit factors, respectively [77,78,79].

Table 4.
PCA results of the six PCs for the investigated variables under study for both untreated and treated palm date types using SDBD plasma.
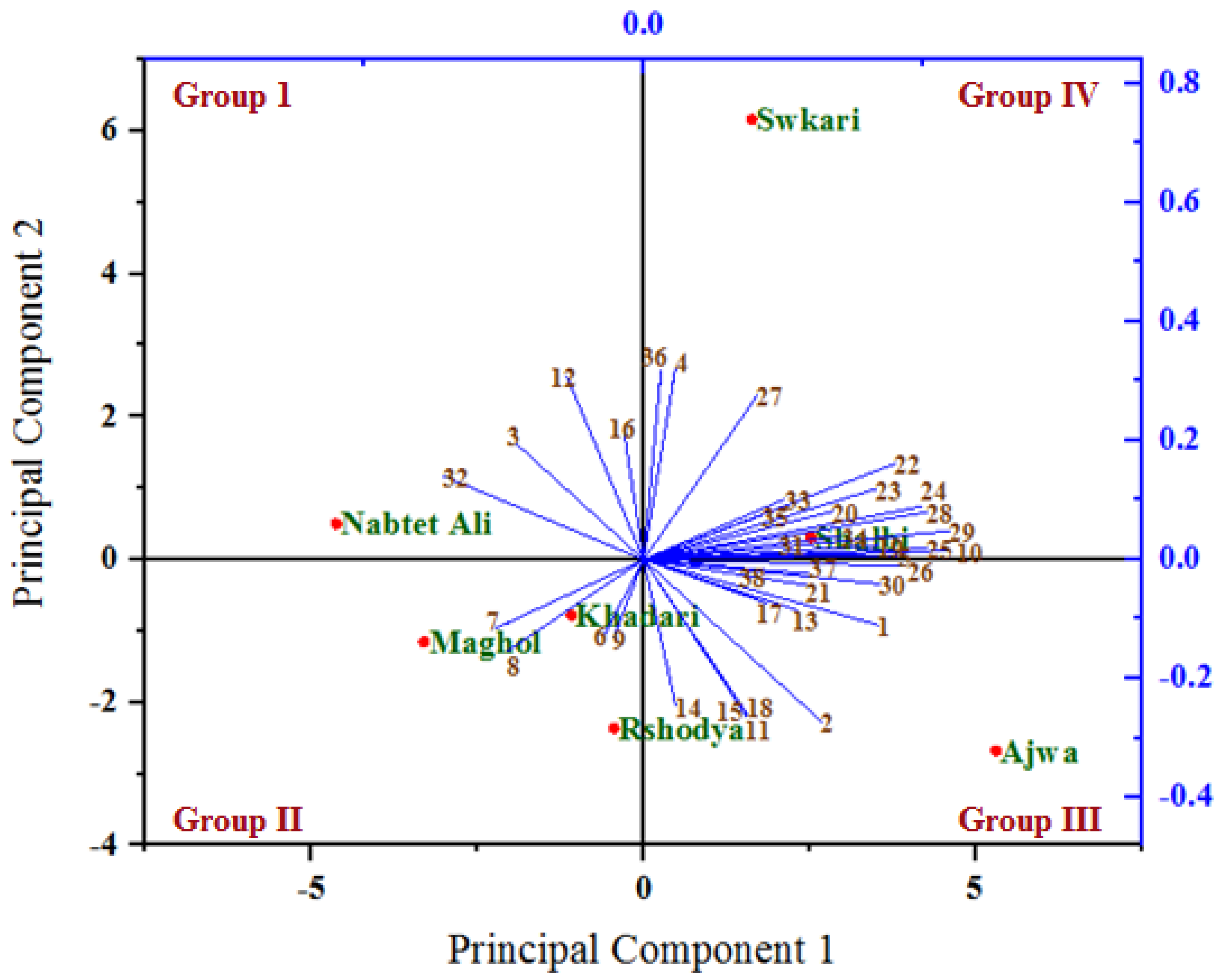
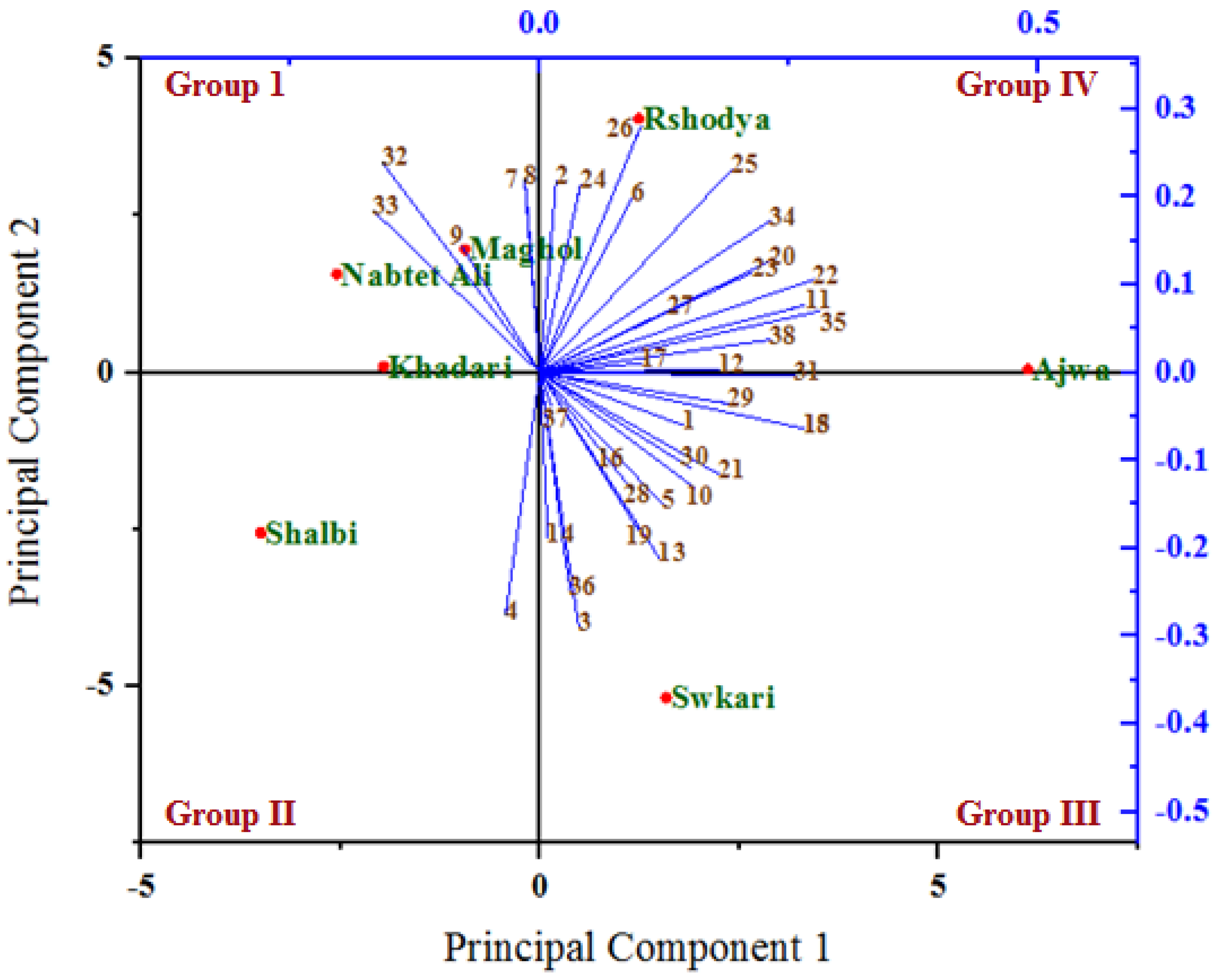
The relationships among the analyzed variables under untreated (Figure 6) and treated (Figure 7) palm date varieties with SDBD plasma were depicted using a biplot created from the first two PCs. Out of 38 variables, 30 and 32 variables with PC1, as well as 21 and 19 variables with PC2, showed positive correlation under both untreated and treated palm date varieties, respectively. The highest positive correlations of factor loadings with PC1 were recorded for methionine (22), lysine (23) in both treatments, for ash % (1), total carbohydrates % (5), B1 (10), Fe (19), isoleucine (24), histidine (25), arginine (26), phenylalanine (28), leucine (29), and glycine (30) under untreated palm date types, and for Na (11), P (15), Mn (18), serine (20), glutamic acid (31), tyrosine (34), threonine (35) and antioxidant RSA % (38) under-treated palm date types. On the other hand, the PC2 has identified total protein % (3), total sugar % (4), Mg (12), Cu (16), alanine (27), and HMF (36) variables as well as total fat (2), B12 (6), B9 (7), B6 (8), isoleucine (24), histidine (25), arginine (26), and aspartic acid (32) as possessing high positive correlations of factor loadings under untreated and treated palm date types, respectively. In the examined date palm cultivars, PC1 and PCA2 were positively associated with most quantitative and qualitative traits [77]. A variety of date palm fruit hydroxycinnamates and flavor flavonoids were discovered to correlate with PCA1 [80]. However, some of them did not. It found that PCA1 has a strong positive relationship with regulatory polyamines, glutathione-mediated antioxidant activity, energy production, lysophospholipids, amino acids, tannins, non-reducing carbohydrates, and hormones [80].
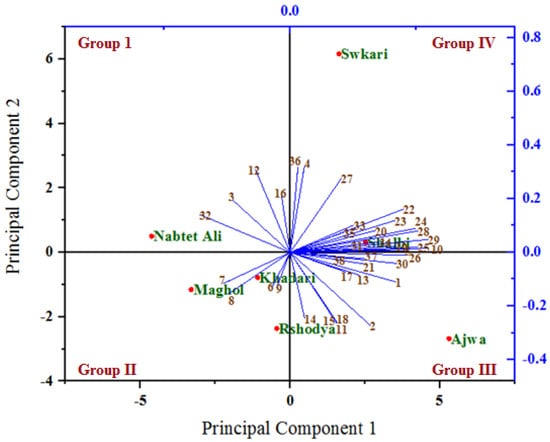
Figure 6.
A biplot diagram based on PC1 and PC2 shows similarities and dissimilarities in relationships among the measured variables across different varieties of palm dates under untreated. 1—Ash%; 2—Total fat %DW; 3—Total protein%; 4—Total sugar %; 5—Total carbohydrates %; 6—B12; 7—B9; 8—B6; 9—B2; 10—B1; 11—Na; 12—Mg; 13—Ca; 14—K; 15—P; 16—Cu; 17—Zn; 18—Mn; 19—Fe; 20—Serine; 21—Proline; 22—Methionine; 23—Lysine; 24—LsoLeucine; 25—Histidine; 26—Arginine; 27—Alanine; 28—Phenylalanine; 29—Leucine; 30—Glycine; 31—Glutamic acid; 32—Aspartic acid; 33—Valine; 34—Tyrosine; 35—Threonine; 36—HMF μg/100g; 37—Polyphenols mg GAE/100 g FW; 38—Antioxidant RSA %.
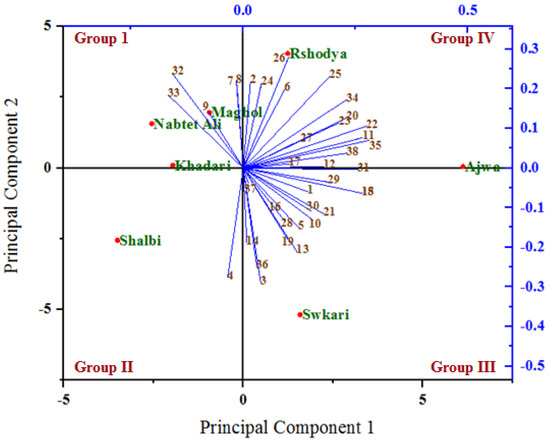
Figure 7.
A biplot diagram based on PC1 and PC2 shows similarities and dissimilarities in relationships among the measured variables across different varieties of palm dates under after SDBD plasma treatment. 1—Ash%; 2—Total fat %DW; 3—Total protein%; 4—Total sugar %; 5—Total carbohydrates %; 6—B12; 7—B9; 8—B6; 9—B2; 10—B1; 11—Na; 12—Mg; 13—Ca; 14—K; 15—P; 16—Cu; 17—Zn; 18—Mn; 19—Fe; 20—Serine; 21—Proline; 22—Methionine; 23—Lysine; 24—LsoLeucine; 25—Histidine; 26—Arginine; 27—Alanine; 28—Phenylalanine; 29—Leucine; 30—Glycine; 31—Glutamic acid; 32—Aspartic acid; 33—Valine; 34—Tyrosine; 35—Threonine; 36—HMF μg/100 g; 37—Polyphenols mg GAE/100 g FW; 38—Antioxidant RSA %.
In both the untreated and treated varieties with SDBD plasma, an acute angle among the most variables studied was found, indicating a positive correlation between these variables. However, the degree and consistency of these associations vary. Our research showed that most of the assessed variables had acute angles (below 90 degrees), indicating a favorable association between these variables. Most variables evaluated were shown to have the highest positive association (smallest sharp angles) throughout either or both treatments. In both the untreated and treated palm date types, the first two PCs primarily distributed and distinguished the analyzed variables into four groups based on the strength of their relationships, according to PCA analysis. The studied factors within each group were significantly inversely or favorably correlated with one another, while showing insignificant correlation for variables between these groups (positive or negative). Previous research reported that the results showed significant heterogeneity, and the sixteen quantitative and qualitative characteristics of the date palm varieties that were assessed showed a potent discriminating factor, according to the PCA model [78].
The group I (the highest PC2 and the lowest PC1) included variables total protein % (3), Mg (12), Cu (16), and aspartic acid (32) under untreated as well as the variables B9 (7), B6 (8), B2 (9), aspartic acid (32), and valine (33) under treated, which are highly positively associated with the Nabtet Ali variety as well as Maghol, Khadari, and Nabtet Ali varieties, respectively. Group II (the lowest PC1 and PC2) included B12 (6), B9 (7), B6 (8), and B2 (9) variables with Khadari, Maghol, and Rshodya types in untreated conditions and only total sugar (4) with Shalbi type in treated conditions. However, group III is related to the highest PC1 and lowest PC2 and consists of variables ash% (1), total fat % (2), Na (11), Ca (13), K (14), P (15), Zn (17), Mn (18), proline (21), arginine (26), glycine (30), polyphenols (37), and antioxidant RSA (38) under untreated varieties as well as variables ash % (1), total protein % (3), total carbohydrates % (5), B1 (10), Ca (13), K (14), Cu (16), Mn (18), Fe (19), phenylalanine (28), leucine (29), glycine (30), hmf (36), and polyphenols (37) under treated varieties, which are located with the varieties Ajwa and Swkari, respectively. The highest PC1 and PC2 formed group IV and consisted of other variables with types Shalbi and Swkari, as well as with types Rshodya and Ajwa under untreated and treated using SDBD plasma, respectively. In contrast, some correlations among variables investigated between four groups were either positive (low) or negative, depending on whether the angles between them were acute (big) or obtuse, respectively. By utilizing PCA to map the positions of main and minor elements for date palm samples by Inductively Coupled Plasma Optical Emission Spectrometry, the correlation among Mg, Na, Ca, and K as well as Fe, Al, Cu, and Zn were found [81].
4. Conclusions
After 3 min of non-thermal plasma treatment with SDBD plasma, A. niger isolated from palm date types is inhibited. The SDBD plasma treatment conditions did not significantly change the nutritional and mineral composition of palm date fruits. The dose and strength of the used SDBD plasma did not have the same impact on the chemical components of dates. However, the plasma concentration and power of SDBD were capable of influencing the amounts of active components, such as HMF, phenolic, and antioxidant activity. Plasma from SDBD may be an effective decontamination method, not only for palm date varieties but also for other fruits and vegetables. It is probably acceptable to conclude that the non-thermal treatments did not significantly change the quality of palm date types. In addition, the HFM values, antioxidant activity, and total phenol contents of palm date varieties treated with SDBD plasma were significantly changed, resulting in an improvement in palm date variety quality. The results of the PCA model could show how the variables of palm date varieties that have been treated with SDBD plasma and those that have not been treated relate to each other.
Author Contributions
Conceptualization, K.L. and S.M.A.-Q.; methodology, K.L., S.M.A.-Q., F.A.B.S., S.A.A. and T.A.M.; software, K.M.E.-A., N.A.A.-H. and S.M.A.-Q.; validation, N.A.A.-H., F.A.B.S. and T.A.M.; formal analysis, K.L., F.A.B.S., S.A.A. and T.A.M.; investigation, K.L., F.A.B.S., S.A.A. and T.A.M.; resources, S.M.A.-Q., F.A.B.S. and S.A.A.; data curation, K.L., F.A.B.S., S.A.A. and T.A.M.; writing—original draft preparation, K.L., S.A.A. and N.A.A.-H.; writing—review and editing, K.L., K.M.E.-A. and T.A.M.; visualization, K.L., S.M.A.-Q., N.A, F.A.B.S., S.A.A. and T.A.M.; supervision, N.A.A.-H. and F.A.B.S.; project administration, K.L.; funding acquisition, F.A.B.S. and S.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Saudi Arabia Ministry of Environment, Water, and Agriculture grant number [2200011540].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors would like to extend their gratitude to the Saudi Arabia Ministry of Environment, Water, and Agriculture for providing financial, technical, and administrative support to fund this work through the initiative of encouraging the agricultural “Applied research project in the field of palm and dates no. 2200011540”.
Conflicts of Interest
All the authors declare no conflict of interest for this work. There is no known competing financial interest and personal relationship that could influence this paper’s reported work.
References
- Fernández, A.; Shearer, N.; Wilson, D.R.; Thompson, A. Effect of microbial loading on the efficiency of cold atmospheric gas plasma inactivation of Salmonella enterica serovar Typhimurium. Int. J. Food Microbiol. 2012, 152, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Jayasena, D.D.; Kim, H.J.; Yong, H.I.; Park, S.; Kim, K.; Choe, W.; Jo, C. Flexible thin-layer dielectric barrier discharge plasma treatment of pork butt and beef loin: Effects on pathogen inactivation and meat-quality attributes. Food Microbiol. 2015, 46, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, E.J.; Choi, E.H.; Kim, Y.J. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov. Food Sci. Emerg. Technol. 2014, 22, 124–130. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, C.; Lozano-Sanchez, J.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Alternatives to conventional thermal treatments in fruit-juice processing. Part 2: Effect on composition, phytochemical content, and physicochemical, rheological, and organoleptic properties of fruit juices. Crit. Rev. Food Sci. Nutr. 2017, 57, 637–652. [Google Scholar] [CrossRef]
- Waghmare, R. Cold plasma technology for fruit based beverages: A review. Trends Food Sci. Technol. 2021, 114, 60–69. [Google Scholar] [CrossRef]
- Silva, R.M.; Campelo, P.H.; Silva, F.E.F.; Zampieri, D.S.; Gramosa, N.V.; Fernandes, F.A.; Rodrigues, S. NMR Spectroscopy and Chemometrics to Evaluate the Effect of Different Non-Thermal Plasma Processing on Sapota-do-Solimões (Quararibea cordata Vischer) Juice Quality and Composition. Food Bioprocess Technol. 2022, 15, 875–890. [Google Scholar] [CrossRef]
- Pohl, P.; Dzimitrowicz, A.; Cyganowski, P.; Jamroz, P. Do we need cold plasma treated fruit and vegetable juices? A case study of positive and negative changes occurred in these daily beverages. Food Chem. 2022, 375, 131831. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.H.; Sun, D.W. Blocking and degradation of aflatoxins by cold plasma treatments: Applications and mechanisms. Trends Food Sci. Technol. 2021, 109, 647–661. [Google Scholar] [CrossRef]
- Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araujo Bezerra, J.; Rodriguese, S.; Fernandesf, F.A.N.; Campeloab, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044. [Google Scholar] [CrossRef]
- Starek, A.; Sagan, A.; Andrejko, D.; Chudzik, B.; Kobus, Z.; Kwiatkowski, M.; Terebun, P.; Pawłat, J. Possibility to extend the shelf life of NFC tomato juice using cold atmospheric pressure plasma. Sci. Rep. 2020, 10, 20959. [Google Scholar] [CrossRef]
- Campelo, P.H.; Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Rodrigues, S.; Fernandes, F.A.N. Modulation of aroma and flavor using glow discharge plasma technology. Innov. Food Sci. Emerg. Technol. 2020, 62, 102363. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Donsì, F.; Yildiz, S.; Candoğan, K.; Pokhrel, P.R.; Guadarrama-Lezama, A.Y. Nonthermal processing technologies for stabilization and enhancement of bioactive compounds in foods. Food Eng. Rev. 2022, 14, 63–99. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Liao, X.; Cullen, P.J.; Liu, D.; Xiang, Q.; Wang, J.; Chen, S.; Ye, X.; Ding, T. Effects of nonthermal plasma technology on functional food components. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1379–1394. [Google Scholar] [CrossRef]
- Tappi, S.; Ragni, L.; Tylewicz, U.; Romani, S.; Ramazzina, I.; Rocculi, P. Browning response of fresh-cut apples of different cultivars to cold gas plasma treatment. Innov. Food Sci. Emerg. Technol. 2019, 53, 56–62. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Ezhilarasi, P.N.; Rajauria, G. Application of cold plasma on food matrices: A review on current and future prospects. J. Food Process. Preserv. 2021, 45, e15070. [Google Scholar] [CrossRef]
- Tang, Z.X.; Shi, L.E.; Aleid, S.M. Date fruit: Chemical composition, nutritional and medicinal values, products. J. Sci. Food Agric. 2013, 9, 2351–2361. [Google Scholar] [CrossRef]
- Al-Ahmadi, S.S.; Ibrahim, R.H.; Ouf, S.A. Application of ozone to control insect pests and moulds of date fruits. Biosci. Biotechnol. Res. Asia 2009, 6, 435–446. [Google Scholar] [CrossRef]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.A.H. Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J. Sci. Food Agric. 2015, 95, 3204–3210. [Google Scholar] [CrossRef]
- Dasan, B.G.; Mutlu, M.; Boyaci, I.H. Decontamination of Aspergillus flavus and Aspergillus parasiticus spores on hazelnuts via atmospheric pressure fluidized bed plasma reactor. Int. J. Food Microbiol. 2016, 216, 50–59. [Google Scholar] [CrossRef]
- Biganzoli, I.; Barni, R.; Riccardi, C. Note: On the use of Rogowski coils as current probes for atmospheric pressure dielectric barrier discharges. Rev. Sci. Instrum. 2013, 84, 016101. [Google Scholar] [CrossRef]
- Piferi, C.; Riccardi, C. A study on propane depletion by surface dielectric barrier discharges. Clean. Eng. Technol. 2022, 8, 100486. [Google Scholar] [CrossRef]
- Piferi, C.; Brescia, A.; Riccardi, C. Intensity comparison between UV lamps and plasma emission for air purification studies. AIP Adv. 2021, 11, 085209. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Acadamic Press: Sydney, Australia, 1985; pp. 1–413. [Google Scholar]
- Raper, K.B.; Fennell, D.J. The Genus Aspergillus; Williams and Wikins: Baltimore, MA, USA, 1965. [Google Scholar]
- Klich, M.A. Identifiction of Common Aspergillus Species; United State Department of Agriculture, Agriculutre Research Service, Southern Regional Research Center: New Oluisiana, LA, USA, 2002; p. 116. [Google Scholar]
- Muranyi, P.; Wunderlich, J.; Heise, M. Sterilization efficiency of a cascaded dielectric barrier discharge. J. Appl. Microbiol. 2007, 103, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists International. AOAC Official Methods of Analysis, 18th ed.; AOAC: Gaithersburg, Maryland, 2012. [Google Scholar]
- Association of Official Analytical Chemists International. Official Method of Analysis Association of Official Analytical Chemists, 15th ed.; AOAC International Publisher: Washington, DC, USA, 1990. [Google Scholar]
- James, C.S. Experimental Methods. In Analytical Chemistry of Foods; Champman and Hall: New York, NY, USA, 1995; p. 28. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Jajic, I.; Krstovic, S.; Glamocis, D.; Jaksis, S.; Abramovic, B. Validation of an HPLC method for the determination of amino acids in feed. J. Serb. Chem. Soc. 2013, 78, 839–850. [Google Scholar] [CrossRef]
- Cheng, K.L.; Bray, R.H. Determination of Calcium and Magnesium in Soil and Plants materials. Soil Sci. 1951, 72, 449–458. [Google Scholar] [CrossRef]
- Kundsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, Sodium and Potassium. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; part 2. Agron. Monogr. 9; American Society of Agronomy: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Walter, W.G. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1989; p. 940. [Google Scholar]
- Raofie, F.; Falsafi, Z. Development of a bimetal–organic framework–polypyrrole composite as a novel fiber coating for direct immersion solid phase microextraction in situ supercritical fluid extraction coupled with gas chromatography for simultaneous determination of furfurals in dates. Anal. Methods 2021, 13, 4941–4948. [Google Scholar]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2022, 50, 7449–7454. [Google Scholar] [CrossRef]
- Zhu, X.B.; Pan, L.; Wei, W.; Pen, J.-Q.; Qi, Y.-W.; Ren, X.-L. Changes in the content of water-soluble vitamins in Actinidia chinensis during cold storage. J. Serb. Chem. Soc. 2016, 81, 623–632. [Google Scholar] [CrossRef]
- Ilahi, I.; Samar, S.; Khan, I.; Ahmad, I. In vitro antioxidant activities of four medicinal plants on the basis of DPPH free radical scavenging. Pk. J. Pharm. Sci. 2013, 26, 949–952. [Google Scholar]
- Kogelschatz, U.; Eliasson, B.; Egli, W. From ozone generators to flat television screens: History and future potential of dielectric-barrier discharges. Pure Appl. Chem. 1999, 71, 1819–1828. [Google Scholar] [CrossRef]
- Goldberg, B.M.; Hoder, T.; Brandenburg, R. Electric field determination in transient plasmas: In-situ & non-invasive methods. Plasma Sources Sci. Technol. 2022.
- Šimek, M.; Homola, T. Plasma-assisted agriculture: History, presence, and prospects—A review. Eur. Phys. J. D 2021, 75, 210. [Google Scholar] [CrossRef]
- Massines, F.; Rabehi, A.; Decomps, P.; Gadri, R.B.; Ségur, P.; Mayoux, C. Experimental and theoretical study of a glow discharge at atmospheric pressure controlled by dielectric barrier. J. Appl. Phys. 1998, 83, 2950–2957. [Google Scholar] [CrossRef]
- Škoro, N.; Živković, S.; Jevremović, S.; Puač, N. Treatment of Chrysanthemum Synthetic Seeds by Air SDBD Plasma. Plants 2022, 11, 907. [Google Scholar] [CrossRef]
- Misra, N.N.; Keener, K.M.; Bourke, P.; Cullen, P.J. Generation of in-package cold plasma and efficacy assessment using methylene blue. Plasma Chem. Plasma Process. 2015, 35, 1043–1056. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Murphy, A.B.; McLean, K.M.; Kong, M.G.; Ostrikov, K. Atmospheric pressure plasmas: Infection control and bacterial responses. Int. J. Antimicrob. Agents 2014, 43, 508–517. [Google Scholar] [CrossRef]
- Kiš, M.; Milošević, S.; Vulić, A.; Herceg, Z.; Vukušić, T.; Pleadin, J. Efficacy of low pressure DBD plasma in the reduction of T-2 and HT-2 toxin in oat flour. Food Chem. 2020, 316, 126372. [Google Scholar] [CrossRef]
- Muranyi, P.; Wunderlich, J.; Heise, M. Influence of relative gas humidity on the inactivation efficiency of a low temperature gas plasma. J. Appl. Microbiol. 2008, 104, 1659–1666. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The effects of cold atmospheric plasma on cell adhesion, differentiation, migration, apoptosis and drug sensitivity of multiple myeloma. Biochem. Biophys. Res. Comm. 2016, 473, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Du, T.; Lu, X.; Cao, Y.; Pan, Y. How deep can plasma penetrate into a biofilm? Appl. Phys. Lett. 2011, 98, 221503. [Google Scholar] [CrossRef]
- Pasquali, F.; Stratakos, A.C.; Koidis, A.; Berardinelli, A.; Cevoli, C.; Ragni, L.; Mancusic, R.; Manfredaa, G.; Trevisanic, M. Atmospheric cold plasma process for vegetable leaf decontamination: A feasibility study on radicchio (red chicory, Cichorium intybus L.). Food Control 2016, 60, 552–559. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Rotolo, C.; Morano, M.; Minafra, A.; Ambrico, M.; Pollastro, S.; Gerin, D.; Faretra, F.; De Miccolis Angelini, R.M. Surface Dielectric Barrier Discharge plasma: A suitable measure against fungal plant pathogens. Sci. Rep. 2020, 10, 3673. [Google Scholar] [CrossRef]
- Kuzminova, A.; Kretková, T.; Kylián, O.; Hanuš, J.; Khalakhan, I.; Prukner, V.; Biederman, H. Etching of polymers, proteins and bacterial spores by atmospheric pressure DBD plasma in air. J. Phys. D Appl. Phys. 2017, 50, 135201. [Google Scholar] [CrossRef]
- Won, M.Y.; Lee, S.J.; Min, S.C. Mandarin Preservation by Microwave-Powered Cold wPlasma Treatment. Innov. Food Sci. Emerg. Technol. 2017, 39, 25–32. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Han, C.; Ji, N.; Jin, P.; Zheng, Y. Physiological and metabolomic analysis of cold plasma treated fresh-cut strawberries. J. Agric. Food Chem. 2019, 67, 4043–4053. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Ji, N.; Jin, P.; Zhang, J.; Zheng, Y.; Zhang, X.; Li, F. Cold plasma treatment induces phenolic accumulation and enhances antioxidant activity in fresh-cut pitaya (Hylocereus undatus) fruit. Lwt 2019, 115, 108447. [Google Scholar] [CrossRef]
- Sarangapani, C.; O’Toole, G.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma dissipation efficiency of agrochemicals on blueberries. Innov. Food Sci. Emerg. Technol. 2017, 44, 235–241. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovacevic, D.B.; Kljusuric, J.G.; Jambrak, A.R.; Zoric, Z.; Dragovic-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Hao, X.; Shishir, M.R.I.; Karim, N.; Chen, W. Cold plasma: An emerging pretreatment technology for the drying of jujube slices. Food Chem. 2021, 337, 127783. [Google Scholar] [CrossRef] [PubMed]
- Paixao, L.M.N.; Fonteles, T.V.; Oliveira, V.S.; Fernandes, F.A.N.; Rodrigues, S. Cold plasma effects on functional compounds of siriguela juice. Food Bioprocess Technol. 2019, 12, 110–121. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Cheng, J.H.; Sun, D.W. Chemical, physical and physiological quality attributes of fruit and vegetables induced by cold plasma treatment: Mechanisms and application advances. Crit. Rev. Food Sci. Nutr. 2020, 60, 2676–2690. [Google Scholar] [CrossRef]
- Berardinelli, A.; Vannini, L.; Ragni, L.; Guerzoni, M.E. Impact of Atmospheric Plasma Generated by a DBD Device on Quality-Related Attributes of “Abate Fetel” Pear Fruit Plasma for Bio-Decontamination, Medicine and Food Security; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bogdanov, T.; Tsonev, I.; Marinova, P.; Benova, E.; Rusanov, K.; Rusanova, M.; Atanassov, I.; Kozáková, Z.; Krčma, F. Microwave plasma torch generated in argon for small berries surface treatment. Appl. Sci. 2018, 8, 1870. [Google Scholar] [CrossRef]
- Ziuzina, D.; Misra, N.; Han, L.; Cullen, P.; Moiseev, T.; Mosnier, J.; Keener, K.; Gastone, E.; Vilaró, I.; Bourke, P. Investigation of a large gap cold plasma reactor for continuous in-package decontamination of fresh strawberries and spinach. Innov. Food Sci. Emerg. Technol. 2020, 59, 102229. [Google Scholar] [CrossRef]
- Pour, A.K.; Khorram, S.; Ehsani, A.; Ostadrahimi, A.; Ghasempour, Z. Atmospheric cold plasma effect on quality attributes of banana slices: Its potential use in blanching process. Innov. Food Sci. Emerg. Technol. 2022, 76, 102945. [Google Scholar] [CrossRef]
- Illera, A.E.; Chaple, S.; Sanz, M.T.; Ng, S.; Lu, P.; Jones, J.; Bourke, P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. 2019, 3, 100049. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Bastos, D.M.; Monaro, E.; Siguemoto, E.; Séfora, M. Maillard Reaction Products in Processed Food: Pros and Cons; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef]
- Onal-Ulusoy, B. Effects of Cold Atmospheric Gliding Arc Discharge Plasma, Non-thermal Ultrasound, and Low-Temperature Oven Treatments on Quality Parameters of Turkish Blossom Honey. Food Bioprocess Technol. 2021, 14, 1763–1771. [Google Scholar] [CrossRef]
- Trivedi, M.H.; Patel, K.; Itokazu, H.; Huynh, N.A.; Kovalenko, M.; Nirenberg, G.; Miller, V.; Fridman, A.; Fridman, G.; Lahne, J.; et al. Enhancing shelf life of bananas by using atmospheric pressure pulsed cold plasma treatment of the storage atmosphere. Plasma Med. 2019, 9, 23–38. [Google Scholar] [CrossRef]
- Zhao, N.; Ge, L.; Huang, Y.; Wang, Y.; Wang, Y.; Lai, H.; Wang, Y.; Zhu, Y.; Zhang, J. Impact of cold plasma processing on quality parameters of packaged fermented vegetable (radish paocai) in comparison with pasteurization processing: Insight into safety and storage stability of products. Innov. Food Sci. Emerg. Technol. 2020, 60, 102300. [Google Scholar] [CrossRef]
- Dong, X.Y.; Yang, Y.L. A novel approach to enhance blueberry quality during storage using cold plasma at atmospheric air pressure. Food Bioprocess Technol. 2019, 12, 1409–1421. [Google Scholar] [CrossRef]
- Suhem, K.; Matan, N.; Nisoa, M.; Matan, N. Low pressure RF plasma effects on the mould control, physical quality, nutritional value, mineral content and trace element content of a brown rice snack bar. J. Food Nutr. Res. 2013, 52, 87–94. [Google Scholar]
- Zhou, R.; Zhou, R.; Yu, F.; Xi, D.; Wang, P.; Li, J.; Wang, X.; Zhang, X.; Bazak, K.; Ostrikov, K.K. Removal of organophosphorus pesticide residues from Lycium barbarum by gas phase surface discharge plasma. Chem. Eng. J. 2018, 342, 401–409. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Principal component analysis (PCA) of physicochemical compounds’ content in different cultivars of peach fruits, including qualification and quantification of sugars and organic acids by HPLC. Eur. Food Res. Technol. 2019, 245, 929–938. [Google Scholar] [CrossRef]
- Abdul-Hamid, N.A.; Mustaffer, N.H.; Maulidiani, M.; Mediani, A.; Ismail, I.S.; Tham, C.L.; Shadid, K.; Abas, F. Quality evaluation of the physical properties, phytochemicals, biological activities and proximate analysis of nine Saudi date palm fruit varieties. J. Saudi Soc. Agric. Sci. 2020, 19, 151–160. [Google Scholar] [CrossRef]
- Elsafy, M.; Gustavsson, L.G.; Mujaju, C. Phenotypic diversity of date palm cultivars (Phoenix dactylifera L.) from sudan estimated by vegetative and fruit characteristics. Int. J. Biodivers. 2015, 2015, 610391. [Google Scholar] [CrossRef]
- Diboun, I.; Mathew, S.; Al-Rayyashi, M.; Elrayess, M.; Torres, M.; Halama, A.; Méret, M.; Mohney, R.P.; Karoly, E.D.; Malek, J.; et al. Metabolomics of dates (Phoenix dactylifera) reveals a highly dynamic ripening process accounting for major variation in fruit composition. BMC Plant Bio. 2015, 15, 291. [Google Scholar] [CrossRef]
- Badarusham, K.; Sabri, N.E.; Salvamani, S.; Hassan, M.S.; Hassan, Z.; Hashim, R. Assessment of Minerals in Phoenix dactylifera L. as Determined by Inductively Coupled Plasma Optical Emission Spectrometry using ANOVA and PCA. Int. J. Recent Technol. Eng. 2019, 8, 336–344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).