Abstract
This study aimed to biosynthesize zinc oxide nanoparticles (ZnO NPs) using Pleurotus ostreatus to achieve a simple ecofriendly method, and further evaluate antimicrobial activity and cytotoxicity towards HepG2 and Hek293 cells. The nanoparticles were characterized through UV-Vis spectroscopy, Fourier transform infrared spectroscopy (FTIR), transmission and scanning electron microscopy (TEM and SEM), selected area electron diffraction (SAED), X-ray diffraction (XRD), and dynamic light scattering (DLS). The minimal inhibitory concentration (MIC) for antimicrobial activity and MTT assay for cytotoxicity were conducted in vitro. The study revealed an efficient, simple, and ecofriendly method for synthesis of ZnO NPs that have antimicrobial activity. UV-Vis showed peaks at 340 and 400 nm, and the bioactive compounds found in the mushroom acted as capping, reducing, and stabilizing agents. TEM characterized NPs as an amorphous nanosheet, with preferential orientation as projected by SAED patterns. The spherical and agglomerated morphology was observed on SEM, with EDX proving the presence of Zn and O, while XRD indicated a crystallite size of 7.50 nm and a stable nature (zeta potential of −23.3 mV). High cytotoxicity on Hek293 and HepG2 cells was noted for ZnO NPs. The study provides an alternative, ecofriendly method for biosynthesis of ZnO NPs that have antibacterial activity and potential use in cancer treatment.
1. Introduction
Nanotechnology is a rapidly growing field of science, with numerous applications in areas such as engineering and biomedical science [1]. Its applications have triggered research on the synthesis of nanomaterials that are biocompatible, environmentally friendly, fast, safe, efficient, and cost-effective [2]. Two methods (traditional and green synthesis) have been used for the synthesis of nanoparticles; however, the traditional system has fallen out of favor due to its adverse effects such as producing excessive carbon dioxide, which contributes to greenhouse effects and also poses danger to the scientists involved in the synthesis [3]. This has caused a major shift towards green-synthesis methods. Hence, microorganisms (bacterium, fungi, including mushrooms, and yeast) and plants are now used for the green synthesis of nanoparticles [4]. Recently, ZnO nanoparticles have been synthesized successfully using plant extracts [5] and mushroom extracts, thus emphasizing the development of green-synthesis methods. Green synthesis has been proven to be a non-toxic, pollution-free, environmentally friendly, economic, sustainable [6], clean, safe, and cost-effective method [3].
Nanoparticles (NPs) are regarded as microscopic particles having a size ranging from 1 to 100 nm [7]. They exhibit unique properties due to the quantum confinement effect; however, the influence of shapes and structures of the nanoparticles affects the reactivity and toughness of the nanomaterial [8]. These attributes of nanoparticles are due to their variations in shape, surface-to-volume ratio, size, and composition. These properties render their unique physical, chemical, and biological properties that may potentially be of benefit in numerous sectors such as medicines, agriculture, pharmaceuticals, electronics, and cosmetics [9]. Hence, nanoparticles have shown great potential in biomedical applications in managing infectious diseases that are usually caused by multidrug resistant organisms [10]. Currently, antimicrobial-resistant diseases, including hospital-acquired Gram-negative bacterial infections, and their associated morbidity and mortality, have grown at an alarming rate [11]. Hence bacteria such as Pseudomonas aeruginosa, Enterococcus faecium, Klebsiella pneumoniae, Staphylococcus aureus, Acinetobacter baumannii, and Enterobacter are considered as multidrug-resistant bacterial species causing nosocomial infections [12]. The rise in multidrug-resistant pathogens is significantly affecting the global economy and causing catastrophic effects in the health care system worldwide, thus resulting in increases in mortality and morbidity, a loss of certainty regarding orthodox drugs, and a rise in treatment costs [12].
Previously, multidrug-resistant pathogens, such as Enterococci and Pseudomonas, were associated with rapid nosocomial infections. Additionally, Enterococci has been recognized as the second-leading cause of nosocomial infections worldwide [13] (Bhonchal Bhardwaj, [13] 2020), whereas P. aeruginosa was found to contribute to 10–11% of all nosocomial infections [14]. There have been many attempts to eradicate multidrug-resistant bacteria; however, one of the major problems is that some organisms such as P. aeruginosa become more virulent when co-cultured with other Gram-positive bacteria [12,15]. Other researchers have suggested that P. aeruginosa is aggravated during coinfection with E. faecalis [16]. Therefore, it is paramount to find alternative effective and safe antibacterial responses, especially those with multiple mechanisms of resistance [17,18,19].
Recently, Gaglio et al. [18] explored the use of mushrooms as an antimicrobial agent since mushrooms produce a large number of bioactive metabolites with therapeutic potential, as reported earlier by [19]. These metabolites produced by mushrooms have immunomodulatory, cardiovascular, antifibrotic, antidiabetic, antitumor, liver protection, anti-inflammatory, antiviral, antioxidant, and, mostly, antimicrobial properties [20]. Thus, mushrooms may be a good alternative source of natural antibiotics having compounds with a low molecular weight (LMW) or high molecular weight (HMW) [21]. The numerous bioactive compounds found in mushrooms render mushrooms a new potential source for the synthesis of NPs [22]. This finding follows a major focus on NPs, which showed that NPs prevent microbial drug-resistance in some cases. This is because their mode of action is via direct contact with the bacterial cell wall, meaning there is no necessity to penetrate the cell. Hence, this provides hope that NPs do not promote bacterial resistance [23]. Zinc oxide (ZnO) NPs are mostly known to have distinct antibacterial, antimicrobial, and antifungal properties compared to other oxides [24]. However, the synthesis of zinc oxide NPs using P. ostreatus mushroom has been rarely explored in the past because ZnO NPs have been mainly produced using approaches such as the sol-gel, solvothermal, hydrothermal, and micro-emulsion methods [25], and solution combustion synthesis [26]. Based on the conditions and method used for synthesis, ZnO can be obtained in the form of knots, needles, nanorods, nanosheets, or nanoplates [26]. Thus, the shape of the nanoparticle usually affects its properties, such as its tensile strength, hardness, and stiffness, and its functions [27].
Hence, the present study focused on the synthesis of ZnO NPs using P. ostreatus mushrooms to evaluate both the antioxidant and the antibacterial effects against the multidrug resistance bacteria (P. aeruginosa and E. faecalis). This study proved to be advantageous in finding ways to eliminate the regime of multidrug-resistance, especially since mushroom-derived NPs are known to have high stability, longer shelf life, water-solubility, and good dispersion properties [22]. This technology of synthesizing nanoparticles using mushrooms is very promising for the synthesis of non-toxic, ecofriendly, and mostly stable nanomaterials [28,29]. Hence, this study promotes mushrooms as an alternative source for the synthesis of nanoparticles using a very cost-effective, non-toxic, and ecofriendly synthesis method for ZnO NPs. Previous researchers have only used green-synthesis methods for the production of CuO NPs using actinomycetes bacteria [30]. Hence the main objective of this study was to synthesize stable and biocompatible ZnO NPs, which would be an efficient antimicrobial and potential anticancer agent, using a fast, safe, cost-effective, and ecofriendly green method. This was achieved through incorporating a rarely used P. ostreatus mushroom as a capping and reducing agent during green synthesis.
2. Materials and Methods
2.1. Cultivation of P. ostreatus Mushrooms
Mushrooms were cultivated using locally available sugar cane bagasse waste material. The modified method outlined by Mkhize et al. [31] was utilized for the whole process of growing mushrooms. Briefly, the mushroom strain was pre-cultured on potato dextrose agar (PDA) and incubated at ambient temperatures, and thereafter maintained as mushroom culture in a refrigerator at 4 °C for further processing. Mushroom spawn was prepared using the modified method adopted from Mkhize et al. [32], whereby bird seed grains were soaked in distilled water overnight, and the excess water was drained. The grains were mixed with gypsum and calcium carbonates at a ratio of 4:1:300 g, and then sterilized in an autoclave. The sterilized grains were aseptically inoculated with previously grown mushroom cultures, and after that incubated in a darkroom set at ±25 °C for 2–3 weeks until mycelia fully colonized the grains. The spawn was inoculated into sterilized sugar cane bagasse, then incubated in the dark until mycelia fully colonized the bagasse. After full colonization, the colonized bags were moved from the dark into a fruiting room, which was constructed from plastic film covered by a single layer of 30% grey shade cloth on the outside. Then, mushrooms were allowed to fruit under ambient temperatures with constant fogging to provide 60% moisture to the mushrooms. The mushroom fruiting bodies were harvested and dried in the same tunnel with 30% shade cloth, with varying temperatures depending on the weather. However, the temperatures did not exceed ±45 °C. Mushrooms were then powdered for further analysis.
2.2. Synthesis of Mushroom ZnO NPs (Zinc Oxide Nanoparticles)
The mushroom powder (10 g) was dissolved in 100 mL of distilled water and allowed to boil for approximately 30 min, then filtered using Whatman filter paper, and the filtrate was stored at 4 °C for further analysis. The modified method of Muhammad et al. [33] was used for mushroom ZnO nanoparticle synthesis, whereby mushroom extract was used as the capping agent/reducing agent. Specifically, 0.2 M zinc nitrates (ZnNO3) and 4 M sodium hydroxide (NaOH) solution were prepared by dissolving ZnNO3 and NaOH at a ratio of 1:1 in distilled water. About 25 mL of 4 M NaOH was pipetted dropwise into 25 mL of 0.2 M ZnNO3 under constant stirring with a magnetic stirrer. The pH of 13 was maintained via the addition of distilled water. A quantity of 10 mL of the mushroom extract was added to the solution mixture and heated at temperatures of 60–80 °C for 2 h. Within intervals of 30 min, a solution mixture of 3 mL was taken out for UV-Vis analysis at the wavelength of 200–800 nm using a Shimadzu spectrophotometer. The color change of the solution was gradually observed, which possibly indicated the formation of mushroom ZnO NPs. After heating while stirring with a magnetic stirrer, the solution mixture was allowed to cool at room temperature, and thereafter centrifuged at a speed of 3000 rpm for 15 min. The precipitate obtained was washed by adding distilled water and again centrifuged. The obtained precipitate was dried at 60 °C overnight. The mushroom ZnO NP powder was used for further analysis, such as analysis of morphology, structure, characterization, and antioxidant and antibacterial activity.
2.3. Characterization of ZnO NPs
For the characterization of NPs, different methods were utilized, as modified from different researchers such as Mickymaray [11] and Sharma et al. [9]. The ZnO NPs were characterized using a UV-visible spectrophotometer set at 300–600 nm with the operating resolution of 1 nm. The Fourier transform infrared (FTIR) spectroscopic analysis of mushroom ZnO NPs was evaluated using a spectrum 65 FTIR spectrometer to determine the vibrational bonding between mushroom ZnO NPs and phyto-compounds attached to surfaces of the NPs. The FTIR spectrum was recorded at the wave number range of 380 to 4000 cm−1 with a spectral resolution of 4 cm−1. X-ray diffraction (XRD) analysis was performed using a powder X-ray diffractometer that had Cu-kα radiation of 1.5418 Å, and a nickel monochromator in the range of 2ϴ from 10° to 80°. XRD was mainly performed to evaluate the phase of ZnO NPs. TEM was performed by dispensing a drop of ZnO NP solution into a carbon-coated 200 mesh copper grid and evaporated at room temperature before examination using TEM. Further characterization was also performed using an energy dispersive X-ray spectrometer (EDS) attached to a scanning electron microscope (SEM), which was used to identify and map elements within the synthesized ZnO NP powder. The dynamic light scattering (DLS) technique using a Zetasizer was employed to determine ZnO NPs’ stability using the zeta potential of the nanoparticles.
2.4. Antimicrobial Activity Assay
The minimum inhibitory concentrations (MICs) of mushroom ZnO NPs were determined for Enterococcus faecalis, Candida albicans, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Moraxella catarrhalis, Mycoplasma hominis, Staphylococcus aureus, and Bacillus cereus using the microplate dilution assay modified from Soyingbe et al. [34]. The 2-fold serial dilution of nanoparticles was prepared in a 96-well microtiter plate using nutrient broth. A quantity of 50 μL of nutrient broth was added to all the 96-well microtiter plates. About 50 μL of mushroom ZnO NPs (5 mg/mL) dissolved in 1% DMSO was thereafter serially diluted downwards throughout the rows in the 96-well microtiter plates. A quantity of 50 μL bacterial culture set at 0.5 McFarland standard was added to all the wells of the 96-well microtiter plates, which were incubated at 37 °C for 24 h. The p-Iodo-nitrotetrazolium violet (INT) solution (40 μL of 0.2 mg/mL) was then added to 96-well microtiter plates and incubated at 37 °C for 30 min. The MIC was recorded as the lowest concentration of mushroom ZnO NPs that completely inhibited the microbial growth of organisms.
2.5. DNA Cleavage Studies
A modified method of Rajabi et al. [35] was followed for the DNA cleavage assay, whereby the ability of synthesized ZnO NPs to digest bacterial DNA was tested. The mushroom-synthesized ZnO NPs of various concentrations (30–100 µg/mL) interacted with the pET30 plasmid DNA. The reaction for DNA cleavage was conducted in a 96-well microtiter plate, whereby the reaction mixture was made up to 10 µL. The mixture was composed of pET30 plasmid DNA (2.4 µg), various concentrations of ZnO NPs (3.0–100 µg/mL), H2O, and H2O2 for the positive control instead of ZnO NPs. The reaction mixtures were incubated at 37 °C for 60 min. After incubation, the mixtures were mixed with 0.25% bromophenol blue dye in 50% glycerol. Then, electrophoresis was carried out at 100 volts for 45 min; after that, the electrophoresis bands were observed under UV light.
2.6. Cytotoxicity Assay
In this study, the MTT assay was used to evaluate the cytotoxic effect of mushroom-synthesized ZnO NPs, and that of methanolic P. ostreatus mushroom extract on two cell lines, namely, human embryonic kidney (Hek293) and hepatocellular carcinoma (HepG2). A modified method from [36] was adopted, whereby the HepG2 and Hek293 cells were grown in 25 cm2 flasks using complete culture medium (CCM; DMEM with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin/fungizone) and incubated at 37 °C and 5% CO2. Confluent flasks were trypsinized to resuspend the cells, and the cells were counted using the trypan blue method. The cells were transferred into a sterile 96-well microtiter plate (15,000 cells/well, 200 µL CCM per well) and allowed to attach overnight. The medium was removed, and adherent cells were treated in triplicate with the prepared extracts (0–2000 µg/mL, 200 µL/well); a 4000 µg/mL stock solution of the mushroom ZnO NPs and P. ostreatus mushroom extract was used to prepare the treatment dilutions. A vehicle control (1% DMSO in CCM) was included. After 24 h, the treatment medium was discarded and replaced with 20 µL MTT salt solution (5 mg/mL in 0.1 M PBS) and 100 µL CCM at 37 °C. After a 4 h incubation, the MTT salt was removed, and the formazan crystals were solubilized by adding 100µL of DMSO to each well and incubated for an hour at 37 °C. The absorbance of the samples was read at 570 nm/690 nm using a Bio-Tek µQuant spectrophotometer (USA). The cell viability was calculated using the following formula:
Cell viability = ((absorbance of sample)/(absorbance of control) × 100)
Furthermore, the cell viability vs. log treatment concentration curve was analyzed using nonlinear regression analysis (GraphPad Prism V5.0, GraphPad PRISM®, La Jolla, CA, USA). The 25% inhibitory concentration (IC25) was extrapolated from the curve; at this concentration, cells retained 75% cell viability. The cell viability at the respective MIC was also extrapolated from the curve.
2.7. Statistical Analysis of Results
All experiments were repeated in triplicate. Statistical analysis data generated were calculated using Graph Pad Prism. The statistical differences were determined using a one-way analysis of variance (ANOVA), followed by a Tukey–Kramer multiple comparison test; results are reported as mean ± S.E.M. The values were considered statistically significant where p ≤ 0.05.
3. Results and Discussion
The UV-Vis spectrum of P. ostreatus-synthesized ZnO NPs displayed absorption peaks in the range between 300 and 600 nm, as displayed in Figure 1A. The observed absorption peaks of 340 and 400 nm confirmed the formation of ZnO NPs. Previous studies also obtained absorption peaks similar to those of our study, obtaining absorption peaks between 300 and 400 nm, which were reported to be typical of ZnO NP peaks [37]. Thus, this confirms the presence of ZnO NPs in our mushroom-synthesized nanoparticles. The results of the study were also in line with the findings of Manimaran et al. [38], who obtained an absorption peak of 350 nm for P. djamor mushroom-synthesized ZnO NPs.
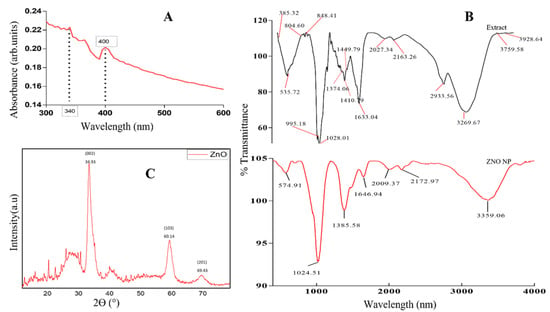
Figure 1.
Characterization of P. ostreatus-synthesized ZNO NPs: (A) UV-Vis spectrum of the synthesized ZnO NPs; (B) FTIR spectrum of ZnO NPs; and (C) XRD pattern of ZnO NPs.
Figure 1B shows the Fourier-transform infrared spectroscopy (FTIR) spectra of the Pleurotus ostreatus mushroom extract and synthesized ZnO NPs. Hence FTIR was used to identify functional groups present within Pleurotus ostreatus mushroom extract and within synthesized ZnO NPs. FTIR spectra showed a broad band at 3291.06 cm−1 for a mushroom extract, which shifted to 3359.06 cm−1 on synthesized ZnO NPs. These broad bands correspond to the O-H stretching vibrations, which were attributed to the alcohol or phenols found in mushrooms that might be transferred to ZnO NPs during synthesis. Furthermore, the mushroom extract had absorption bands of 2117.60 cm−1, which also shifted to 2172.97 and 2009.37 cm−1 within ZnO NPs due to C=C interactions. The presence of C=O (amide1) was observed for the absorption band of 1633.04 cm−1 in the mushroom extract. This absorption band was slightly shifted to 1646.94 cm−1 in ZnO NPs. This shift was an indication that mushroom extract contained functional groups that could act as a capping and reducing agent in the synthesis of ZnO NPs. The peaks of 1410.79 and 1028.01 cm−1 were noted in the mushroom extract, which shifted to 138.58 and 1024.51 cm−1 within the spectra of mushroom-synthesized ZnO NPs, indicating the bending alkane vibrations of C-H (138.58 cm−1) and amine C-N (1024.51 cm−1) stretching vibrations. The peaks (1024.51 and 574.91 cm−1) observed on the fingerprint region of ZnO NPs probably corresponded to the ZnO stretching vibrations; metal oxides are known to have absorption peaks between 600 and 400 cm−1 [39]. Such findings corroborate with other findings reported by Daumann et al. [40], who also established a deep absorption band from 610 cm−1 to a lower wavelength corresponding to ZnO. The observed peak at 574.91 cm−1 was attributed to the ZnO stretching band, meaning the P. ostreatus extracts successfully acted as capping and stabilizing agents in the synthesis of ZnO NPs. The mushroom-synthesized ZnO NPs were further analyzed with XRD to determine the crystallinity and purity of the nanoparticles, as indicated in Figure 1C. The diffraction pattern of ZnO NPs at three theta angles of 34.93, 60.14, and 69.43, which are indexed to (002), (103) and (201) planes, were observed, as shown in Figure 1C. Thus, the XRD pattern of the study was in line with the research conducted by Manimaran et al. [38], where P. djamor mushrooms were used for the synthesis of ZnO NPs. The average particle size of the sample was found to be 7.50 nm, derived from the full width at half-maximum (FWHM) of the more intense peak; hence, this corresponded to the 002 plane located at 34.93°.
The elemental composition of the green-synthesized ZnO NPs was determined using EDX spectrum analysis. The EDX spectrum shown in Figure 2C revealed the presence of Zn and O within the samples, as expected, with no traces of impurities. Hence, a strong signal in Zn and O regions was observed, confirming that mushroom extracts were able to reduce and stabilize Zn+ ions, and that ZnO NPs were synthesized. Previous research conducted by Preethi et al. [41] also proved that mushrooms can act as a reducing agent in synthesizing ZnO NPs. The study indicated that the percentage composition of zinc was 65.93% and that of oxygen was 34.07%. This result agrees with the findings of Mohana and Sumathi [42], who found the percentage composition of zinc was 66.16% and that of oxygen was 33.84% for ZnO NPs synthesized by Agaricus bisporus mushroom extract. The results of ZnO mapping shown in Figure 2A,B proved that zinc and oxygen were present in the synthesized ZnO NPs, which further verifies that the mushroom-synthesized ZnO NPs had no impurities.
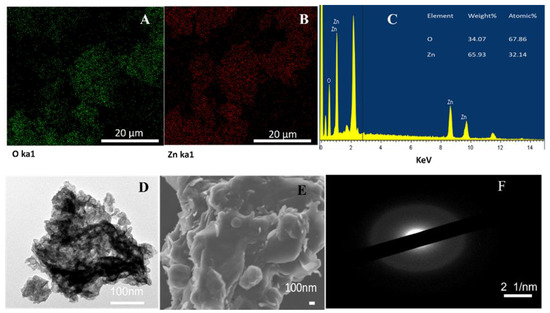
Figure 2.
Structural characterization of ZnO NPs by electron microscopy prepared from P. ostreatus: (A,B) are EDS elemental maps (20 kV) of oxygen (Ka1) and zinc (Ka1) emission lines, respectively; (C) EDX analysis (20 kV) of O Ka1 and Zn Ka1; (D) TEM images of synthesized ZnO NPs; (E) SEM micrograph image of synthesized ZnO NPs; and (F) SAED pattern of ZnO NPs.
Figure 2D shows the transmission electron microscopy (TEM) images of the amorphous ZnO NPs, which had dark spots that are probably due to the stacking of a large number of layers of nanosheets. This indicates that the synthesized ZnO NPs were probably agglomerated, since ZnO nanoparticles are also prone to forming agglomerations that are either hard or soft, where hard agglomeration is caused by the chemical reaction of the surface groups and soft agglomeration is caused by other physical effects [43]. Furthermore, the selected area electron diffraction (SAED) pattern in Figure 2F shows bright rings, which confirm the preferential orientation of mushroom-synthesized ZnO crystals.
The SEM images in Figure 2E indicate that the synthesized ZnO NPs have a spherical morphology and are agglomerated together. Furthermore, as expected, the green-synthesis method is known to usually cause agglomeration in NPs because the green-synthesized NPs mostly have a high surface area, allowing NPs to stick together [44]. However, polarity and the electrostatic attraction of ZnO NPs may also have contributed to the agglomeration of ZnO NPs [39]. The surface charge and stability of Pleurotus ostreatus-mediated ZnO NPs were examined using the zeta potential (Figure 3). In this study, the Pleurotus ostreatus-synthesized ZnO NPs were observed to be negatively charged with good stability; thus, the zeta potential was found to be −23.3 mV. These results suggest that the synthesized ZnO NPs had negatively charged capping molecules, which are responsible for the stability of the synthesized nanoparticles [45]. These findings corroborate well with the results reported by Mohana and Sumathi [42], who found the zeta potential of Agaricus bisporus-mediated ZnO NPs to be −20.5 mV, with good stability and a negative charge. Other researchers also confirmed that particles with charges of or above −25 mV and +25 mV tend to be more stable [46].
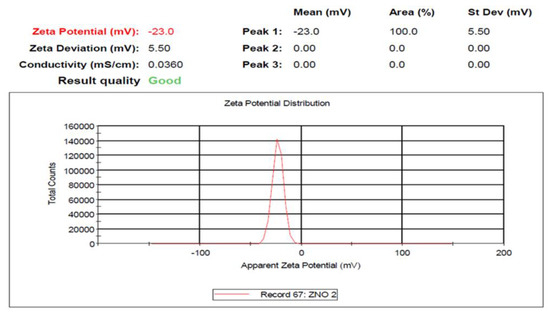
Figure 3.
Zeta potential analysis of the biosynthesized ZnO NPs.
Most pathogenic microorganisms, such as those of clinical isolates, have been reported to exhibit a higher level of resistance towards antibiotics that are particularly used in medical care facilities [47]. In addition to the clinical isolates, the bacterial strains associated with foodborne outbreak infections also proved to have higher levels of resistance towards antibiotics [48]. The literature states that zinc oxide nanoparticles can be used as antimicrobial agents against pathogenic microorganisms [49]. In the present study, the antibacterial activity of mushroom-synthesized ZnO NPs was investigated for both Gram-positive (C. albicans, B. cereus, S. aureus, and E. faecalis) and Gram-negative (K. pneumoniae, P. aeruginosa, P. vulgaris, E. coli, and M. catarrhalis) bacteria using the 96-well microtiter method to determine the MIC, as indicated in Table 1. The results in Table 1 indicate that both the mushroom extract and Pleurotus ostreatus-synthesized ZnO NPs exhibit antimicrobial activity against all tested microorganisms (Gram-positive and Gram-negative).

Table 1.
Antimicrobial activity of mushroom ZnO NPs (MIC in mg/mL) against selected pathogenic microorganisms.
However, when comparing mushroom extract with mushroom-synthesized ZnO NPs, the study showed that mushroom-synthesized ZnO NPs had enhanced antimicrobial activity for almost all the tested microorganisms. Therefore, this means that mushroom antibacterial activity is enhanced through nanoparticle synthesis. This activity of mushroom-synthesized ZnO NPs may be attributed to the fact that both Gram-positive and Gram-negative microorganisms contain a cell wall that is negatively charged, hence causing interactions of the bacterial cell wall with positively charged ions released by nanoparticles [50]. Previous researchers have also noted that the ZnO NPs interact with the plasma membrane of bacteria through electrostatic attraction; hence, membrane permeability may be disrupted through metal ions (Zn2+), resulting in damage to bacterial DNA leading to cell death [51]. The results indicate that different microorganisms have varying susceptibility towards mushroom-synthesized ZnO NPs, probably due to differences in the polarity of the cell membranes of these bacteria [52].
To further confirm possible antibacterial activity of synthesized ZnO NPs, DNA cleavage assay was conducted in our study. Figure 4 shows that mushroom-synthesized ZnO NPs potentially damage bacterial DNA in a concentration-dependent manner. Lane C1 indicates pure bacterial DNA, whereas lane C2 shows DNA treated with H2O2 (completely denatured DNA), and the remainder of the lanes indicate DNA treated with mushroom-synthesized ZnO NPs at increasing concentrations of 3.0, 6.25, 12.5, 25.0, 50.0, and 100 µg/mL. It can be noted from Figure 4 that mushroom-synthesized ZnO NPs were able to digest/damage bacterial DNA; hence, higher concentrations of ZnO NPs, such as 50 and 100 µg/mL, degraded the DNA structure. This observed DNA cleavage mechanism may be responsible for the antibacterial effects attained in other microorganisms. As expected, other researchers also found that DNA digestion can potentially be considered as an antimicrobial mechanism employed by nanoparticles [53]. These results suggest that ZnO NPs may compromise membrane permeability of bacteria, therefore causing Zn2+ to enter the bacterial cytoplasm and causing damage to bacterial DNA, as reported by Murali et al. [51].
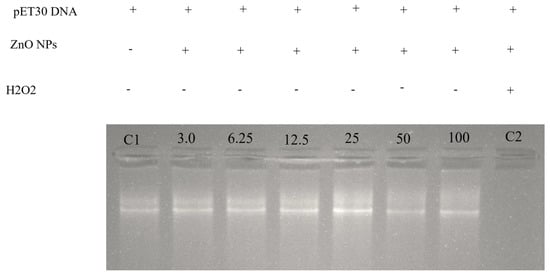
Figure 4.
The gel electrophoresis image of DNA cleavage of P. ostreatus-synthesized ZnO NPs. C1 (control with pure pET30 DNA), C2 (control with DNA+ H2O2), lanes 3.0–100 represent increasing concentrations of P. ostreatus-synthesized ZnO NPs in µg/mL.
The cytotoxicity of mushroom-synthesized ZnO NPs and mushroom extract was evaluated in vitro using HepG2 and Hek293 cell lines, which are the two frequently used cell lines for cytotoxicity testing. These cell lines were selected due to the vital role of liver cells in cellular metabolism and glucose homeostasis. In addition, the liver also detoxifies xenobiotics (including drugs) to water-soluble metabolites for excretion by the kidney [54]. The conversion of the yellow tetrazolium salt into purple formazan crystals by mitochondrial dehydrogenases only occurs in viable cells; thus, the intensity of formazan absorbance is directly proportional to the percentage of viable cells in the sample [55]. The mushroom extract (0–2000 µg/mL) caused a variable response in HepG2 cells, with cell viability above 100% for all treatments (Figure 5A). In contrast, the mushroom extract induced a dose-dependent decline in cell viability for the Hek293 cells, with the lowest cell viability of 22% recorded at 2000 µg/mL (Figure 5B). The mushroom-synthesized ZnO NPs induced similar cytotoxic responses for the HepG2 (Figure 5C) and Hek293 (Figure 5D) cells. There was a sharp decline in cell viability from approximately 97% at 16 µM to 12% at 100 µM in HepG2 cells, and 94% and 22% cell viability was observed in Hek293 cells at the same concentrations. The observed results for mushroom-synthesized ZnO NPs are consistent with the findings of Anitha et al. [56], who stipulated that ZnO NPs have the potential to cause cytotoxicity in many cells, including HepG2 cells.
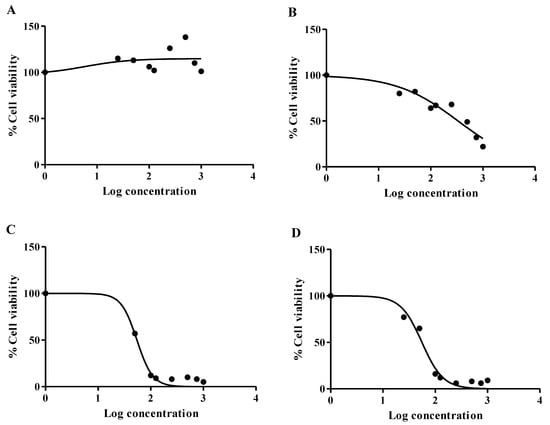
Figure 5.
The dose response curves derived from the MTT assay. (A) The mushroom extract was not cytotoxic to HepG2, with increased cell viability occurring with increased concentrations. (B) Cell viability decreased gradually in Hek293 cells treated with increasing mushroom extract concentration. (C) ZnO mushroom nanoparticles decreased cell viability significantly between 10 and 100 µg/mL in HepG2 cells, with more than 80% cell death from 1000 to 2000 µg/mL. (D) The Hek293 cell response to ZnO mushroom nanoparticles indicates cytotoxicity at all concentrations.
Cell viabilities below 20% were recorded from the concentrations of 1000–2000 µg/mL treatments in both HepG2 (Figure 5C) and Hek293 cells (Figure D). The decreased cell viability indicates a diminished capacity of mitochondrial dehydrogenases to convert the yellow tetrazolium salt into purple formazan crystals [55]. Thus, the lower IC50 values in Hek293 and HepG2 cells following ZnO treatment (Table 2) suggest increased sensitivity of the liver and kidney cells to this exposure (Figure 5C,D). In particular, these values denote mitochondrial toxicity since cell viability decreased. Recently, Li et al. [57] showed that ZnO NPs promote ROS generation, inducing mitochondrial dysfunction, and may impair mitochondrial biogenesis in cardiomyocytes [55,57]. The ROS generation may be attributed to interference at complex III of the electron transport chain [58]. The acute cytotoxicity of the mushroom-synthesized ZnO NPs may be due to parameters such as particle size, surface area, and surface reactivity. Previous studies have identified these parameters for other nanoparticles [59]. For example, Panda et al. [60] noted that smaller-sized nanoparticles are more toxic when compared to larger nanoparticles; however, it should also be considered that other factors can influence the toxicity of nanoparticles, as in the case of metallic particles [61].

Table 2.
The extrapolated IC25 and IC50 concentrations for HepG2 and Hek293 cells.
In this study, the ISO norm that requires >75% cell viability for biomedical products was applied [61]. Thus, an IC25 where HepG2 and Hek293 cells retained 75% viability was applied for the mushroom ZnO NPs and mushroom extract to be deemed safe. The IC25 for the P. ostreatus mushroom extract was 0.074 mg/mL in Hek293 cells (Table 2) and could not be calculated for HepG2 cells (Table 2), which retained cell viability (Figure 5A), indicating the concentrations retained 75% cell viability in both cell lines in accordance with the ISO norm. At the MIC for P. aeruginosa (0.04 mg/mL), the Hek293 and HepG2 cell viability was around 83% and 112%, respectively. Thus, the P. ostreatus mushroom extracts conformed to the ISO standard for the P. aeruginosa MIC, indicating that the mushroom extract would be safe to use for treating P. aeruginosa infections. However, the MIC for P. vulgaris exposed to mushroom extract (0.160 mg/mL) yielded 63% cell viability in Hek293, scoring below the ISO standard. Interestingly, the mushroom extract was not cytotoxic to HepG2 cells at the P. vulgaris MIC. Since all other MIC values for the mushroom extract were higher than the P. vulgaris MIC, the data suggest that a cytotoxic effect can manifest in Hek293 cells, but not HepG2 cells.
Acceptable ISO values for the Hek293 and HepG2 cells were 0.034 and 0.037 mg/mL, respectively (Table 2). Thus, the liver and kidney cells appear more sensitive to the mushroom-synthesized ZnO NPs (Figure 5C,D). Therefore, it was not surprising that the 67% and 70% cell viability for Hek293 and HepG2 cells, respectively, at the P. aeruginosa MIC (0.04 mg/mL), was slightly below the ISO norm. Similarly, approximately 33% and 24% cell viability was extrapolated for the MIC of E. faecalis (0.08 mg/mL) in Hek293 cells and HepG2 cells, respectively. The mushroom-synthesized ZnO NPs induced an MIC of 0.160 mg/mL for K. pneumonia, E. coli, and M. hominis, corresponding to cell viabilities of 10% (Hek293 cells) and 4% (HepG2 cells). Further increases in MIC, as indicated for other pathogenic microorganisms (Table 1), were also overtly cytotoxic to both cell lines. This suggests that the mushroom-synthesized ZnO NPs may be a potential anticancer agent if appropriately administered so that it can have specific activity; hence, future studies should confirm the anticancer property and cell proliferation as influenced by the ZnO NPs. Other mushrooms have been reported to be able to synthesize ZnO NPs, such as P. djamor [38], P. floridanus [62], and Agarius bisporus [41], in addition to P. ostreatus. To the best of our knowledge, this is one of the first reports demonstrating the biotechnological potential for synthesizing stable, biocompatible, ecofriendly, and antimicrobial ZnO NPs using P. ostreatus.
4. Conclusions
This paper presents the successful synthesis of ZnO NPs from P. ostreatus mushrooms, and characterization of ZnO NPs on UV-Vis spectra confirmed the peaks of ZnO NPs at 340 and 400 nm. The synthesized ZnO NPs were observed to be spherical and agglomerated with high stability. Thus, the mushroom was able to act as a reducing, capping, and stabilizing agent in the synthesis of ZnO NPs. The green-synthesis method proved beneficial in fighting against Gram-positive and Gram-negative bacteria, which are a major concern in the health sector. This is the first report of ZnO NP biosynthesis using the edible P. ostreatus mushroom. Hence, an alternative safe, cost-effective, and ecofriendly biosynthesis method to produce ZnO NPs can be achieved through mushroom-mediated synthesis. Further research can be conducted on other nanoparticles using the P. ostreatus-mediated biosynthesis method. Overall, minimal cytotoxicity to HepG2 cells (cell viability above 75% in accordance with the ISO standard) was observed for the P. ostreatus mushroom extract. Thus, the liver function of xenobiotic metabolism possibly resulted in the detoxification of the cytotoxic compounds in the mushroom extract. However, cell viability below the ISO standard at MIC above 0.160 mg/mL indicates that the mushroom extract should be used with caution due to its potential cytotoxicity to kidney cells. The mushroom-synthesized ZnO NPs were acutely cytotoxic to both Hek293 and HepG2 cells. Further studies should investigate the cytotoxic effects of the P. ostreatus mushroom extracts in vivo using a rat or mouse model, since metabolism in the liver may reduce the toxic effects in kidney cells that were elucidated in this study. The cytotoxicity data also demonstrate antiproliferative activity of the mushroom-synthesized ZnO NPs in HepG2 cells. Thus, further investigation of potential anticancer effects of the P. ostreatus mushroom extract and mushroom-synthesized ZnO NPs is warranted and recommended.
Author Contributions
The manuscript was written and read by all the authors who were mentioned on title page. The authors S.S.M., M.B.C.S. and O.J.P. structured and designed the experiments, and wrote and proofread the paper for possible corrections. S.S.M., I.N.M. and R.K. conducted the laboratory experiments, and collected and analyzed data; S.K. also proofread the paper, and analyzed and interpreted part of the data. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the National Research Foundation (NRF) of South African [grant number: 138414] to M.B.C. and [grant number: 145396] awarded to O.J.P. Some of the work reported herein was made possible through funding by the South African Medical Research Council through its Division of Research Capacity Development under the Early Investigators Programme awarded to O.J.P. from funding received from the South African National Treasury.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
All the authors declare no conflict of interest for this work. There is no known competing financial interest and personal relationship that could influence this paper’s reported work.
References
- Ye, F.; Zhao, Y.; El-Sayed, R.; Muhammed, M.; Hassan, M. Advances in nanotechnology for cancer biomarkers. Nano Today 2018, 18, 103–123. [Google Scholar] [CrossRef]
- Ovais, M.; Raza, A.; Naz, S.; Islam, N.U.; Khalil, A.T.; Ali, S.; Khan, M.A.; Shinwari, Z.K. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl. Microbiol. Biotechnol. 2017, 101, 3551–3565. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.; Debella, M.; Dibella, M.; Gupta, A. Green synthesis of nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Owaid, M.N. Biomedical Applications of Nanoparticles Synthesized from Mushrooms. In Green Nanoparticles. Nanotechnology in the Life Sciences; Patra, J., Fraceto, L., Das, G., Campos, E., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 289–303. [Google Scholar] [CrossRef]
- Mthana, M.S.; Mthiyane, D.M.N.; Onwudiwe, D.C.; Singh, M. Biosynthesis of ZnO Nanoparticles Using Capsicum chinense Fruit Extract and Their In Vitro Cytotoxicity and Antioxidant Assay. Appl. Sci. 2022, 12, 4451. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Begum, S.J.P.; Pratibha, S.; Rawat, J.M.; Venugopal, D.; Sahu, P.; Gowda, A.; Qureshi, K.A.; Jaremko, M. Recent Advances in Green Synthesis, Characterization, and Applications of Bioactive Metallic Nanoparticles. Pharmaceuticals 2022, 15, 455. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Sharma, G.; Nam, J.S.; Sharma, A.R.; Lee, S.S. Antimicrobial potential of silver nanoparticles synthesized using medicinal herb coptidis rhizome. Molecules 2018, 23, 2268. [Google Scholar] [CrossRef]
- Muzammil, S.; Hayat, S.; Fakhar-e-Alam, M.; Aslam, B.; Siddique, M.H.; Nisar, M.A.; Saqalein, M.; Atif, M.; Sarwar, A.; Khurshid, A.; et al. Nanoantibiotics: Future nanotechnologies to combat antibiotic resistance. Front. Biosci. Elite 2018, 10, 352–374. [Google Scholar] [CrossRef] [Green Version]
- Mickymaray, S. One-step synthesis of silver nanoparticles using Saudi arabian desert seasonal plant Sisymbrium irio and antibacterial activity against multidrug-resistant bacterial strains. Biomolecules 2019, 9, 662. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed]
- Bhonchal Bhardwaj, S. Enterococci: An Important Nosocomial Pathogen. In Pathogenic Bacteria; IntechOpen: London, UK, 2020; ISBN 978-1-78985-988-1. [Google Scholar] [CrossRef]
- Labovská, S. Pseudomonas aeruginosa as a Cause of Nosocomial Infections. In Pseudomonas aeruginosa—Biofilm Formation, Infections and Treatments; IntechOpen: London, UK, 2021; ISBN 978-1-83968-648-1. [Google Scholar] [CrossRef]
- Reece, E.; Doyle, S.; Greally, P.; Renwick, J.; McClean, S. Aspergillus fumigatus inhibits Pseudomonas aeruginosa in co-culture: Implications of a mutually antagonistic relationship on virulence and inflammation in the CF airway. Front. Microbiol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Lee, K.; Lee, K.M.; Kim, D.; Yoona, S.S. Molecular determinants of the thickened matrix in a dual-species Pseudomonas aeruginosa and Enterococcus faecalis biofilm. Appl. Environ. Microbiol. 2017, 83, e01182-17. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Guarcello, R.; Venturella, G.; Palazzolo, E.; Francesca, N.; Moschetti, G.; Settanni, L.; Saporita, P.; Gargano, M.L. Microbiological, chemical and sensory aspects of bread supplemented with different percentages of the culinary mushroom Pleurotus eryngii in powder form. Int. J. Food Sci. Technol. 2019, 54, 1197–1205. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Gonçalves, O.; Pereira, R.; Gonçalves, F.; Mendo, S.; Coimbra, M.A.; Rocha, S.M. Evaluation of the mutagenicity of sesquit-erpenic compounds and their influence on the susceptibility towards antibiotics of two clinically relevant bacterial strains. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 723, 18–25. [Google Scholar] [CrossRef]
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Kalia, A.; Kaur, G. Biosynthesis of Nanoparticles Using Mushrooms. In Biology of Macrofungi; Springer Nature: Cham, Switzerland, 2018; pp. 351–360. [Google Scholar]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Mahamuni, P.P.; Patil, P.M.; Dhanavade, M.J.; Badiger, M.V.; Shadija, P.G.; Lokhande, A.C.; Bohara, R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019, 17, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Khaliullin, S.M.; Zhuravlev, V.D.; Ermakova, L.V.; Buldakova, L.Y.; Yanchenko, M.Y.; Porotnikova, N.M. Solution Combustion Synthesis of ZnO Using Binary Fuel (Glycine + Citric Acid). Int. J. Self Propagating High Temp. Synth. 2019, 28, 226–232. [Google Scholar] [CrossRef]
- Morin, J.; Fujimoto, K.; Preston, A.; Guillen, D.P. Synthesis Methods for Nanoparticle Morphology Control in Energy Applications. In The Minerals, Metals and Materials Series; Springer Nature: Cham, Switzerland, 2022; pp. 21–31. [Google Scholar] [CrossRef]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Banerjee, K.; Ravishankar Rai, V. A Review on Mycosynthesis, Mechanism, and Characterization of Silver and Gold Nanoparticles. Bionanoscience 2018, 8, 17–31. [Google Scholar] [CrossRef]
- Nabila, M.I.; Kannabiran, K. Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal. Agric. Biotechnol. 2018, 15, 56–62. [Google Scholar] [CrossRef]
- Mkhize, S.S.; Cloete, J.; Basson, A.K.; Zharare, G.E. Performance of Pleurotus ostreatus mushroom grown on maize stalk residues supplemented with various levels of maize flour and wheat bran. Food Sci. Technol. 2016, 36, 598–605. [Google Scholar] [CrossRef] [Green Version]
- Mkhize, S.S.; Simelane, M.B.C.; Gasa, N.L.; Pooe, O.J. Evaluating the antioxidant and heavy metal content of Pleurotus ostreatus mushrooms cultivated using sugar cane agro-waste. Pharmacogn. J. 2021, 13, 844–852. [Google Scholar] [CrossRef]
- Muhammad, F.R.; Nurgaha, E.S.; Fahim, M.T. Synthesis of ZnO nanoparticles by precipitation method with their antibacterial effect. Indones. J. Chem. 2016, 16, 117–123. [Google Scholar] [CrossRef]
- Soyingbe, O.S.; Mongalo, N.I.; Makhafola, T.J. In vitro antibacterial and cytotoxic activity of leaf extracts of Centella asiatica (L.) Urb, Warburgia salutaris (Bertol. F.) Chiov and Curtisia dentata (Burm. F.) C.A.Sm—Medicinal plants used in South Africa. BMC Complement. Altern. Med. 2018, 18, 315. [Google Scholar] [CrossRef]
- Rajabi, H.R.; Naghiha, R.; Kheirizadeh, M.; Sadatfaraji, H.; Mirzaei, A.; Alvand, Z.M. Microwave assisted extraction as an efficient approach for biosynthesis of zinc oxide nanoparticles: Synthesis, characterization, and biological properties. Mater. Sci. Eng. C 2017, 78, 1109–1118. [Google Scholar] [CrossRef]
- Kongsema, M.; Tadakittisarn, S.; Chumnanpuen, P. Riceberry rice bran protein hydrolyzed fractions 2 induced apoptosis, senescence and G1/S cell cycle 3 arrest in human colon cancer cell lines 4 5 Vichugorn Wattayagorn. Appl. Sci. 2022, 12, 6917. [Google Scholar] [CrossRef]
- Aldalbahi, A.; Alterary, S.; Ali Abdullrahman Almoghim, R.; Awad, M.A.; Aldosari, N.S.; Fahad Alghannam, S.; Nasser Alabdan, A.; Alharbi, S.; Ali Mohammed Alateeq, B.; Abdulrahman Al Mohsen, A.; et al. Greener Synthesis of Zinc Oxide Nanoparticles: Characterization and Multifaceted Applications. Molecules 2020, 25, 4198. [Google Scholar] [CrossRef] [PubMed]
- Manimaran, K.; Balasubramani, G.; Ragavendran, C.; Natarajan, D.; Murugesan, S. Biological Applications of Synthesized ZnO Nanoparticles Using Pleurotus djamor Against Mosquito Larvicidal, Histopathology, Antibacterial, Antioxidant and Anticancer Effect. J. Clust. Sci. 2021, 32, 1635–1647. [Google Scholar] [CrossRef]
- Fakhari, S.; Jamzad, M.; Kabiri Fard, H. Green synthesis of zinc oxide nanoparticles: A comparison. Green Chem. Lett. Rev. 2019, 12, 19–24. [Google Scholar] [CrossRef]
- Daumann, S.; Andrzejewski, D.; Di Marcantonio, M.; Hagemann, U.; Wepfer, S.; Vollkommer, F.; Bacher, G.; Epple, M.; Nannen, E. Water-free synthesis of ZnO quantum dots for application as an electron injection layer in light-emitting electrochemical cells. J. Mater. Chem. C 2017, 5, 2344–2351. [Google Scholar] [CrossRef]
- Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Abilaji, S.; Prakash, C.; Rajasekar, A.; Nanthini, A.U.R.; Valli, G. Myco-Synthesis of Zinc Oxide Nanoparticles as Potent Anti-corrosion of Copper in Cooling Towers. J. Clust. Sci. 2019, 30, 1583–1590. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Synthesis of zinc oxide using Agaricus bisporus and its in-vitro biological activities. J. Environ. Chem. Eng. 2020, 8, 104192. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Sun, S. Preparation and characterization of ZnO nanoparticles supported on amorphous SiO2. Nanomaterials 2017, 7, 217. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ambika, S.; Bharathi, K. Plant-extract mediated synthesis of ZnO nanoparticles using Pongamia pinnata and their activity against pathogenic bacteria. Adv. Powder Technol. 2015, 26, 1294–1299. [Google Scholar] [CrossRef]
- Alamdari, S.; Ghamsari, M.S.; Lee, C.; Han, W.; Park, H.H.; Tafreshi, M.J.; Afarideh, H.; Ara, M.H.M. Preparation and characterization of zinc oxide nanoparticles using leaf extract of Sambucus ebulus. Appl. Sci. 2020, 10, 3620. [Google Scholar] [CrossRef]
- Mahobia, S.; Bajpai, J.; Bajpai, A.K. An in-vitro investigation of swelling controlled delivery of insulin from egg albumin nanocarriers. Iran. J. Pharm. Res. 2016, 15, 695–711. [Google Scholar] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Venkitanarayanan, K.; Johny, A.K. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, K.S.; ur Rahman, A.; Tajuddin; Husen, A. Properties of Zinc Oxide Nanoparticles and Their Activity Against Microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Murali, M.; Kalegowda, N.; Gowtham, H.G.; Ansari, M.A.; Alomary, M.N.; Alghamdi, S.; Shilpa, N.; Singh, S.B.; Thriveni, M.C.; Aiyaz, M.; et al. Plant-mediated zinc oxide nanoparticles: Advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics 2021, 13, 1662. [Google Scholar] [CrossRef]
- Espitia, P.J.; Soares, N.D.; Coimbra, J.S.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A. Zinc Oxide Nanoparticles: Synthesis, Antimicrobial Activity and Food Packaging Applications. Food Bioprocess Technol. 2012, 5, 1447–1464. [Google Scholar] [CrossRef]
- Mousavi-Khattat, M.; Keyhanfar, M.; Razmjou, A. A comparative study of stability, antioxidant, DNA cleavage and antibacterial activities of green and chemically synthesized silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1022–S1031. [Google Scholar] [CrossRef]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Anitha, R.; Ramesh, K.V.; Ravishankar, T.N.; Sudheer Kumar, K.H.; Ramakrishnappa, T. Cytotoxicity, antibacterial and an-tifungal activities of ZnO nanoparticles prepared by the Artocarpus gomezianus fruit mediated facile green combustion method. J. Sci. Adv. Mater. Devices 2018, 3, 440–451. [Google Scholar] [CrossRef]
- Li, Y.; Li, F.; Zhang, L.; Zhang, C.; Peng, H.; Lan, F.; Peng, S.; Liu, C.; Guo, J. Zinc oxide nanoparticles induce mitochondrial biogenesis impairment and cardiac dysfunction in human ipsc-derived cardiomyocytes. Int. J. Nanomed. 2020, 15, 2669–2683. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Bae, H.C.; Kim, J.; Jeong, S.H.; Yang, S.I.; Son, S.W. Zinc oxide nanoparticles induce HIF-1α protein stabilization through increased reactive oxygen species generation from electron transfer chain complex III of mitochondria. J. Dermatol. Sci. 2018, 91, 104–107. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- De Lima, R.; Feitosa, L.; Pereira, A.D.; De Moura, M.R.; Aouada, F.A.; Mattoso, L.H.; Fraceto, L.F. Evaluation of the genotoxicity of chitosan nanoparticles for use in food packaging films. J. Food Sci. 2010, 75, N89–N96. [Google Scholar] [CrossRef]
- Rafeeq, C.M.; Paul, E.; Vidya Saagar, E.; Manzur Ali, P.P. Mycosynthesis of zinc oxide nanoparticles using Pleurotus floridanus and optimization of process parameters. Ceram. Int. 2021, 47, 12375–12380. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).