Anaerobic Digestion in the Presence of Antimicrobials—Characteristics of Its Parameters and the Structure of Methanogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Anaerobic Digestion Process
2.3. Sampling
2.4. Genomic DNA Isolation from Digestate Samples
2.5. Analysis of Gene Characteristics for Methanogens
2.6. Data Analysis
3. Results
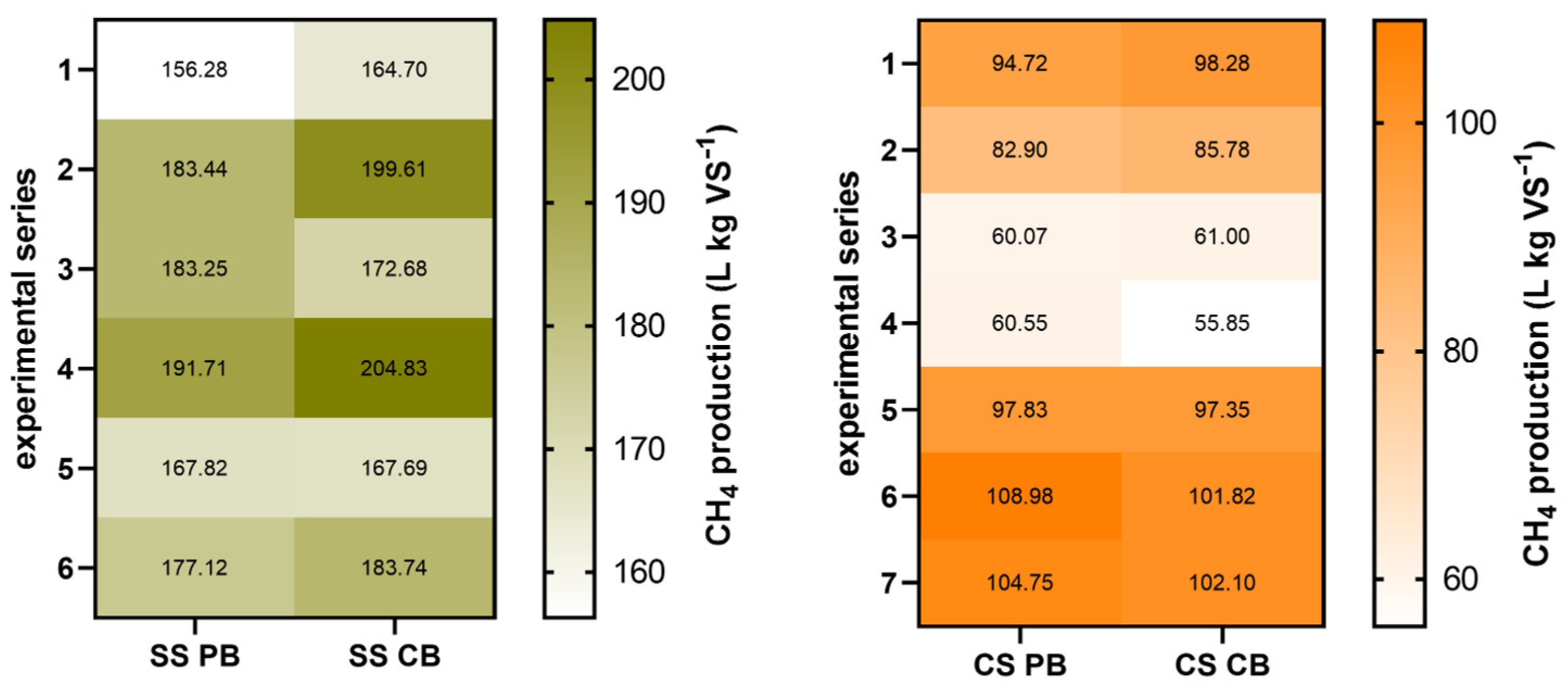
3.1. The Long-Term Impact of Antibiotics on CH4 Production and mcrA Gene Abundance
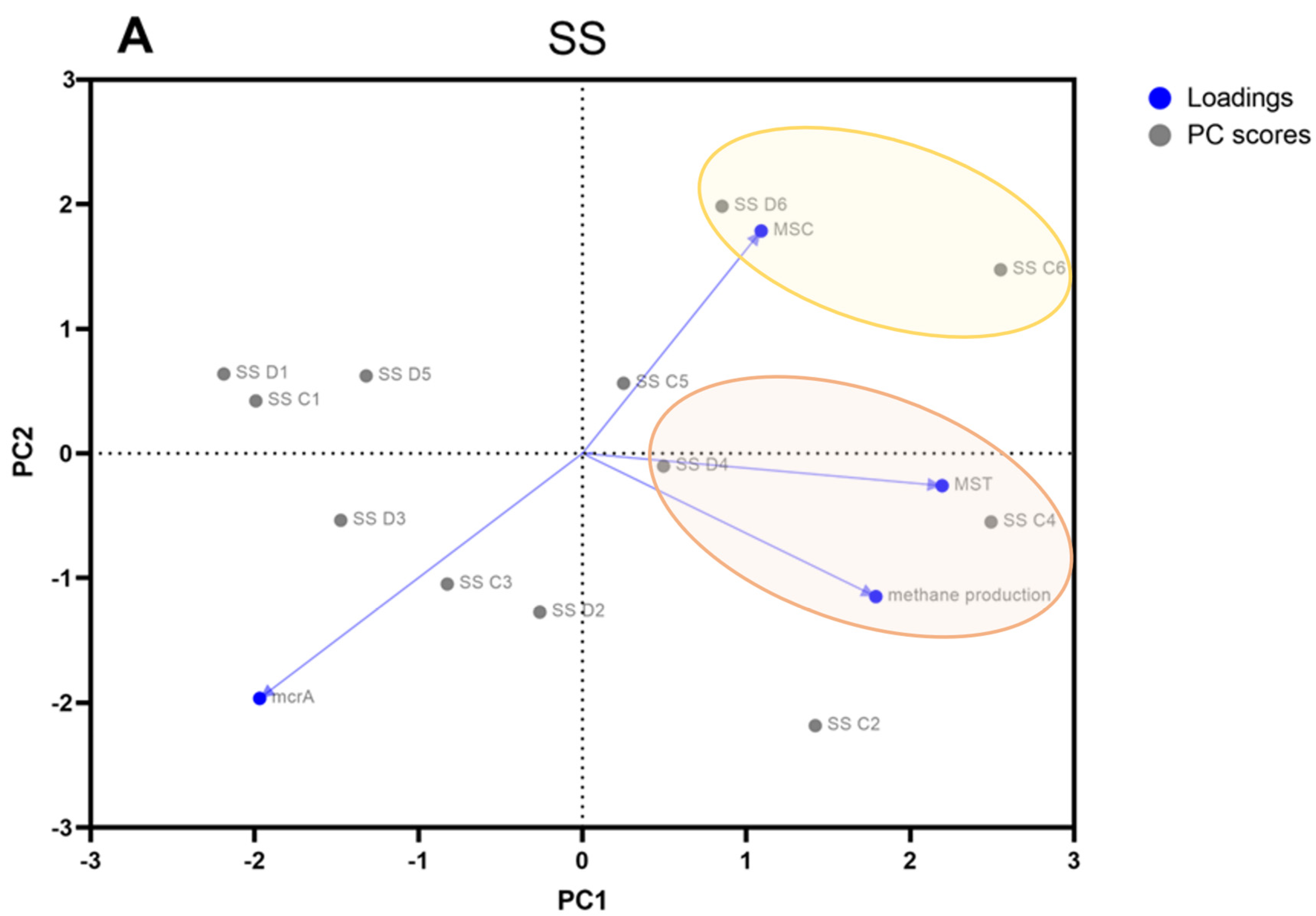
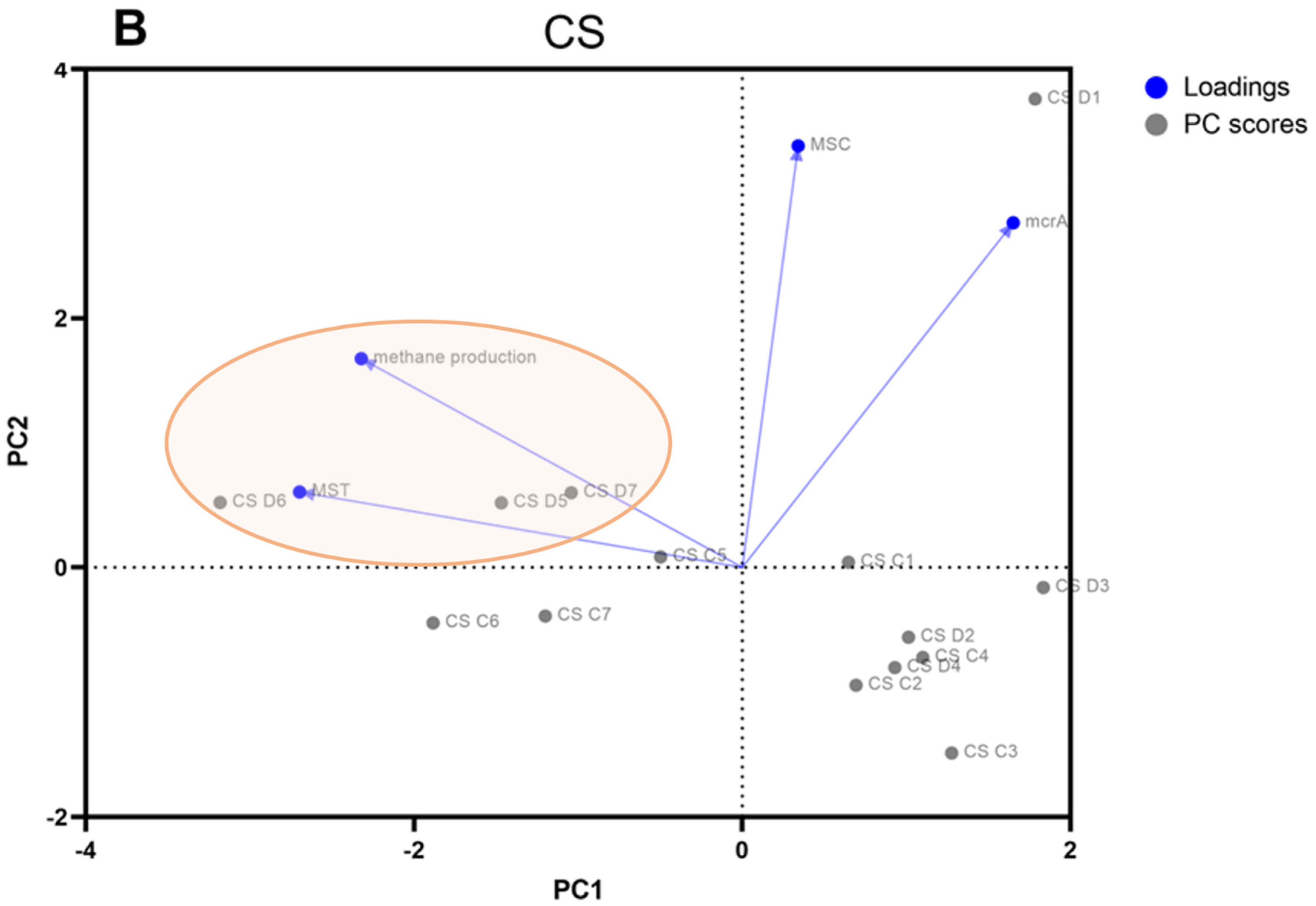
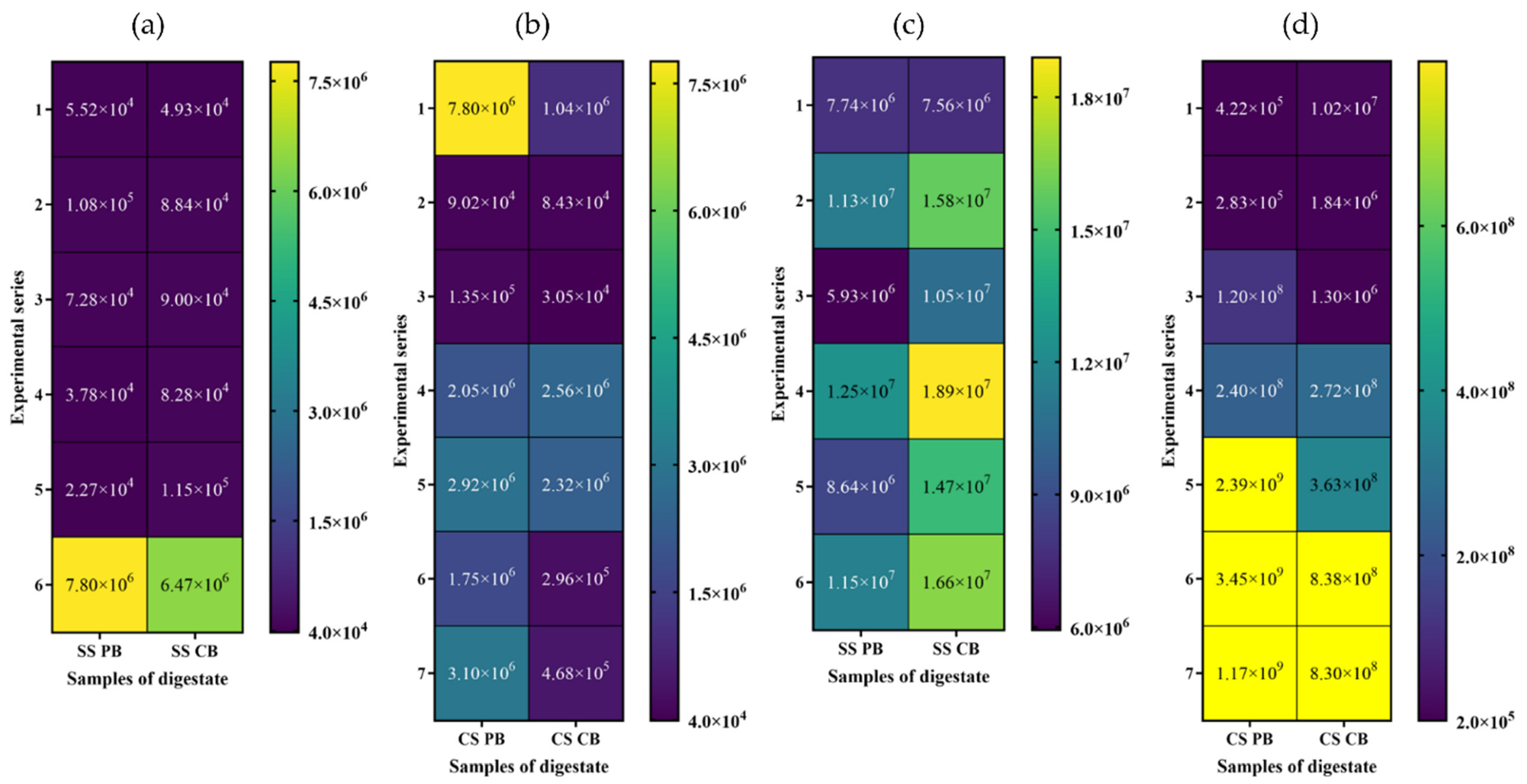
3.2. Real-Time PCR Quantification of Genes Specific to Methanogens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collivignarelli, M.C.; Abba, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef] [Green Version]
- Stiborova, H.; Kracmarova, M.; Vesela, T.; Biesiekierska, M.; Cerny, J.; Balik, J.; Demnerova, K. Impact of Long-Term Manure and Sewage Sludge Application to Soil as Organic Fertilizer on the Incidence of Pathogenic Microorganisms and Antibiotic Resistance Genes. Agronomy 2021, 11, 1423. [Google Scholar] [CrossRef]
- Degueurce, A.; Trémier, A.; Peu, P. Dynamic effect of leachate recirculation on batch mode solid state anaerobic digestion: Influence of recirculated volume, leachate to substrate ratio and recirculation periodicity. Bioresour. Technol. 2016, 2016, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, Y.; Lichtfouse, E.; Li, Z.; Kumar, P.S.; Liu, J.; Feng, D.; Yang, Q.; Liu, F. Effect of Antibiotics on the Microbial Efficiency of Anaerobic Digestion of Wastewater: A Review. Front. Microbiol. 2021, 11, 611613. [Google Scholar] [CrossRef] [PubMed]
- Koniuszewska, I.; Czatzkowska, M.; Harnisz, M.; Korzeniewska, E. The impact of antimicrobial substances on the methanogenic community during methane fermentation of sewage sludge and cattle slurry. Appl. Sci. 2021, 11, 369. [Google Scholar] [CrossRef]
- Stone, J.J.; Clay, S.A.; Zhu, Z.; Wong, K.L.; Porath, L.R.; Spellman, G.M. Effect of antimicrobial compounds tylosin and chlortetracycline during batch anaerobic swine manure digestion. Water Res. 2009, 43, 4740–4750. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.H.; Zheng, Y.; Wang, Q.P.; Bai, Y.; Liu, J.B.; Zhang, Y.R.; Xiong, W.P.; Lu, Y.; Fan, C.Z. Metagenomic analysis reveals the effects of long-term antibiotic pressure on sludge anaerobic digestion and antimicrobial resistance risk. Bioresour. Technol. 2019, 282, 179–188. [Google Scholar] [CrossRef]
- Lueders, T.; Chin, K.J.; Conrad, R.; Friedrich, M. Molecular analyses of methyl-coenzyme M reductase α-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 2001, 3, 194–204. [Google Scholar] [CrossRef]
- Hashemi, H.; Amin, M.; Afshin, E.; Asghar, E. Effects of oxytetracycline, tylosin, and amoxicillin antibiotics on specific methanogenic activity of anaerobic biomass. Int. J. Environ. Health Eng. 2012, 1, 37. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment-Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- Yu, Y.; Kim, J.; Hwang, S. Use of real-time PCR for group-specific quantification of aceticlastic methanogens in anaerobic processes: Population dynamics and community structures. Biotechnol. Bioeng. 2006, 93, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Ince, B.; Ince, O. Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res. 2015, 76, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Osińska, A.; Korzeniewska, E.; Harnisz, M.; Niestȩpski, S. Quantitative occurrence of antibiotic resistance genes among bacterial populations from wastewater treatment plants using activated sludge. Appl. Sci. 2019, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Chen, H.; Wei, S.; Gu, J. Antibiotic contamination in animal manure, soil, and sewage sludge in Shenyang, northeast China. Environ. Earth Sci. 2015, 74, 5077–5086. [Google Scholar] [CrossRef]
- Kies, F.K.; Boutchebak, S.; Bendaida, N. Soil Contamination by Pharmaceutical Pollutants: Adsorption of an Antibiotic (Amoxicillin) on an Agricultural Land. Proceedings 2020, 30, 60. [Google Scholar] [CrossRef]
- Berendsen, B.J.A.; Wegh, R.S.; Memelink, J.; Zuidema, T.; Stolker, L.A.M. The analysis of animal faeces as a tool to monitor antibiotic usage. Talanta 2015, 132, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Huerta, B.; Marti, E.; Gros, M.; López, P.; Pompêo, M.; Armengol, J.; Barceló, D.; Balcázar, J.L.; Rodríguez-Mozaz, S.; Marcé, R. Exploring the links between antibiotic occurrence, antibiotic resistance, and bacterial communities in water supply reservoirs. Sci. Total Environ. 2013, 456, 161–170. [Google Scholar] [CrossRef]
- Klein, E.Y.; Van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef] [Green Version]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Rusanowska, P.; Bajkacz, S.; Felis, E.; Jastrzębski, J.P.; Paukszto, Ł.; Koniuszewska, I. The impact of antimicrobials on the efficiency of methane fermentation of sewage sludge, changes in microbial biodiversity and the spread of antibiotic resistance. J. Hazard. Mater. 2021, 416, 125773. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Harnisz, M.; Korzeniewska, E.; Czatzkowska, M.; Jastrzębski, J.P.; Paukszto, Ł.; Bajkacz, S.; Felis, E.; Rusanowska, P. The Effect of Antibiotics on Mesophilic Anaerobic Digestion Process of Cattle Manure. Energies 2021, 14, 1125. [Google Scholar] [CrossRef]
- Spielmeyer, A. Occurrence and fate of antibiotics in manure during manure treatments: A short review. Sustain. Chem. Pharm. 2018, 9, 76–86. [Google Scholar] [CrossRef]
- Kisielewska, M.; Dębowski, M.; Zieliński, M. Improvement of biohydrogen production using a reduced pressure fermentation. Bioprocess Biosyst. Eng. 2015, 38, 1925–1933. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-Specific Primer and Probe Sets to Detect Methanogenic Communities Using Quantitative Real-Time Polymerase Chain Reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Denman, S.E.; Tomkins, N.W.; McSweeney, C.S. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 2007, 62, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Chen, Y.; Zhang, D. High-solid Anaerobic Co-digestion of Sewage Sludge and Cattle Manure: The Effects of Volatile Solid Ratio and pH. Sci. Rep. 2016, 6, 35194. [Google Scholar] [CrossRef] [Green Version]
- Edelmann, W.; Engeli, H.; Gradenecker, M. Co-digestion of organic solid waste and sludge from sewage treatment. Water Sci. Technol. 2000, 41, 213–221. [Google Scholar] [CrossRef]
- Nikiema, M.; Narcis, B.; Ynoussa, M.; Marius, K.S.; Emilian, M.; Cheik, A.T.O.; Dayeri, D.; Alfred, S.T.; Valentin, N.; Aboubakar, S.O. Optimization of Biogas Production from Sewage Sludge: Impact of Combination with Bovine Dung and Leachate from Municipal Organic Waste. Sustainability 2022, 14, 4380. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Breier, B.; Groißmeier, K.; Hamscher, G. Elimination patterns of worldwide used sulfonamides and tetracyclines during anaerobic fermentation. Bioresour. Technol. 2015, 193, 307–314. [Google Scholar] [CrossRef]
- Lagator, M.; Uecker, H.; Neve, P. Adaptation at different points along antibiotic concentration gradients. Biol. Lett. 2021, 17, 20200913. [Google Scholar] [CrossRef]
- Lwin, K.O.; Matsui, H. Comparative analysis of the methanogen diversity in horse and pony by using mcra gene and archaeal 16S rRNA Gene clone libraries. Archaea 2014, 2014, 483574. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, A.; Montañez-hernández, L.E.; Palacio-molina, S.L.; Oropeza-navarro, R.; Miriam, P. Microbial trophic interactions and mcrA gene expression in monitoring of anaerobic digesters. Front. Microbiol. 2014, 5, 597. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Conrad, R.; Lu, Y. Responses of methanogen mcrA genes and their transcripts to an alternate dry/wet cycle of paddy field soil. Appl. Environ. Microbiol. 2012, 78, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, R.; Schauer-Gimenez, A.; Bhattad, U.; Kearney, C.; Struble, C.A.; Zitomer, D.; Maki, J.S. Methyl coenzyme M reductase (mcrA) gene abundance correlates with activity measurements of methanogenic H2/CO2-enriched anaerobic biomass. Microb. Biotechnol. 2014, 7, 77–84. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, J.; Hennebel, T.; Van den Brande, J.; Bilad, R.M.; Bruton, T.A.; Vankelecom, I.F.J.; Verstraete, W.; Boon, N. Anaerobic digestion of molasses by means of a vibrating and non-vibrating submerged anaerobic membrane bioreactor. Biomass Bioenergy 2014, 68, 95–105. [Google Scholar] [CrossRef]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Wolak, I.; Rusanowska, P.; Paukszto, Ł.; Jastrzębski, J.P.; Bajkacz, S. Long-Term, Simultaneous Impact of Antimicrobials on the Efficiency of Anaerobic Digestion of Sewage Sludge and Changes in the Microbial Community. Energies 2022, 15, 1826. [Google Scholar] [CrossRef]
- Lackner, N.; Hintersonnleitner, A.; Wagner, A.O.; Illmer, P. Hydrogenotrophic Methanogenesis and Autotrophic Growth of Methanosarcina thermophila. Archaea 2018, 1826, 4712608. [Google Scholar] [CrossRef] [Green Version]
- Kurth, J.M.; Op den Camp, H.J.M.; Welte, C.U. Several ways one goal—Methanogenesis from unconventional substrates. Appl. Microbiol. Biotechnol. 2020, 104, 6839–6854. [Google Scholar] [CrossRef]
- Ho, D.P.; Jensen, P.D.; Batstone, D.J. Methanosarcinaceae and Acetate-Oxidizing Pathways Dominate in High-Rate Thermophilic Anaerobic Digestion of Waste-Activated Sludge. Appl. Environ. Microbiol. 2013, 79, 6491–6500. [Google Scholar] [CrossRef] [Green Version]
- Lins, P.; Reitschuler, C.; Illmer, P. Methanosarcina spp., the key to relieve the start-up of a thermophilic anaerobic digestion suffering from high acetic acid loads. Bioresour. Technol. 2014, 152, 347–354. [Google Scholar] [CrossRef]
- Ike, M.; Inoue, D.; Miyano, T.; Liu, T.T.; Sei, K.; Soda, S.; Kadoshin, S. Microbial population dynamics during startup of a full-scale anaerobic digester treating industrial food waste in Kyoto eco-energy project. Bioresour. Technol. 2010, 101, 3952–3957. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Hwang, K.; O’Flaherty, V.; Hwang, S. Quantitative analysis of methanogenic community dynamics in three anaerobic batch digesters treating different wastewaters. Water Res. 2009, 43, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Donnelly, J.; Oliveira, I.; Anthony, R.; Wilson, V.; Esteves, S.R. Enhancement of microbial density and methane production in advanced anaerobic digestion of secondary sewage sludge by continuous removal of ammonia. Bioresour. Technol. 2017, 232, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Nordgård, A.S.R.; Bergland, W.H.; Vadstein, O.; Mironov, V.; Bakke, R.; Østgaard, K.; Bakke, I. Anaerobic digestion of pig manure supernatant at high ammonia concentrations characterized by high abundances of Methanosaeta and non-euryarchaeotal archaea. Sci. Rep. 2017, 7, 15077. [Google Scholar] [CrossRef] [PubMed]
- Weiß, S.; Tauber, M.; Somitsch, W.; Meincke, R.; Müller, H.; Berg, G.; Guebitz, G.M. Enhancement of biogas production by addition of hemicellulolytic bacteria immobilised on activated zeolite. Water Res. 2010, 44, 1970–1980. [Google Scholar] [CrossRef]
- Traversi, D.; Villa, S.; Acri, M.; Pietrangeli, B.; Degan, R.; Gilli, G. The role of different methanogen groups evaluated by real-time qPCR as high-efficiency bioindicators of wet anaerobic co-digestion of organic waste. AMB Express 2011, 1, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| TS a gD−1 b (mg) | VS c gD−1 (mg) | pH | TP d gTS−1 e (mg) | TN f gTS−1 (mg) | |
|---|---|---|---|---|---|
| SS g | 52.0 ± 11.37 | 39.92 ± 9.59 | 6.35 ± 0.3 | 2.02 ± 0.48 | 4.32 ± 1.61 |
| CS h | 107.46 ± 29.01 | 84.23 ± 22.17 | 7.75 ± 0.42 | 0.95 ± 0.26 | 4.10 ± 1.64 |
| Inoculum | 38.8 ± 5.2 | 25.2 ± 3.8 | 8.1 ± 0.5 | 0.9 ± 0.4 | 5.5 ± 1.9 |
| Antibiotic Concentrations (µg mL−1) | ||||||
|---|---|---|---|---|---|---|
| Substrate | Series | Dose ID | AMO | CIP | MET | ENR |
| SS | 1 | D1 | 1 | 0.25 | 0.25 | X |
| 2 | D2 | 2 | 0.5 | 0.5 | ||
| 3 | D3 | 4 | 1 | 1 | ||
| 4 | D4 | 8 | 4 | 4 | ||
| 5 | D5 | 16 | 8 | 8 | ||
| 6 | D6 | 36 | 16 | 16 | ||
| CS | 1 | D1 | 1 | X | 0.25 | 0.25 |
| 2 | D2 | 2 | 0.5 | 0.5 | ||
| 3 | D3 | 2.5 | 0.75 | 0.75 | ||
| 4 | D4 | 5 | 1.5 | 1.5 | ||
| 5 | D5 | 10 | 3 | 3 | ||
| 6 | D6 | 16 | 4 | 4 | ||
| 7 | D7 | 32 | 8 | 8 | ||
| SS | CS | ||||
|---|---|---|---|---|---|
| Series | Samples | PB a | CB b | PB | CB |
| Sample ID | |||||
| 1 | 1 | SS D1.1 | SS C1.1 | CS D1.1 | CS C1.1 |
| 2 | SS D1.2 | SS C1.2 | CS D1.2 | CS C1.2 | |
| 3 | SS D1.3 | SS C1.3 | CS D1.3 | CS C1.3 | |
| 4 | SS D1.4 | SS C1.4 | CS D1.4 | CS C1.4 | |
| 5 | x | CS D1.5 | CS C1.5 | ||
| 6 | CS D1.6 | CS C1.6 | |||
| 2 | 1 | SS D2.1 | SS C2.1 | CS D2.1 | CS C2.1 |
| 2 | SS D2.2 | SS C2.2 | CS D2.2 | CS C2.2 | |
| 3 | SS D2.3 | SS C2.3 | CS D2.3 | CS C2.3 | |
| 4 | SS D2.4 | SS C2.4 | CS D2.4 | CS C2.4 | |
| 5 | SS D2.5 | SS C2.5 | CS D2.5 | CS C2.5 | |
| 6 | x | CS D2.6 | CS C2.6 | ||
| 3 | 1 | SS D3.1 | SS C3.1 | CS D3.1 | CS C3.1 |
| 2 | SS D3.2 | SS C3.2 | CS D3.2 | CS C3.2 | |
| 3 | SS D3.3 | SS C3.3 | CS D3.3 | CS C3.3 | |
| 4 | SS D3.4 | SS C3.4 | CS D3.4 | CS C3.4 | |
| 5 | x | CS D3.5 | CS C3.5 | ||
| 6 | CS D3.6 | CS C3.6 | |||
| 4 | 1 | SS D4.1 | SS C4.1 | CS D4.1 | CS C4.1 |
| 2 | SS D4.2 | SS C4.2 | CS D4.2 | CS C4.2 | |
| 3 | SS D4.3 | SS C4.3 | CS D4.3 | CS C4.3 | |
| 4 | SS D4.4 | SS C4.4 | CS D4.4 | CS C4.4 | |
| 5 | SS D4.5 | SS C4.5 | CS D4.5 | CS C4.5 | |
| 6 | x | CS D4.6 | CS C4.6 | ||
| 5 | 1 | SS D5.1 | SS C5.1 | CS D5.1 | CS C5.1 |
| 2 | SS D5.2 | SS C5.2 | CS D5.2 | CS C5.2 | |
| 3 | SS D5.3 | SS C5.3 | CS D5.3 | CS C5.3 | |
| 4 | SS D5.4 | SS C5.4 | CS D5.4 | CS C5.4 | |
| 5 | SS D5.5 | SS C5.5 | CS D5.5 | CS C5.5 | |
| 6 | x | CS D5.6 | CS. C5.6 | ||
| 6 | 1 | SS D6.1 | SS C6.1 | CS D6.1 | CS C6.1 |
| 2 | SS D6.2 | SS C6.2 | CS D6.2 | CS C6.2 | |
| 3 | SS D6.3 | SS C6.3 | CS D6.3 | CS C6.3 | |
| 4 | SS D6.4 | SS C6.4 | CS D6.4 | CS C6.4 | |
| 5 | SS D6.5 | SS C6.5 | CS D6.5 | CS C6.5 | |
| 6 | x | CS D6.6 | CS C6.6 | ||
| 7 | 1 | x | CS D7.1 | CS C7.1 | |
| 2 | CS D7.2 | CS C7.2 | |||
| 3 | CS D7.3 | CS C7.3 | |||
| 4 | CS D7.4 | CS C7.4 | |||
| 5 | CS D7.5 | CS C7.5 | |||
| 6 | CS D7.6 | CS C7.6 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czatzkowska, M.; Wolak, I.; Korzeniewska, E.; Harnisz, M. Anaerobic Digestion in the Presence of Antimicrobials—Characteristics of Its Parameters and the Structure of Methanogens. Appl. Sci. 2022, 12, 8422. https://doi.org/10.3390/app12178422
Czatzkowska M, Wolak I, Korzeniewska E, Harnisz M. Anaerobic Digestion in the Presence of Antimicrobials—Characteristics of Its Parameters and the Structure of Methanogens. Applied Sciences. 2022; 12(17):8422. https://doi.org/10.3390/app12178422
Chicago/Turabian StyleCzatzkowska, Małgorzata, Izabela Wolak, Ewa Korzeniewska, and Monika Harnisz. 2022. "Anaerobic Digestion in the Presence of Antimicrobials—Characteristics of Its Parameters and the Structure of Methanogens" Applied Sciences 12, no. 17: 8422. https://doi.org/10.3390/app12178422
APA StyleCzatzkowska, M., Wolak, I., Korzeniewska, E., & Harnisz, M. (2022). Anaerobic Digestion in the Presence of Antimicrobials—Characteristics of Its Parameters and the Structure of Methanogens. Applied Sciences, 12(17), 8422. https://doi.org/10.3390/app12178422







