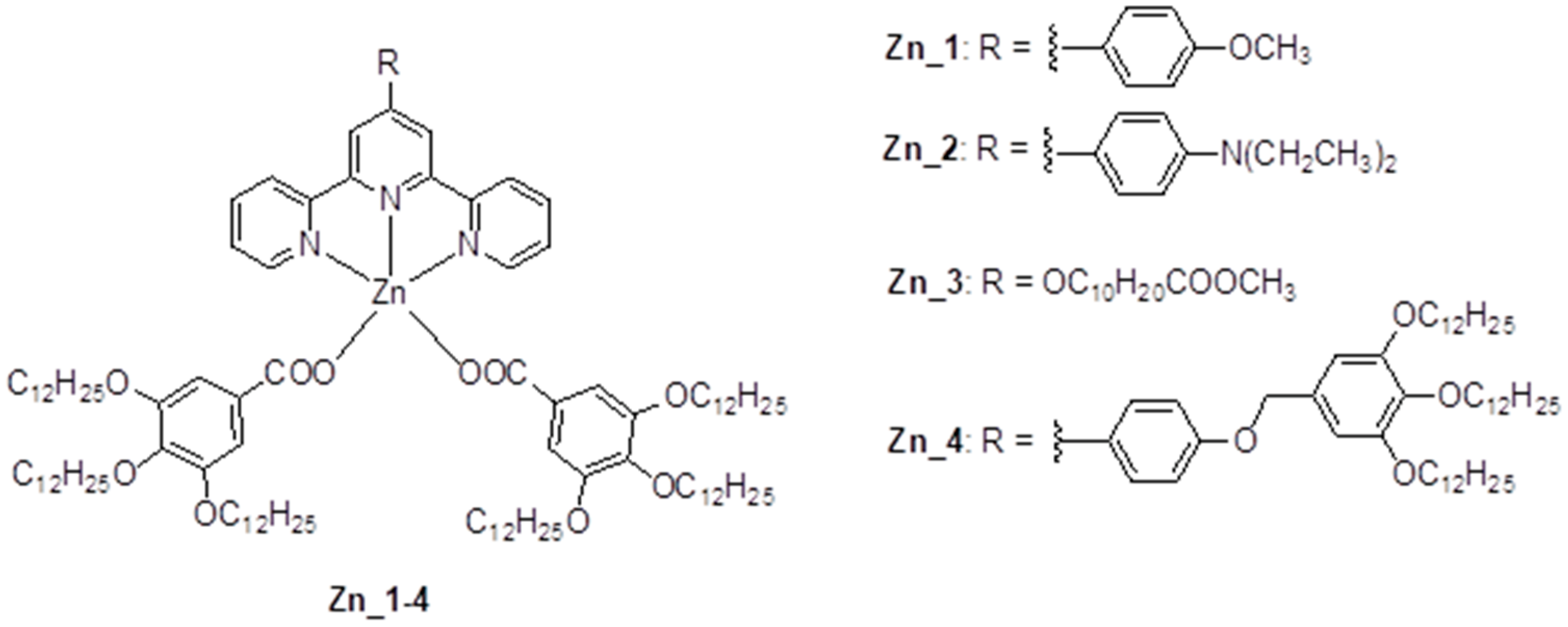
Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
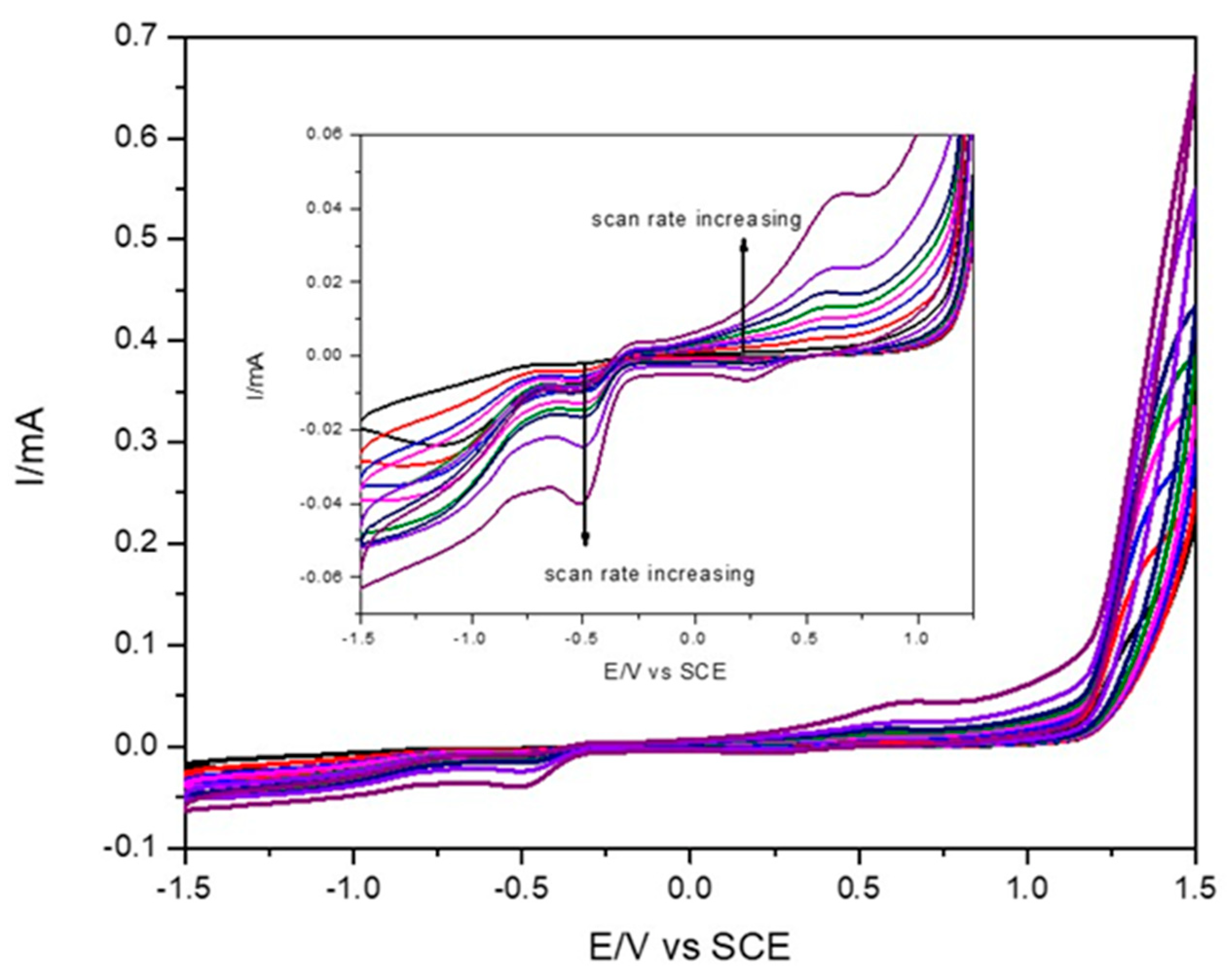
2.2. Electrochemical Characterization
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Thermal and Mesomorphic Properties
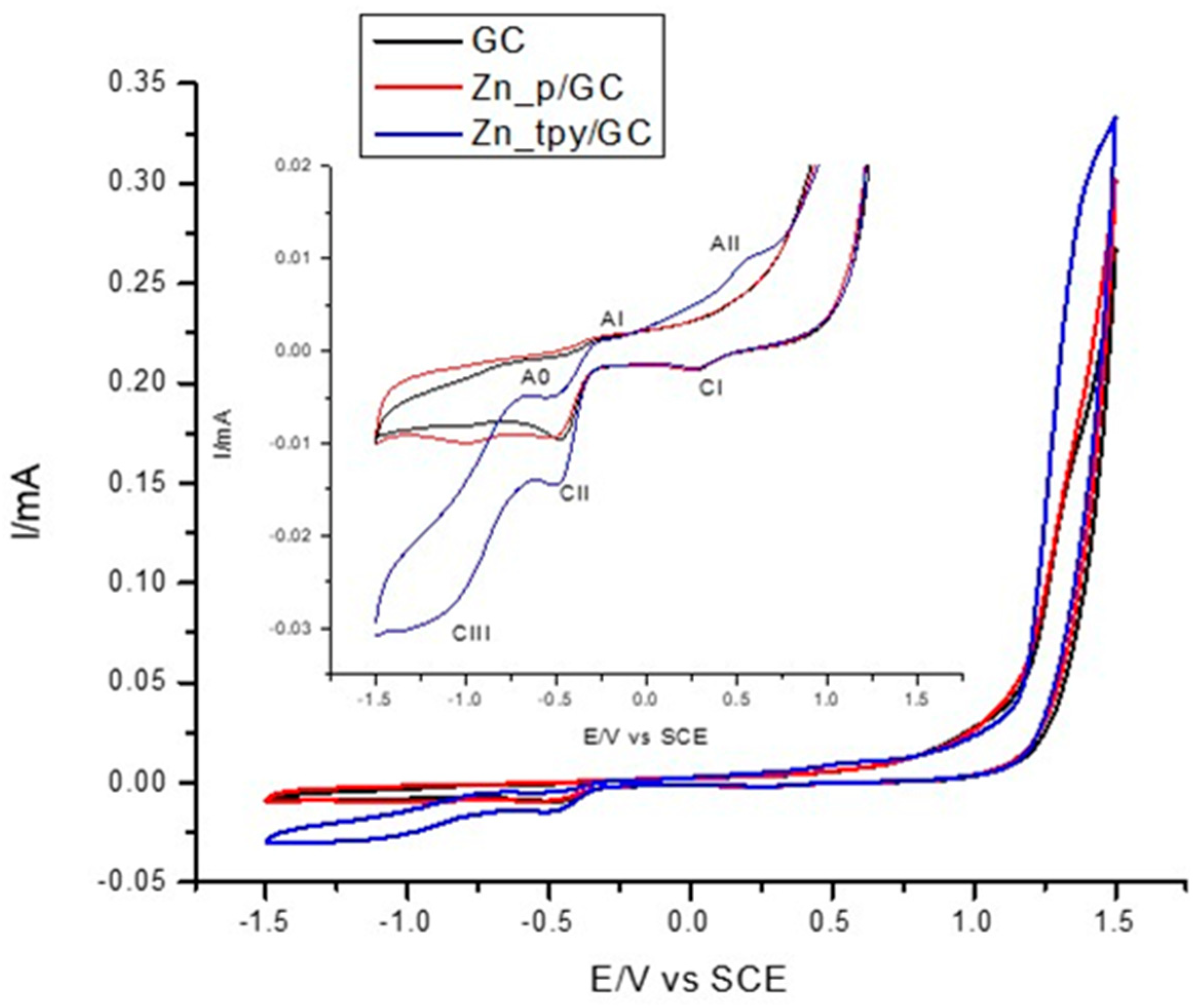
3.3. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Housecroft, C.E.; Constable, E.C. Solar energy conversion using first row d-block metal coordination compound sensitizers and redox mediators. Chem. Sci. 2022, 13, 1225–1262. [Google Scholar] [CrossRef]
- Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Light harvesting with Earth abundant d-block metals: Development of sensitizers in dye-sensitized solar cells (DSCs). Coord. Chem. Rev. 2013, 257, 3089–3106. [Google Scholar] [CrossRef]
- Howarth, A.J.; Majewski, M.B.; Wolf, M.O. Photophysical properties and applications of coordination complexes incorporating pyrene. Coord. Chem. Rev. 2015, 282, 139–149. [Google Scholar] [CrossRef]
- Greenfield, T.J.; Julve, M.; Doyle, R.P. Exploring the biological, catalytic, and magnetic properties of transition metal coordination complexes incorporating pyrophosphate. Coord. Chem. Rev. 2019, 384, 37–64. [Google Scholar] [CrossRef]
- Pan, M.; Liao, W.-M.; Yin, S.-Y.; Sun, S.-S.; Su, C.-Y. Single-Phase White-Light-Emitting and Photoluminescent Color-Tuning Coordination Assemblies. Chem. Rev. 2018, 118, 8889–8935. [Google Scholar] [CrossRef]
- Kumar, N.; Bhalla, V.; Kumar, M. Beyond zinc coordination: Bioimaging applications of Zn(II)-complexes. Coord. Chem. Rev. 2021, 427, 213550. [Google Scholar] [CrossRef]
- Gupta, D.; Sathiyendiran, M. Rhenium-Carbonyl-Based Supramolecular Coordination Complexes: Synthesis, Structure and Properties. Chem. Sel. 2018, 3, 7439–7458. [Google Scholar] [CrossRef]
- Cargnelutti, R.; Schumacher, R.F.; Belladona, A.L.; Kazmierczak, J.C. Coordination chemistry and synthetic approaches of pyridyl-selenium ligands: A decade update. Coord. Chem. Rev. 2021, 426, 213537. [Google Scholar] [CrossRef]
- Marchetti, F.; Pettinari, C.; Di Nicola, C.; Tombesi, A.; Pettinari, R. Coordination chemistry of pyrazolone-based ligands and applications of their metal complexes. Coord. Chem. Rev. 2019, 401, 213069. [Google Scholar] [CrossRef]
- Malinowski, J.; Zych, D.; Jacewicz, D.; Gawdzik, B.; Drzezdzon, J. Application of Coordination Compounds with Transition Metal Ions in the Chemical Industry—A Review. Int. J. Mol. Sci. 2020, 21, 5443. [Google Scholar] [CrossRef]
- Chalkley, M.J.; Drover, M.W.; Peters, J.C. Catalytic N2-to-NH3 (or -N2H4) Conversion by Well-Defined Molecular Coordination Complexes. Chem. Rev. 2020, 120, 5582–5636. [Google Scholar] [CrossRef] [PubMed]
- Chylewska, A.; Biedulska, M.; Sumczynski, P.; Makowski, M. Metallopharmaceuticals in Therapy—A New Horizon for Scientific Research. Curr. Med. Chem. 2018, 25, 1729–1791. [Google Scholar] [CrossRef]
- Pervaiz, M.; Sadiq, S.; Sadiq, A.; Younas, U.; Ashraf, A.; Saeed, Z.; Zuber, M.; Adnan, A. Azo-Schiff base derivatives of transition metal complexes as antimicrobial agents. Coord. Chem. Rev. 2021, 447, 214128. [Google Scholar] [CrossRef]
- Doistau, B.; Jimenez, J.R.; Piguet, C. Beyond Chiral Organic (p-block) Chromophores for Circularly Polarized Luminescence: The Success of d-Block and f-Block Chiral Complexes. Front. Chem. 2020, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Maiuolo, L.; Calandra, P.; Lombardo, D.; Szerb, E.I. Amphiphiles-metals interactions for applications in modern technologies: Recent developments and future perspectives. Rev. Roum. Chim. 2020, 65, 647–671. [Google Scholar] [CrossRef]
- Cretu, C.; Maiuolo, L.; Lombardo, D.; Szerb, E.I.; Calandra, P. Luminescent Supramolecular Nano- or Microstructures Formed in Aqueous Media by Amphiphile-Noble Metal Complexes. J. Nanomater. 2020, 2020, 5395048. [Google Scholar] [CrossRef]
- Pellei, M.; Del Bello, F.; Porchia, M.; Santini, C. Zinc coordination complexes as anticancer agents. Coord. Chem. Rev. 2021, 445, 214088. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, J.; Yang, X.-J.; Wu, B. Anion-coordination-directed self-assemblies. Org. Chem. Front. 2018, 5, 662–690. [Google Scholar] [CrossRef]
- Kameo, H.; Nakazawa, H. Recent Developments in the Coordination Chemistry of Multidentate Ligands Featuring a Boron Moiety. Chem. Asian J. 2013, 8, 1720–1734. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, Y.; Xiao, X.; Chen, X.; Hu, M.; Geng, X.; Wang, Z.; Zhao, J. Recent development of the transition metal complexes showing strong absorption of visible light and long-lived triplet excited state: From molecular structure design to photophysical properties and applications. Coord. Chem. Rev. 2020, 417, 21337. [Google Scholar] [CrossRef]
- Dey, N.; Haynes, C.J.E. Supramolecular Coordination Complexes as Optical Biosensors. Chem. Plus Chem. 2021, 86, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Cuerva, C.; Cano, M.; Lodeiro, C. Advanced Functional Luminescent Metallomesogens: The Key Role of the Metal Center. Chem. Rev. 2021, 121, 12966–13010. [Google Scholar] [CrossRef]
- Pastor, M.J.; Cuerva, C.; Fernández-Lodeiro, A.; Lodeiro, C.; Campo, J.A.; Cano, M. Designing Zn(II) complexes as a support of bifunctional liquid crystal and luminescent materials. Dyes Pigm. 2018, 149, 37–50. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Zinc(II)-salphen complexes bearing long alkoxy side arms: Synthesis, solvent dependent aggregation, and spacer group substituent effect on mesomorphism and photophysical property. J. Molec. Liq. 2017, 246, 290–301. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mondal, P.; Prasad, S.K.; Rao, D.S.S.; Bhattacharjee, C.R. Induction of Mesomorphism through Supramolecular Assembly in Metal Coordination Compounds of “salphen”-Type Schiff Bases: Photoluminescence and Solvatochromism. Eur. J. Inorg. Chem. 2016, 28, 4604–4614. [Google Scholar] [CrossRef]
- Kumar, N.S.S.; Shafikov, M.Z.; Whitwood, A.C.; Donnio, B.; Karadakov, P.B.; Kozhevnikov, V.N.; Bruce, D.W. Mesomorphism and Photophysics of Some Metallomesogens Based on Hexasubstituted 2,2′:6′, 2″-Terpyridines. Chem. Eur. J. 2016, 22, 8215–8233. [Google Scholar] [CrossRef] [PubMed]
- Andelescu, A.A.; Heinrich, B.; Spirache, M.A.; Voirin, E.; La Deda, M.; Di Maio, G.; Szerb, E.I.; Donnio, B.; Costisor, O. Playing with PtII and ZnII Coordination to Obtain Luminescent Metallomesogens. Chem. Eur. J. 2020, 26, 4850–4860. [Google Scholar] [CrossRef]
- La Deda, M.; Di Maio, G.; Candreva, A.; Heinrich, B.; Andelescu, A.A.; Popa, E.; Voirin, E.; Badea, V.; Amati, M.; Costisor, O.; et al. Very intense polarized emission in self-assembled room temperature metallomesogens based on Zn(II) coordination complexes: An experimental and computational study. J. Mater. Chem. C 2022, 10, 115–125. [Google Scholar] [CrossRef]
- Cavero, E.; Uriel, S.; Romero, P.; Serrano, J.L.; Giménez, R. Tetrahedral Zinc Complexes with Liquid Crystalline and Luminescent Properties: Interplay Between Nonconventional Molecular Shapes and Supramolecular Mesomorphic Order. J. Am. Chem. Soc. 2007, 129, 11608–11618. [Google Scholar] [CrossRef]
- Pucci, D.; Crispini, A.; Ghedini, M.; La Deda, M.; Liguori, P.F.; Pettinari, C.; Szerb, E.I. ‘‘Green light’’ for Zn(II) mesogens. RSC Adv. 2012, 2, 9071–9078. [Google Scholar] [CrossRef]
- Keerthiga, R.; Kaliyappan, T.; Kannan, P. Studies on Twist Bent Core Zinc (II) Methacrylate Supramolecular Columnar Hexagonal Phase Mesogen derived from azobenzene moiety and its Photoluminescent Behaviours. Inorg. Chem. Comm. 2019, 99, 26–30. [Google Scholar] [CrossRef]
- Morale, F.; Date, R.W.; Guillon, D.; Bruce, D.W.; Finn, R.L.; Wilson, C.; Blake, A.J.; Schröder, M.; Donnio, B. Columnar mesomorphism from hemi-disklike metallomesogens derived from 2,6-bis[3′,4′,5′-tri(alkoxy)phenyliminomethyl]pyridines (L): Crystal and molecular structures of [M(L)Cl2 (M = Mn, Ni, Zn). Chem. Eur. J. 2003, 9, 2484–2501. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, C.R.; Das, G.; Mondal, P.; Prasad, S.K.; Rao, D.S.S. Novel Green Light Emitting Nondiscoid Liquid Crystalline Zinc(II) Schiff-Base Complexes. Eur. J. Inorg. Chem. 2011, 2011, 1418–1424. [Google Scholar] [CrossRef]
- Bhattacharjee, C.R.; Chakraborty, S.; Das, G.; Mondal, P. Emissive ‘zinc(II)-salphen’ core: Building block for columnar liquid crystals. Liq. Cryst. 2012, 39, 1435–1442. [Google Scholar] [CrossRef]
- Cuerva, C.; Ovejero, P.; Campo, J.A.; Cano, M. Tetrahedral and octahedral metallomesogenic Zn(II) complexes supported by pyridine-functionalised pyrazole ligands. New J. Chem. 2014, 38, 511–517. [Google Scholar] [CrossRef]
- Pucci, D.; Aiello, I.; Bellusci, A.; Crispini, A.; Ghedini, M.; La Deda, M. Coordination Induction of Nonlinear Molecular Shape in Mesomorphic and Luminescent ZnII Complexes Based on Salen-Like Frameworks. Eur. J. Inorg. Chem. 2009, 2009, 4274–4281. [Google Scholar] [CrossRef]
- Ames, K.A.; Collinson, S.R.; Blake, A.J.; Wilson, C.; Love, J.B.; Bruce, D.W.; Donnio, B.; Guillon, D.; Schröder, M. Design of Neutral Metallomesogens from 5,5-Dimethyldipyrromethane: Metal Ion Mediated Control of Folding and Hairpin Structures. Eur. J. Inorg. Chem. 2008, 2008, 5056–5066. [Google Scholar] [CrossRef]
- Giménez, R.; Manrique, A.B.; Uriel, S.; Barberá, J.; Serrano, J.L. Mesomorphism of a tetrahedral zinc complex. Chem. Commun. 2004, 18, 2064–2065. [Google Scholar] [CrossRef]
- Chakraborty, S.; Bhattacharjee, C.R.; Mondal, P.; Prasad, S.K.; Rao, D.S.S. Synthesis and aggregation behaviour of luminescent mesomorphic zinc(II) complexes with ‘salen’ type asymmetric Schiff base ligand. Dalton Trans. 2015, 44, 7477–7488. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Chen, J.; Yuan, C. Micro/Nano Electrode Array Sensors: Advances in Fabrication and Emerging Applications in Bioanalysis. Front. Chem. 2020, 8, 573865. [Google Scholar] [CrossRef]
- Hannah, S.; Damion, E.B.; Corrigan, K. Developments in Micro and Nanoscale Sensors for Biomedical Sensing. Curr. Opin. Electrochem. 2020, 23, 7–15. [Google Scholar] [CrossRef]
- Karimian, N.; Ugo, P. Recent advances in sensing and biosensing with arrays of nanoelectrodes. Curr. Opin. Electrochem. 2019, 16, 106–116. [Google Scholar] [CrossRef]
- Yu, J.; Huang, T.; Jiang, Z.; Sun, M.; Tang, C. Synthesis and Characterizations of Zinc Oxide on Reduced Graphene Oxide for High Performance Electrocatalytic Reduction of Oxygen. Molecules 2018, 23, 3227. [Google Scholar] [CrossRef] [PubMed]
- Sofianos, M.V.; Lee, J.; Silvester, D.S.; Samanta, P.K.; Paskevicius, M.; English, N.J.; Buckley, C.E. Diverse morphologies of zinc oxide nanoparticles and their electrocatalytic performance in hydrogen production. J. Energy Chem. 2020, 56, 162–170. [Google Scholar] [CrossRef]
- Kelly, S.R.; Shi, X.; Back, S.; Vallez, L.; Park, S.Y.; Siahrostami, S.; Zheng, X.; Norskov, J.K. ZnO as an Active and Selective Catalyst for Electrochemical Water Oxidation to Hydrogen Peroxide. ACS Catal. 2019, 9, 4593–4599. [Google Scholar] [CrossRef]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Rajendar, V.; Park, S.H. Novel synthesis and structural analysis of zinc oxide nanoparticles for the non-enzymatic glucose biosensor. Mater. Sci. Eng. C 2017, 75, 1472–1479. [Google Scholar] [CrossRef]
- Ramya, B.; Priya, P.G. Rapid phytochemical microwave-assisted synthesis of zinc oxide nano flakes with excellent electrocatalytic activity for non-enzymatic electrochemical sensing of uric acid. J. Mater. Sci. Mater. Electron. 2021, 32, 21406–21424. [Google Scholar] [CrossRef]
- Motoc, S.; Cretu, C.; Costisor, O.; Baciu, A.; Manea, F.; Szerb, E.I. Cu(I) Coordination Complex Precursor for Randomized CuOx Microarray Loaded on Carbon Nanofiber with Excellent Electrocatalytic Performance for Electrochemical Glucose Detection. Sensors 2019, 9, 5353. [Google Scholar] [CrossRef]
- Motoc, S.; Schinteie, B.; Pop, A.; Negrea, S.; Cretu, C.; Szerb, E.I.; Manea, F. Graphene Quantum Dots and Cu(I) Liquid Crystal for Advanced Electrochemical Detection of Doxorubicine in Aqueous Solutions. Nanomaterials 2021, 11, 2788. [Google Scholar] [CrossRef]
- Szerb, E.I.; Pucci, D.; Crispini, A.; La Deda, M. Soft Luminescent Materials Based on Ag(I) Coordination Complexes. Mol. Cryst. Liq. Cryst. 2013, 573, 34–45. [Google Scholar] [CrossRef]
- Pucci, D.; Barberio, G.; Bellusci, A.; Crispini, A.; Donnio, B.; Giorgini, L.; Ghedini, M.; La Deda, M.; Szerb, E.I. Silver Coordination Complexes as Room-Temperature Multifunctional Materials. Chem. Eur. J. 2006, 12, 6738–6747. [Google Scholar] [CrossRef] [PubMed]
- The Cambridge Structural Database; Ma, Z.; Lu, W.; Liang, B.; Pombeiro, A.J.L. Synthesis, Characterization, Photoluminescent and Thermal Properties of Zinc(II) 4′-phenyl-terpyridine Compounds. New J. Chem. 2013, 37, 1529–1537. [Google Scholar] [CrossRef]
- Soliman, A.B.; Abdel-Samad, H.S.; Abdel-Rehim, S.S.; Hassan, H.H. Surface functionality and electrochemical investigations of a graphitic electrode as a candidate for alkaline energy conversion and storage devices. Sci. Rep. 2016, 6, 22056. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.P. The Electrochemical Behavior of Zinc in Alkaline Media. J. Electrochem. Soc. 1969, 116, 757. [Google Scholar] [CrossRef]
- Dumitru, C.-S.; Cojocaru, A.; Anicai, L.; Cotarta, A.; Visan, T. Electrodeposition of zinc oxide films from choline chloride based ionic liquid media containing zinc and nitrate ions. UPB Sci. Bull. Ser. B 2016, 78, 59. [Google Scholar]
- Anwar, S.; Zhang, Y.; Khan, F. Electrochemical behaviour and analysis of Zn and Zn–Ni alloy anti-corrosive coatings deposited from citrate baths. RSC Adv. 2018, 8, 28861–28873. [Google Scholar] [CrossRef]
- Renuka, R.; Srinivasan, L.; Ramamurthy, S.; Veluchamy, A.; Venkatakrishnan, N. Cyclic voltammetric study of zinc and zinc oxide electrodes in 5.3 M KOH. J. Appl. Electrochem. 2001, 31, 655–661. [Google Scholar] [CrossRef]

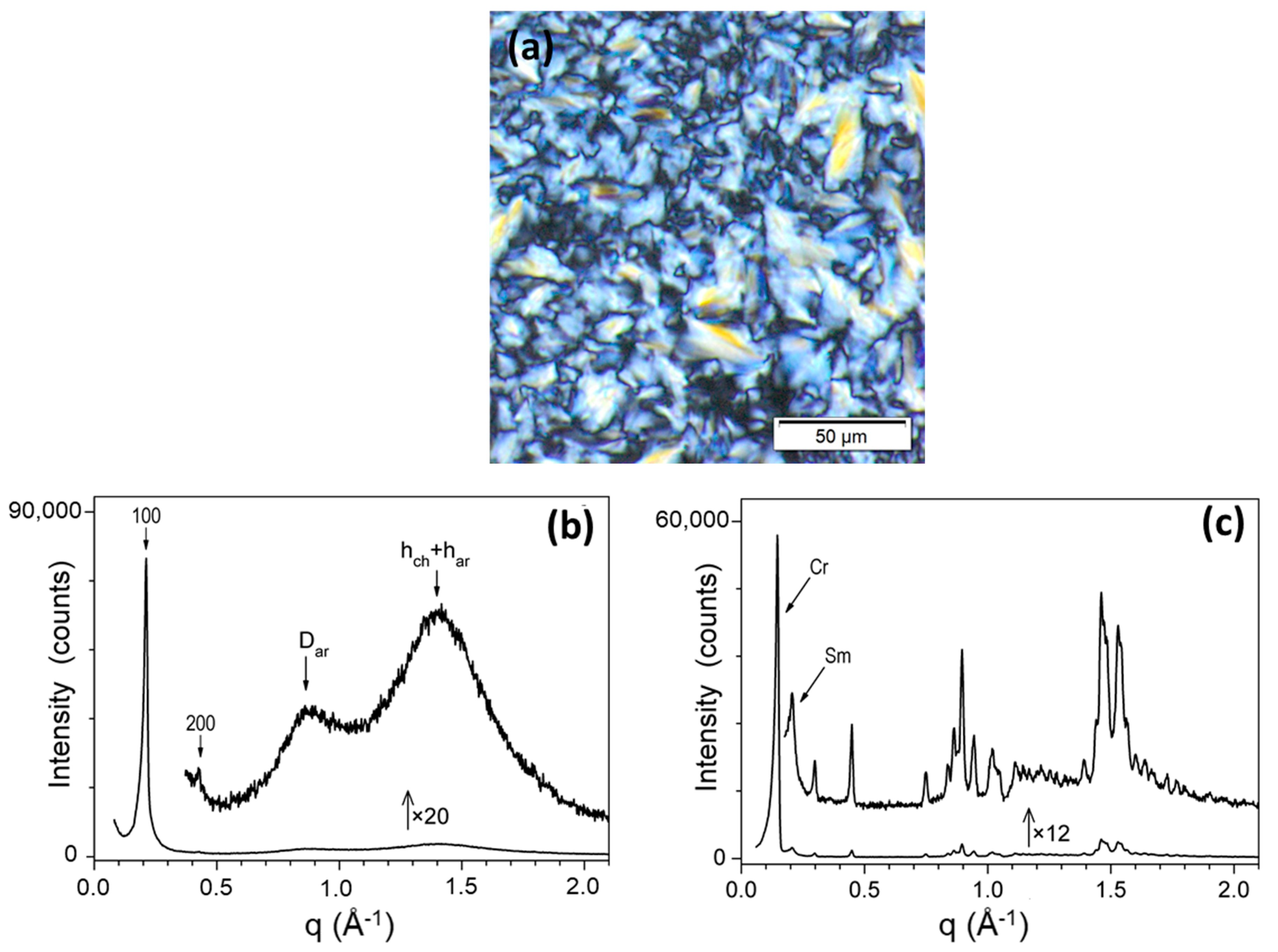


| Tdec5% [a] [°C] | Mesophases [b], Transition Temperatures (°C) and Enthalpies [ΔH (kJ·mol−1)] [c] |
|---|---|
| 245 | Cr 89 [12.0] SmA 109 [2.5] Isso/Isso 114 [2.6] SmA 73 [10.2] Sm + Cr |
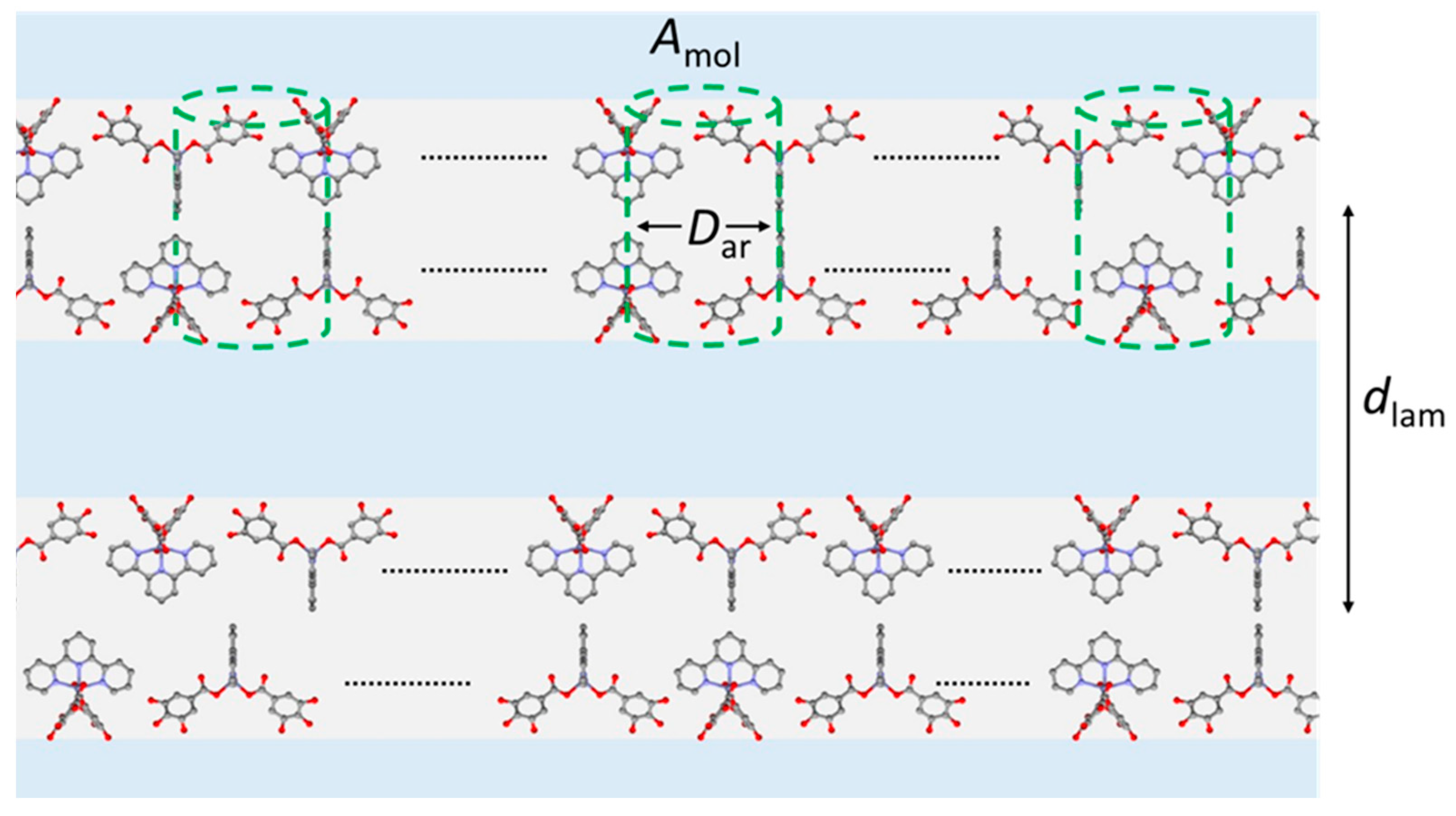
| Sample Phase, T | Vmol ρ | dlam | NmolL Amol | NarL SZnAr | NchL Sch | qch |
|---|---|---|---|---|---|---|
| Zn_tpy SmA, 85 | 27,530.99 | 29.31 | 2 | 1 | 2 | 1.41 |
| 188 | 94 | 31.3 | ||||
| Zn_tpy SmA, 100 | 2781 | 28.89 | 2 | 1 | 2 | 1.43 |
| 0.98 | 192 | 96 | 32.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andelescu, A.A.; Ilies, S.; Cretu, C.; Popa, E.; Marinescu, S.; Heinrich, B.; Manea, F.; Negrea, S.; Donnio, B.; Szerb, E.I. Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Appl. Sci. 2022, 12, 8306. https://doi.org/10.3390/app12168306
Andelescu AA, Ilies S, Cretu C, Popa E, Marinescu S, Heinrich B, Manea F, Negrea S, Donnio B, Szerb EI. Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Applied Sciences. 2022; 12(16):8306. https://doi.org/10.3390/app12168306
Chicago/Turabian StyleAndelescu, Adelina A., Sorina Ilies (b. Motoc), Carmen Cretu, Evelyn Popa, Sorin Marinescu, Benoît Heinrich, Florica Manea, Sorina Negrea, Bertrand Donnio, and Elisabeta I. Szerb. 2022. "Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties" Applied Sciences 12, no. 16: 8306. https://doi.org/10.3390/app12168306
APA StyleAndelescu, A. A., Ilies, S., Cretu, C., Popa, E., Marinescu, S., Heinrich, B., Manea, F., Negrea, S., Donnio, B., & Szerb, E. I. (2022). Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Applied Sciences, 12(16), 8306. https://doi.org/10.3390/app12168306




.png)