Aqueous Synthesis of the Tiopronin-Capped Gold Nanoclusters/Nanoparticles with Precise Size Control via Deprotonation of the Ligand
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
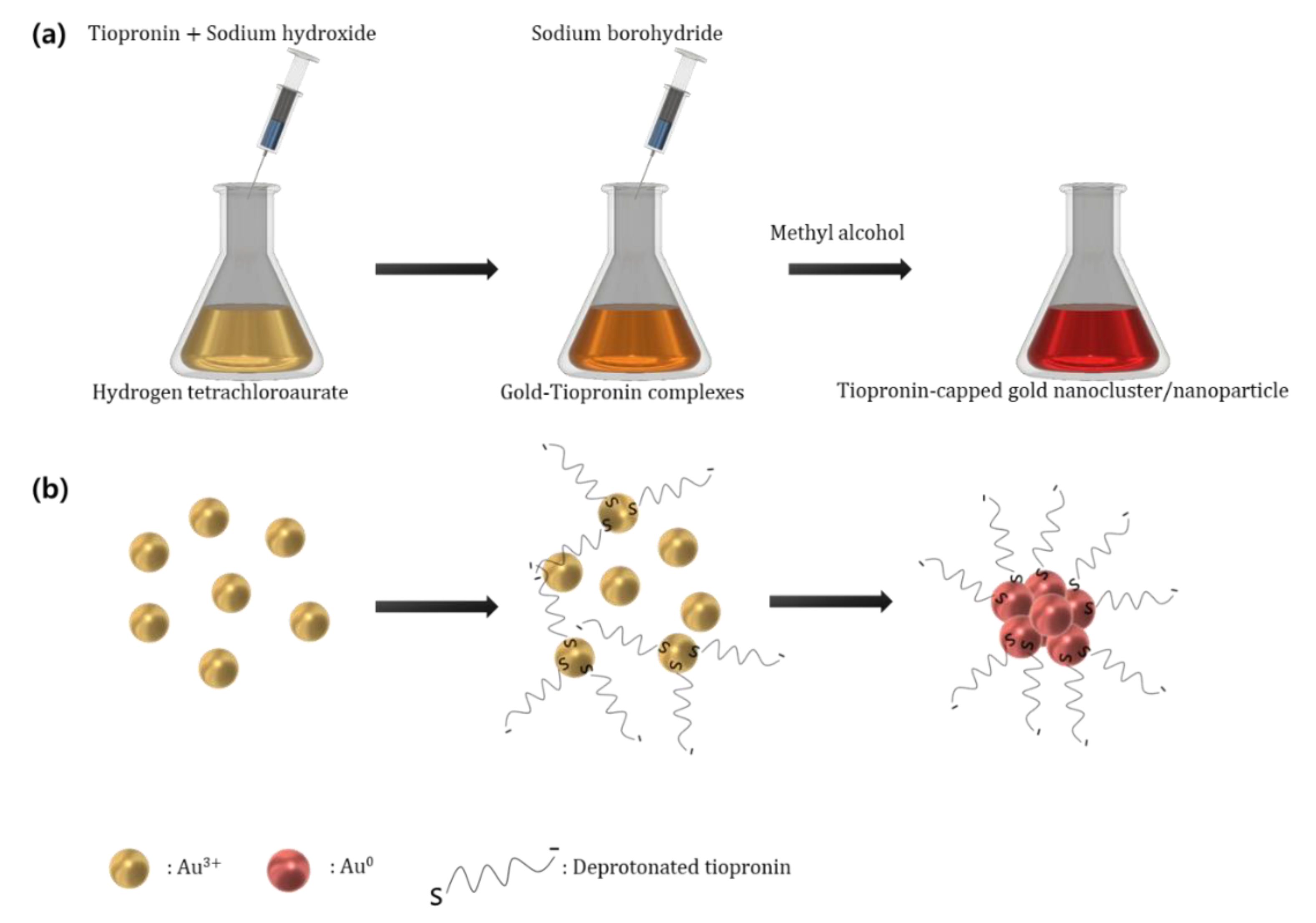
2.2. Synthesis of Tiopronin-Capped Gold Nanoclusters/Nanoparticles
2.3. Measurements
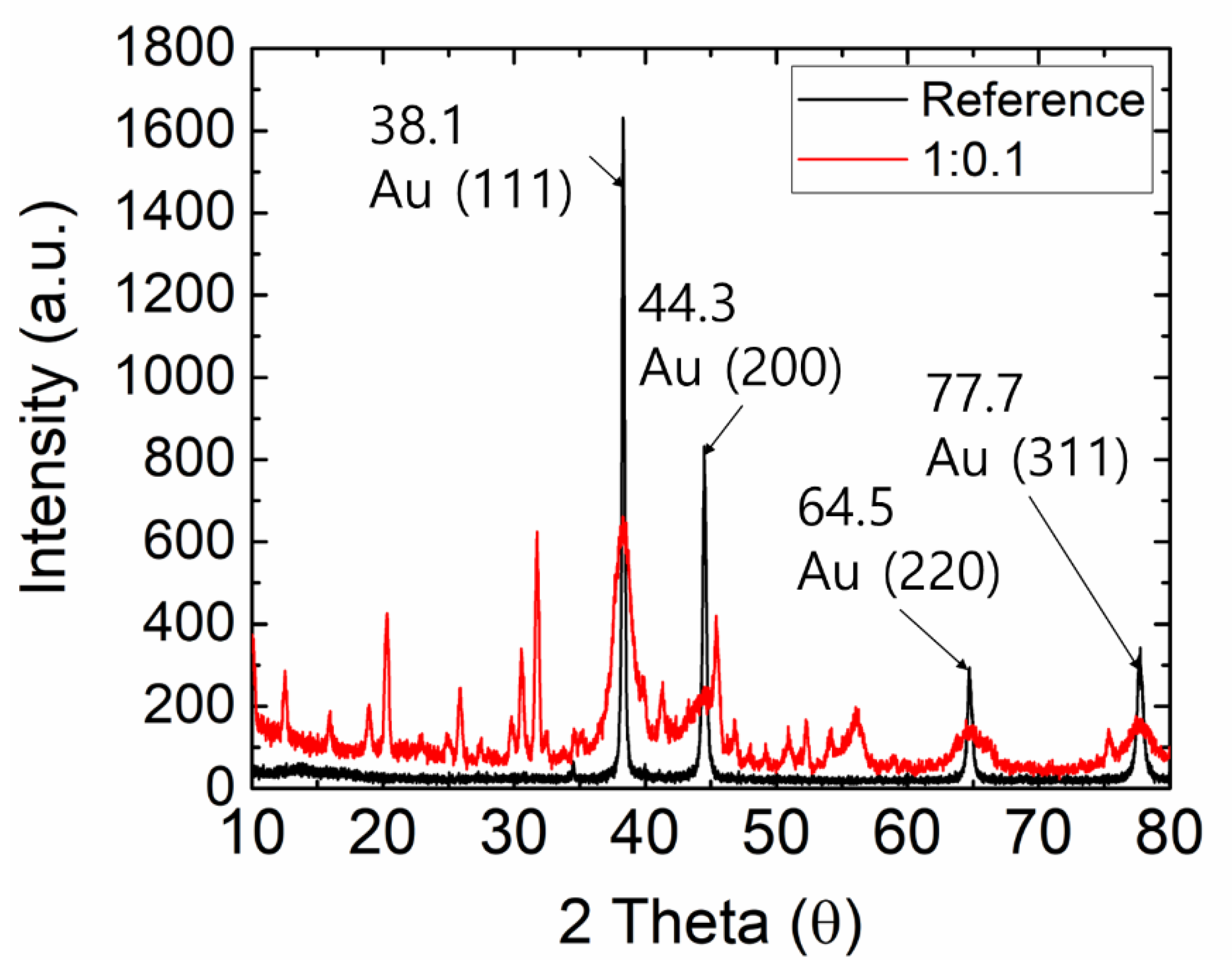
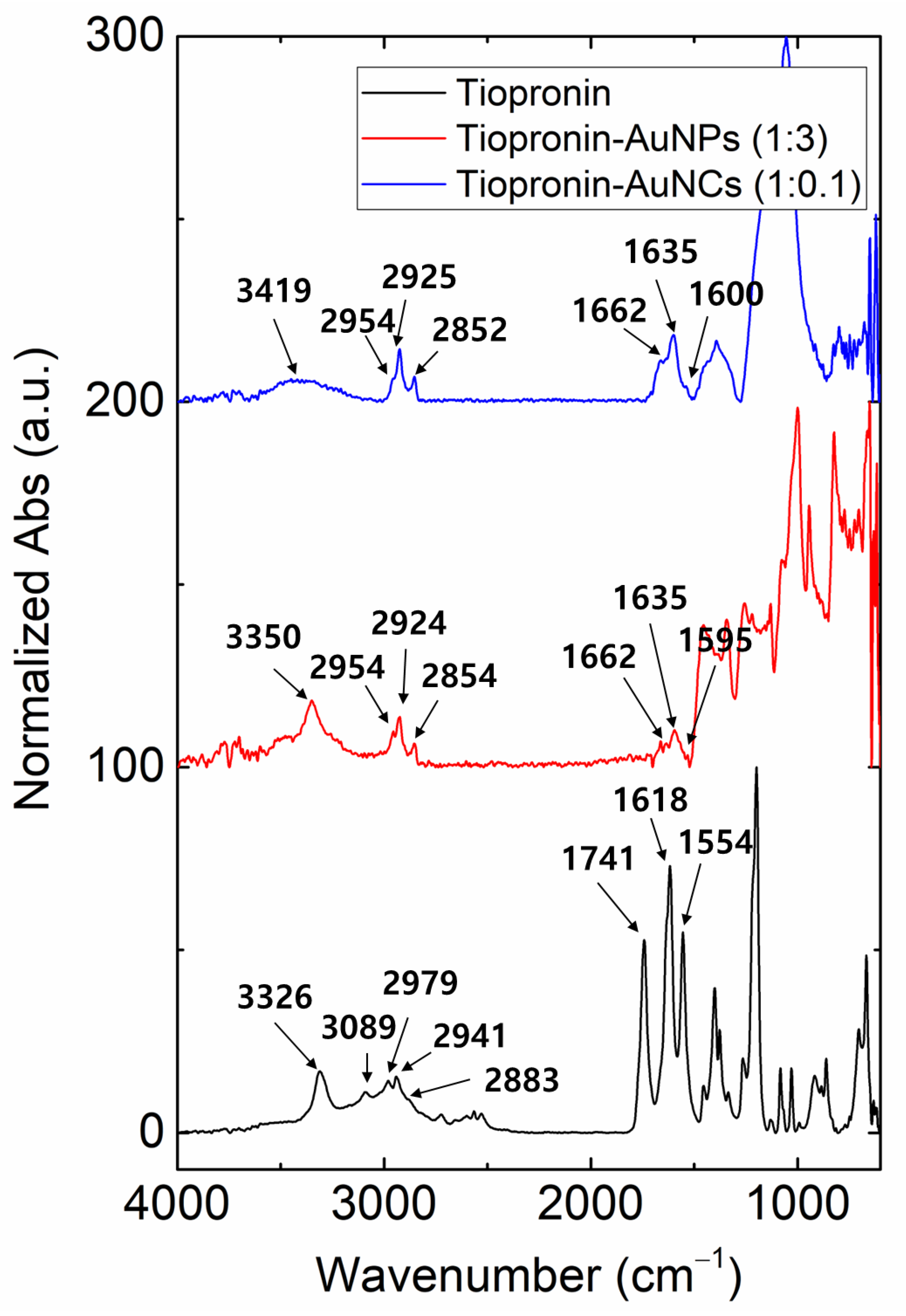
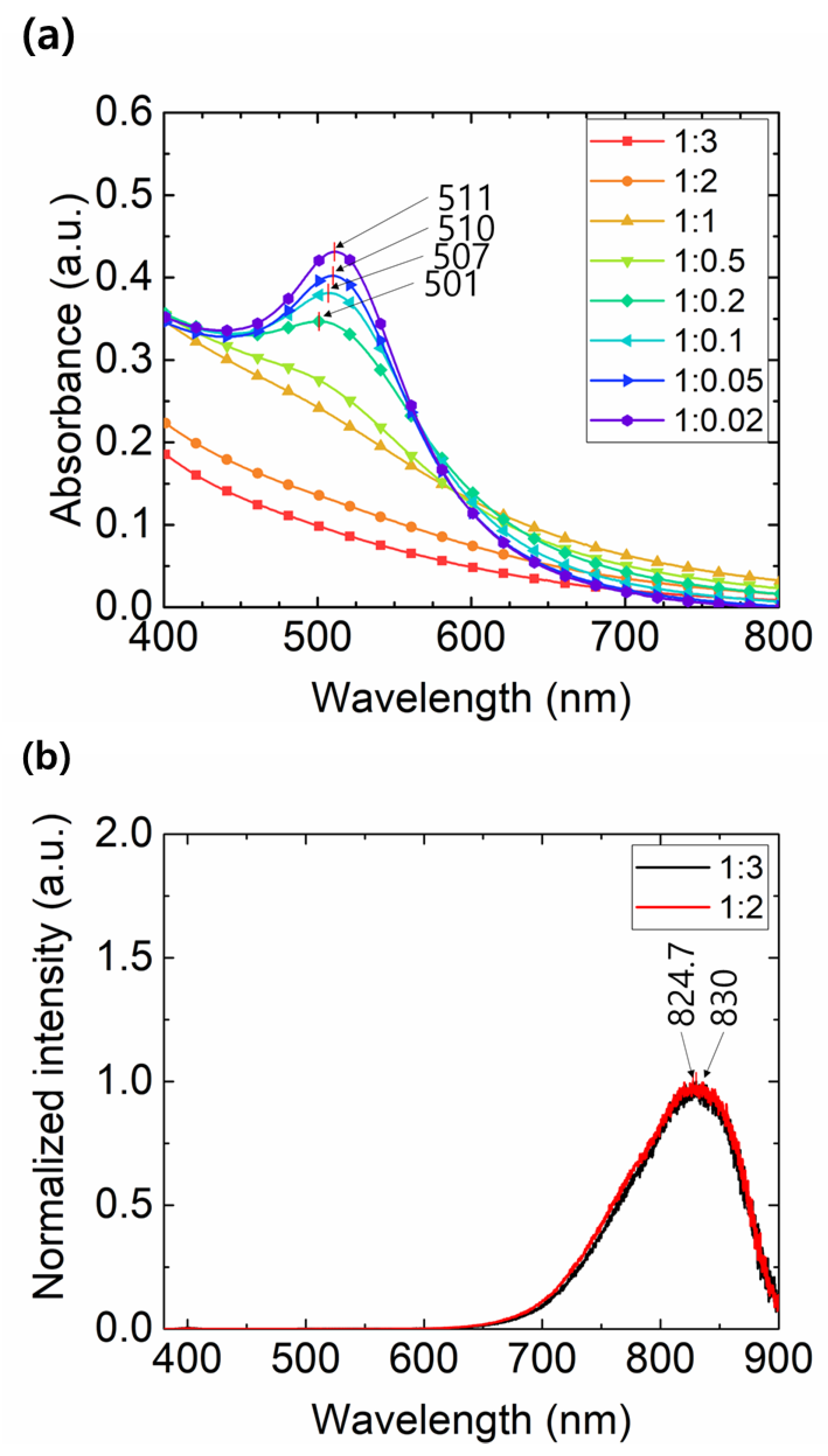
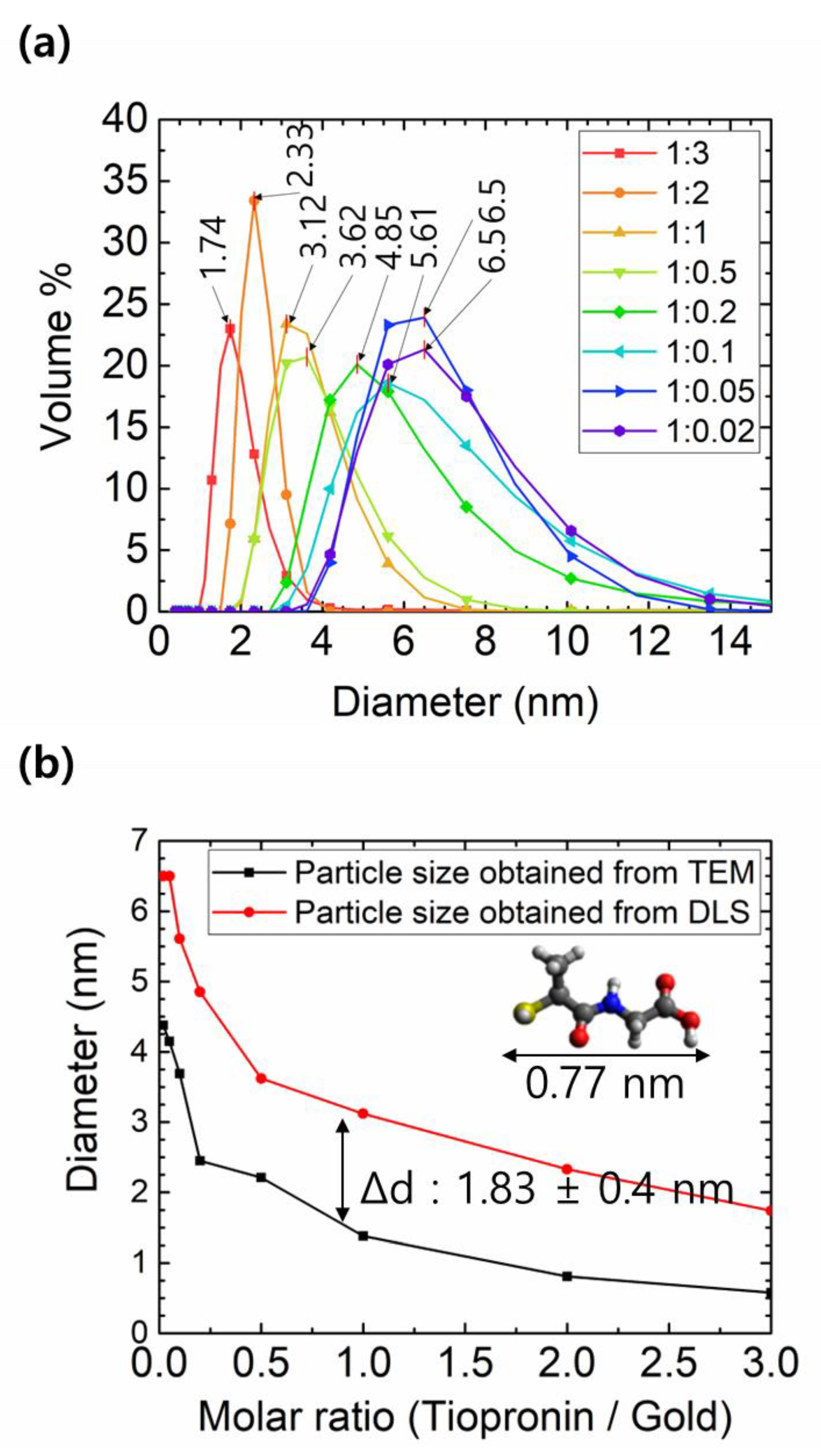
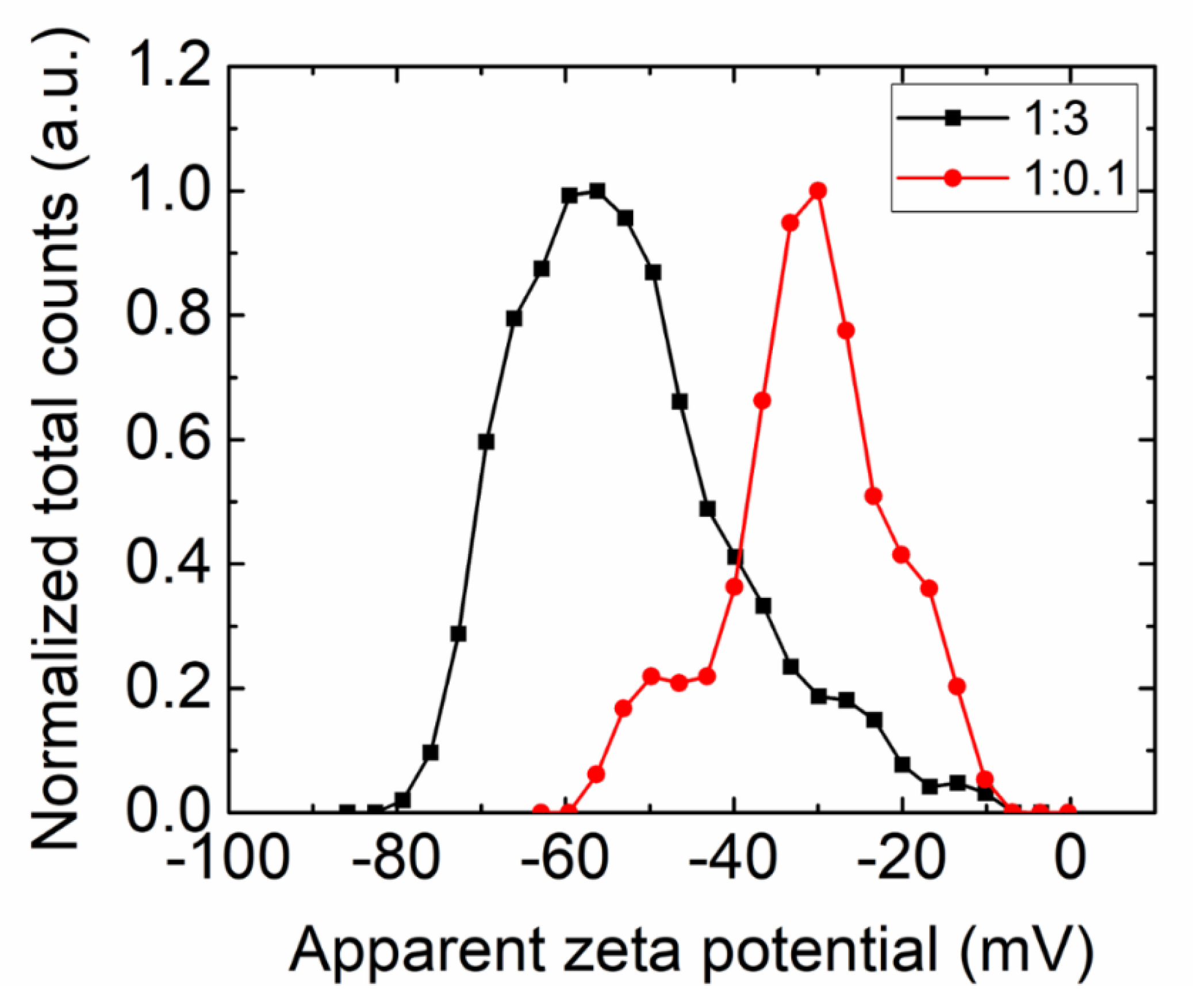
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Cho, H.Y.; Choi, H.K.; Lee, J.Y.; Choi, J.W. Application of Gold Nanoparticle to Plasmonic Biosensors. Int. J. Mol. Sci. 2018, 19, 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mazouzi, Y.; Salmain, M.; Liedberg, B.; Boujday, S. Antibody-Gold Nanoparticle Bioconjugates for Biosensors: Synthesis, Characterization and Selected Applications. Biosens. Bioelectron. 2020, 165, 112370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhong, S.; Wang, K.; Huang, J. Gold Nanoparticle Based Fluorescent Oligonucleotide Probes for Imaging and Therapy in Living Systems. Analyst 2019, 144, 1052–1072. [Google Scholar] [CrossRef]
- Wu, Y.; Ali, M.R.K.; Chen, K.C.; Fang, N.; El-Sayed, M.A. Gold Nanoparticles in Biological Optical Imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Sharifi, M.; Attar, F.; Saboury, A.A.; Akhtari, K.; Hooshmand, N.; Hasan, A.; El-Sayed, M.A.; Falahati, M. Plasmonic Gold Nanoparticles: Optical Manipulation, Imaging, Drug Delivery and Therapy. J. Control. Release 2019, 311–312, 170–189. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yañez-Sedeño, P.; González-Cortés, A.J.E.A. Gold Nanoparticle-Based Electrochemical Biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Zeng, S.W.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Sharma, P.; Brown, S.; Walter, G.; Santra, S.; Moudgil, B. Nanoparticles for Bioimaging. Adv. Colloid Interface Sci. 2006, 123–126, 471–485. [Google Scholar] [CrossRef]
- Yong, K.T.; Swihart, M.T.; Ding, H.; Prasad, P.N. Preparation of Gold Nanoparticles and Their Applications in Anisotropic Nanoparticle Synthesis and Bioimaging. Plasmonics 2009, 4, 79–93. [Google Scholar] [CrossRef]
- Cheng, Y.; Samia, A.C.; Meyers, J.D.; Panagopoulos, I.; Fei, B.; Burda, C. Highly Efficient Drug Delivery with Gold Nanoparticle Vectors for in Vivo Photodynamic Therapy of Cancer. J. Am. Chem. Soc. 2008, 130, 10643–10647. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of Gold Nanoparticles in Biomedical and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized Gold Nanoparticles for Drug Delivery. Nanomedicine 2007, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Duncan, B.; Kim, C.; Rotello, V.M. Gold Nanoparticle Platforms as Drug and Biomacromolecule Delivery Systems. J. Control. Release 2010, 148, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, T.; Chen, J.; Shi, J.; Wang, L.; Shen, J.; Liu, X. Water-dispersible Gold Nanoclusters: Synthesis Strategies, Optical Properties, and Biological Applications. Chem. Eur. J. 2022, 28, e202103736. [Google Scholar] [CrossRef]
- Chen, W.; Deng, H.H.; Hong, L.; Wu, Z.Q.; Wang, S.; Liu, A.L.; Lin, X.H.; Xia, X.H. Bare Gold Nanoparticles as Facile and Sensitive Colorimetric Probe for Melamine Detection. Analyst 2012, 137, 5382–5386. [Google Scholar] [CrossRef]
- Ijaz, M.; Zafar, M.; Iqbal, T. Green Synthesis of Silver Nanoparticles by Using Various Extracts: A review. Inorg. Nano-Met. Chem. 2020, 51, 744–755. [Google Scholar] [CrossRef]
- Iqbal, T.; Tufail, S.; Ghazal, S. Synthesis of Silver, Chromium, Manganese, Tin and Iron Nano Particles by Different Techniques. Int. J. Nanosci. Nanotechnol. 2017, 13, 19–52. [Google Scholar]
- Wang, Z.L.; Zong, S.F.; Wang, Y.J.; Li, N.; Li, L.; Lu, J.; Wang, Z.Y.; Chen, B.A.; Cui, Y.P. Screening and Multiple Detection of Cancer Exosomes Using an SERS-Based Method. Nanoscale 2018, 10, 9053–9062. [Google Scholar] [CrossRef]
- Pang, Y.; Wang, C.; Lu, L.; Wang, C.; Sun, Z.; Xiao, R. Dual-SERS Biosensor for One-Step Detection of MicroRNAs in Exosome and Residual Plasma of Blood Samples for Diagnosing Pancreatic Cancer. Biosens. Bioelectron. 2019, 130, 204–213. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, L.; Yang, X.; Liu, X.; Nie, W.; Zheng, Y.; Cheng, Q.; Wang, K. Direct Quantification of Cancerous Exosomes via Surface Plasmon Resonance with Dual Gold Nanoparticle-Assisted Signal Amplification. Biosens. Bioelectron. 2019, 135, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, N.; Borah, G.; Gogoi, P.K.; Chetia, T.R. TiO2 Supported Gold Nanoparticles: An Efficient Photocatalyst for Oxidation of Alcohol to Aldehyde and Ketone in Presence of Visible Light Irradiation. Chem. Phys. Lett. 2018, 692, 224–231. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Gatard, S.; Ciganda, R.; Hernandez, R.; Ruiz, J.; Astruc, D. Sodium Borohydride Stabilizes Very Active Gold Nanoparticle Catalysts. Chem. Commun. 2014, 50, 14194–14196. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Simon, T.; Sun, K.W.; Ko, F.H. Simple Bare Gold Nanoparticles for Rapid Colorimetric Detection of Cr3+ Ions in Aqueous Medium with Real Sample Applications. Sens. Actuators B Chem. 2016, 226, 44–51. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Sun, K.W.; Wu, S.P. A pH Cooperative Strategy for Enhanced Colorimetric Sensing of Cr(III) Ions Using Biocompatible L-Glutamic Acid Stabilized Gold Nanoparticles. Microchem. J. 2021, 160, 105754. [Google Scholar] [CrossRef]
- Chu, Y.Z.; Schonbrun, E.; Yang, T.; Crozier, K.B. Experimental Observation of Narrow Surface Plasmon Resonances in Gold Nanoparticle Arrays. Appl. Phys. Lett. 2008, 93, 181108. [Google Scholar] [CrossRef]
- Zayats, M.; Kharitonov, A.B.; Pogorelova, S.P.; Lioubashevski, O.; Katz, E.; Willner, I. Probing Photoelectrochemical Processes in Au-CdS Nanoparticle Arrays by Surface Plasmon Resonance: Application for the Detection of Acetylcholine Esterase Inhibitors. J. Am. Chem. Soc. 2003, 125, 16006–16014. [Google Scholar] [CrossRef]
- Briñas, R.P.; Hu, M.; Qian, L.; Lymar, E.S.; Hainfeld, J.F. Gold Nanoparticle Size Controlled by Polymeric Au(I) Thiolate Precursor Size. J. Am. Chem. Soc. 2008, 130, 975–982. [Google Scholar] [CrossRef]
- Karimi, S.; Moshaii, A.; Nikkhah, M. Controlled Synthesis of Colloidal Monodisperse Gold Nanoparticles in a Wide Range of Sizes; Investigating the Effect of Reducing Agent. Mater. Res. Express 2019, 6, 1150f2. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Brust, M.; Fink, J.; Bethell, D.; Schiffrin, D.; Kiely, C. Synthesis and Reactions of Functionalised Gold Nanoparticles. J. Chem. Soc. Chem. Commun. 1995, 16, 1655–1656. [Google Scholar] [CrossRef]
- Templeton, A.C.; Chen, S.W.; Gross, S.M.; Murray, R.W. Water-Soluble, Isolable Gold Clusters Protected by Tiopronin and Coenzyme A Monolayers. Langmuir 1999, 15, 66–76. [Google Scholar] [CrossRef]
- Daood, U.; Akram, Z.; Matinlinna, J.P.; Fawzy, A.S. Dentine Collagen Cross-Linking Using Tiopronin-Protected Au/EDC Nanoparticles Formulations. Dent. Mater. 2019, 35, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Grueso, E.; Perez-Tejeda, P.; Giráldez-Pérez, R.M.; Prado-Gotor, R.; Muriel-Delgado, F. Ethanol Effect on Gold Nanoparticle Aggregation State and Its Implication in the Interaction Mechanism with DNA. J. Colloid Interface Sci. 2018, 529, 65–76. [Google Scholar] [CrossRef]
- Muraca, F.; Boselli, L.; Castagnola, V.; Dawson, K.A. Ultrasmall Gold Nanoparticle Cellular Uptake: Influence of Transient Bionano Interactions. ACS Appl. Bio Mater. 2020, 3, 3800–3808. [Google Scholar] [CrossRef]
- Dahan, I.; Sorrentino, S.; Boujemaa-Paterski, R.; Medalia, O. Tiopronin-Protected Gold Nanoparticles as a Potential Marker for Cryo-EM and Tomography. Structure 2018, 26, 1408–1413. [Google Scholar] [CrossRef]
- Ramakrishna, G.; Varnavski, O.; Kim, J.; Lee, D.; Goodson, T. Quantum-Sized Gold Clusters as Efficient Two-Photon Absorbers. J. Am. Chem. Soc. 2008, 130, 5032–5033. [Google Scholar] [CrossRef]
- Wilcoxon, J.P.; Martin, J.E.; Parsapour, F.; Wiedenman, B.; Kelley, D.F. Photoluminescence from Nanosize Gold Clusters. J. Chem. Phys. 1998, 108, 9137–9143. [Google Scholar] [CrossRef]
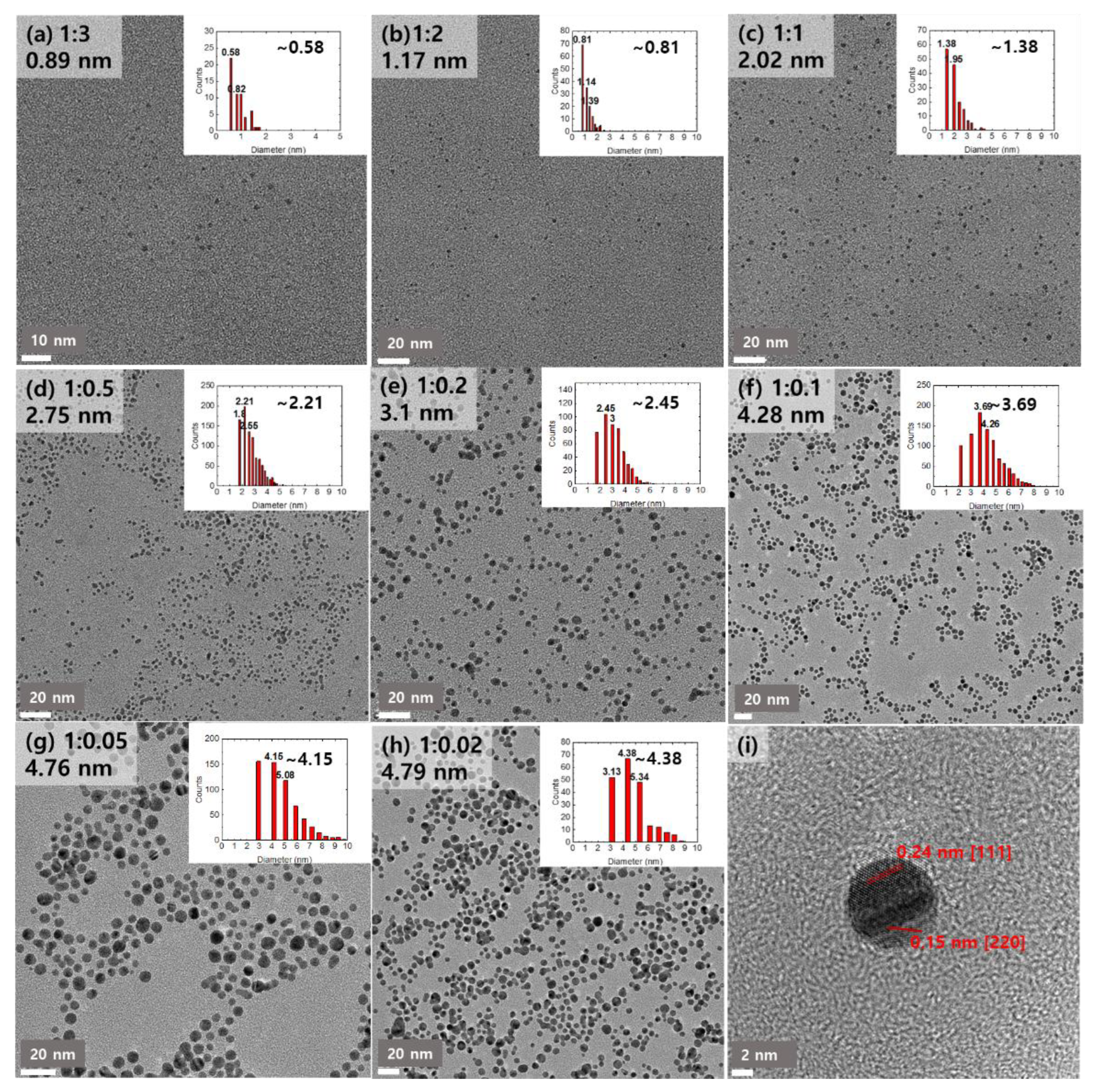
| Au:Tiopronin | 1:3 | 1:2 | 1:1 | 1:0.5 | 1:0.2 | 1:0.1 | 1:0.05 | 1:0.02 |
|---|---|---|---|---|---|---|---|---|
| dmode (nm) | 0.58 | 0.81 | 1.38 | 2.21 | 2.45 | 3.69 | 4.15 | 4.38 |
| dmean (nm) | 0.89 | 1.17 | 2.02 | 2.75 | 3.1 | 4.28 | 4.76 | 4.79 |
| σ (nm) | ±0.32 | ±0.42 | ±0.66 | ±0.76 | ±0.94 | ±1.32 | ±1.55 | ±1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, D.; Son, C.; Lee, H.; Park, H.; Kim, T.; Park, J. Aqueous Synthesis of the Tiopronin-Capped Gold Nanoclusters/Nanoparticles with Precise Size Control via Deprotonation of the Ligand. Appl. Sci. 2022, 12, 8263. https://doi.org/10.3390/app12168263
Kang D, Son C, Lee H, Park H, Kim T, Park J. Aqueous Synthesis of the Tiopronin-Capped Gold Nanoclusters/Nanoparticles with Precise Size Control via Deprotonation of the Ligand. Applied Sciences. 2022; 12(16):8263. https://doi.org/10.3390/app12168263
Chicago/Turabian StyleKang, Daekyung, Changhee Son, Hakseon Lee, Hongsik Park, Taewan Kim, and Jonghoo Park. 2022. "Aqueous Synthesis of the Tiopronin-Capped Gold Nanoclusters/Nanoparticles with Precise Size Control via Deprotonation of the Ligand" Applied Sciences 12, no. 16: 8263. https://doi.org/10.3390/app12168263
APA StyleKang, D., Son, C., Lee, H., Park, H., Kim, T., & Park, J. (2022). Aqueous Synthesis of the Tiopronin-Capped Gold Nanoclusters/Nanoparticles with Precise Size Control via Deprotonation of the Ligand. Applied Sciences, 12(16), 8263. https://doi.org/10.3390/app12168263






