Optimization of Common Iliac Artery Sonography Images via an Indigenous Water Phantom and Taguchi’s Analysis: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Taguchi Analysis
2.3. Orthogonal Arrays
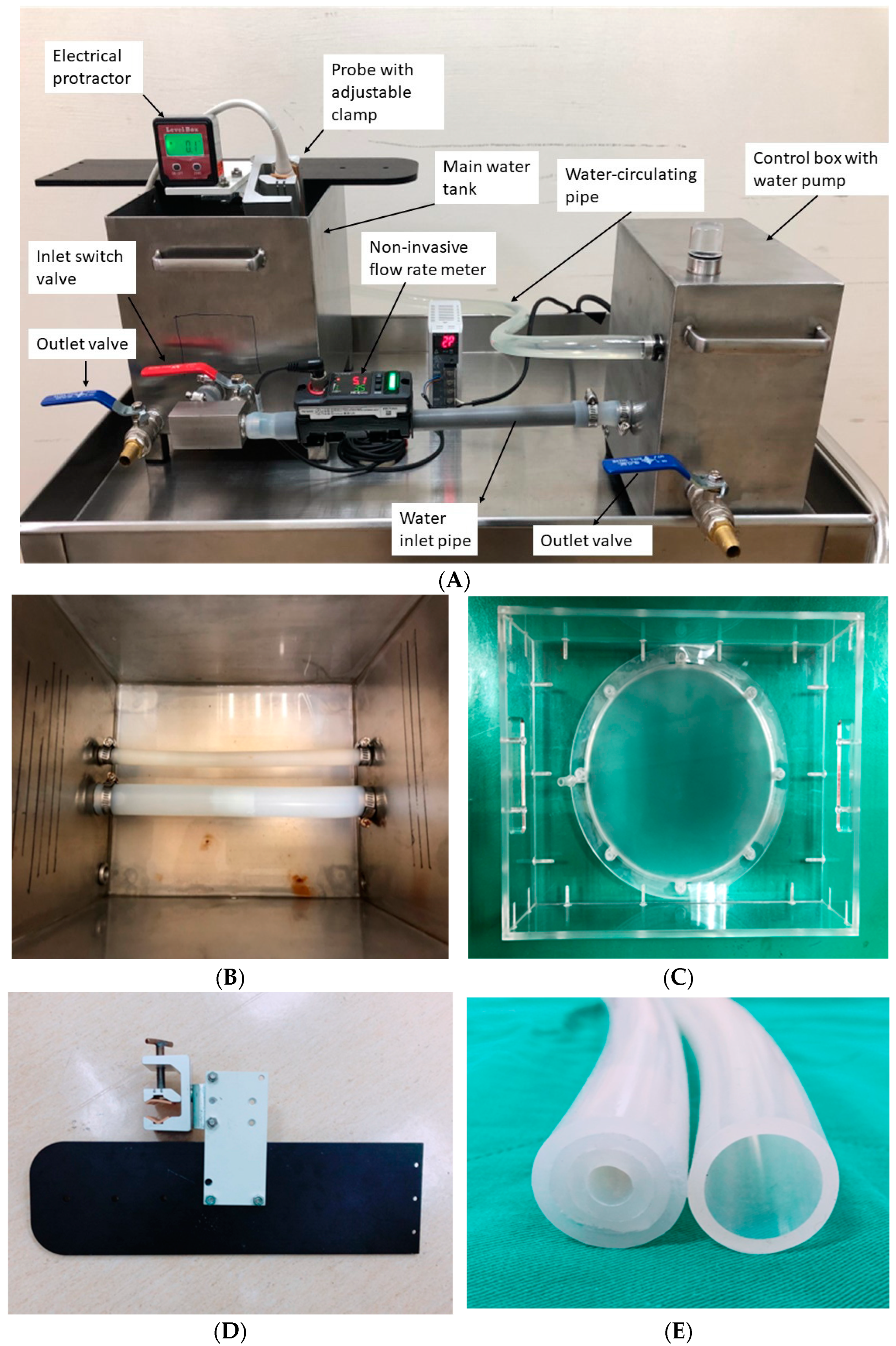
2.4. The Customized Water Phantom
2.5. Analysis of Variance (ANOVA)
2.6. Sonography Images Scoring
3. Results
3.1. Raw Data Analysis
3.2. Inspecting the ANOVA
4. Discussion
4.1. Taguchi’s Approach Verification
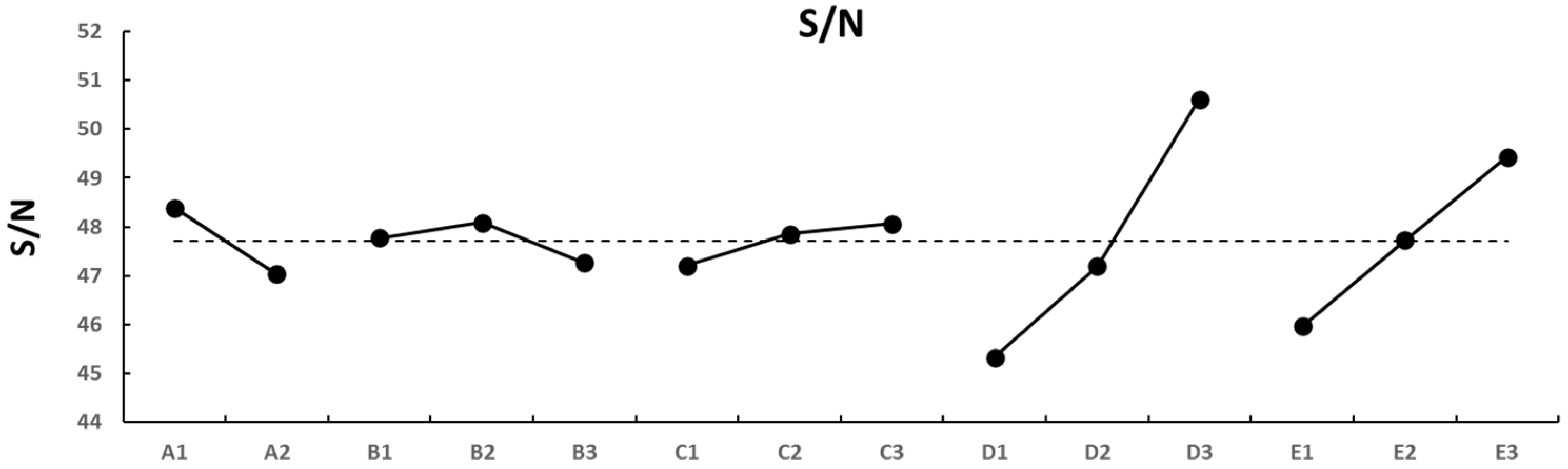
4.2. Manipulating the S/N Ratio
4.3. The S/N Focused on the Difference in Area or Diameter
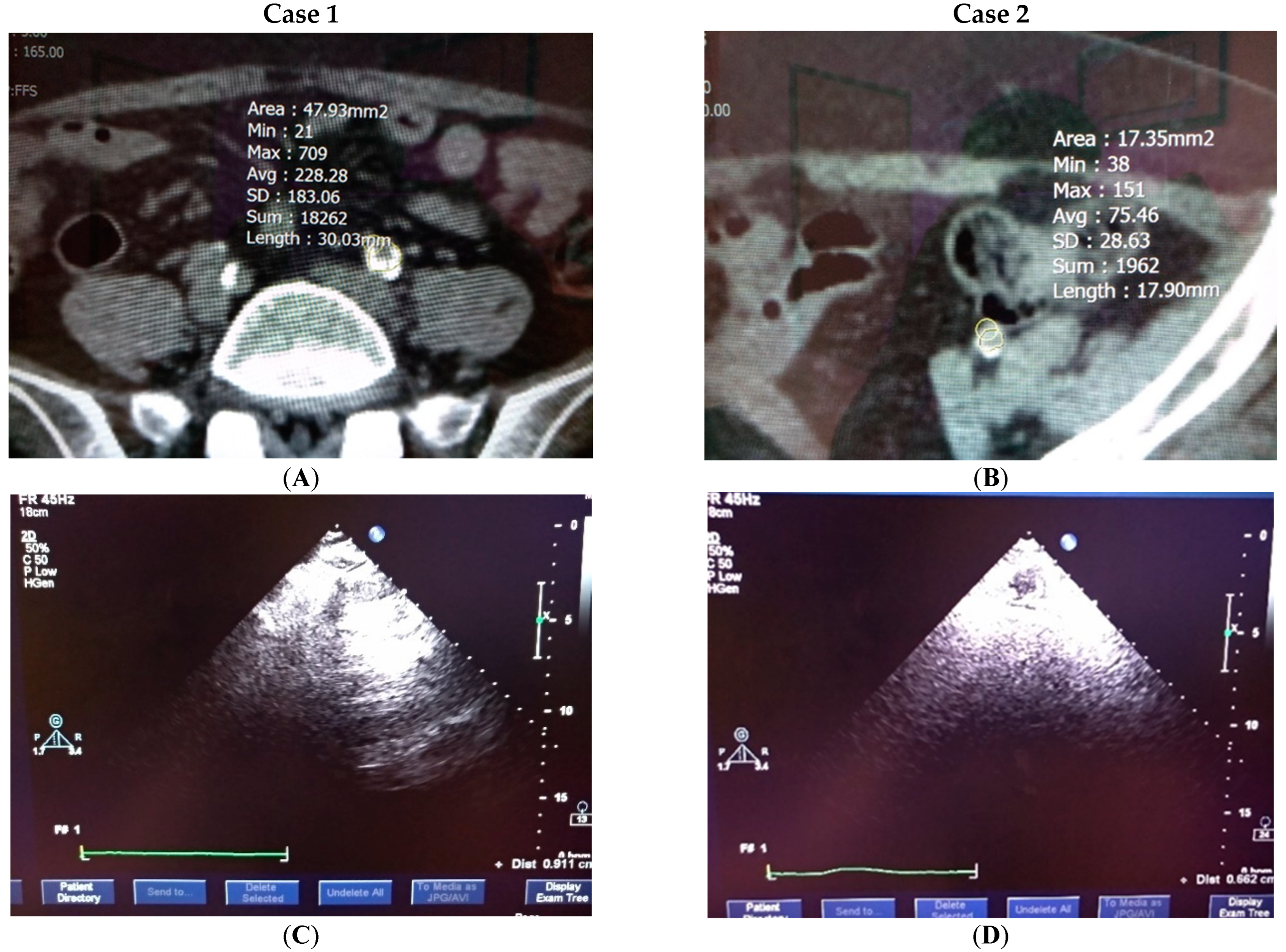
4.4. Clinical Testification
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hertzberg, Y.; Volovick, A.; Zur, Y.; Medan, Y.; Vitek, S.; Navon, G. Ultrasound focusing using magnetic resonance acoustic radiation force imaging: Application to ultrasound transcranial therapy. Med. Phys. 2010, 37, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Geng, Y.; Han, D.; Lu, M.; Li, R.; Li, Y.; Zhang, Q.; Wan, M. Viscoelastic characterization of HIFU ablation with shear wave by using K-space analysis combined with model-fitting correction method. Ultrasonics 2020, 108, 106179. [Google Scholar] [CrossRef]
- Marquet, F.; Aubry, J.-F.; Pernot, M.; Fink, M.; Tanter, M. Optimal transcostal high-intensity focused ultrasound with combined real-time 3D movement tracking and correction. Phys. Med. Biol. 2011, 56, 7061–7080. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Anuraj, B.; Dupont, P.E. Varying ultrasound power level to distinguish surgical instruments and tissue. Med. Biol. Eng. Comput. 2017, 56, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Grishenkov, D.; Gonon, A.; Janerot-Sjoberg, B. In Search of the Optimal Heart Perfusion Ultrasound Imaging Platform. J. Ultrasound Med. 2015, 34, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- King, D.M.; Hangiandreou, N.J.; Tradup, D.J.; Stekel, S.F. Evaluation of a low-cost liquid ultrasound test object for detection of transducer artifacts. Phys. Med. Biol. 2010, 55, N557–N570. [Google Scholar] [CrossRef]
- Lo, M.D.; Ackley, S.H.; Solari, P. Homemade ultrasound phantom for teaching identification of superficial soft tissue abscess. Emerg. Med. J. 2011, 29, 738–741. [Google Scholar] [CrossRef]
- Pan, L.-F.; Chu, K.H.; Sher, H.-F. Optimizing left anterior oblique (LAO) caudal imaging in coronary angiography using the Taguchi method: A phantom study with clinical verification. Int. J. Cardiovasc. Imaging 2017, 130, 185–1295. [Google Scholar] [CrossRef]
- Zander, D.; Huske, S.; Hoffmann, B.; Cui, X.W.; Dong, Y.; Lim, A.; Jenssen, C.; Lowe, A.; Koch, J.B.H.; Dietrich, C.F. Ultrasound image optimization (“Knobology”): B-Mode. Ultrasound Int. Open 2020, 6, E14–E24. [Google Scholar] [CrossRef]
- Coffey, K.R.; Marx, R.G.; Neumaier, J.F. DeepSqueak: A deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology 2019, 44, 859–868. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, S. Research on Ultrasonic Image Recognition Based on Optimization Immune Algorithm. Comput. Math. Methods Med. 2021, 2021, 5868949. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Zhu, Y.; Hedhli, J.; Dobrucki, L.W.; Insana, M.F. Multidimensional Clutter Filter Optimization for Ultrasonic Perfusion Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2018, 65, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Hsun-Nan, K.; Juei-En, Y.; Chia-Hui, C.; Lung-Fa, P.; Lung-Kwang, P. Taguchi dynamic analysis application to computer tomography number-mass density linear dependence optimization. Comput. Assist. Surg. 2017, 22, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.-K.; Wu, K.-Y.; Chen, K.-L.; Kittipayak, S. Taguchi method-based optimization of the minimum detectable difference of a cardiac X-ray imaging system using a precise line pair gauge. J. Mech. Med. Biol. 2019, 19, 1940030. [Google Scholar] [CrossRef]
- Taguchi, G.; Rafanelli, A.J. Taguchi on Robust Technology Development: Bringing Quality Engineering Upstream. J. Electron. Packag. 1994, 116, 161. [Google Scholar] [CrossRef][Green Version]
- Votino, A.; Bosch, T.V.D.; Installé, A.; Van Schoubroeck, D.; Kaijser, J.; Kacem, Y.; De Moor, B.; Van Pachterbeke, C.; Timmerman, D. Optimizing the ultrasound visualization of the endometrial-myometrial junction (EMJ). Facts Views Vis. ObGyn 2015, 7, 60–63. [Google Scholar]
- Peng, B.-R.; Kittipayak, S.; Pan, L.-F. Optimizing the minimum detectable difference of computed tomography scanned images via the taguchi analysis: A feasibility study with an indigenous hepatic phantom and a line group gauge. J. Mech. Med. Biol. 2019, 19, 1940048. [Google Scholar] [CrossRef]
- Fisher, R.A. Design and Analysis of Experiments; Olive and Boyd: London, UK, 1925. [Google Scholar]
- Sharma, S.; Rangaiah, G.; Cheah, K. Multi-objective optimization using MS Excel with an application to design of a falling-film evaporator system. Food Bioprod. Process. 2012, 90, 123–134. [Google Scholar] [CrossRef]
- Kenny, D.A.; Mannetti, L.; Pierro, A.; Livi, S.; Kashy, D.A. The statistical analysis of data from small groups. J. Personal. Soc. Psychol. 2002, 83, 126–137. [Google Scholar] [CrossRef]
- Pan, L.K.; Wang, C.C.; Hsiao, Y.C.; Ho, K.C. Optimization of Nd:YAG laser welding onto magnesium alloy via Taguchi analysis. Opt. Laser Technol. 2005, 37, 33–42. [Google Scholar] [CrossRef]
- Yeh, D.M.; Wang, T.H.; Pan, L.K. Evaluating the quality characteristics of TLD-100T and TLD-100H exposed to diagnostic X-rays and 64 multislice CT using Taguchi’s quality loss function. Radiat. Meas. 2015, 80, 17–22. [Google Scholar] [CrossRef]
- Chang, C.-F.; Chen, C.-F.; Wang, T.-H.; Chiang, F.-T.; Wu, H.-T.; Pan, L.-F. Semi-quantification of the minimum detectable difference of imaging quality of gamma camera SPET for four radionuclides via an innovative PMMA phantom with a V-shaped slit: Interpretation of a feasibility study. Hell. J. Nucl. Med. 2019, 22, 49–57. [Google Scholar] [PubMed]



| Group | Factor | ||||
|---|---|---|---|---|---|
| A | B | C | D | E | |
| 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 1 | 2 | 2 | 2 |
| 3 | 1 | 1 | 3 | 3 | 3 |
| 4 | 1 | 2 | 1 | 1 | 2 |
| 5 | 1 | 2 | 2 | 2 | 3 |
| 6 | 1 | 2 | 3 | 3 | 1 |
| 7 | 1 | 3 | 1 | 2 | 1 |
| 8 | 1 | 3 | 2 | 3 | 2 |
| 9 | 1 | 3 | 3 | 1 | 3 |
| 10 | 2 | 1 | 1 | 3 | 3 |
| 11 | 2 | 1 | 2 | 1 | 1 |
| 12 | 2 | 1 | 3 | 2 | 2 |
| 13 | 2 | 2 | 1 | 2 | 3 |
| 14 | 2 | 2 | 2 | 3 | 1 |
| 15 | 2 | 2 | 3 | 1 | 2 |
| 16 | 2 | 3 | 1 | 3 | 2 |
| 17 | 2 | 3 | 2 | 1 | 3 |
| 18 | 2 | 3 | 3 | 2 | 1 |
| Factor | Levels | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| (A) angle of probe (degree) | 0 | 30 | |
| (B) water depth (cm) | 5 | 6 | 7 |
| (C) frame rate (/sec) | 45 | 50 | 55 |
| (D) amplitude gain (%) | 50 | 60 | 70 |
| (E) compress ratio (%) | 45 | 50 | 55 |
| Group | Area Difference (cm2) | Ave. | Stdev | S/N | ||
|---|---|---|---|---|---|---|
| #1 | #2 | #3 | ||||
| 1 | −0.074 | −0.181 | −0.067 | −0.107 | 0.064 | 19.39 |
| 2 | −0.189 | −0.175 | −0.134 | −0.166 | 0.029 | 15.60 |
| 3 | −0.231 | −0.236 | −0.223 | −0.230 | 0.007 | 12.77 |
| 4 | −0.143 | −0.092 | −0.075 | −0.103 | 0.035 | 19.72 |
| 5 | −0.191 | −0.169 | −0.156 | −0.172 | 0.018 | 15.29 |
| 6 | −0.165 | −0.164 | −0.196 | −0.175 | 0.018 | 15.14 |
| 7 | −0.107 | −0.149 | −0.199 | −0.152 | 0.046 | 16.38 |
| 8 | −0.246 | −0.229 | −0.226 | −0.234 | 0.011 | 12.63 |
| 9 | −0.147 | −0.203 | −0.195 | −0.182 | 0.030 | 14.81 |
| 10 | −0.135 | −0.164 | −0.111 | −0.137 | 0.027 | 17.29 |
| 11 | −0.268 | −0.243 | −0.224 | −0.245 | 0.022 | 12.22 |
| 12 | −0.237 | −0.205 | −0.245 | −0.229 | 0.021 | 12.80 |
| 13 | −0.133 | −0.073 | −0.116 | −0.107 | 0.031 | 19.39 |
| 14 | −0.204 | −0.181 | −0.226 | −0.204 | 0.023 | 13.82 |
| 15 | −0.276 | −0.227 | −0.226 | −0.243 | 0.029 | 12.29 |
| 16 | −0.147 | −0.165 | −0.120 | −0.144 | 0.023 | 16.83 |
| 17 | −0.245 | −0.228 | −0.202 | −0.225 | 0.022 | 12.96 |
| 18 | −0.222 | −0.184 | −0.217 | −0.208 | 0.021 | 13.65 |
| Ave.= | −0.181 | 0.026 | 15.16 | |||
| Factor | SS | DOF | Contribution | Var | F | Probability | Confidence Level | Significance * |
|---|---|---|---|---|---|---|---|---|
| A | 0.0081 | 1 | 5.4% | 0.0081 | 9.5347 | 0.39% | 99.61% | Yes |
| B | 0.0053 | 2 | 3.6% | 0.0027 | 3.1643 | 5.42% | 94.58% | No |
| C | 0.0852 | 2 | 56.6% | 0.0426 | 50.1001 | 0.00% | 100.00% | Yes |
| D | 0.0022 | 2 | 1.5% | 0.0011 | 1.3147 | 28.11% | 71.89% | No |
| E | 0.0011 | 2 | 0.7% | 0.0006 | 0.6502 | 52.80% | 47.20% | No |
| Others | 0.0178 | 8 | 11.9% | 0.0022 | 2.6245 | 2.25% | 97.75% | No |
| Error | 0.0306 | 36 | 20.3% | 0.0009 | S = 0.029174761 | |||
| Total | 0.1506 | 53 | 100.0% | * Note: At least 99% confidence level | ||||
| Factor | Conventional (The 1st Group) | Combined with The Highest S/N | Optimal Setting (The 4th Group) |
|---|---|---|---|
| (A) angle of probe (degree) | 0 | 0 | 0 |
| (B) water depth (cm) | 5 | 6 | 6 |
| (C) frame rate (/s) | 45 | 45 | 45 |
| (D) amplitude gain (%) | 50 | 60 | 50 |
| (E) compress ratio (%) | 45 | 55 | 50 |
| S/N (dB) | 19.39 | 16.63 | 19.72 |
| Group | Diameter Difference (cm) | Ave. | Stdev | S/N | ||
|---|---|---|---|---|---|---|
| #1 | #2 | #3 | ||||
| 1 | −0.238 | −0.238 | −0.195 | −0.224 | 0.02 | 13.01 |
| 2 | −0.092 | −0.082 | −0.044 | −0.073 | 0.03 | 22.77 |
| 3 | −0.072 | −0.039 | −0.039 | −0.050 | 0.02 | 26.02 |
| 4 | −0.022 | −0.022 | 0.021 | −0.008 | 0.02 | 42.31 |
| 5 | −0.044 | −0.119 | −0.119 | −.094 | 0.04 | 20.54 |
| 6 | −0.006 | 0.027 | −0.006 | 0.005 | 0.02 | 46.02 |
| 7 | 0.021 | −0.065 | −0.022 | −0.022 | 0.04 | 33.15 |
| 8 | −0.006 | −0.044 | −0.006 | −0.019 | 0.02 | 34.58 |
| 9 | −0.072 | −0.138 | −0.138 | −0.116 | 0.04 | 18.71 |
| 10 | −0.022 | −0.065 | −0.065 | −0.051 | 0.02 | 25.91 |
| 11 | −0.044 | −0.082 | −0.044 | −0.057 | 0.02 | 24.93 |
| 12 | −0.072 | −0.039 | −0.105 | −0.072 | 0.03 | 22.85 |
| 13 | −0.022 | −0.109 | 0.021 | −0.037 | 0.07 | 28.71 |
| 14 | −0.006 | −0.119 | −0.044 | −0.056 | 0.06 | 24.98 |
| 15 | −0.204 | −0.138 | −0.171 | −0.171 | 0.03 | 15.34 |
| 16 | 0.064 | 0.021 | −0.022 | 0.021 | 0.04 | 33.56 |
| 17 | −0.119 | −0.006 | −0.044 | −0.056 | 0.06 | 24.98 |
| 18 | −0.138 | −0.105 | −0.105 | −0.116 | 0.02 | 18.71 |
| Ave.= | −0.066 | 0.03 | 26.51 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.-Y.; Liang, C.-C.; Chuang, C.-H.; Pan, L.-F.; Pan, L.-K. Optimization of Common Iliac Artery Sonography Images via an Indigenous Water Phantom and Taguchi’s Analysis: A Feasibility Study. Appl. Sci. 2022, 12, 8197. https://doi.org/10.3390/app12168197
Wu K-Y, Liang C-C, Chuang C-H, Pan L-F, Pan L-K. Optimization of Common Iliac Artery Sonography Images via an Indigenous Water Phantom and Taguchi’s Analysis: A Feasibility Study. Applied Sciences. 2022; 12(16):8197. https://doi.org/10.3390/app12168197
Chicago/Turabian StyleWu, Keng-Yi, Chun-Chieh Liang, Chao-Hsun Chuang, Lung-Fa Pan, and Lung-Kwang Pan. 2022. "Optimization of Common Iliac Artery Sonography Images via an Indigenous Water Phantom and Taguchi’s Analysis: A Feasibility Study" Applied Sciences 12, no. 16: 8197. https://doi.org/10.3390/app12168197
APA StyleWu, K.-Y., Liang, C.-C., Chuang, C.-H., Pan, L.-F., & Pan, L.-K. (2022). Optimization of Common Iliac Artery Sonography Images via an Indigenous Water Phantom and Taguchi’s Analysis: A Feasibility Study. Applied Sciences, 12(16), 8197. https://doi.org/10.3390/app12168197







