Featured Application
The results of this study may be implemented in recommendations for the calculation of the delay in clock time for the weekday waking time required to reduce sleep loss on weekdays.
Abstract
Background: Our work/study culture is biased towards the circadian clocks of “morning types”, whereas “evening types” are forced to advance their weekday waking times relative to weekend waking times. Since the experimental research consistently reveals a >2 h difference between these two chronotypes in the positions of their endogenous circadian phases, we hypothesized the necessity to permit a >2 h difference between them in weekday waking times to equalize their irrecoverable loss in sleep on weekdays. Methods: A total of 659 and 1106 participants of online surveys identified themselves as morning and evening types, respectively. The hypothesis was tested by applying a model of sleep–wake regulation for simulating sleep times reported by 245 lecturers of these two types, and by comparison of sleep times of these types among these lecturers and 1520 students. Results: The hypothesis was supported by results showing that, if, on weekdays, an “average” morning type wakes at 6 a.m., the equalization of the weekday sleep loss of the two chronotypes would require the waking time of an “average” evening type to be no earlier than 8 a.m. Conclusions: These results may be implemented in a model-based methodology for the correction of weekday waking times to equalize weekday sleep loss.
1. Introduction
Research in the fields of chronobiology and sleep science distinguishes between people of two distinct chronotypes: morning and evening types or, in short, M- and E-types [1,2]. If M-types are most alert in the morning, E-types are most alert in the evening, and, in addition, they prefer early awakening–early bedtime and late awakening–late bedtime, respectively [3]. Links are presented in the literature between the chronotype and individual differences in various domains, including the domains of neurophysiology, psychiatry, cognitive psychology, personality, and mental and physical health (reviewed in [4]).
According to a growing body of experimental research, the positions of endogenous circadian phases show, at least, a 2 h difference between chronotypes [5,6,7,8,9,10]. When workers/students work/study Monday to Friday, and then have two days without work/study, they might be tempted to “catch up” on sleep. However, the simulations of weekday and weekend sleep times with a sleep–wake regulation model [11] suggest that, despite complete freedom to sleep in and nap during the two weekend days, the reduction in sleep during the week cannot be reversed by the extension of weekend sleep beyond its normal, adequate duration [12,13,14,15,16]. The simulations also suggest a more profound weekday sleep loss in E-types than M-types because E-types have to advance their weekday times by more relative to weekend waking times [12,13].
It appears that negative impacts of the disparity between chronotypes in weekday sleep loss underlie the complaints about “the tyranny of the early risers/birds” (e.g., [17,18]). This disparity rests upon the tradition of setting working and school start-times too early. Consequently, given that our work/study culture is biased towards the circadian clocks of M-types, E-types are forced to sacrifice a larger amount of sleep on weekdays to arrive at their place of work/study at the M-type-oriented start-times. The recent “natural experiment” during “lockdown” demonstrated that, when home-workers/students were suddenly able to choose their own waking times, most slept later. Notably, this “experiment” also confirmed the model-based prediction of a failure to decrease the weekend sleep duration during lockdown, in response to the increase in weekday sleep duration leading to the decrease in weekday sleep loss [14].
Therefore, a question arises of whether the simulations of data on sleep timing of M- and E-types can help in the overthrowing of such a “tyranny of the early risers” by changing sleep times of E-types; that is, can the simulations based on a sleep–wake regulating model be implicated in the development of a method to correct the sleep times of E-types required to equalize the irrecoverable weekday sleep loss of the two chronotypes?
Consequently, the aim of this paper was to apply the model-based simulations of sleep times self-reported by M- and E-types for the development of a methodology for the estimation of the delay in the waking times of E-types relative to the waking times of M-types required to overthrow the “tyranny of the early risers”. Our hypothesis was that, since the literature suggested a >2 h difference between the two chronotypes in the positions of their endogenous circadian phases, such estimates would indicate that permitting a >2 h difference in weekday waking times of M- and E-types can be recommended to equalize the irrecoverable sleep losses on weekdays.
2. Materials and Methods
Lecturers from several Russian universities invited their students and colleagues (and some other workers of their universities) to respond, using their smartphones, to questions concerning their sleep–wake behavior. To collect such responses, two web sites were developed in Moscow and Novosibirsk. Table 1 briefly describes the collected samples. More details on selection, exclusion criteria, and additional chronobiological and somnological characteristics of these samples are given in Supplementary Materials (Table S1).

Table 1.
Participants of four online surveys and their divisions into early and late risers.
The survey participants were asked to report clock hours for bed- and risetimes on weekdays and weekends. Single-Item Chronotyping (SIC) was used for the selection of M- and E-types from the whole sample (Table 1). It was designed for self-choosing chronotype (CT) from 7 options [19]. The options for M-, E-, and other types were either illustrated by simple graphs (the first Moscow survey and two Novosibirsk surveys) or accompanied by short descriptions of daily pattern of activity (the second Moscow survey), e.g., for the options of M- and E-types: “morning type: high level in the morning, middle in the afternoon, low in the evening” and “evening type: low level in the morning, middle in the afternoon, high in the evening”, respectively [19]. Table 1 provides information on the number of survey participants classified into either “morning type” or “evening type” (they were included in further analysis; Table 2, Table 3, Table 4 and Table 5) and the number of participants of these two chronotypes who reported either early or late weekday risetime (RT, either before 7 a.m. or at 7 a.m., or later, respectively).
The Statistical Package for the Social Sciences (SPSS23, IBM, Armonk, NY, USA) was used for statistical analysis of empirical data. Two-, three-, and four-way ANOVAs of self-reported sleep times (Table 3, Table 4 and Table 5) were performed with up to four independent factors, such as self-chosen chronotype (“CT”, either M- or E-type, Table 3), self-reported weekday risetime (“RT”, either <7 or ≥7 a.m., Table 3), the combination of these two subdivisions (“CT&RT”, either MT or ET with RT either <7 or ≥7 a.m., Table 4), “Age” (either lecturers or university students, Table 3 and Table 4), the combination of three subdivisions (“CT&RT&Age”, either lecturers or university students of either MT or ET with RT either <7 or ≥7 a.m., Table 5), and “Survey” (four online surveys, 1–4, see Table 1 and Table S1). Post hoc pairwise Bonferroni comparisons were applied for testing the significance of the differences in sleep times between two of four “CT&RT” subdivisions (M-types with early and E-types with late weekday RT, Table 4) and between several “CT&RT&Age” subdivisions (Table 5).
The simulations of rise- and bedtimes self-reported by M- and E-types, and by early and late weekday risers, <7 a.m. and ≥7 a.m. (Figure 1, Figure 2, Figure 3 and Figure 4), were performed with a variant of the two-process model of sleep–wake regulation postulating the circadian modulation of the parameters of the sleep homeostatic process, S(t) [11]. If t1 and t2 are the initial times for the buildup and decay phases of the 24 h sleep–wake cycle (rise- and bedtime, respectively), the sleep–wake regulating process S(t) can be simulated using the following equations:
where:
is a periodic function with a period τ assigned to 24 h [11].
S(t) = [Su + C(t)] − {[Su + C(t)] − Sb} ∗ e(−(t − t1)/[Tb − k ∗ C(t)])
S(t) = [Sl + C(t)] − {Sd − [Sl + C(t)]} ∗ e(−(t − t2)/[Td − k ∗ C(t)])
C(t) = A ∗ sin(2π ∗ t/τ + φ0)

Table 2.
Parameters of the model and output of preliminary and final simulations of sleep times.
Table 2.
Parameters of the model and output of preliminary and final simulations of sleep times.
| Simulation | Initial | Preliminary | RT < 7 | RT ≥ 7 | MT | ET | <7 MT | ≥7 ET | |
|---|---|---|---|---|---|---|---|---|---|
| Shift in φmax, t2 and t1, h | 0.00 | 0.00 | 0.10 | 1.80 | −0.30 | 1.20 | −1.00 | 1.20 | |
| Advance RT on Weekdays, h | 3.00 | 2.00 | 3.00 | 2.00 | 2.00 | 3.00 | 2.00 | 2.00 | |
| Sleep times as output of simulations of the sleep–wake regulator, S(t): | |||||||||
| Weekday bedtime (BT), clock h | 22.69 | 23.26 | 22.79 | 24.06 | 22.96 | 23.89 | 22.26 | 24.46 | |
| Weekend bedtime (BT), clock h | 23.98 | 23.99 | 24.08 | 24.79 | 23.69 | 1.18 | 22.99 | 1.19 | |
| Weekday risetime (RT), clock h | 6.00 | 7.00 | 6.10 | 7.80 | 6.70 | 7.20 | 6.00 | 8.20 | |
| Weekend risetime (RT), clock h | 8.74 | 8.85 | 8.84 | 9.65 | 8.55 | 9.94 | 7.85 | 10.05 | |
| Weekday time in bed (TIB), h | 7.31 | 7.74 | 7.31 | 7.74 | 7.74 | 7.31 | 7.74 | 7.74 | |
| Weekend time in bed (TIB), h | 8.76 | 8.86 | 8.76 | 8.86 | 8.86 | 8.76 | 8.86 | 8.86 | |
| Initial times for buildup (1) and decay phases (2) of S(t): | |||||||||
| t2 (bedtime on free day), clock h | 23.00 | 24.00 | 24.00 | 24.10 | 24.80 | 23.70 | 1.20 | 23.00 | 1.20 |
| t1 (risetime on free day), clock h | 7.00 | 9.00 | 9.00 | 9.10 | 9.80 | 8.70 | 10.20 | 8.00 | 10.20 |
| Sine wave-form circadian modulation (3) of S(t): | |||||||||
| φmax (circadian peak), clock h | 15.00 | 16.00 | 16.00 | 16.10 | 16.80 | 15.70 | 17.20 | 15.00 | 17.20 |
| A (circadian amplitude), rSWA | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| τ (entrained circadian period), h | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 | 24.00 |
| k (twofold impact for this term) | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Inverse exponential buildup (1) and exponential decay phases (2) of S(t): | |||||||||
| Sl (lower asymptote), rSWA | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 |
| Sb (lowest decay), rSWA | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Sd (highest buildup), rSWA | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| Su (upper asymptote), rSWA | 4.50 | 4.51 | 4.51 | 4.51 | 4.51 | 4.51 | 4.51 | 4.51 | 4.51 |
| Td (decay phase constant), h | 1.95 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 | 2.30 |
| Tb (buildup phase constant), h | 27.04 | 24.75 | 24.75 | 24.75 | 24.75 | 24.75 | 24.75 | 24.75 | 24.75 |
Notes. Parameters of the model of the sleep regulating process, S(t), applied for the simulation of sleep times. Initial: Parameters of the model derived in the original publication [11] from data on sleep duration after extended wakefulness and relative Slow-Wave Activity (rSWA) in naps and extended sleep episodes (mean SWA = 1 in a baseline night episode). Sleep–wake regulating mechanisms were proposed to be identical in all simulations of lecturers’ data. Preliminary: Sleep times for free days (t2 and t1) were suggested to be identical, with the only difference between these two simulations in the amount of advance in risetime (RT) on weekdays relative to free day RT (3.00 h and 2.00 h). In the next 6 (final) simulations, the difference in shift in φmax, t2 and t1 was additionally proposed to account for the differences between two chronotypes (evening and morning types, MT and ET, respectively), between two weekday RTs (<7 a.m. and ≥7 a.m.), and between both chronotypes and RTs (MT with RT < 7 and ET with RT ≥ 7, <7 MT and ≥7 ET, respectively). See also illustrations of these 6 simulations of sleep times of lecturers in Figure 1, Figure 2 and Figure 3, their fit to data in Figure 4, and more details on these simulated sleep times in Table 3 (upper part).
In the initial simulations, S(t) was represented by the time course of Slow-Wave Activity (SWA), in relative SWA (SWA = 1 in the baseline conditions of experiments published by Dijk et al. [20,21,22]). All parameters of this model are explained in Table 2. Initial parameters were derived from data on the durations of recovery sleep after 6 gradually increasing intervals of extended wakefulness [23], and on the levels of SWA obtained during 10 naps [20] and two recovery sleep episodes [21,22]. The model was validated by comparing a model prediction [16] with data from the two latest “natural experiments” [14,15].
In order to account for a larger sleep duration reported by the participants of the present study (Table 1 and Table S1) compared to that of the participants of the study of Åkerstedt and Gillberg [23], the parameters of the model were slightly modified (Table 2 and Table 3). The sleep times for free days (t1 and t2) in the present simulations resemble the estimates of rise- and bedtimes on weekends reported by lecturers (Table 2, middle part, and Figure 4). For the sake of simplicity and clarity, these sleep times in simulations were rounded to yield the free day sleep duration of 9.0 h (Table 2 and Figure 1, Figure 2, Figure 3 and Figure 4). The simulations (Table 2 and Table 3, upper part) were performed to account for the differences in: (1) an advance in weekday risetime (RT) relative to free day RT, of either 2.0 or 3.0 h (Figure 1 and Figure 4, left graphs); (2) an advance in both weekday RT and chronotype (CT), either 2.0 h advance for M-type or 3.0 h advance for E-type (Figure 2 and Figure 4, middle graphs); and (3) either M- or E-type with the same (2.0 h) advance (Figure 3 and Figure 4, right graphs).
3. Results
Table 2 shows the parameters of preliminary simulations based on only one difference between the two simulations, in the advance shift in weekday RT relative to free day RT, of either 2.0 or 3.0 h. These simulations are called “preliminary” because they did not account for the phase-delaying effect of a smaller (2.0 h) advance shift. Such a smaller shift leads to a later phase of light exposure of the circadian clocks on weekdays that, in turn, leads to a delayed shift in the phase of these clocks, that, in turn, leads to a shift in weekend sleep timing at a later hour. Such a shift in weekend sleep timing is a cause of a phase difference between earlier and later weekday risers (i.e., in the present study we compared the subdivisions in accord with weekday RT, either earlier than 7:00 or later (i.e., either RT < 7 or RT ≥ 7, respectively). Therefore, such real-world data on the sleep timing of lecturers having early and late weekday RTs (Table 3, left columns, and Figure 4, left) were used to correct sleep timing of the preliminary simulations (Table 2). This correction yielded a 0.7 h difference in sleep timing on free days between lecturers with RT < 7 and those with RT ≥ 7 (Figure 1).
The difference between M- and E-types in the position of their circadian phases (>2.0 h), as mentioned in the Introduction, translates into the difference between their sleep timing on weekends. However, the difference in weekend sleep timing appears to be smaller than 2.0 h [12,13] (i.e., on average, a 1.8 h difference was obtained in the analysis of data on sleep times of as many as 50 pairs of samples of M- and E-types reported in the literature [12]). It seems that the major cause of such a reduction in the phase difference between chronotypes in weekend sleep timing is their difference in the advance in weekday waking times relative to weekend waking times. The circadian clocks of E-types are delayed by ≥2.0 h relative to the clocks of M-types, and, in turn, these types have later weekend waking times, forcing them to advance their weekday waking times by more. Such a larger advance in weekday waking times is expected to lead to an earlier phase of light exposure of their circadian clocks on weekdays that, in turn, can lead to an advancing shift in the phase of these clocks that, in turn, can lead to a shift in their weekend sleep timing at an earlier hour.
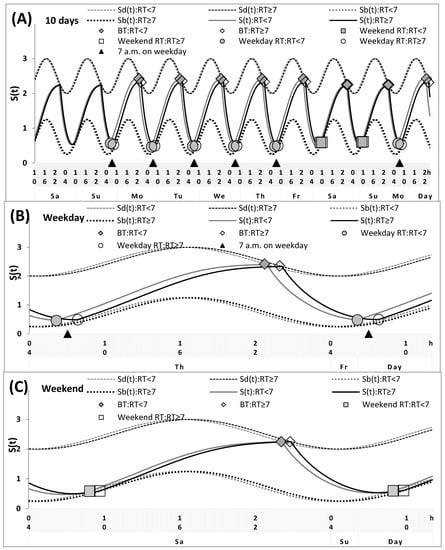
Figure 1.
Simulations of the sleep–wake cycles of early and late risers. Simulations of the whole sequence of 10 sleep–wake cycles (A), and two subintervals for a weekday and a weekend, (B,C), respectively, for the participants with different weekday risetime (RT), either <7 or ≥7 a.m. See Table 3 and Figure 4 (left graphs) for the comparison with sleep times self-reported by lecturers. The sequence of 10 sleep–wake cycles includes two last free days (e.g., at the end of a hypothetical vacation), the following week consisting of five weekdays and two weekends, and the first weekday of the next week (i.e., Sa-Su, Mo-Fr, Sa-Su, and Mo are the days of the week, namely, Saturday, Sunday, Monday, Tuesday, Wednesday, Thursday, Friday). S(t): Alternations between buildup and decay phases of the process of sleep–wake regulation. The parameters of these buildups and decays are modulated by a sine-form function with a 24 h period (i.e., this function represents the circadian pacemaker). Sd(t) and Sb(t): The time course of predicted highest and lowest buildups and decays of S(t), respectively. The simulations suggested that, in general, the parameters of the sleep–wake regulatory processes may be practically identical in two subdivisions with RT either <7 a.m. or ≥7 a.m. The only postulated differences were in sleep times on free days and in an advance in weekday waking times relative to free day waking times. The latter difference led to the difference in the timing of light exposure on weekdays and, as a consequence, to the difference in the circadian phase (0.7 h).
Since such an advance in weekend sleep timing led to a delay of less than 2.0 h in the sleep timing of E-types relative to the timing of M-types, the real-world data on sleep timing of lecturers of M- and E-types (Table 3, middle columns, and Figure 4, middle graphs) were used to correct sleep times of the preliminary simulations that differed only in the advance in weekday RT, i.e., either a 2.0 or 3.0 h advance in weekday RT relative to free day RT (Table 2 and Table 3, upper part). This correction yielded the 1.5 h difference in sleep timing on free days between the subdivisions into M- and E-types (Table 2 and Figure 2).
These two corrections of preliminary simulations enabled the estimation of the differences in sleep timing caused by the phase shifts of the circadian phase in two opposite directions. One of these differences is the difference in an advance in weekday RT relative to free day RT, of either 2.0 or 3.0 h (Figure 1), and the other difference is the difference caused by the combination of this effect (i.e., the difference in the advance shift) with the effect of the difference between two chronotypes, i.e., either M-types with a smaller advance shift (2.0 h) or E-types with a larger advance shift (3.0 h) (Figure 2). The estimates of the differences caused by these bidirectional shifts enabled determination of the difference in the sleep timing of M- and E-types with the same (2.0 h) phase advance in weekday RT relative to free day RT. As shown in Table 3 (middle columns), this difference between chronotypes with a 2.0 h advance is expected to be equal to the sum of the difference caused by a larger weekday advance and the difference caused by the combination of this larger advance with the effect of chronotype on sleep timing. That is, such summation allows the subtraction of the effect of a larger advance in weekday RT in E-types for the estimation of a pure effect of the difference between M– and E-types in the internal circadian phases of their sleep–wake cycles. The summation of bidirectional differences yielded a difference of 1.5 + 0.7 = 2.2 h between MT with RT <7 and ET with RT ≥7 in sleep timing on free days (Table 2 and Figure 3). The sums obtained for other sleep times were the same: 1.27 + 0.93 = 2.20 h and 0.71 + 1.49 = 2.20 h for weekday and weekend bedtimes, and 1.70 + 0.50 = 2.20 h and 0.81 + 1.39 = 2.20 for weekday and weekend RTs (Table 2, upper part). These sums (Table 2, upper part) were found to be rather close to the sums obtained for the simulated lecturers’ self-reports, of 2.20, 2.22, 2.16 and 2.16 h, respectively (Table 3, middle part). They were also close to the sums obtained for students’ self-reports, of 1.67, 2.03, 2.04, and 2.25 h, respectively (Table 2, lower part).
The same estimates could be obtained by applying another approach for the calculation of the differences in internal sleep timing of M- and E-types with the identical (2.0 h) phase advance in weekday RT relative to free day RT. These estimates (Table 3, right columns) were provided by the calculation of differences between two of four subdivisions of survey participants (i.e., in accord with two criteria, CT and weekday RT, the first division was into two CTs, M- and E-types, and the second division was into two weekday RTs, RT < 7 and RT ≥ 7). The differences were calculated between M-types with early weekday RT and E-types with late weekday RT (see the illustration of simulations of their sleep times in Figure 3). The last column of Table 3 confirms the expectation of the zero differences between the estimates of sleep times based on the two methodologies for calculation of the differences in internal sleep timing of M- and E-types with the identical (2.0 h) phase advance in weekday RT relative to free day RT (left and right parts of Table 3).
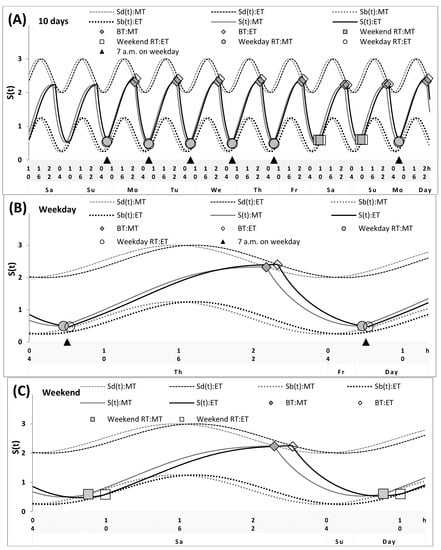
Figure 2.
Simulations of the sleep–wake cycles of morning and evening types. Simulations of the whole sequence of 10 sleep–wake cycles (A), and two subintervals for a weekday and a weekend, (B,C), respectively, for the morning- and evening-type participants (MT and ET, respectively). See Table 3 and Figure 4 (middle graphs) for the comparison with sleep times self-reported by lecturers. The simulations suggested that, in general, the parameters of the sleep–wake regulatory processes may be practically identical in MT and ET. The only two suggested differences were in sleep times on free days and in an advance in weekday waking times relative to weekend waking times. The latter difference led to the difference in the timing of light exposure and, as a consequence, to the difference between MT and ET in the weekend sleep phase (1.5 h). This difference was, however, shorter than their difference in the endogenous circadian phase (>2.0 h) because a larger advance in weekday waking times in ET led to an earlier phase of light exposure of their circadian clocks on weekdays that, in turn, led to an advance in the phase of these clocks that, in turn, led to a shift in their weekend sleep timing at an earlier hour (see Figure 1).
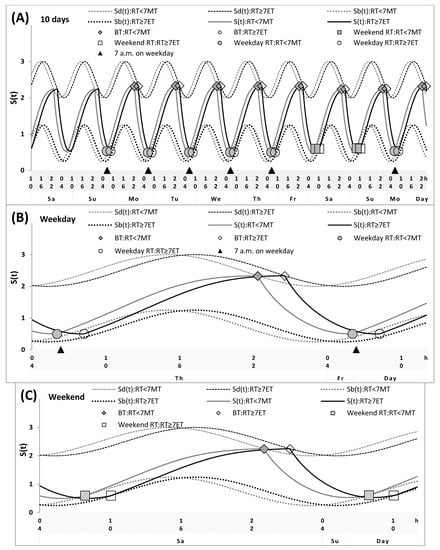
Figure 3.
Simulations of the sleep–wake cycles of early morning types and late evening types. Simulations of the whole sequence of 10 sleep–wake cycles (A), and two subintervals for a weekday and a weekend, (B,C), respectively, for the participants with different chronotypes and risetimes (RTs), MT with early RT and ET with late RT (RT <7 MT and RT ≥7 ET, respectively. See Table 3 and Figure 4 (right graphs) for the comparison of these simulations with sleep times self-reported by lecturers. The simulations suggested that, in general, the parameters of the sleep–wake regulatory processes may be practically identical in these two subdivisions. The only postulated difference was the difference in sleep times on free days, whereas the advances in weekday waking times were suggested to become equal in these MTs and ETs due to the early weekday RT of the former and the late weekday RT of the latter (2.0 h). Therefore, the difference in the circadian phase (2.2 h) was the only contributor to the difference between these subdivisions of MT and ET in sleep timing.

Table 3.
Differences in sleep times: early and late risers.
Table 3.
Differences in sleep times: early and late risers.
| Division | Two RT | Two CT | Differences | Their | RT < 7 | RT ≥ 7 | Difference | With | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sleep Time | <7 | ≥7 | MT | ET | ≥7-<7 | ET-MT | Sum | MT | ET | ≥7 ET-<7 MT | Sum |
| Simulation: | |||||||||||
| Weekday BT | 22.79 | 24.06 | 22.96 | 23.89 | 1.27 | 0.93 | 2.20 | 22.26 | 24.46 | 2.20 | 0.00 |
| Weekend BT | 24.08 | 24.79 | 23.69 | 1.18 | 0.71 | 1.49 | 2.20 | 22.99 | 1.19 | 2.20 | 0.00 |
| Difference BT | 1.29 | 0.73 | 0.73 | 1.29 | −0.56 | 0.56 | 0.00 | 0.73 | 0.73 | 0.00 | 0.00 |
| Weekday RT | 6.10 | 7.80 | 6.70 | 7.20 | 1.70 | 0.50 | 2.20 | 6.00 | 8.20 | 2.20 | 0.00 |
| Weekend RT | 8.84 | 9.65 | 8.55 | 9.94 | 0.81 | 1.39 | 2.20 | 7.85 | 10.05 | 2.20 | 0.00 |
| Difference RT | 2.74 | 1.85 | 1.85 | 2.74 | −0.89 | 0.89 | 0.00 | 1.85 | 1.85 | 0.00 | 0.00 |
| Weekday TIB | 7.31 | 7.74 | 7.74 | 7.31 | 0.43 | −0.43 | 0.00 | 7.74 | 7.74 | 0.00 | 0.00 |
| Weekend TIB | 8.76 | 8.86 | 8.86 | 8.76 | 0.10 | −0.10 | 0.00 | 8.86 | 8.86 | 0.00 | 0.00 |
| Difference TIB | 1.45 | 1.12 | 1.12 | 1.45 | −0.32 | 0.32 | 0.00 | 1.12 | 1.12 | 0.00 | 0.00 |
| Lecturers (n): | (138) | (107) | (138) | (107) | (98) | (67) | |||||
| Weekday BT | 23.16 | 24.17 | 23.07 | 24.26 | 1.01 | 1.20 | 2.20 | 22.65 | 24.85 | 2.20 | 0.00 |
| Weekend BT | 23.97 | 24.73 | 23.62 | 1.08 | 0.77 | 1.45 | 2.22 | 23.13 | 1.35 | 2.22 | 0.00 |
| Difference BT | 0.81 | 0.57 | 0.56 | 0.82 | −0.24 | 0.26 | 0.02 | 0.49 | 0.50 | 0.02 | 0.00 |
| Weekday RT | 6.17 | 7.84 | 6.76 | 7.25 | 1.67 | 0.49 | 2.16 | 6.01 | 8.17 | 2.16 | 0.00 |
| Weekend RT | 8.74 | 9.50 | 8.41 | 9.82 | 0.76 | 1.40 | 2.16 | 7.98 | 10.14 | 2.16 | 0.00 |
| Difference RT | 2.57 | 1.65 | 1.65 | 2.57 | −0.91 | 0.91 | 0.00 | 1.97 | 1.97 | 0.00 | 0.00 |
| Weekday TIB | 7.01 | 7.68 | 7.70 | 6.99 | 0.67 | −0.71 | −0.04 | 7.36 | 7.32 | −0.04 | 0.00 |
| Weekend TIB | 8.77 | 8.76 | 8.79 | 8.74 | −0.01 | −0.05 | −0.06 | 8.85 | 8.79 | −0.06 | 0.00 |
| Difference TIB | 1.76 | 1.09 | 1.10 | 1.75 | −0.67 | 0.66 | −0.02 | 1.48 | 1.47 | −0.02 | 0.00 |
| Students (n): | (773) | (747) | (521) | (999) | (296) | (522) | |||||
| Weekday BT | 23.83 | 24.22 | 23.39 | 24.66 | 0.40 | 1.28 | 1.67 | 23.24 | 24.91 | 1.67 | 0.00 |
| Weekend BT | 24.50 | 1.04 | 24.02 | 1.51 | 0.54 | 1.49 | 2.03 | 23.87 | 1.91 | 2.03 | 0.00 |
| Difference BT | 0.67 | 0.82 | 0.64 | 0.85 | 0.15 | 0.22 | 0.36 | 0.63 | 1.00 | 0.36 | 0.00 |
| Weekday RT | 6.09 | 7.93 | 6.90 | 7.11 | 1.84 | 0.21 | 2.04 | 6.02 | 8.06 | 2.04 | 0.00 |
| Weekend RT | 9.49 | 10.28 | 9.16 | 10.62 | 0.79 | 1.46 | 2.25 | 8.80 | 11.05 | 2.25 | 0.00 |
| Difference RT | 3.41 | 2.36 | 2.25 | 3.51 | −1.05 | 1.26 | 0.21 | 2.78 | 2.99 | 0.21 | 0.00 |
| Weekday TIB | 6.26 | 7.67 | 7.52 | 6.41 | 1.40 | −1.11 | 0.30 | 6.78 | 7.08 | 0.30 | 0.00 |
| Weekend TIB | 9.00 | 9.21 | 9.14 | 9.07 | 0.21 | −0.07 | 0.14 | 8.93 | 9.07 | 0.15 | 0.00 |
| Difference TIB | 2.74 | 1.55 | 1.62 | 2.66 | −1.19 | 1.04 | −0.15 | 2.15 | 2.00 | −0.15 | 0.00 |
Notes. RT: risetime; BT: bedtime; TIB: time in bed. Difference BT (or RT or TIB): Difference between weekend and weekday in BT (or in RT or in TIB). Sleep times from four-way ANOVAs for subdivisions into two weekday RTs (<7 and ≥7 a.m.), two chronotypes (CTs: morning type and evening type, MT and ET, respectively), and into two CT × RT (MT with RT <7 and ET with RT ≥7); Differences (≥7-<7, ET-MT, and <7 ET-≥7 MT): difference between these subdivisions in sleep time; Their sum: sum of two differences for subdivisions into two RTs and into two CTs; (n): number of participants. The independent factors: “RT” (either <7 or ≥7 a.m.), “CT” (either MT or ET), “Age” (either lecturers or university students), and “Survey” (see online surveys 1–4, in Table 1 and Table S1). See also illustrations of the paired simulations in Figure 1 and their fit with data in Figure 4.
Note that, as we expected (see Introduction), only one of the sleep times, i.e., the time in bed on weekends, did not show any tendency to be significantly different in M- and E-types or in early and late risers (RT < 7 and RT ≥ 7), indicating that the negative effects on E-types/early risers of less sleep during the week cannot be reversed by the extension of their sleep on weekends (Table 4).

Table 4.
Statistical differences in sleep times: early morning types and late evening types.
Table 4.
Statistical differences in sleep times: early morning types and late evening types.
| Division: CT | Two MT | Two ET | Two-way | Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Division: RT | <7 | ≥7 | <7 | ≥7 | ANOVA | ≥7 ET-<7 MT | |||||
| Sleep Time | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | F | Mean | SEM |
| Lecturers (n): | (98) | (40) | (40) | (67) | |||||||
| Weekday BT | 22.65 | 0.13 | 23.49 | 0.22 | 23.67 | 0.19 | 24.85 | 0.19 | 31.95 *** | 2.20 *** | 0.18 |
| Weekend BT | 23.13 | 0.14 | 24.12 | 0.25 | 24.80 | 0.22 | 1.35 | 0.22 | 29.83 *** | 2.22 *** | 0.20 |
| Difference BT | 0.49 | 0.13 | 0.63 | 0.22 | 1.13 | 0.19 | 0.50 | 0.19 | 2.80 * | 0.02 | 0.18 |
| Averaged BT | 22.78 | 0.12 | 23.67 | 0.21 | 24.00 | 0.18 | 24.99 | 0.18 | 37.91 *** | 2.21 *** | 0.17 |
| Weekday RT | 6.01 | 0.08 | 7.51 | 0.15 | 6.33 | 0.13 | 8.17 | 0.13 | 80.22 *** | 2.16 *** | 0.12 |
| Weekend RT | 7.98 | 0.18 | 8.85 | 0.31 | 9.49 | 0.27 | 10.14 | 0.27 | 17.66 *** | 2.16 *** | 0.25 |
| Difference RT | 1.97 | 0.18 | 1.34 | 0.31 | 3.17 | 0.27 | 1.97 | 0.27 | 7.40 *** | 0.00 | 0.25 |
| Averaged RT | 6.57 | 0.09 | 7.90 | 0.15 | 7.23 | 0.13 | 8.73 | 0.13 | 67.86 *** | 2.16 *** | 0.12 |
| Weekday TIB | 7.36 | 0.12 | 8.03 | 0.20 | 6.66 | 0.18 | 7.32 | 0.18 | 8.77 *** | −0.04 | 0.16 |
| Weekend TIB | 8.85 | 0.18 | 8.73 | 0.32 | 8.69 | 0.28 | 8.79 | 0.28 | 0.08 | −0.06 | 0.26 |
| Difference TIB | 1.48 | 0.21 | 0.71 | 0.36 | 2.04 | 0.32 | 1.47 | 0.31 | 2.54 | −0.02 | 0.29 |
| Averaged TIB | 7.79 | 0.10 | 8.23 | 0.18 | 7.24 | 0.16 | 7.74 | 0.16 | 5.97 ** | −0.05 | 0.14 |
| Students (n): | (296) | (225) | (477) | (552) | |||||||
| Weekday BT | 23.24 | 0.13 | 23.53 | 0.13 | 24.41 | 0.09 | 24.91 | 0.07 | 60.94 *** | 1.67 *** | 0.14 |
| Weekend BT | 23.87 | 0.13 | 24.17 | 0.13 | 1.12 | 0.10 | 1.91 | 0.08 | 82.19 *** | 2.03 *** | 0.15 |
| Difference BT | 0.63 | 0.12 | 0.64 | 0.12 | 0.71 | 0.09 | 1.00 | 0.07 | 4.03 ** | 0.36 | 0.14 |
| Averaged BT | 23.42 | 0.11 | 23.72 | 0.11 | 24.61 | 0.09 | 1.20 | 0.07 | 82.78 *** | 1.78 *** | 0.13 |
| Weekday RT | 6.02 | 0.08 | 7.79 | 0.07 | 6.16 | 0.06 | 8.06 | 0.04 | 356.57 *** | 2.04 *** | 0.09 |
| Weekend RT | 8.80 | 0.13 | 9.51 | 0.13 | 10.18 | 0.10 | 11.05 | 0.08 | 86.40 *** | 2.25 *** | 0.15 |
| Difference RT | 2.78 | 0.14 | 1.72 | 0.14 | 4.03 | 0.10 | 2.99 | 0.08 | 65.19 *** | 0.21 | 0.16 |
| Averaged RT | 6.81 | 0.07 | 8.28 | 0.07 | 7.31 | 0.05 | 8.92 | 0.04 | 302.29 *** | 2.10 *** | 0.08 |
| Weekday TIB | 6.78 | 0.13 | 8.26 | 0.13 | 5.75 | 0.10 | 7.08 | 0.08 | 84.81 *** | 0.30 | 0.15 |
| Weekend TIB | 8.93 | 0.14 | 9.35 | 0.14 | 9.07 | 0.10 | 9.07 | 0.08 | 1.68 | 0.15 | 0.16 |
| Difference TIB | 2.15 | 0.16 | 1.09 | 0.16 | 3.32 | 0.12 | 2.00 | 0.09 | 48.51 *** | −0.15 | 0.18 |
| Averaged TIB | 7.39 | 0.11 | 8.57 | 0.11 | 6.70 | 0.08 | 7.65 | 0.06 | 63.69 *** | 0.25 | 0.13 |
Notes. Results of two-way ANOVAs of data on either lecturers or students with independent factors “RT&CT” (self-reported weekday risetime, either <7 or ≥7 a.m., and self-chosen chronotype, CT, either morning or evening (MT or ET, respectively) and “Survey” (four online surveys, 1–4, see Table 1 and Table S1); Sleep times from ANOVAs for subdivisions: MT and ET with weekday RT < 7 and ≥ 7 a.m.; Difference <7 ET-≥7 MT: Difference between two of four such subdivisions; BT: bedtime; TIB: time in bed; Averaged: weekly averaged sleep time. Difference BT (or RT or TIB): Difference between weekend and weekday in BT (or in RT or in TIB); * p < 0.05, ** p < 0.01, *** p < 0.001 for F-ratio of independent factor “RT&CT” (F3/229 and F3/1504 for lecturers and students, respectively) and for t from post hoc pairwise Bonferroni comparisons of two of four subdivisions. See also self-reported sleep times in Figure 4 and comparisons of ages for these times in Table 5.
The right column of Table 4 shows the results of a statistical comparison of sleep times in two subdivisions with an identical advance in weekday RT relative to free day RT (e.g., 2.0 h in the simulations). These results indicated that, when the weekday sleep loss of M- and E-types was similar, none of their times in bed (on weekdays, on weekends, and averaged over 7 days) statistically differed between them (Table 4, last columns of upper part). This implies that the equalization of the weekday sleep loss of the two distinct chronotypes can be achieved by setting a >2 h difference between the chronotypes’ weekday waking times (Table 2, Table 3 and Table 4 and Figure 3 and Figure 4).

Table 5.
Differences between lecturers and students in some of the subdivisions.
Table 5.
Differences between lecturers and students in some of the subdivisions.
| CT&RT Division | The Same CT and RT | The Opposite CT and RT | ||||||
|---|---|---|---|---|---|---|---|---|
| Lecturers-Students | <7 MT-<7 MT | ≥7 ET-≥7 ET | <7 MT-≥7 ET | ≥7 ET-<7 MT | ||||
| Sleep Time | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Weekday BT | −0.59 | 0.21 | −0.06 | 0.27 | −2.27 *** | 0.18 | 1.61 *** | 0.29 |
| Weekend BT | −0.74 * | 0.22 | −0.56 | 0.28 | −2.78 *** | 0.20 | 1.48 *** | 0.30 |
| Difference BT | −0.15 | 0.21 | −0.49 | 0.26 | −0.51 | 0.18 | −0.13 | 0.28 |
| Averaged BT | −0.64 * | 0.19 | −0.21 | 0.24 | −2.41 *** | 0.17 | 1.57 *** | 0.26 |
| Weekday RT | −0.01 | 0.13 | 0.11 | 0.16 | −2.05 *** | 0.11 | 2.15 *** | 0.17 |
| Weekend RT | −0.83 ** | 0.23 | −0.91 * | 0.29 | −3.08 *** | 0.20 | 1.34 ** | 0.31 |
| Difference RT | −0.81 * | 0.23 | −1.02 * | 0.30 | −1.02 *** | 0.20 | −0.82 | 0.31 |
| Averaged RT | −0.24 | 0.12 | −0.18 | 0.16 | −2.35 *** | 0.11 | 1.92 *** | 0.17 |
| Weekday TIB | 0.58 | 0.22 | 0.25 | 0.28 | 0.29 | 0.19 | 0.54 | 0.29 |
| Weekend TIB | −0.08 | 0.24 | −0.29 | 0.30 | −0.23 | 0.21 | −0.14 | 0.32 |
| Difference TIB | −0.67 | 0.27 | −0.53 | 0.34 | −0.51 | 0.24 | −0.68 | 0.37 |
| Averaged TIB | 0.39 | 0.19 | 0.09 | 0.24 | 0.14 | 0.16 | 0.35 | 0.25 |
Notes. Some of the results of post hoc pairwise Bonferroni comparisons of sleep times in lecturers and students from two-way ANOVAs with independent factors “Survey” (four online surveys, 1–4, see Table 1 and Table S1) and “RT&CT&Age” (self-reported weekday risetime, either <7 or ≥7 a.m., self-chosen chronotype, either MT or ET, and Age, either lecturers or students); Sleep times from ANOVAs for four pairwise comparisons of lecturers with students: the same two subdivisions (<7 MT-<7 MT, ≥7 ET-≥7 ET): MT with RT < 7 (n = 98 and 296 for lecturers and students, respectively), ET with RT ≥7 (n = 67 and 522 for lecturers and students, respectively), and the two opposite subdivisions (<7 MT-≥7 ET and ≥7 ET-<7 MT): MT with RT <7 for lecturers and ET with RT ≥ 7 for students, ET with RT ≥ 7 for lecturers and MT with RT <7 for students; * p < 0.05, ** p < 0.01, *** p < 0.001 for t from four pairwise comparisons. In order to calculate the differences shown in this table, sleep times of students (Table 4, lower part) were subtracted from sleep times of lecturers (Table 4, upper part).
As shown in Table 5, only non-significant differences were found between times in bed of young and older adults (university students and lecturers, respectively) from the same two subdivisions characterized by similar advances in weekday RT. This implies a possibility to use the estimates obtained either in the simulations (Table 3, upper part) or in the statistical analysis of the simulated lecturers’ self-reports (Table 3, middle part) for equalizing the weekday sleep loss of university students of M- and E-types (Table 3, lower part).
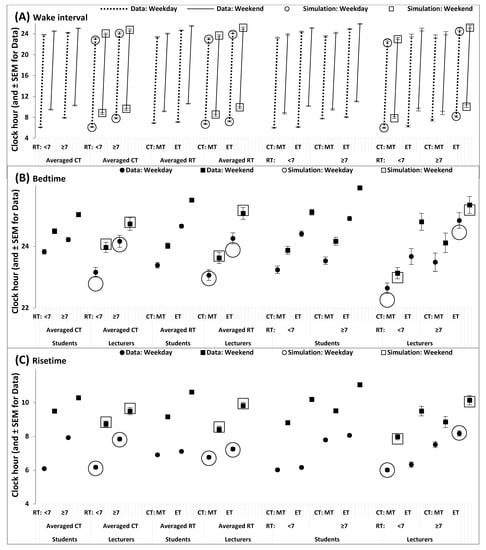
Figure 4.
Simulated and self-reported wake intervals and bed- and risetimes. (A). Wake interval, (B) bedtime, and (C) risetime self-reported by survey participants (Table 3, middle and lower parts) in comparison with those obtained as output of simulations of the sequence of 10 sleep–wake cycles for two intervals, the interval of 5 weekdays, and the interval of two following weekends (Figure 1, Figure 2 and Figure 3 and Table 2 and Table 3, upper part).
4. Discussion
When people wake up earlier on weekdays, they miss out on the last portion of their sleep, but often believe they can make up for it during the following weekend. However, there is no way of minimizing the negative effects of insufficient weekday sleep by relying on a weekend “catch up”. The simulations of weekday and weekend sleep timing suggested that, if people lose sleep by waking earlier during the week, they cannot make up for it on the weekends [11,12,13,14,15,16]. An earlier waking time on weekdays leads to an earlier start and termination of light exposure of the internal circadian clock. This earlier exposure, in turn, leads to an advance in sleep timing and a larger loss in sleep on weekdays. This loss cannot be compensated for by the extension of weekend sleep duration [15]. Given that our work/study culture is biased towards the circadian clocks of M-types, the tradition of setting working and school start-times too early forces E-types to sacrifice a larger amount of sleep on weekdays to arrive at their place of work/study at the M-type-oriented start-times. As a consequence, E-types cannot simply catch up on sleep during the weekends, and this might be the major reason for complaints about “the tyranny of the early risers/birds” [17,18]. Here, we applied a model of sleep–wake regulation to simulate sleep times of M- and E-types on weekdays and weekends to test the hypothesis that, since the literature indicates a >2 h difference between chronotypes in the positions of their endogenous circadian phases [5,6,7,8,9,10], permitting a >2 h difference in the weekday waking times between M- and E-types would be necessary to equalize their irrecoverable loss in weekday sleep. The support for this hypothesis was provided by the simulations of sleep times reported by lecturers, and by the statistical analyses of sleep times reported by either lecturers or university students. We found that, to overthrow “the tyranny of the early risers”, the >2 h difference between M- and E-types in waking times would ensure the equalization of their irrecoverable loss in sleep on weekdays. If, on weekdays, an “average” M-type university student or an “average” M-type lecturer wakes at 6 a.m., equality between the two distinct chronotypes in terms of weekday sleep losses would be achieved by enabling an “average” E-type university student or an “average” E-type lecturer to wake up no earlier than 8 a.m.
The estimates of the difference in weekday sleep losses between E- and M-types appear to be comparable with the estimates reported in the literature for people before and after retirement [24] and for people living in adjacent counties on either side of a time-zone border [25]. The latter study also evaluated the negative consequences for health resulting from an additional reduction in sleep after a larger advance in weekday waking times reported by people living on the late sunset side of the border. The health index dropped by 0.3 standard deviations when people lived on this side of the border compared to the index of people living on the early sunset side [25]. Moreover, other reports indicated that these people are at an increased risk of cancer [26,27].
The association of poor health with evening preference and late weekend sleep timing was previously noted [28,29]. However, little is known about the contribution of a larger weekday sleep insufficiency in E-types compared to M-types. The association of being an E-type with poorer health can be a consequence of this type’s tendency to develop unhealthy behavior [30,31,32,33]. By comparison, results recently reported by Maultsby and co-workers [34] suggested the roles of both scheduled day-sleep duration and chronotype in shaping health outcomes. Since we did not ask survey participants to report their health and sleep problems, further studies are required to examine whether weekday sleep insufficiency is an independent and important contributor to the association of E-type with poorer health, poorer night sleep quality, etc.
In the light of the reports on negative health outcomes of weekday sleep insufficiency and delayed weekend sleep timing, the present results may be recommended for calculation of the desired delay in weekday waking times of an individual having a certain delay in waking times on weekends and free days.
The limitations of the present study include the method of recruitment of the participants of the online surveys (see Supplementary Materials). This approach did not allow the examination of possible differences between non-responders and responders in their chronobiological and somnological characteristics. An example of such potential differences may be an increased proportion of people who have problems with their sleep, relative to those who do not. Although it is important to recognize this, our aim was not to report the prevalence of sleep problems and patterns. As mentioned in the Supplementary Materials, any potential difference between non-responders and responders does not seem to be critical for achieving the main purposes of the study, i.e., to collect self-reported sleep times from M- and E-types, and to simulate these times with a two-process model of sleep regulation for the development of a methodology for equalizing weekday sleep losses of different chronotypes (e.g., by calculation of the delay in the waking times of E-types relative to the waking times of M-types).
5. Conclusions
As indicated by our previous research, the negative effects of insufficient sleep during the week cannot be reversed by the attempt to extend weekend sleep. Given that our work/study culture is biased towards the circadian clocks of M-types, the irrecoverable loss in weekday sleep is larger in E- than in M-types. We hypothesized that it would be necessary to permit a >2 h difference between M- and E-types in weekday waking times to equalize this loss between the two types. This hypothesis was tested by simulating sleep times reported by 245 lecturers of M- and E-types using a model of sleep–wake regulation, and by the statistical analyses of sleep times of these lecturers and 1520 M- and E-type students. The hypothesis was supported by the results of the simulation and statistical analyses. As predicted, these results suggested the need for a >2 h difference between the waking times of M- and E-types to equalize their irrecoverable weekday sleep loss. Such results may be implemented in a methodology for reducing the inequality of the loss between chronotypes via the development of model-based recommendations for the correction of weekday waking times of E-types.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12168092/s1, Supplementary Materials contains more details on selection, exclusion criteria, and chronobiological and somnological characteristics of the participants from 8 samples briefly described in Table S1. Refs. [11,12,14,15,16,19,35,36,37,38,39,40,41] are cited in the Supplementary Materials.
Author Contributions
A.A.P.: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing—original draft, review and editing. D.S.S.: Investigation; Methodology; Software; Writing—review and editing. Z.V.B.: Data curation; Investigation; Writing—review and editing. E.B.Y.: Data curation; Investigation; Writing—review and editing. Y.P.S.: Data curation; Investigation; Writing—review and editing. V.I.T.: Data curation; Investigation; Writing—review and editing. E.A.T.: Data curation; Investigation; Writing—review and editing. M.M.L.: Data curation; Investigation; Writing—review and editing. Z.N.L.: Data curation; Investigation; Writing—review and editing. R.O.B.: Data curation; Investigation; Writing—review and editing. E.V.B.: Data curation; Investigation; Writing—review and editing. E.L.T.: Data curation; Investigation; Writing—review and editing. M.P.D.: Data curation; Investigation; Writing—review and editing. L.P.C.: Data curation; Investigation; Writing—review and editing. O.G.D.: Investigation; Project administration; Software; Writing—review and editing. A.N.P.: Methodology; Data curation; Writing—review and editing. V.B.D.: Funding acquisition; Data curation; Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The Russian Science Foundation provided financial support for VBD in the form of grant # 22-28-01769. Moreover, the research projects of ROB and EVB were supported by the North-Caucasus Federal University. The sponsors had no role in the design or conduct of this research.
Institutional Review Board Statement
All procedures performed in surveys of human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments and in accordance with the ethical standards of each of the participated universities and the institutional research committees (the Ethics Committees of the Institute of Higher Nervous Activity and Neurophysiology of the Russian Academy of Sciences, the approved protocols of the studies #2 from 3 June 2019 and #5 from 15 December 2019).
Informed Consent Statement
Informed consent was obtained from all individual participants of the study in the form of response “Yes” to the first question of a survey.
Data Availability Statement
The dataset is available on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Horne, J.A.; Östberg, O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Adan, A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction 1994, 89, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, L.; Adan, A.; di Milia, L.; Randler, C.; Natale, V. Measures of circadian preference in childhood and adolescence: A review. Eur. Psychiatry 2015, 30, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; di Milia, L.; Natale, V.; Randler, C. Circadian typology: A comprehensive review. Chronobiol. Int. 2012, 299, 1153–1175. [Google Scholar] [CrossRef]
- Baehr, E.K.; Revelle, W.; Eastman, C.I. Individual differences in the phase and amplitude of the human circadian temperature rhythm: With an emphasis on morningness-eveningness. J. Sleep Res. 2000, 9, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Duffy, J.F.; Dijk, D.J.; Hall, E.F.; Czeisler, C.A. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J. Investig. Med. 1999, 47, 141–150. [Google Scholar]
- Duffy, J.F.; Rimmer, D.W.; Czeisler, C.A. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci. 2001, 115, 895–899. [Google Scholar] [CrossRef]
- Kerkhof, G.A.; van Dongen, H.P.A. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci. Lett. 1996, 218, 153–156. [Google Scholar] [CrossRef]
- Lack, L.; Bailey, M.; Lovato, N.; Wright, H. Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat. Sci. Sleep 2009, 1, 1–8. [Google Scholar] [CrossRef]
- Paine, S.J.; Gander, P.H. Differences in circadian phase and weekday/weekend sleep patterns in a sample of middle-aged morning types and evening types. Chronobiol. Int. 2016, 33, 1009–1017. [Google Scholar] [CrossRef]
- Putilov, A.A. The timing of sleep modelling: Circadian modulation of the homeostatic process. Biol. Rhythm Res. 1995, 26, 1–19. [Google Scholar] [CrossRef]
- Putilov, A.A.; Donskaya, O.G. What can make the difference between chronotypes in sleep duration? Testing similarity of their homeostatic processes. Front. Neurosci. 2022, 16, 832807. [Google Scholar] [CrossRef] [PubMed]
- Putilov, A.A.; Verevkin, E.G.; Donskaya, O.G.; Tkachenko, O.N.; Dorokhov, V.B. Model-based simulations of weekday and weekend sleep times self-reported by larks and owls. Biol. Rhythm Res. 2020, 51, 709–726. [Google Scholar] [CrossRef]
- Putilov, A.A. Sleep during “lockdown” highlighted the need to rethink the concept of weekend catch-up sleep. Sleep Breath. 2022, 1–7. Available online: https://link.springer.com/article/10.1007%2Fs11325-021-02492-z#Sec6 (accessed on 28 June 2022). [CrossRef] [PubMed]
- Putilov, A.A. Weekend sleep after early and later school start times confirmed a model-predicted failure to catch up sleep missed on weekdays. Sleep Breath 2022, 1–11. Available online: https://link.springer.com/article/10.1007/s11325-022-02648-5 (accessed on 28 June 2022). [CrossRef] [PubMed]
- Putilov, A.A.; Verevkin, E.G. Simulation of the ontogeny of social jet lag: A shift in just one of the parameters of a model of sleep-wake regulating process accounts for the delay of sleep phase across adolescence. Front. Physiol. 2018, 9, 1529. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R. Late and great. The Guardian, 21 May 2007. Available online: https://www.theguardian.com/money/2007/may/21/careers.theguardian1 (accessed on 28 June 2022).
- Kuper, S. Overthrowing the tyranny of the early birds with new sleep rules that give workers more shuteye. Financial Post, 2 November 2021. Available online: https://financialpost.com/fp-work/overthrowing-the-tyranny-of-the-early-birds-with-new-sleep-rules-that-give-workers-more-shuteye (accessed on 28 June 2022).
- Putilov, A.A.; Sveshnikov, D.S.; Puchkova, A.N.; Dorokhov, V.B.; Bakaeva, Z.B.; Yakunina, E.B.; Starshinov, Y.P.; Torshin, V.I.; Alipov, N.N.; Sergeeva, O.V.; et al. Single-Item Chronotyping (SIC), a method to self-assess diurnal types by using 6 simple charts. Pers. Ind. Differ. 2021, 168, 110353. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Beersma, D.G.M.; Daan, S. EEG power density during nap sleep: Reflection of an hourglass measuring the duration of prior wakefulness. J. Biol. Rhythm. 1987, 2, 207–220. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Brunner, D.P.; Borbély, A.A. Time course of EEG power density during long sleep in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1990, 258, R650–R661. [Google Scholar] [CrossRef]
- Dijk, D.-J.; Brunner, D.P.; Borbély, A.A. EEG power density during recovery sleep in the morning. Electroencephalogr. Clin. Neurophysiol. 1991, 78, 203–214. [Google Scholar] [CrossRef]
- Åkerstedt, T.; Gillberg, M. The circadian variation of experimentally displaced sleep. Sleep 1981, 4, 159–169. [Google Scholar] [CrossRef]
- Garefelt, J.; Gershagen, S.; Kecklund, G.; Westerlund, H.; Platts, L.G. How does cessation of work affect sleep? Prospective analyses of sleep duration, timing and efficiency from the Swedish Retirement Study. J. Sleep Res. 2021, 30, e13157. [Google Scholar] [CrossRef] [PubMed]
- Giuntella, O.; Mazzonna, F. Sunset time and the economic effects of social jetlag evidence from US time zone borders. J. Health Econ. 2019, 65, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Xu, S.; Devesa, S.S.; Zhang, F.; Klerman, E.B.; Graubard, B.I.; Caporaso, N.E. Longitude position in a time zone and cancer risk in the United States. Cancer Epidemiol. Prev. Biomark. 2017, 26, 1306–1311. [Google Scholar] [CrossRef]
- VoPham, T.; Weaver, M.D.; Vetter, C.; Hart, J.E.; Tamimi, R.M.; Laden, F.; Bertrand, K.A. Circadian misalignment and hepatocellular carcinoma incidence in the United States. Cancer Epidemiol. Prev. Biomark. 2018, 27, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Mito, N.; Fujimoto, E.; Sasaki, S. Three-generation Study of Women on Diets and Health Study Group. Association of chronotype as assessed by the midpoint of sleep with the dietary intake and health-related quality of life for elderly Japanese women. J. Nutr. Sci. 2021, 10, e25. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.C.; Tan, J.; Lai, C.; Lim, S.; Chandramoghan, Y.; Gooley, J. University-wide chronotyping shows late-type students have lower grades, shorter sleep, and more absenteeism. Sleep 2022, 45, A3–A4. [Google Scholar] [CrossRef]
- Fernando, J.; Stochl, J.; Ersche, K.D. Drug use in night owls may increase the risk for mental health problems. Front. Neurosci. 2022, 15, 819566. [Google Scholar] [CrossRef]
- Makarem, N.; Paul, J.; Giardina, E.V.; Liao, M.; Aggarwal, B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol. Int. 2020, 37, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Kronholm, E.; Peltonen, M.; Laatikainen, T.; Lahti, T.; Partonen, T. Relation of chronotype to sleep complaints in the general Finnish population. Chronobiol. Int. 2012, 29, 311–317. [Google Scholar] [CrossRef]
- Patterson, F.; Malone, S.K.; Lozano, A.; Grandner, M.A.; Hanlon, A.L. Smoking, screen-based sedentary behavior, and diet associated with habitual sleep duration and chronotype: Data from the UK Biobank. Ann. Behav. Med. 2016, 50, 715–726. [Google Scholar] [CrossRef]
- Maultsby, K.D.; Temmen, C.D.; Lewin, D.; Sita, K.R.; Luk, J.W.; Simons-Morton, B.G.; Haynie, D.L. Longitudinal associations between high school sleep characteristics and young adult health outcomes. J. Clin. Sleep Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [PubMed]
- Daan, S.; Beersma, D.G.M.; Borbély, A.A. Timing of human sleep: Recovery process gated by a circadian pacemaker. Am. J. Physiol. Regul. Integr. Comp. Physiol 1984, 246, R161–R178. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Putilov, A.A.; Budkevich, E.V.; Tinkova, E.L.; Dyakovich, M.P.; Sveshnikov, D.S.; Donskaya, O.G.; Budkevich, R.O. A six-factor structure of individual variation in the tendencies to become sleepy and to sleep at different times of the day. Acta Psychol. 2021, 217, 103327. [Google Scholar] [CrossRef]
- Putilov, A.A.; Sveshnikov, D.S.; Bakaeva, Z.B.; Yakunina, E.B.; Starshinov, Y.P.; Torshin, V.I.; Alipov, N.N.; Sergeeva, O.V.; Trutneva, E.A.; Lapkin, M.M.; et al. Differences between male and female university students in sleepiness, weekday sleep loss, and weekend sleep duration. J. Adolesc. 2021, 88, 84–96. [Google Scholar] [CrossRef]
- Putilov, A.A.; Nechunaev, V.V.; Budkevich, R.O.; Budkevich, E.V.; Kolomeichuk, S.N.; Morozov, A.V.; Plusnin, J.M.; Donskaya, O.G.; Verevkin, E.G.; Puchkova, A.N.; et al. Overlap between individual variation in personality traits and sleep-wake behavior. Curr. Psychol. 2021. [Google Scholar] [CrossRef]
- Putilov, A.A. Three-dimensional structural representation of the sleep-wake adaptability. Chronobiol. Int. 2016, 33, 169–180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).