Titanium Surface Analysis after Instrumentation with Different Burs Simulating the Implantoplasty Technique: A Pilot In Vitro Experimental Study
Abstract
1. Introduction
2. Materials and Methods
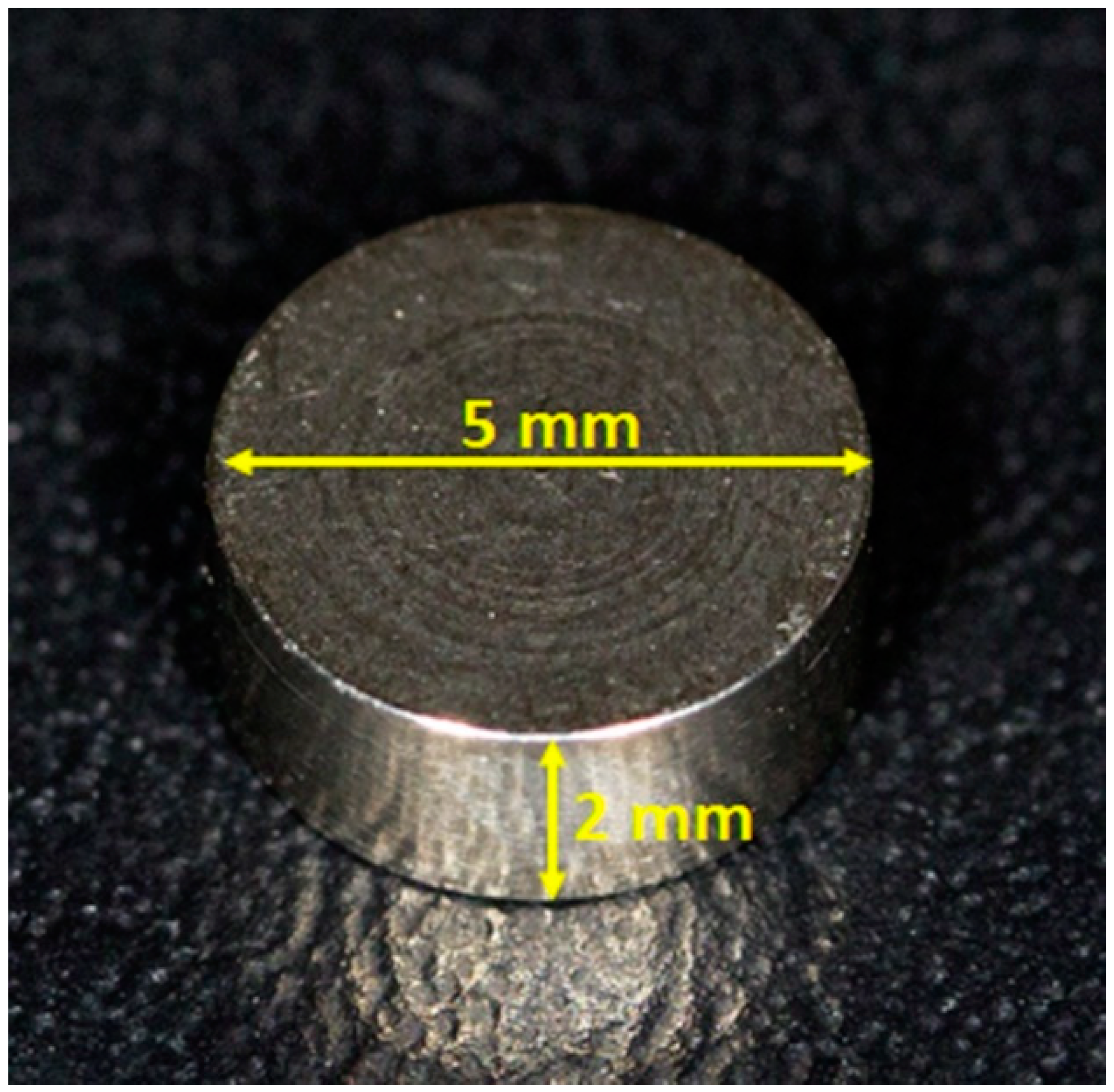
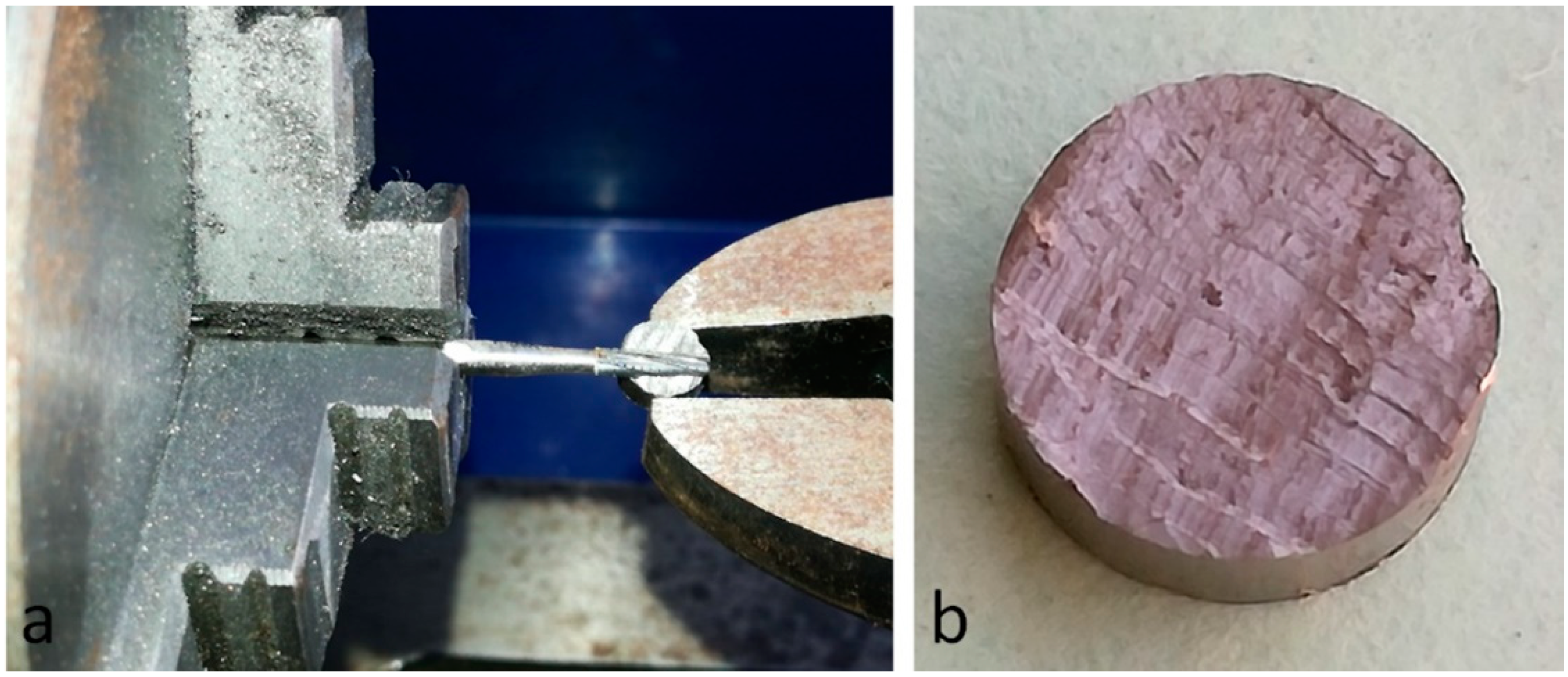
2.1. Titanium Disks and Group Formation
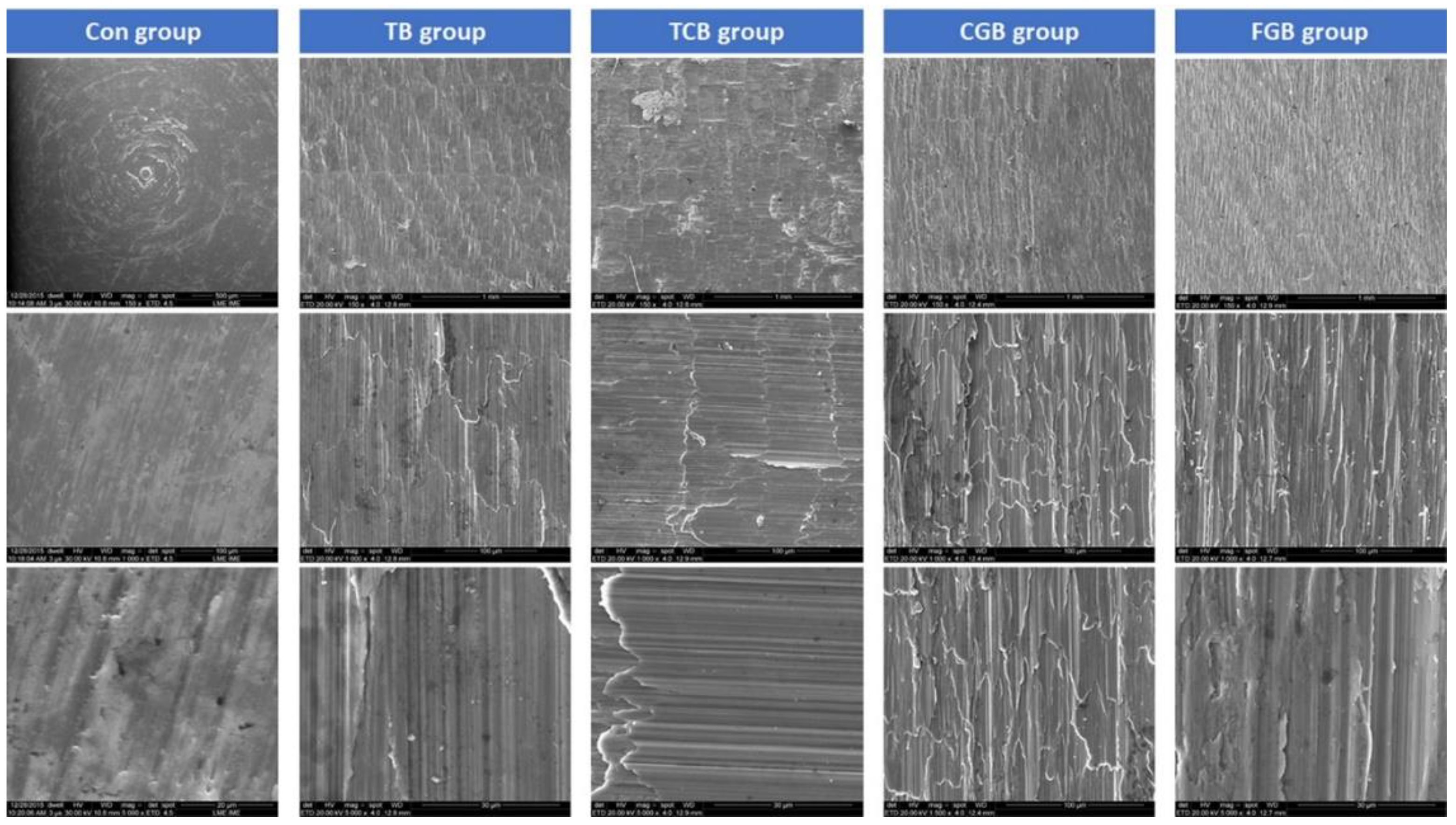
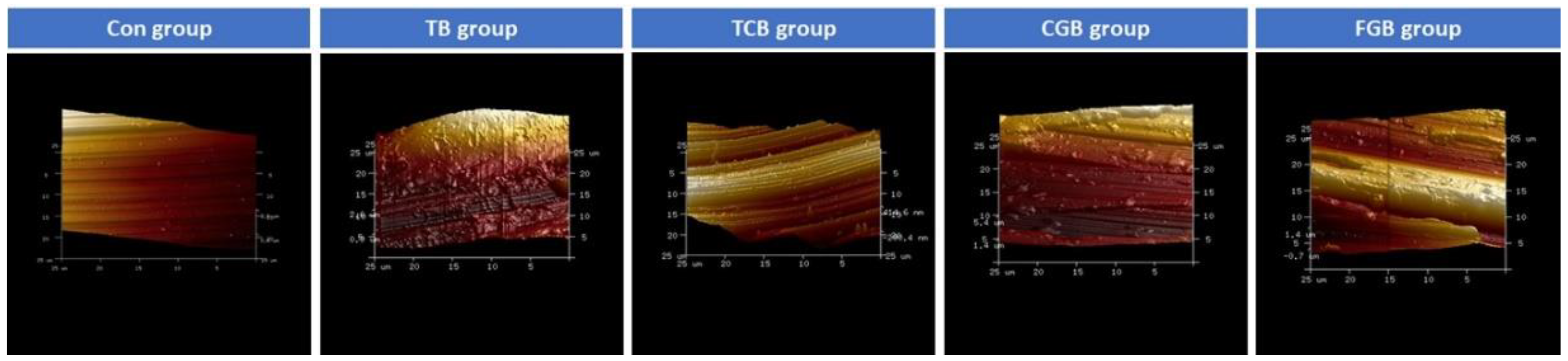
2.2. Morphological Surface Analysis
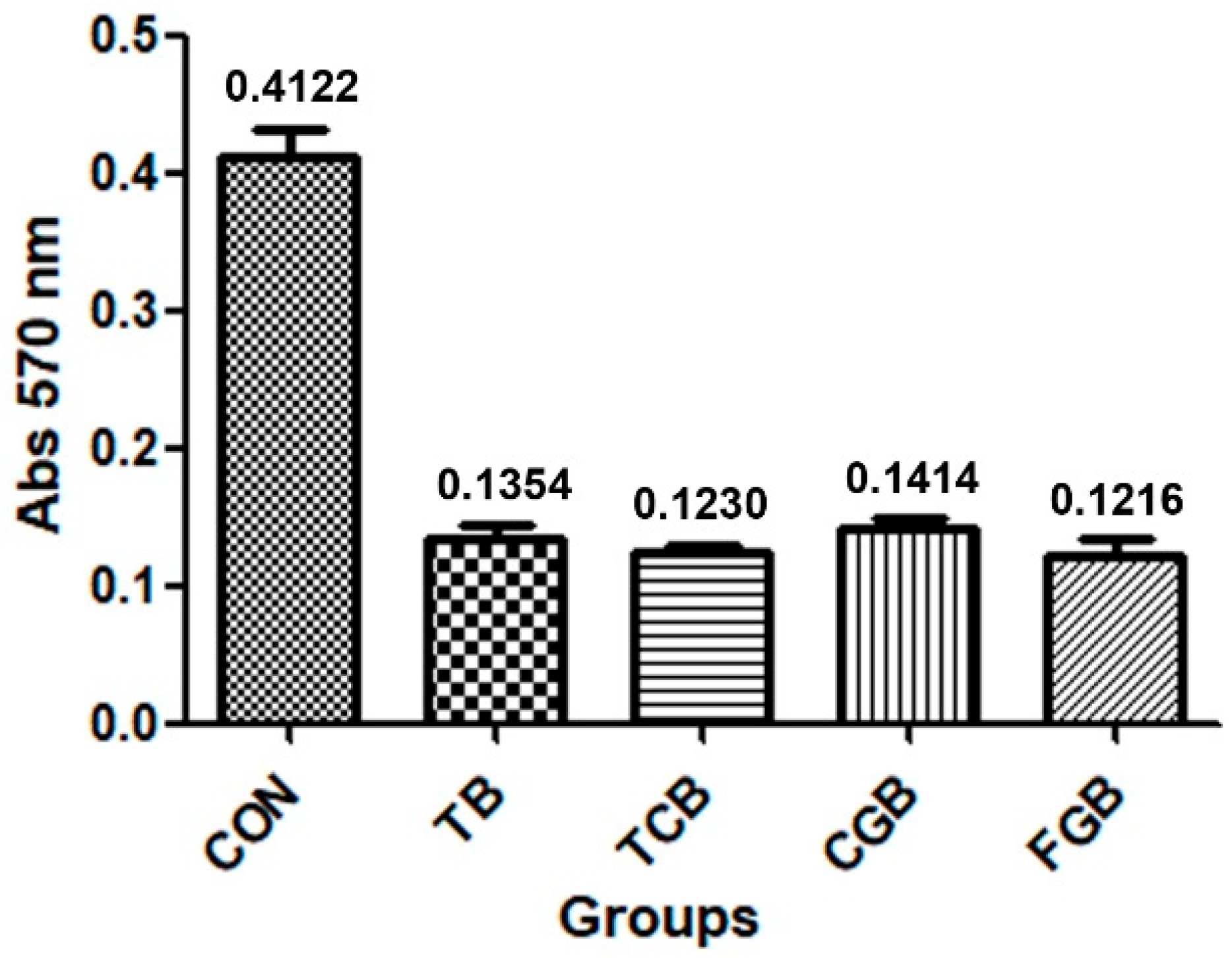
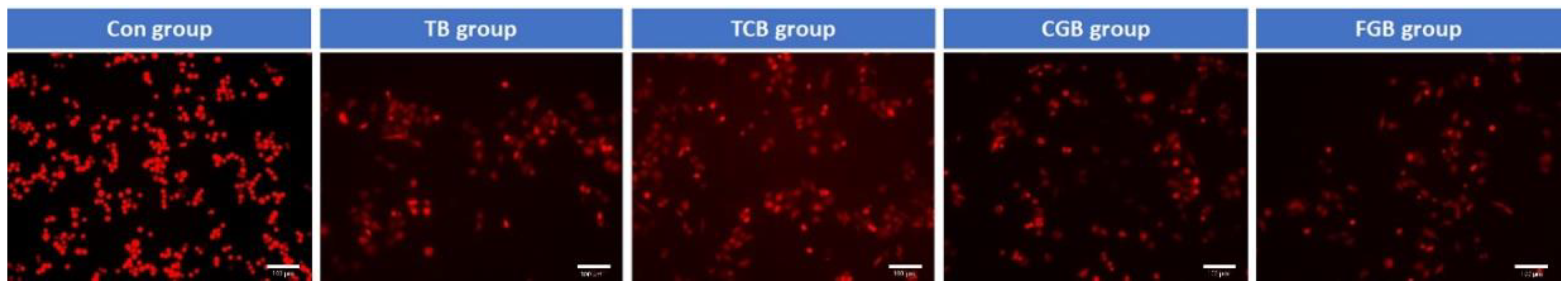
2.3. Cellular Assay
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rokaya, D.; Srimaneepong, V.; Wisitrasameewon, W.; Humagain, M.; Thunyakitpisal, P. Peri-implantitis Update: Risk Indicators, Diagnosis, and Treatment. Eur. J. Dent. 2020, 14, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Elemek, E.; Agrali, O.B.; Kuru, B.; Kuru, L. Peri-implantitis and Severity Level. Eur. J. Dent. 2020, 14, 24–30. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porenczuk, A.; Górski, B. Peri-implantitis: A Serious Problem of Dental Implantology. In Advances in Dental Implantology Using Nanomaterials and Allied Technology Applications; Chaughule, R.S., Dashaputra, R., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89, S267–S290. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef]
- Valente, N.A.; Andreana, S. Peri-implant disease: What we know and what we need to know. J. Periodontol. Implant. Sci. 2016, 46, 136–151. [Google Scholar] [CrossRef]
- Cosgarea, R.; Sculean, A.; Shibli, J.A.; Salvi, G.E. Prevalence of peri-implant diseases—A critical review on the current evidence. Braz. Oral Res. 2019, 33, e063. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef]
- Wada, M.; Mameno, T.; Otsuki, M.; Kani, M.; Tsujioka, Y.; Ikebe, K. Prevalence and risk indicators for peri-implant diseases: A literature review. Jpn. Dent. Sci. Rev. 2021, 57, 78–84. [Google Scholar] [CrossRef]
- Salmeron, S.; Rezende, M.L.; Consolaro, A.; Sant’ana, A.C.; Damante, C.A.; Greghi, S.L.; Passanezi, E. Laser therapy as an effective method for implant surface decontamination: A histomorphometric study in rats. J. Periodontol. 2013, 84, 641–649. [Google Scholar] [CrossRef]
- El Chaar, E.; Almogahwi, M.; Abdalkader, K.; Alshehri, A.; Cruz, S.; Ricci, J. Decontamination of the Infected Implant Surface: A Scanning Electron Microscope Study. Int. J. Periodontics Restor. Dent. 2020, 40, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Linden, E.; Cobb, C.M.; Fletcher, P.; Zhao, D. SEM Evaluation of the Effects of Laser-Mediated Implant Surface Decontamination: An In Situ Human Pilot Study. Int. J. Periodontics Restor. Dent. 2021, 41, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Wheelis, S.E.; Gindri, I.M.; Valderrama, P.; Wilson, T.G., Jr.; Huang, J.; Rodrigues, D.C. Effects of decontamination solutions on the surface of titanium: Investigation of surface morphology, composition, and roughness. Clin. Oral Implant. Res. 2016, 27, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.M.; Pfammatter, C.; Zitzmann, N.U.; Filippi, A.; Kühl, S. Surface quality after implantoplasty. Schw. Mon. Zahnmed. 2012, 122, 714–724. [Google Scholar]
- Sahrmann, P.; Luso, S.; Mueller, C.; Ender, A.; Attin, T.; Stawarczyk, B.; Schmidlin, P.R. Titanium Implant Characteristics After Implantoplasty: An In Vitro Study on Two Different Kinds of Instrumentation. Int. J. Oral Maxillofac. Implant. 2019, 34, 1299–1305. [Google Scholar] [CrossRef]
- Costa-Berenguer, X.; García-García, M.; Sánchez-Torres, A.; Sanz-Alonso, M.; Figueiredo, R.; Valmaseda-Castellón, E. Effect of implantoplasty on fracture resistance and surface roughness of standard diameter dental implants. Clin. Oral Implant. Res. 2018, 29, 46–54. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Aramburú Júnior, J.S.; Dedavid, B.A.; Shibli, J.A. Analysis of Implant Strength After Implantoplasty in Three Implant-Abutment Connection Designs: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2016, 31, e65–e70. [Google Scholar] [CrossRef]
- Perez-Diaz, L.; Dedavid, B.A.; Gehrke, S.A. Evaluation of Fibroblasts Cells Viability and Adhesion on Six Different Titanium Surfaces: An in vitro Experimental Study. Recent Pat. Biotechnol. 2018, 12, 145–153. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Yu, S.H.; Wang, H.L. Non-Surgical Therapy for Peri-Implant Diseases: A Systematic Review. J. Oral Maxillofac. Res. 2016, 7, e13. [Google Scholar] [CrossRef]
- Newman, M.G.; Essex, G.; Laughter, L.; Elangovan, S. Clinical Periodontology for the Dental Hygienist; Elsevier Health Sciences: New York, NY, USA, 2020; pp. 651–659. [Google Scholar]
- Roccuzzo, M.; Layton, D.M.; Roccuzzo, A.; Heitz-Mayfield, L.J. Clinical outcomes of peri-implantitis treatment and supportive care: A systematic review. Clin. Oral Implant. Res. 2018, 2, 331–350. [Google Scholar] [CrossRef]
- Khoshkam, V.; Suarez-Lopez Del Amo, F.; Monje, A.; Lin, G.H.; Chan, H.L.; Wang, H.L. Long-term radiographic and clinical outcomes of regenerative approach for treating peri-implantitis: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Wu, X.; Wang, L. Management of peri-implantitis: A systematic review, 2010–2015. Springerplus 2016, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Pons, R.; Insua, A.; Nart, J.; Wang, H.L.; Schwarz, F. Morphology and severity of peri-implantitis bone defects. Clin. Implant. Dent. Relat. Res. 2019, 21, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, M.A.; Galarraga-Vinueza, M.E.; Bedoya, K.A.; Correa, B.B.; de Souza Magini, R.; Schwarz, F. Implantoplasty Enhancing Peri-implant Bone Stability Over a 3-Year Follow-up: A Case Series. Int. J. Periodontics Restor. Dent. 2020, 40, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.A.F.; Balderrama, Í.F.; Karam, P.S.B.H.; de Oliveira, R.C.; de Oliveira, F.A.; Grandini, C.R.; Vicente, F.B.; Stavropoulos, A.; Zangrando, M.S.R.; Sant’Ana, A.C.P. Surface roughness of titanium disks influences the adhesion, proliferation and differentiation of osteogenic properties derived from human. Int. J. Implant. Dent. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Milleret, V.; Lienemann, P.S.; Gasser, A.; Bauer, S.; Ehrbar, M.; Wennerberg, A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin. Implant. Dent. Relat. Res. 2019, 21, 15–24. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Souza Dos Santos Vianna, M.; Dedavid, B.A. Influence of bone insertion level of the implant on the fracture strength of different connection designs: An in vitro study. Clin. Oral Investig. 2014, 18, 715–720. [Google Scholar] [CrossRef]
- Prathapachandran, J.; Suresh, N. Management of peri-implantitis. Dent. Res. J. 2012, 9, 516–521. [Google Scholar] [CrossRef]
- Renvert, S.; Roos-Jansåker, A.M.; Claffey, N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J. Clin. Periodontol. 2008, 35, 305–315. [Google Scholar] [CrossRef]
- Pita, P.P.C.; Rodrigues, J.A.; Ota-Tsuzuki, C.; Miato, T.F.; Zenobio, E.G.; Giro, G.; Figueiredo, L.C.; Gonçalves, C.; Gehrke, S.A.; Cassoni, A.; et al. Oral Streptococci Biofilm Formation on Different Implant Surface Topographies. Biomed. Res. Int. 2015, 2015, 159625. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Boligon, J.; Shibli, J.A. Evaluation of the Cleaning and Alterations in Titanium Surfaces with Different Mechanical Instruments Using an Artificial Calculus. Oral Health Dent. Manag. 2014, 13, 1029–1033. [Google Scholar]
- Schwarz, F.; Becker, K.; Renvert, S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: A systematic review. J. Clin. Periodontol. 2015, 42, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; John, G.; Mainusch, S.; Sahm, N.; Becker, J. Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination. A two-year clinical follow up report. J. Clin. Periodontol. 2012, 39, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Ichioka, Y.; Derks, J.; Dahlén, G.; Berglundh, T.; Larsson, L. In vitro evaluation of chemical decontamination of titanium discs. Sci. Rep. 2021, 11, 22753. [Google Scholar] [CrossRef]
- Amid, R.; Kadkhodazadeh, M.; Mojahedi, S.M.; Gilvari Sarshari, M.; Zamani, Z. Physicochemical Changes of Contaminated Titanium Discs Treated With Erbium-Doped Yttrium Aluminum Garnet (Er:YAG) Laser Irradiation or Air-Flow Abrasion: An In Vitro Study. J. Lasers Med. Sci. 2021, 12, e67. [Google Scholar] [CrossRef]
- Ichioka, Y.; Derks, J.; Dahlén, G.; Berglundh, T.; Larsson, L. Mechanical removal of biofilm on titanium discs: An in vitro study. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1044–1055. [Google Scholar] [CrossRef]
- Tawse-Smith, A.; Kota, A.; Jayaweera, Y.; Vuuren, W.J.; Ma, S. The effect of standardised implantoplasty protocol on titanium surface roughness: An in-vitro study. Braz. Oral Res. 2016, 30, e137. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; Sánchez-Garcés, M.Á.; Gay-Escoda, C.; Valmaseda-Castellón, E.; Camps-Font, O.; Verdeguer, P.; Molmeneu, M.; Gil, F.J. Mechanical Properties and Corrosion Behavior of Ti6Al4V Particles Obtained by Implantoplasty: An In Vitro Study. Part II. Materials 2021, 14, 6519. [Google Scholar] [CrossRef]

| Roughness Parameters | CON Group | TB Group | TCB Group | CGB Group | FGB Group |
|---|---|---|---|---|---|
| Ra | 0.77 ± 0.14 | 0.45 ± 0.10 | 0.75 ± 0.16 | 0.83 ± 0.16 | 0.51 ± 0.14 |
| Rz | 4.32 ± 0.66 | 3.68 ± 0.64 | 5.61 ± 0.74 | 6.03 ± 0.71 | 3.98 ± 0.67 |
| Group Comparison | Mean of Diff. | p-Value | 95% CI |
|---|---|---|---|
| CON vs. TB | 0.2768 | 0.0022 * | 0.2262 to 0.3274 |
| CON vs. TCB | 0.2892 | 0.0022 * | 0.2385 to 0.3398 |
| CON vs. CGB | 0.2708 | 0.0022 * | 0.2201 to 0.3214 |
| CON vs. FGB | 0.2906 | 0.0022 * | 0.2400 to 0.3412 |
| TB vs. TCB | 0.01238 | 0.2403 | −0.03825 to 0.06302 |
| TB vs. CGB | −0.006019 | 0.6991 | −0.05666 to 0.04462 |
| TB vs. FGB | 0.01382 | 0.4848 | −0.03682 to 0.06446 |
| TCB vs. CGB | −0.01840 | 0.1727 | −0.06904 to 0.03223 |
| TCB vs. FGB | 0.001436 | 0.6884 | −0.04920 to 0.05207 |
| CGB vs. FGB | 0.01984 | 0.3939 | −0.03080 to 0.07048 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehrke, S.A.; Dedavid, B.A.; Odella Colla, G.; De Aza, P.N.; Pérez-Díaz, L. Titanium Surface Analysis after Instrumentation with Different Burs Simulating the Implantoplasty Technique: A Pilot In Vitro Experimental Study. Appl. Sci. 2022, 12, 7920. https://doi.org/10.3390/app12157920
Gehrke SA, Dedavid BA, Odella Colla G, De Aza PN, Pérez-Díaz L. Titanium Surface Analysis after Instrumentation with Different Burs Simulating the Implantoplasty Technique: A Pilot In Vitro Experimental Study. Applied Sciences. 2022; 12(15):7920. https://doi.org/10.3390/app12157920
Chicago/Turabian StyleGehrke, Sergio Alexandre, Berenice Anina Dedavid, Germán Odella Colla, Piedad N. De Aza, and Leticia Pérez-Díaz. 2022. "Titanium Surface Analysis after Instrumentation with Different Burs Simulating the Implantoplasty Technique: A Pilot In Vitro Experimental Study" Applied Sciences 12, no. 15: 7920. https://doi.org/10.3390/app12157920
APA StyleGehrke, S. A., Dedavid, B. A., Odella Colla, G., De Aza, P. N., & Pérez-Díaz, L. (2022). Titanium Surface Analysis after Instrumentation with Different Burs Simulating the Implantoplasty Technique: A Pilot In Vitro Experimental Study. Applied Sciences, 12(15), 7920. https://doi.org/10.3390/app12157920








