ATP-Binding Cassette (ABC) Transporters in Fusarium Specific Mycoparasite Sphaerodes mycoparasitica during Biotrophic Mycoparasitism
Abstract
1. Introduction
2. Materials and Methods
2.1. Co-Cultures
2.2. Transmission Electron Microscopy Analysis
2.3. Transciptomic Analyses
2.4. Phylogenetic Analyses
3. Results
3.1. Investigation of Morphological Changes during Biotrophic Mycoparasitism
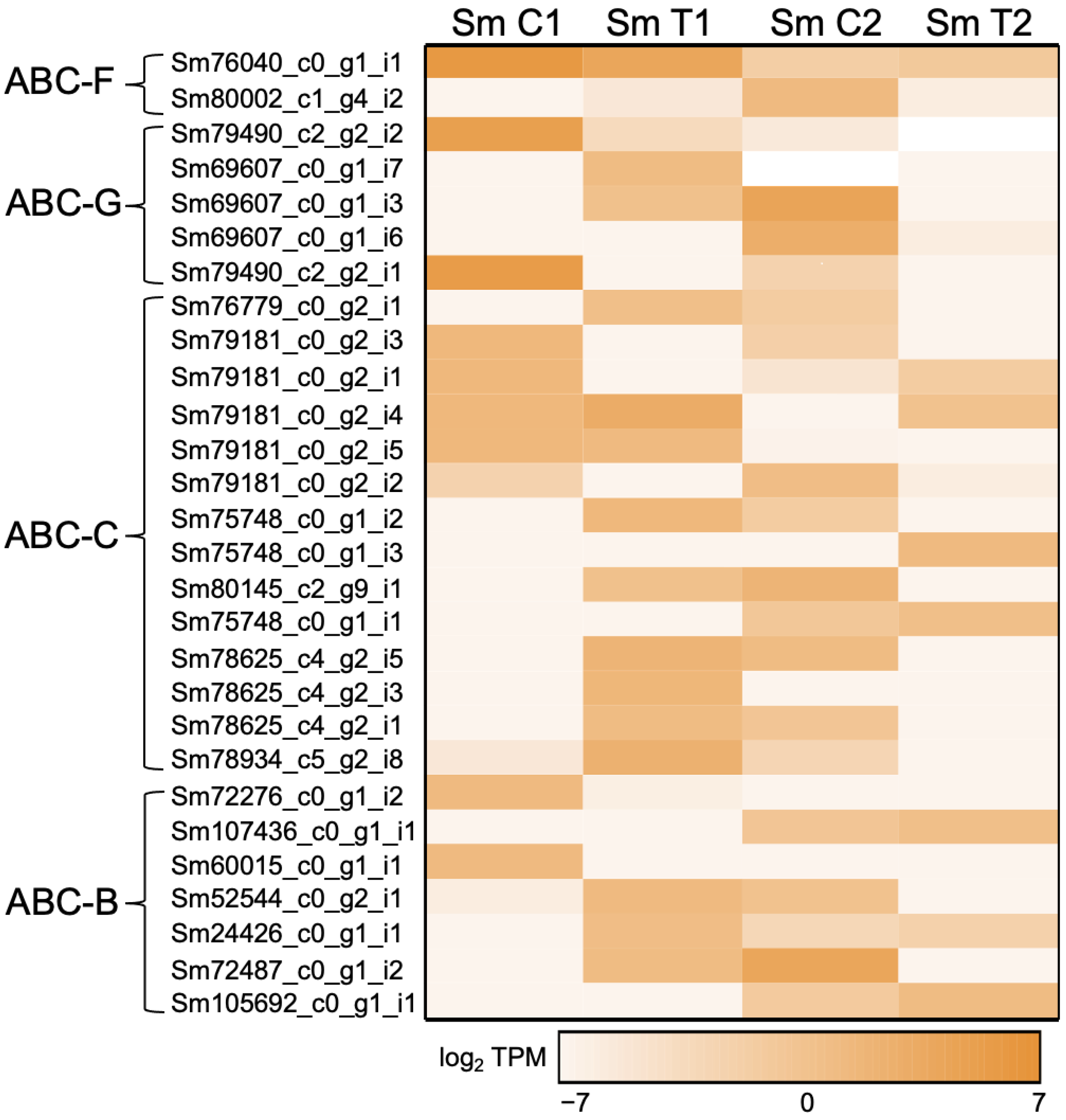
3.2. Phylogenetic Evolutionary Analyses Based on the Interactive Transcriptome
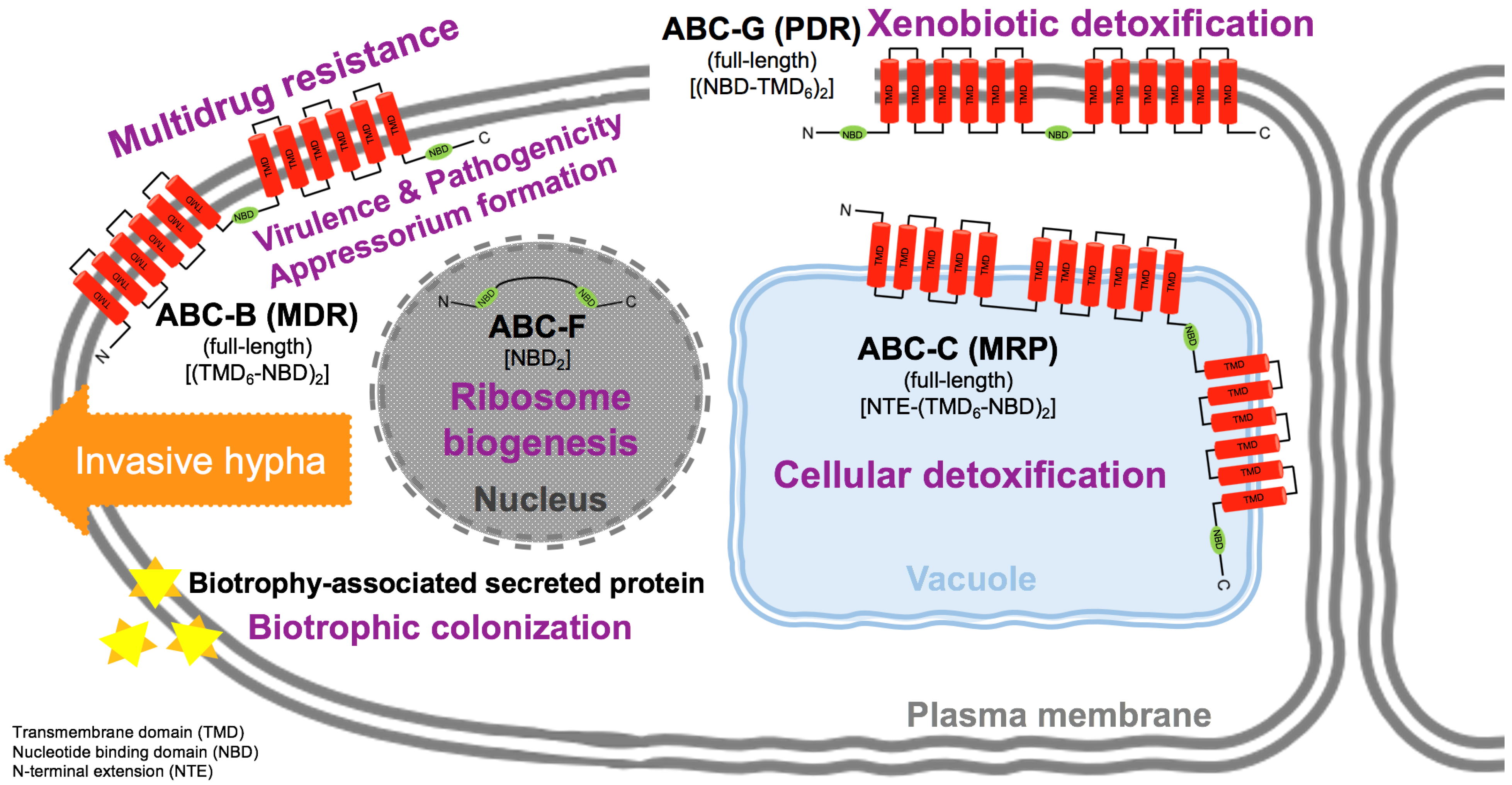
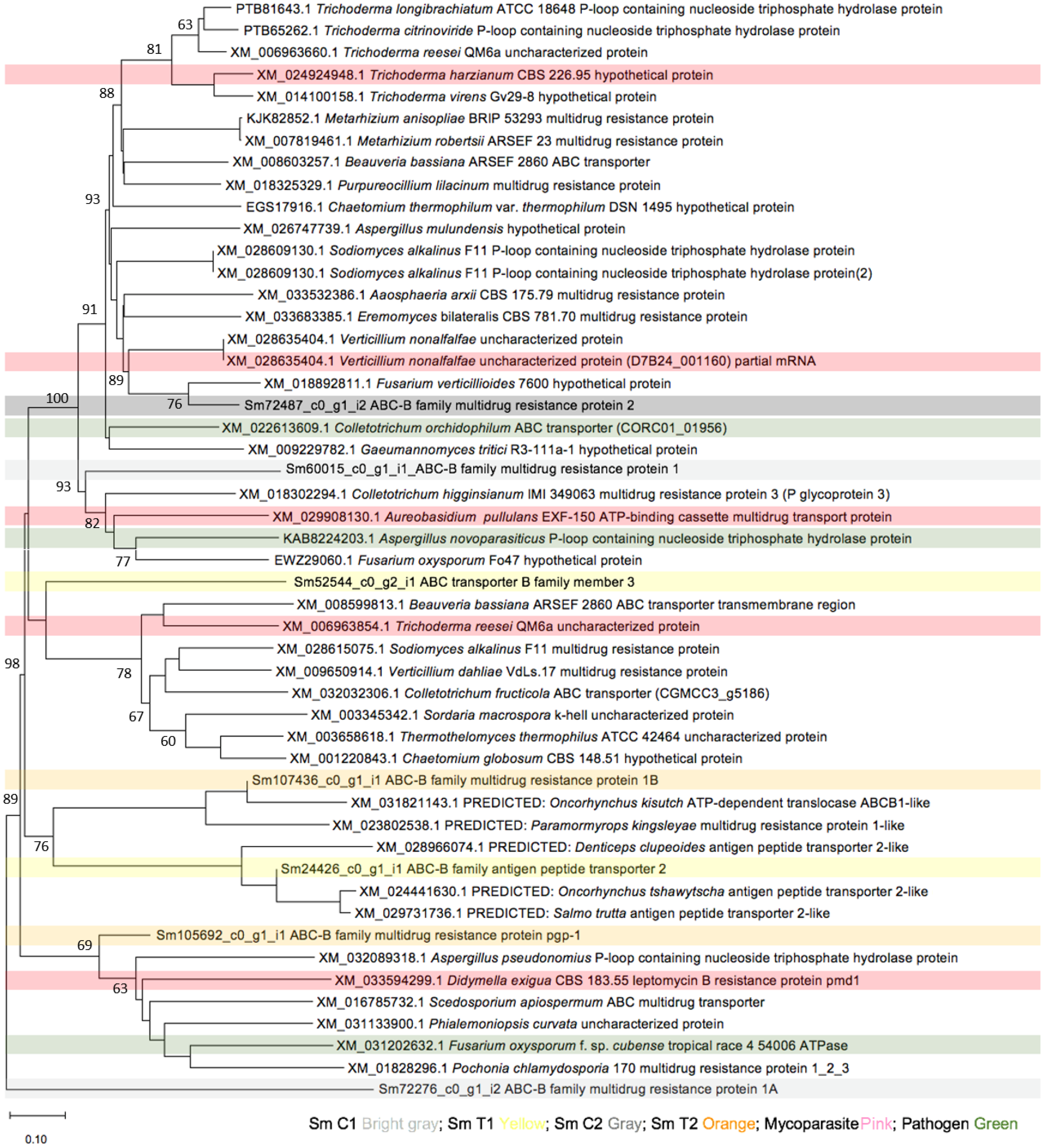
3.2.1. Multidrug Resistance (MDR) Exporter Subfamily (ABC-B Family)
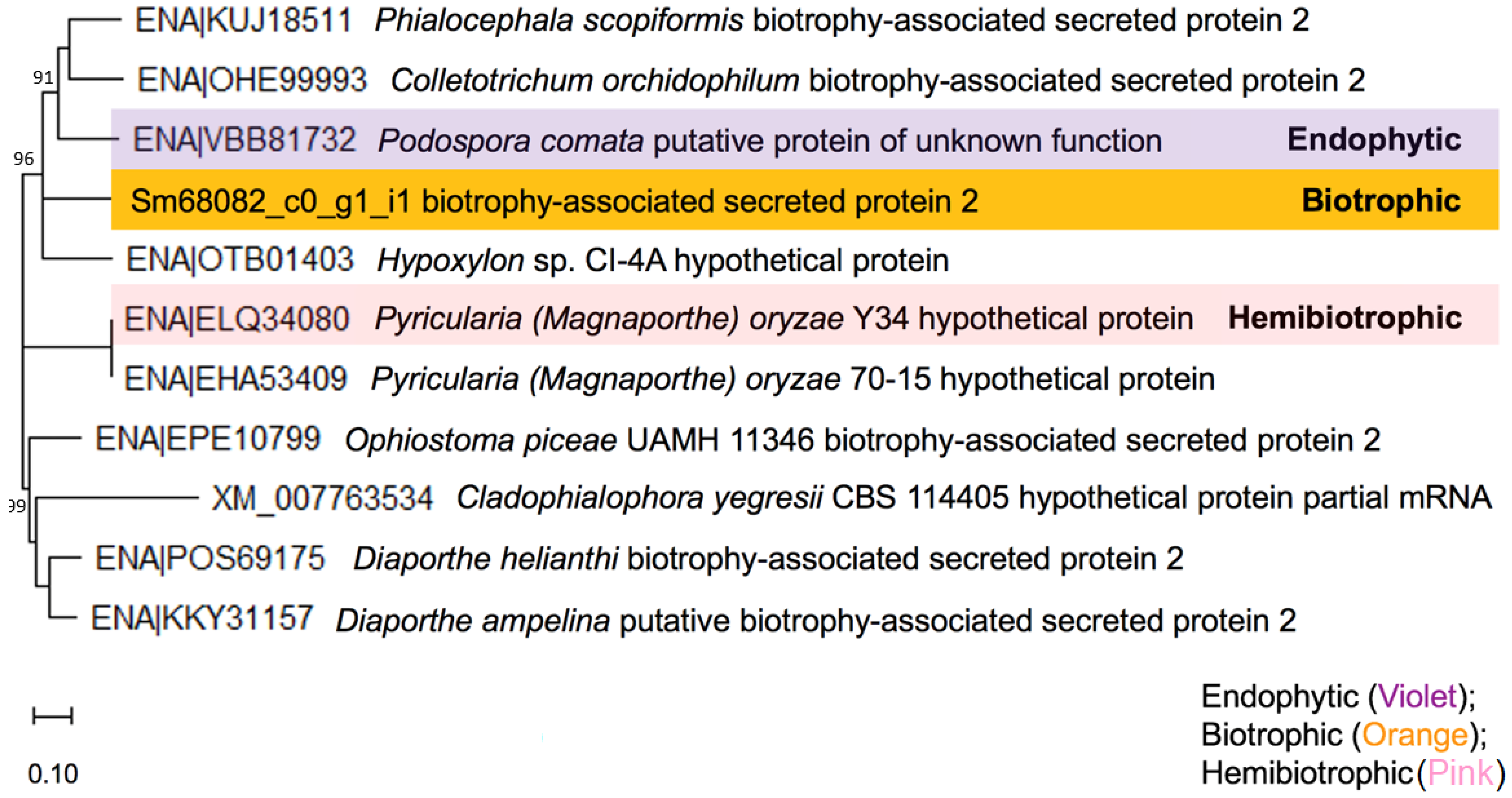
3.2.2. Conjugate Transporter Subfamily (ABC-C Family)
3.2.3. EF3 Subfamily (ABC-F Family)
3.2.4. Pleiotropic Drug Resistance (PDR) Subfamily (ABC-G Family)
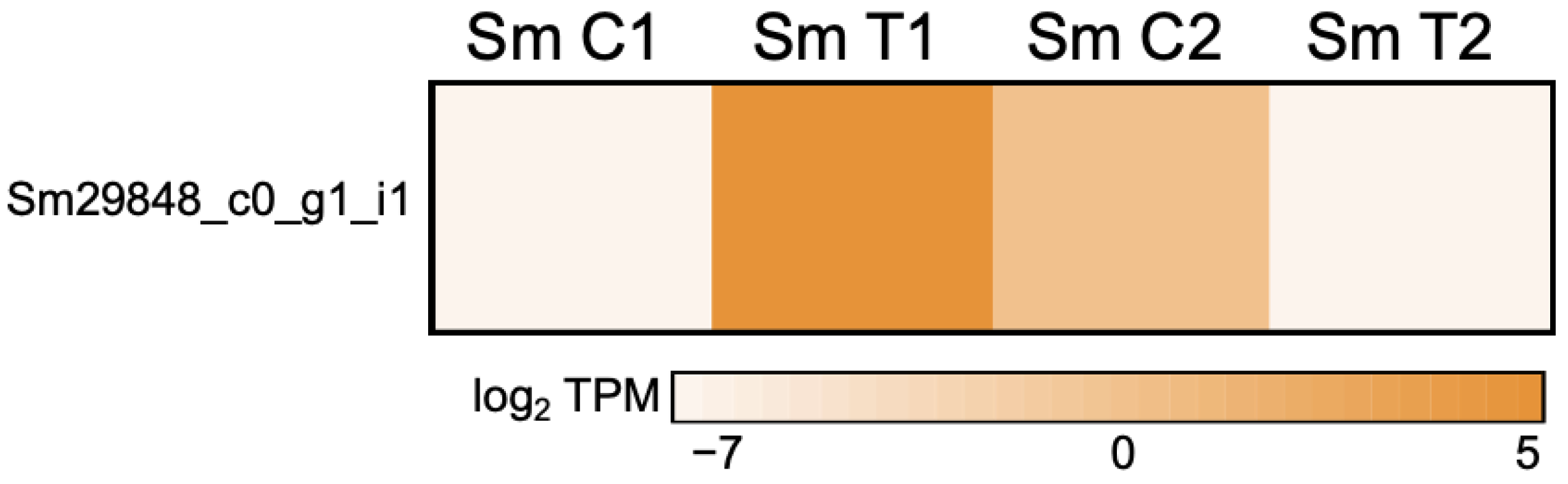
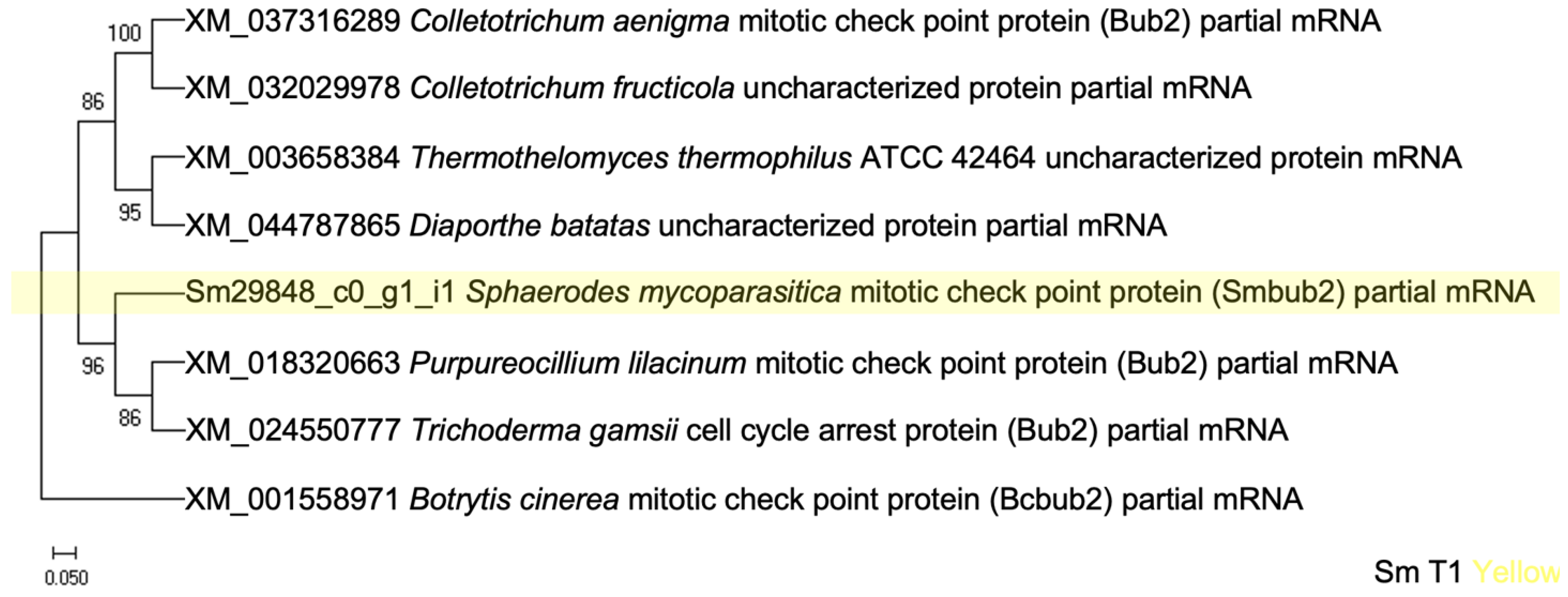
3.2.5. Mitotic Checkpoint Protein (Bub2) Interacting with ABC in Appressoria Formation
4. Discussion
4.1. Investigation of Morphological Changes during Biotrophic Mycoparasitism
4.2. Phylogenetic Evolutionary Analysis Based on the Interactive Transcriptome
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, V.; Kim, S.H. Adaptability of mitosporic stage in Sphaerodes mycoparasitica towards its mycoparasitic-polyphagous lifestyle. Mycologia 2018, 109, 701–709. [Google Scholar] [CrossRef]
- Goh, Y.K.; Vujanovic, V. Biotrophic mycoparasitic interactions between Sphaerodes mycoparasitica and phytopathogenic Fusariums species. Biocontrol. Sci. Technol. 2010, 20, 891–902. [Google Scholar] [CrossRef]
- Inglis, G.D.; Kawchuk, L.M. Comparative degradation of oomycete, ascomycete, and basidiomycete cell walls by mycoparasitic and biocontrol fungi. Can. J. Microbiol. 2002, 48, 60–70. [Google Scholar] [CrossRef]
- Atanasova, L.; Crom, S.L.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative transcriptomics reveals different strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef]
- Quandt, C.A.; Bushley, K.E.; Spatafora, J.W. The genome of the truffle-parasite Tolypocladium ophioglossoides and the evolution of antifungal peptaibiotics. BMC Genom. 2015, 16, 553. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Vujanovic, V. Relationship between mycoparasites lifestyles and biocontrol behaviors against Fusarium spp. and mycotoxins production. Appl. Microbiol. Biotechnol. 2016, 100, 5257–5272. [Google Scholar] [CrossRef]
- Vujanovic, V.; Goh, Y.K. Sphaerodes mycoparasitica biotrophic mycoparasite of 3-acetyldeoxynivalenol- and 15-acetyldeoxynivalenol-producing toxigenic Fusarium graminearum chemotypes. FEMS Microbiol. Lett. 2011, 316, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Vujanovic, V. Biodegradation and biodetoxification of Fusarium mycotoxins by Sphaerodes mycoparasitica. AMB Express 2017, 7, 145. [Google Scholar] [CrossRef]
- Qualhato, T.F.; Lopes, F.A.C.; Steindorff, A.S.; Brandão, R.S.; Jesuino, R.S.A.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma species against three phytopathogenic fungi: Evaluation of antagonism and hydrolytic enzyme production. Biotechnol. Lett. 2013, 35, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, S.; Punja, Z.K. Chitinase and β-1,3-glucanase enzyme production by the mycoparasite Clonostachys rosea f. catenulata against fungal plant pathogens. Can. J. Microbiol. 2009, 55, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Elamathi, E.; Malathi, P.; Viswanathan, R.; Ramesh Sundar, A. Expression analysis on mycoparasitism related genes during antagonism of Trichoderma with Colletotrichum falcatum causing red rot in sugarcane. J. Plant Biochem. Biotechnol. 2018, 27, 351–361. [Google Scholar] [CrossRef]
- Steindorff, A.S.; Ramada, M.H.S.; Coelho, A.S.G.; Miller, R.N.G.; Pappas, G.J.; Ulhoa, C.J.; Noronha, E.F. Identification of mycoparasitism-related genes against the phytopathogen Sclerotinia sclerotiorum through transcriptome and expression profile analysis in Trichoderma harzianum. BMC Genom. 2014, 15, 204. [Google Scholar] [CrossRef]
- Reithner, B.; Schuhmacher, R.; Stoppacher, N.; Pucher, M.; Brunner, K.; Zeilinger, S. Signaling via the Trichoderma atroviride mitogen-activated protein kinase Tmk 1 differentially affects mycoparasitism and plant protection. Fungal Genet. Biol. 2007, 44, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-B.; Wang, Q.; Sun, M.-H.; Li, S.-D. The mitogen-activated protein kinase gene Crmapk is involved in Clonostachys chloroleuca mycoparasitism. Mol. Plant Microbe Interact. 2020, 33, 902–910. [Google Scholar] [CrossRef]
- Gruber, S.; Zeilinger, S. The transcription factor Ste12 mediates the regulatory role of the Tmk1 MAP kinase in mycoparasitism and vegetative hyphal fusion in the filamentous fungus Trichoderma atroviride. PLoS ONE 2014, 9, e111636. [Google Scholar] [CrossRef]
- Sun, Z.B.; Wang, Q.; Zhang, J.; Jiang, W.Z.; Wang, Q.; Li, S.D.; Ma, G.Z.; Sun, M.H. The transcription factor-encoding gene crtf is involved in Clonostachys chloroleuca mycoparasitism on Sclerotinia sclerotiorum. Microbiol. Res. 2018, 210, 6–11. [Google Scholar] [CrossRef]
- Seidl, V.; Song, L.; Lindquist, E.; Gruber, S.; Koptchinskiy, A.; Zeilinger, S.; Schmoll, M.; Martínez, P.; Sun, J.; Grigoriev, I.; et al. Transcriptomic response of the mycoparasitic fungus Trichoderma atroviride to the presence of a fungal prey. BMC Genom. 2009, 10, 567. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-B.; Wang, Q.; Sun, M.-H.; Li, S.-D. The heat shock protein 70 gene is involved for colony morphology, sporulation and mycoparasitism of Clonostachys rosea. FEMS Microbiol. Lett. 2019, 366, fnz188. [Google Scholar] [CrossRef] [PubMed]
- Demissie, Z.A.; Foote, S.J.; Tan, Y.; Loewen, M.C. Profiling of the transcriptomic responses of Clonostachys rosea upon treatment with Fusarium graminearum secretome. Front. Microbiol. 2018, 9, 1061. [Google Scholar] [CrossRef]
- Wu, B.; Cox, M.P. Comparative genomics reveals a core gene toolbox for lifestyle transitions in Hypocreales fungi. Environ. Microbiol. 2021, 23, 3251–3264. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Ambrosino, P.; Carbone, V.; Vinale, F.; Woo, S.L.; Ruocco, M.; Ciliento, R.; Lanzuise, S.; Ferraioli, S.; Soriente, I.; et al. Study of the three-way interaction between Trichoderma atroviride, plant and fungal pathogens by using a proteomic approach. Curr. Genet. 2006, 50, 307–321. [Google Scholar] [CrossRef]
- Ruocco, M.; Lanzuise, S.; Vinale, F.; Marra, R.; Turrà, D.; Woo, S.L.; Lorito, M. Identification of a new biocontrol gene in Trichoderma atroviride: The role of an ABC transporter membrane pump in the interaction with different plant-pathogenic Fungi. Mol. Plant Microbe Interact. 2009, 22, 291–301. [Google Scholar] [CrossRef]
- Piecuch, A.; Obłąk, E. Yeast ABC proteins involved in multidrug resistance. Cell. Mol. Biol. Lett. 2014, 19, 1–22. [Google Scholar] [CrossRef]
- Banerjee, D.; Lelandais, G.; Shukla, S.; Mukhopadhyay, G.; Jacq, C.; Devaux, F.; Prasad, R. Responses of pathogenic and nonpathogenic yeast species to steroids reveal the functioning and evolution of multidrug resistance transcriptional networks. Eukaryot. Cell 2008, 7, 68. [Google Scholar] [CrossRef]
- Mahé, Y.; Lemoine, Y.; Kuchler, K. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in Vivo. J. Biol. Chem. 1996, 271, 25167–25172. [Google Scholar] [CrossRef]
- Dubey, M.K.; Jensen, D.F.; Karlsson, M. An ATP-binding cassette pleiotropic drug transporter protein is required for xenobiotic tolerance and antagonism in the fungal biocontrol agent Clonostachys rosea. Mol. Plant Microbe Interact. 2014, 27, 725–732. [Google Scholar] [CrossRef]
- Kosawang, C.; Karlsson, M.; Jensen, D.F.; Dilokpimol, A.; Collinge, D.B. Transcriptomic profiling to identify genes involved in Fusarium mycotoxin deoxynivalenol and zearalenone tolerance in the mycoparasitic fungus Clonostachys rosea. BMC Genom. 2014, 15, 55. [Google Scholar] [CrossRef]
- Nygren, K.; Dubey, M.; Zapparata, A.; Iqbal, M.; Tzelepis, G.D.; Durling, M.B.; Jensen, D.F.; Karlsson, M. The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol. Appl. 2018, 11, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lahlali, R.; Karunakaran, C.; Vujanovic, V. Specific mycoparasite-Fusarium graminearum molecular signatures in germinating seeds disabled fusarium head blight pathogen’s infection. Int. J. Mol. Sci. 2021, 22, 2461. [Google Scholar] [CrossRef]
- Powell, A.J.; Vujanovic, V. Evolution of fusarium head blight management in wheat: Scientific perspectives on biological control agents and crop genotypes protocooperation. Appl. Sci. 2021, 11, 8960. [Google Scholar] [CrossRef]
- Kim, S.H.; Vujanovic, V. Early transcriptomic response of the mycoparasite Sphaerodes mycoparasitica to the mycotoxigenic Fusarium graminearum 3-ADON, the cause of Fusarium head blight. Bioresour. Bioprocess. 2021, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Vujanovic, V.; Goh, Y.K. Sphaerodes mycoparasitica sp. nov., a new biotrophic mycoparasite on Fusarium avenaceum, F. graminearum and F. oxysporum. Mycol. Res. 2009, 113, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.K.; Vujanovic, V. Ascospore germination patterns revealed ascomycetous biotrophic mycoparasite specificity to Fusarium hosts. Botany 2010, 88, 1033–1043. [Google Scholar] [CrossRef]
- Xiao, M.; Zhang, Y.; Chen, X.; Lee, E.J.; Barber, C.J.; Chakrabarty, R.; Desgagné-Penix, I.; Haslam, T.M.; Kim, Y.B.; Liu, E.; et al. Transcriptome analysis based on next-generation sequencing of non-model plants producing specialized metabolites of biotechnological interest. J. Biotechnol. 2013, 166, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Rzhetsky, A.; Nei, M. A simple method for estimating and testing minimum-evolution trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK; New York, NY, USA, 2000. [Google Scholar]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Silar, P.; Dauget, J.-M.; Gautier, V.; Grognet, P.; Chablat, M.; Hermann-Le Denmat, S.; Couloux, A.; Wincker, P.; Debuchy, R. A gene graveyard in the genome of the fungus Podospora comata. Mol. Genet. Genom. 2019, 294, 177–190. [Google Scholar] [CrossRef]
- Eichhorn, H.; Lessing, F.; Winterberg, B.; Schirawski, J.; Kämper, J.; Müller, P.; Kahmann, R. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell 2006, 18, 3332–3345. [Google Scholar] [CrossRef] [PubMed]
- Winterberg, B.; Uhlmann, S.; Linne, U.; Lessing, F.; Marahiel, M.A.; Eichhorn, H.; Kahmann, R.; Schirawski, J. Elucidation of the complete ferrichrome A biosynthetic pathway in Ustilago maydis. Mol. Microbiol. 2010, 75, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-S.; Szczypka, M.; Lu, Y.-P.; Thiele, D.J.; Rea, P.A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 1996, 271, 6509–6517. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.; Pascolo, L.; Gallo, R.; Cupelli, F.; Ostrow, J.D.; Goffeau, A.; Tiribelli, C.; Bruschi, C.V. The products of YCF1 and YLL015w (BPT1) cooperate for the ATP-dependent vacuolar transport of unconjugated bilirubin in Saccharomyces cerevisiae. Yeast 2000, 16, 561–571. [Google Scholar] [CrossRef]
- Martín, J.P.; Di Pietro, A. Morphogenesis and Pathogenicity in Fungi; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Kuhn, H.; Kwaaitaal, M.; Kusch, S.; Acevedo-Garcia, J.; Wu, H.; Panstruga, R. Biotrophy at its best: Novel findings and unsolved mysteries of the Arabidopsis-powdery mildew pathosystem. Arabidopsis Book 2016, 14, e0184. [Google Scholar] [CrossRef] [PubMed]
- Micali, C.; Göllner, K.; Humphry, M.; Consonni, C.; Panstruga, R. The powdery mildew disease of Arabidopsis: A paradigm for the interaction between plants and biotrophic fungi. Arabidopsis Book 2008, 6, e0115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Henderson, C.; Perfect, E.; Carver, T.L.W.; Thomas, B.J.; Skamnioti, P.; Gurr, S.J. Of genes and genomes, needles and haystacks: Blumeria graminis and functionality. Mol. Plant Pathol. 2005, 6, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.S.; Chen, Y.-J.; Konta, S.; Tibpromma, S.; Abeywickrama, P.D.; Gomdola, D.; Balasuriya, A.; Xu, J.; Lumyong, S. Diversity and Function of Appressoria. Pathogens 2021, 10, 746. [Google Scholar] [CrossRef]
- Kong, L.-A.; Li, G.-T.; Liu, Y.; Liu, M.G.; Zhang, S.-J.; Yang, J.; Zhou, X.-J.; Peng, Y.-L.; Xu, J.-R. Differences between appressoria formed by germ tubes and appressorium-like structures developed by hyphal tips in Magnaporthe oryzae. Fungal Genet. Biol. 2013, 56, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Carraway, K.L.; Ramsauer, V.P.; Haq, B.; Carothers Carraway, C.A. Cell signaling through membrane mucins. BioEssays News Rev. Mol. Cell. Dev. Biol. 2003, 25, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Pinzón Martín, S.; Seeberger, P.H.; Varón Silva, D. Mucins and pathogenic mucin-like molecules are immunomodulators during infection and targets for diagnostics and vaccines. Front. Chem. 2019, 7, 710. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. Mucins: The old, the new and the promising factors in hepatobiliary carcinogenesis. Int. J. Mol. Sci. 2019, 20, 1288. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, X.; Li, G.; Li, L.; Kong, L.; Wang, C.; Zhang, H.; Xu, J.-R. Multiple plant surface signals are sensed by different mechanisms in the rice blast fungus for appressorium formation. PLoS Pathog. 2011, 7, e1001261. [Google Scholar] [CrossRef]
- Khang, C.H.; Berruyer, R.; Giraldo, M.C.; Kankanala, P.; Park, S.-Y.; Czymmek, K.; Kang, S.; Valent, B. Translocation of Magnaporthe oryzae effectors into rice cells and their subsequent cell-to-cell movement. Plant Cell 2010, 22, 1388–1403. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, G.; Giraldo, M.C.; Khang, C.H.; Coughlan, S.; Valent, B. Interaction transcriptome analysis identifies Magnaporthe oryzae BAS1-4 as biotrophy-associated secreted proteins in rice blast disease. Plant Cell 2009, 21, 1273–1290. [Google Scholar] [CrossRef]
- An, B.; Wang, W.; Guo, Y.; Wang, Q.; Luo, H.; He, C. BAS2 is required for conidiation and pathogenicity of Colletotrichum gloeosporioides from Hevea brasiliensis. Int. J. Mol. Sci. 2018, 19, 1860. [Google Scholar] [CrossRef]
- Karlsson, M.; Durling, M.B.; Choi, J.; Kosawang, C.; Lackner, G.; Tzelepis, G.D.; Nygren, K.; Dubey, M.K.; Kamou, N.; Levasseur, A.; et al. Insights on the evolution of mycoparasitism from the genome of Clonostachys rosea. Genome Biol. Evol. 2015, 7, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.H.; Mayfield, J.A.; Rine, J.; Sil, A. Histoplasma requires SID1, a member of an iron-regulated siderophore gene cluster, for host colonization. PLoS Pathog. 2008, 4, e1000044. [Google Scholar] [CrossRef]
- Silva-Bailão, M.G.; Bailão, E.F.; Lechner, B.E.; Gauthier, G.M.; Lindner, H.; Bailão, A.M.; Haas, H.; de Almeida Soares, C.M. Hydroxamate production as a high affinity iron acquisition mechanism in Paracoccidioides spp. PLoS ONE 2014, 9, e105805. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, G. Structures and functions of fungal siderophores containing hydroxamate and complexone type iron binding ligands. Mycol. Res. 1992, 96, 529–534. [Google Scholar] [CrossRef]
- Kim, Y.; Park, S.-Y.; Kim, D.; Choi, J.; Lee, Y.-H.; Lee, J.-H.; Choi, W. Genome-scale analysis of ABC transporter genes and characterization of the ABCC type transporter genes in Magnaporthe oryzae. Genomics 2013, 101, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chattoo, B.B. Functional analysis of a novel ABC transporter ABC4 from Magnaporthe grisea. FEMS Microbiol. Lett. 2008, 278, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Bhargava, T.; Hamer, J.E. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J. 1999, 18, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Yamamoto, K.; Hamamoto, H.; Nakaune, R.; Hibi, T. A novel ABC transporter gene ABC2 involved in multidrug susceptibility but not pathogenicity in rice blast fungus, Magnaporthe grisea. Pestic. Biochem. Physiol. 2005, 81, 13–23. [Google Scholar] [CrossRef]
- Sun, C.B.; Suresh, A.; Deng, Y.Z.; Naqvi, N.I. A multidrug resistance transporter in Magnaporthe is required for host penetration and for survival during oxidative stress. Plant Cell 2006, 18, 3686. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Choudhury, D.; Prasad, R. Complete inventory of ABC proteins in human pathogenic yeast, Candida albicans. J. Mol. Microbiol. Biotechnol. 2005, 9, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Theiss, S.; Kretschmar, M.; Nichterlein, T.; Hof, H.; Agabian, N.; Hacker, J.; Köhler, G.A. Functional analysis of a vacuolar ABC transporter in wild-type Candida albicans reveals its involvement in virulence. Mol. Microbiol. 2002, 43, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.; Van Nistelrooy, J.G.M.; Peery, R.B.; Skatrud, P.L.; De Waard, M.A. The role of ABC transporters from Aspergillus nidulans in protection against cytotoxic agents and in antibiotic production. Mol. Gen. Genet. 2000, 263, 966–977. [Google Scholar] [CrossRef] [PubMed]
- Sasser, T.L.; Lawrence, G.; Karunakaran, S.; Brown, C.; Fratti, R.A. The yeast ATP-binding cassette (ABC) transporter Ycf1p enhances the recruitment of the soluble SNARE Vam7p to vacuoles for efficient membrane fusion. J. Biol. Chem. 2013, 288, 18300–18310. [Google Scholar] [CrossRef] [PubMed]
- Miner, G.E.; Starr, M.L.; Hurst, L.R.; Sparks, R.P.; Padolina, M.; Fratti, R.A. The central polybasic region of the soluble SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) Vam7 affects binding to phosphatidylinositol 3-phosphate by the PX (phox homology) domain. J. Biol. Chem. 2016, 291, 17651–17663. [Google Scholar] [CrossRef] [PubMed]
- Sasser, T.L.; Fratti, R.A. Class C ABC transporters and Saccharomyces cerevisiae vacuole fusion. Cell. Logist. 2014, 4, e943588. [Google Scholar] [CrossRef][Green Version]
- Paul, S.; Diekema, D.; Moye-Rowley, W.S. Contributions of Aspergillus fumigatus ATP-binding cassette transporter proteins to drug resistance and virulence. Eukaryot. Cell 2013, 12, 1619–1628. [Google Scholar] [CrossRef]
- Dong, J.; Lai, R.; Jennings, J.L.; Link, A.J.; Hinnebusch, A.G. The novel ATP-binding cassette protein ARB1 is a shuttling factor that stimulates 40S and 60S ribosome biogenesis. Mol. Cell Biol. 2005, 25, 9859–9873. [Google Scholar] [CrossRef]
- Fostier, C.R.; Monlezun, L.; Ousalem, F.; Singh, S.; Hunt, J.F.; Boël, G. ABC-F translation factors: From antibiotic resistance to immune response. FEBS Lett. 2021, 595, 675–706. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, P.; Mosquera, J.; Anderson, M.; Birch, M.; Bromley, M.; Denning, D.W. Identification of novel genes conferring altered azole susceptibility in Aspergillus fumigatus. FEMS Microbiol. Lett. 2012, 332, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.G.; Bromley, M.; Buied, A.; Moore, C.B.; Rajendran, R.; Rautemaa, R.; Ramage, G.; Denning, D.W.; Bowyer, P. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2013, 68, 1486–1496. [Google Scholar] [CrossRef]
- Paul, S.; Diekema, D.; Moye-Rowley, W.S. Contributions of both ATP-binding cassette transporter and Cyp51A proteins are essential for azole resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2017, 61, e02748-16. [Google Scholar] [CrossRef]
- Ferreira, M.E.d.S.; Malavazi, I.; Savoldi, M.; Brakhage, A.A.; Goldman, M.H.S.; Kim, H.S.; Nierman, W.C.; Goldman, G.H. Transcriptome analysis of Aspergillus fumigatus exposed to voriconazole. Curr. Genet. 2006, 50, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Balzi, E.; Wang, M.; Leterme, S.; Van Dyck, L.; Goffeau, A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J. Biol. Chem. 1994, 269, 2206–2214. [Google Scholar] [CrossRef]
- Bissinger, P.H.; Kuchler, K. Molecular cloning and expression of the Saccharomyces cerevisiae STS1 gene product. A yeast ABC transporter conferring mycotoxin resistance. J. Biol. Chem. 1994, 269, 4180–4186. [Google Scholar] [CrossRef]
- Hirata, D.; Yano, K.; Miyahara, K.; Miyakawa, T. Saccharomyces cerevisiae YDR1, which encodes a member of the ATP-binding cassette (ABC) superfamily, is required for multidrug resistance. Curr. Genet. 1994, 26, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, A.; Kochler, A.; Martin, F.; Asiegbu, F.O. Diversity and evolution of ABC proteins in mycorrhiza-forming fungi. BMC Evol. Biol. 2015, 15, 249. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Vujanovic, V. ATP-Binding Cassette (ABC) Transporters in Fusarium Specific Mycoparasite Sphaerodes mycoparasitica during Biotrophic Mycoparasitism. Appl. Sci. 2022, 12, 7641. https://doi.org/10.3390/app12157641
Kim SH, Vujanovic V. ATP-Binding Cassette (ABC) Transporters in Fusarium Specific Mycoparasite Sphaerodes mycoparasitica during Biotrophic Mycoparasitism. Applied Sciences. 2022; 12(15):7641. https://doi.org/10.3390/app12157641
Chicago/Turabian StyleKim, Seon Hwa, and Vladimir Vujanovic. 2022. "ATP-Binding Cassette (ABC) Transporters in Fusarium Specific Mycoparasite Sphaerodes mycoparasitica during Biotrophic Mycoparasitism" Applied Sciences 12, no. 15: 7641. https://doi.org/10.3390/app12157641
APA StyleKim, S. H., & Vujanovic, V. (2022). ATP-Binding Cassette (ABC) Transporters in Fusarium Specific Mycoparasite Sphaerodes mycoparasitica during Biotrophic Mycoparasitism. Applied Sciences, 12(15), 7641. https://doi.org/10.3390/app12157641





