Special Issue “Thermochemical Conversion Processes for Solid Fuels and Renewable Energies: Volume II”
1. Introduction
- Gasification and combustion of biomass, refuse-derived fuel, tire-derived fuel, solid recovered fuel, sewage sludge, and low-rank coal;
- Technological combinations of thermochemical conversion processes based on renewable sources (power-to-fuel);
- Carbon capture, storage, and utilisation (CCS/U) technologies (carbon-capture-to-fuel);
- Renewable energy for heating and cooling to reduce peak demand, with energy storage systems to mitigate grid imbalances;
- CFD and process simulations of thermochemical conversion processes for solid fuels and renewables.
2. Special Issue Findings
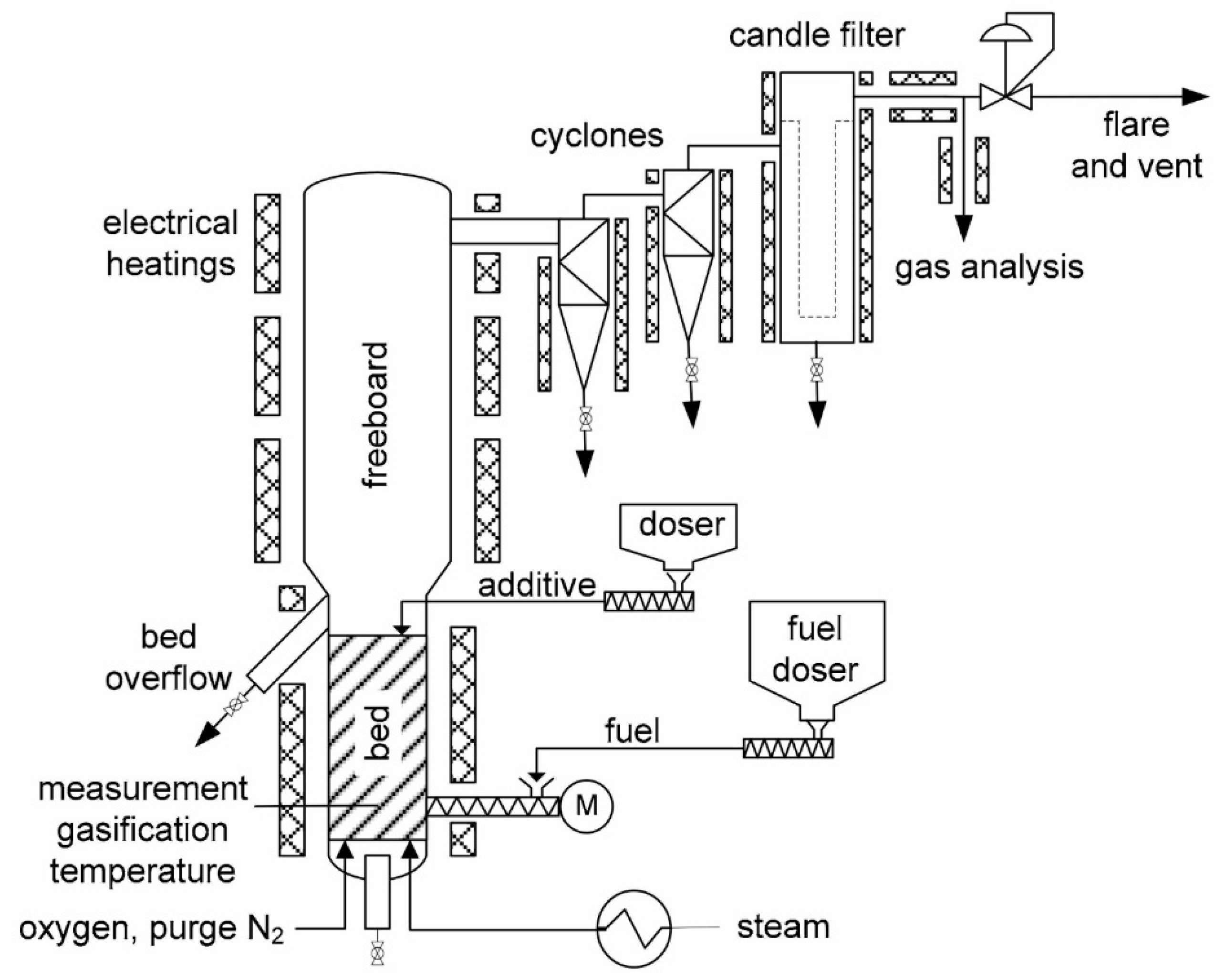
- The first contribution included in this Special Issue was submitted by Schmid, M.; Hafner, S.; and Scheffknecht, G., from the University of Stuttgart, Institute of Combustion and Power Plant Technology, Germany [2]. In this work, sewage sludge gasification was investigated using a 20 kW bubbling fluidised bed test facility (see Figure 1). Steam and oxygen were used as gasification agents, and in situ generated sewage sludge ash was applied as bed material. The influence of the main operating parameters such as gasification temperature, oxygen ratio, steam to carbon ratio, and fluidisation velocity on the composition of the produced raw gas (CO, H2, CO2, H2S, CH4, CxHy, COS, NH3, and tar) was determined. The authors found that the raw gas had high H2 and CO concentrations of up to 0.37 m3/m3 and 0.18 m3/m3, respectively, making it suitable for the synthesis of fuels and chemicals. By adjusting the steam-to-carbon ratio (by the water gas shift reaction), the H2/CO ratio of the syngas could be targeted for different synthesis products such as synthetic natural gas (H2/CO = 3) or Fischer–Tropsch products (H2/CO = 2). The analysis of the bed ash showed that the cadmium and mercury content was drastically reduced by the gasification process. Ash is appropriate as a secondary raw material to produce phosphorus or phosphate fertilisers.
- The second article, which was written by Kubonova, L.; Janakova L.; Malikova P.; Drabinova S.; Dej M.; Smelik R.; Skalny P.; and Heviankova S., from the VSB-Technical University of Ostrava, Czech Republic, dealt with the thermochemical utilisation of sewage sludge based on thermogravimetric analysis (TGA) [3]. Since sewage sludge is difficult to use as a feedstock due to its high moisture and ash content, its energy utilisation is increased by adding suitable waste materials (various types of plastics, used tires, and paper waste). Fifty-five waste mixtures were prepared in different volume ratios, a visual comparison was made, and high and low calorific values were measured. It was found that thermoplastics with sewage sludge and low-density polyethylene with sewage sludge had the lowest residual masses and the highest weight loss rates. Higher pyrolysis temperatures have always resulted in higher gas volume yields, as observed in many previous studies, e.g., [4]. The authors claimed that the highest average mass yield was reached in the pyrolysis of plastics. The most suitable material and temperature combinations to produce pyrolysis oils and gases were the blends of thermoplastics with sewage sludge and low-density polyethylene with sewage sludge.
- Solar absorption cooling systems are more advantageous than other cooling systems such as vapour compression because the peak cooling load nearly coincides with the available solar energy. The most common working fluid pairs are water/lithium bromide (H2O-LiBr) (water as refrigerant and lithium bromide as a solvent, especially for air conditioning and process cooling) and ammonia/water (NH3-H2O) (ammonia as refrigerant and water as a solvent, especially for frozen food applications). The authors of the third article (Al-Falahi, A.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany) carried out a thermo-economic analysis to compare different configurations of solar absorption cooling systems used for thermal cooling processes [5]. While the first solar absorption cooling system used H2O-LiBr as the working fluid pair, the second absorption cooling system adopted NH3-H2O. Parabolic trough collectors and evacuated tube collectors were applied under the same operating conditions as a source of thermal energy for both systems. The results showed that parabolic trough collectors combined with H2O-LiBr provided lower design aspects and minimum rates of hourly costs (5.2 USD/h), followed by evacuated tube collectors with H2O-LiBr (5.6 USD/h). Furthermore, it was found that H2O-LiBr provided a lower thermo-economic product cost (0.14 USD/GJ) compared with NH3-H2O (0.16 USD/GJ). The thermal ratio (absorption refrigeration cycle coefficient of performance) was between 0.5 and 0.9.
- The authors of the fourth article (Nguyen, N.M.; Alobaid F.; and Epple, B., from the Technical University of Darmstadt, Germany, and Can Tho University, Vietnam) developed an Aspen plus model for steam gasification of biomass to investigate the influence of operating parameters on the gasification process under steady-state conditions [6]. To consider the hydrodynamic, reaction kinetic decomposition of biomass and pyrolysis yield distribution, external FORTRAN codes were written and implemented in the developed model. The numerical results were compared with the measurement obtained from a 5 kWth bubbling fluidised bed test rig, showing good agreement. A slight discrepancy between the simulation model and the measured data was observed, which is due to the limitations of the model, i.e., simplified calculations of the hydrodynamics and kinetics of the bed and the absence of tar decomposition reactions. The authors claimed that the gasification process is favoured at higher temperatures. Under such conditions, hydrogen production and carbon conversion efficiency increased, while the percentage of carbon monoxide and methane in the product gas decreased. Increasing the amount of steam in the reactor promoted biomass gasification performance. The ratio of steam to biomass increased the content and yield of hydrogen in the product gas and significantly improved the gas yield and carbon conversion efficiency.
- The fifth contribution included in this Special Issue is by Lucantonio, S.; Di Giuliano, A.; and Gallucci, K., from the University of L’Aquila, Italy [7]. This study investigated the chemical looping gasification of wheat straw pellets as part of the European research project entitled Chemical Looping Gasification for Sustainable Production of Biofuels (CLARA; G.A. 817841 [8]). The focus was on wheat straw pellet pre-treatment, i.e., torrefaction and torrefaction-washing. After pre-treatment, the devolatilisation of individual pellets was carried out in a laboratory-scale fluidised bed of sand at 700, 800, and 900 °C to both quantify and analyse the product gas released from differently pre-treated types of biomass. The experimental data were evaluated using integral average parameters such as gas yield (H2/CO mole ratio) and carbon conversion. For all biomasses, increasing the devolatilisation temperature between 700 and 900 °C improved the thermochemical conversion in terms of gas yield, carbon conversion, and H2/CO mole ratio in the product gas. As for the pre-treatments, the main evidence was observed in the overall improvement in product gas quality (i.e., composition) and quantity compared with the untreated pellets. Negligible differences were observed between the different pre-treatments, mainly owing to the peak quantities that showed an improvement in the H2/CO mole ratio in correlation with the increase in torrefaction temperature from 250 to 270 °C.
- The authors of the sixth article published by Al-Maliki, A. K. W.; Mahmoud S. N.; Al-Khafaji, M. H. H.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq, developed a dynamic process simulation model to improve the efficiency of parabolic trough power plants and their associated control structures [9]. The model was created using advanced process simulation software (APROS) [10] and validated against an existing Andasol II solar power plant in Spain. The study focused on control circuits used in the thermal storage system of a parabolic trough power plant during the charging/discharging cycle. In the charging phase, the heat transfer fluid (Therminol VP-1) from the solar field at a temperature of 393 °C heated the molten salt (sodium and potassium nitrates), which was pumped from a cold tank into a hot tank and stored in a temperature of about 386 °C. The charging phase continued until the maximum capacity of stored thermal energy of about 1025 MWth h was reached. During the discharge phase, the hot molten salt heated the heat transfer fluid (Therminol VP-1), to be used in the power block via the heat exchangers. The molten salt left the heat exchangers and entered the cold tank at a temperature of approximately 292 °C. Accordingly, the thermal storage system provides the thermal power at the nominal load (125.75 MWth) for about 7.5 h in the evening time. The authors stated that the main results of this study should help researchers and designers understand the advanced control structures used in parabolic trough power plants. For more studies on the dynamic simulation of solar power plants, see this review paper [11].
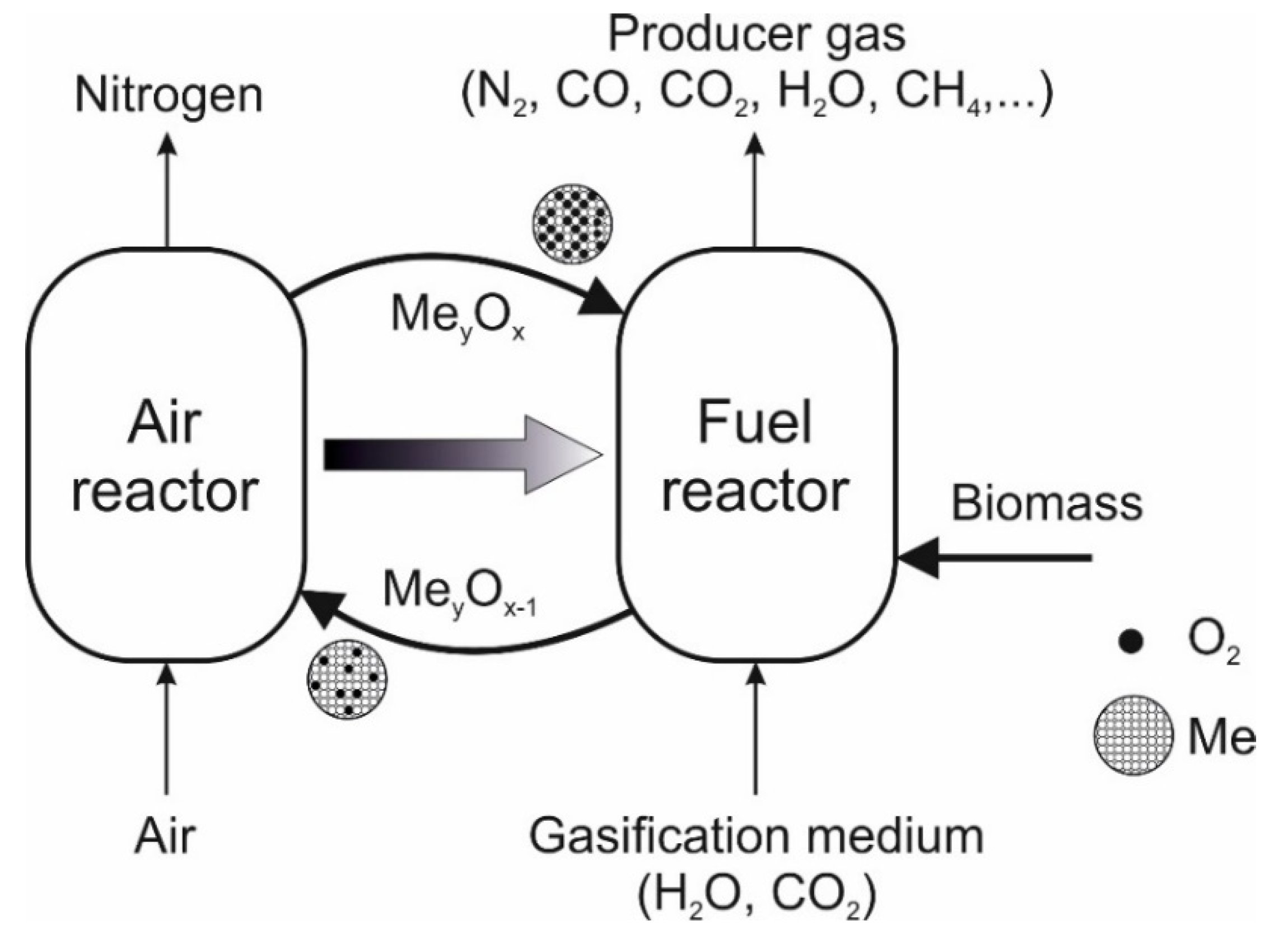
- The seventh article in this Special Issue addressed an important topic currently under research as an effective technology for the gasification of solid fuels such as biomass and refuse-derived fuels to produce valuable products, e.g., methanal or Fischer–Tropsch synthesis (see Figure 2). Here, recent developments related to the biomass-based chemical looping gasification process were reported by the authors (Nguyen, N. M.; Alobaid F.; Dieringer, P.; and Epple, B., from the Technical University of Darmstadt, Germany, and Can Tho University, Vietnam) [12]. The influence of process parameters such as gasification temperature, steam-to-biomass ratio, oxygen-carrier-to-biomass ratio, and biomass characteristics on the performance of the chemical looping gasification process was discussed. Furthermore, improvements in syngas purification technologies were shown to reduce problems associated with breakdowns and plugging, as well as efficiency losses. The latest experimental and simulation/modelling studies and their basic assumptions were described in detail. The authors pointed out that the proof of concept has been carried out for a pilot scale system from a few kW up to one MW. This is the basis for the scaling up of the process, which is required for commercial power plant and syngas production together with CO2 capture. Finally, the review paper emphasised current research topics, highlighted research gaps, and identified opportunities for future applications of the biomass-based chemical looping gasification process.
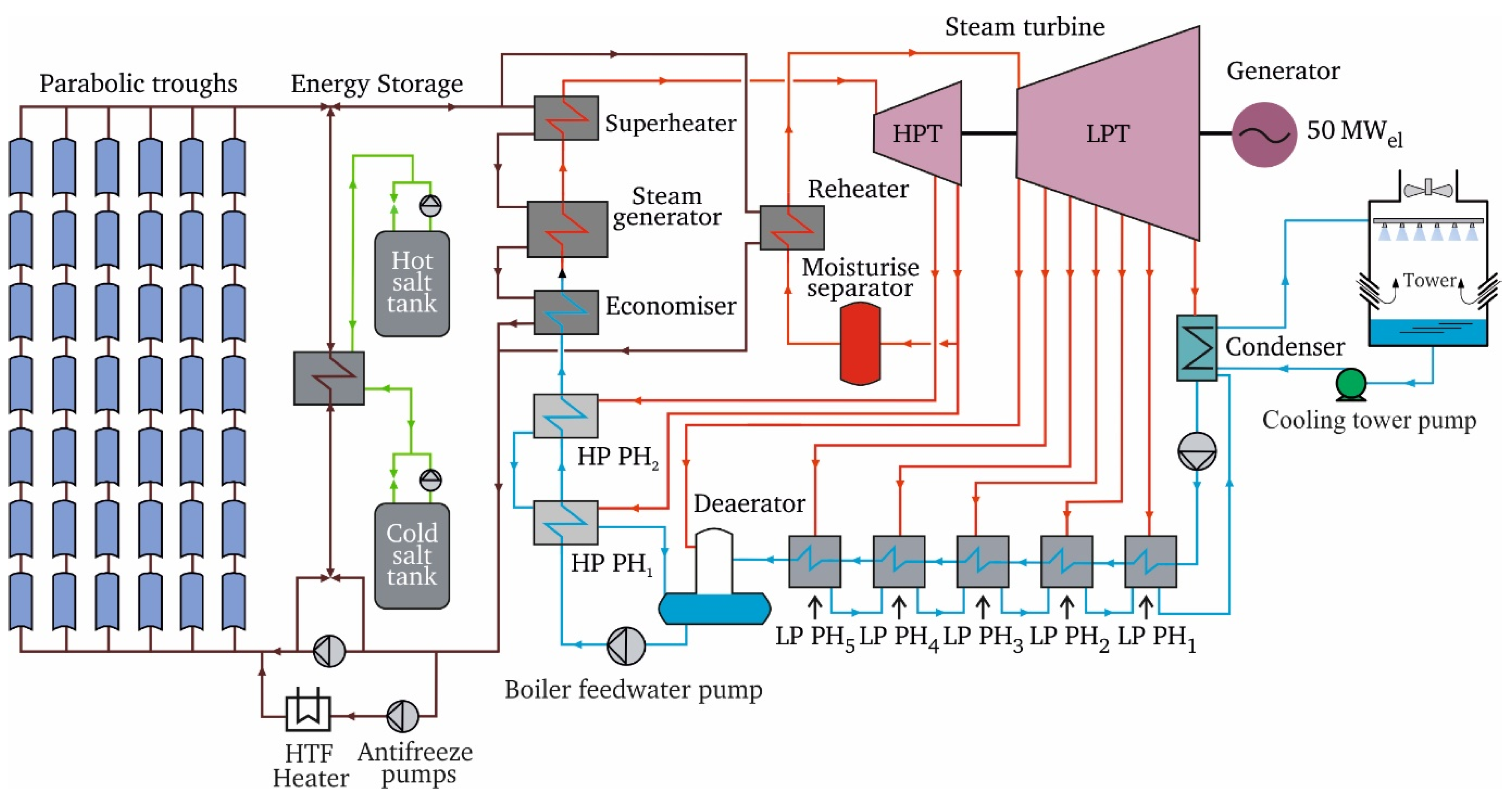
- The authors of the eighth article (Al-Maliki, A.K.W.; Al-Hasnawi, G.T.A.; Abdul Wahhab, A.H.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq) presented a detailed dynamic model of an existing parabolic trough solar power plant using the advanced process simulation software (APROS) [13]. The authors claimed that the developed model is the first in the literature that analyses the dynamic interaction of all parts of a parabolic trough solar power plant, including the solar field, thermal storage system, and power block (see Figure 3), and described the heat transfer fluid and steam-water paths in detail. Furthermore, all control circuits such as drum level and steam bypass controllers were included in the developed model. The model was first validated using measured data from an existing Andasol II solar power plant in Spain. In the next step, the validated model was applied to determine the best operating strategy based on direct normal irradiance variations during the day. The operating strategy used in this model was effective compared with the decisions made by operators during cloudy conditions, as it improved the performance of the power plant and increased the operating hours.
- The authors of the ninth article, Almoslh, A.; Alobaid, F.; Heinze, C.; and Epple, B., from the Technical University of Darmstadt, Germany, built and operated an absorber test rig for CO2 capture [14]. The study aimed to assess the effect of gas flow rate on the hydrodynamic characteristics of the sieve tray including wet tray pressure drop, total tray pressure drops, dry tray pressure drops, liquid holdup, clear liquid height, and froth height. It was found that the inlet gas flow rate has a considerable influence on the hydrodynamic characteristics of the sieve tray. While increasing the inlet gas flow rate up to a certain value improved the liquid retention capacity, raising the inlet gas flow rate above this value did not increase the liquid holdup. There was a relationship between absorber performance and the liquid holdup on the sieve tray. The performance of the CO2 absorber increased due to the rise in liquid holdup caused by the increase in inlet flow rate. The interface between the gaseous and liquid phases varied with a change in gas flow rate. The increase in froth height is a parameter that provides information about how large the interface is between the gaseous and liquid phases. Finally, the authors found that the hydrodynamic properties of the shell are crucial for the choice of optimal absorber operating conditions.
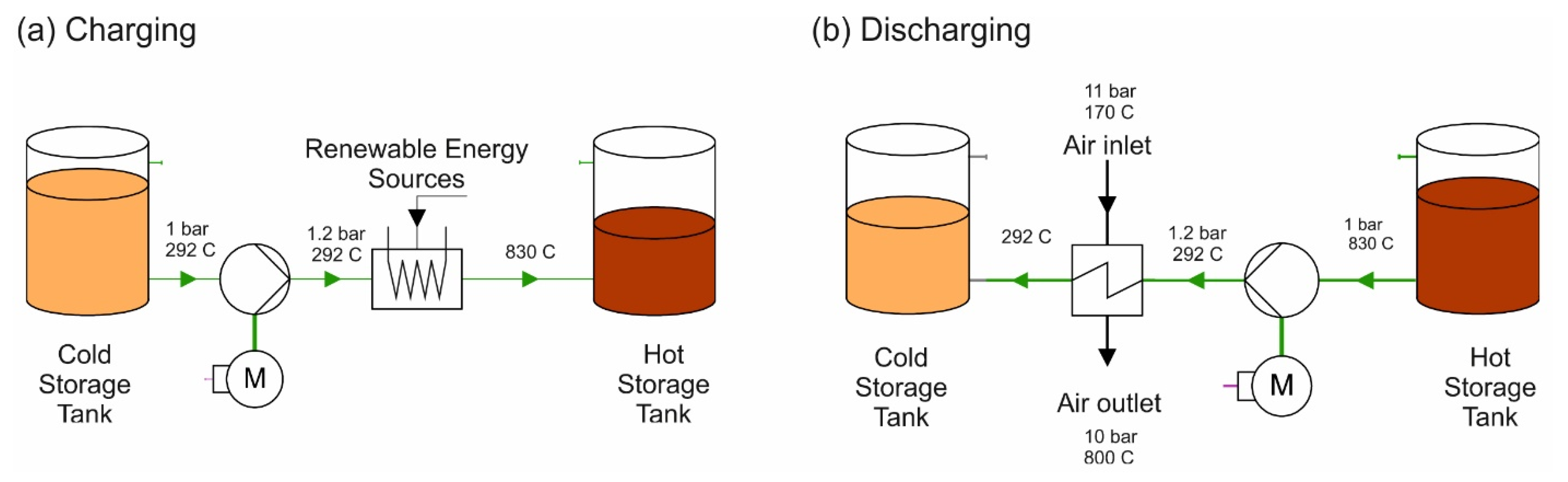
- The main contribution of the tenth article is related to molten salt energy storage systems. The authors (Al-Maliki, A.K.W.; Alobaid, F.; Keil, A.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq) created a numerical model of a molten salt energy storage system used in existing Andasol II solar power plant in Spain [15]. The storage model was validated by comparing the results with measured data from the real power plant. A system analysis and system optimisation were then performed, and the stand-alone concept of the thermal storage system was introduced. Stand-alone means an isolated deployment of the storage system without a solar power plant. At lower peak power demands, the storage medium was heated with surplus electrical energy and later fed back into the electrical grid via a steam cycle at higher peak power demands (see Figure 4). The system was then further optimised by modelling four different types of storage medium (Andasol 2 (base), SSalt max, Hitec, and carbonate salt) with different temperature differences in the cold and hot tanks. From the comparison, the most preferred storage medium was found to be carbonate salt, as it increased both the efficiency and the capacity. The largest increase in efficiency in terms of electricity generation could also be achieved with carbonate salt (18.2%), while the increase for SSalt max and Hitec was 9.5% and 7.4%, respectively.
- In the eleventh article of this Special Issue, the authors Judran, K. H.; Al-Hasnawi, G. T. A.; Al Zubaid, N. F.; Al-Maliki, A. K. W.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq, prepared a new nanofluid that has the potential to be used in various applications such as working fluids in cooling systems [16]. A novel, two-step technique (co-precipitation method) was used to prepare an efficient magnesium oxide–deionised water (MgO-DW) nanofluid without using surfactants and/or organic base fluids. Then, the influence of the volume concentration of the nanopowder and the duration of the ultrasonic treatment on the stability and thermophysical properties of the MgO-DW nanofluid at room temperature was investigated. Based on the experimental assessment, the co-precipitation method was successfully applied to prepare crystalline and pure MgO nanopowder, with an average particle size of 33 nm. Scanning electron microscopy (SEM) images demonstrated unique feathery or fluffy-like nanostructures of the MgO nanopowder that was prepared with volume concentrations ranging from 0.05% to 0.25% and ultrasonic treatment times ranging from 45 to 180 min at ambient temperature. The introduction of MgO nanoparticles to conventional liquid such as deionised water improved the thermal conductivity, with the highest value of thermal conductivity improvement of 25.08% observed at a volume concentration of 0.25% and ultrasonic treatment time of 180 min. The impact of ultrasonic treatment duration on the improvement of thermal conductivity was relatively like the impact of nanoparticle volume concentration. However, this conductivity improvement was limited after an ultrasonication duration of 135 min. The measurements of dynamic viscosity showed that it was directly proportional to the volume concentration of MgO-DW nanofluid until the highest value of 0.0052 [Pa s] was reached at a higher volume solid content of 0.25% of MgO-DW nanofluid. By contrast, increasing the ultrasonic treatment time resulted in a sharp decrease in the dynamic viscosity of the nanofluid samples. It was found that a MgO-DW nanofluid with good dispersion stability, high thermal conductivity, and low viscosity could be generated by controlling the ultrasonic time and/or volume concentration.
- The authors of the twelfth article in this Special Issue (Aghel, B.; Janati, S.; Alobaid, F.; Almoslh, A.; and Epple, B., from the Technical University of Darmstadt, Germany, and Kermanshah University of Technology, Iran) provided a review article on the possible application of nanofluids in CO2 absorption [17]. Fossil fuel conversion (e.g., combustion or gasification) and industrial processes are now the main sources of CO2 emissions, along with forestry and land use, including livestock. Reducing greenhouse gas emissions is, therefore, essential to mitigate climate change and its devastating environmental consequences. Carbon capture and storage (CCS) or carbon dioxide utilisation (CCU) technologies are one of the most environmentally friendly options for reducing anthropogenic greenhouse gas emissions. However, these technologies are currently associated with significant efficiency losses, resulting in higher CO2 avoidance costs. Owing to the advent of nanotechnology and its unique advantages in various fields, a new approach was introduced using suspended particles in a base fluid (suspension) to achieve maximum absorption efficiency and minimum energy consumption through CO2 capture. In this review article, the performance of nanofluids, preparation methods, and their stability, which is one of the most important factors to prevent sedimentation of nanofluids, were discussed. The objective of this article was to present the factors contributing to CO2 absorption by nanofluids in an easy-to-understand manner, focusing on the role of base fluids and the reasons for their selection. Based on research in the literature, the authors claimed that nanofluids are an effective way to increase CO2 absorption compared with the base fluid, which can reduce host plant energy consumption.
- The thirteenth article in this Special Issue focused on evaluating the thermal performance of a tubular heat exchanger equipped with combined basket-twisted tape inserts. Here, the authors, Khafaji, Q.A.H.; Wahhab, A.A.H.; Alsaedi, S.S; Maliki, A.K.W.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq, experimentally investigated the properties of tubular heat exchangers [18]. For this purpose, a test rig was built, equipped with novel inserts, and finally operated under different conditions. Air was used as the working medium, and a constant wall heat flux was provided for turbulent discharge conditions (6000 ≤ Re ≤ 19,500). Two inserts were used to improve heat transfer: First, bascule turbulators were inserted into the heat exchanger with a constant pitch ratio (PR = 150 mm), and second, basket turbulators together with a twisted tape were installed in the core of the basket turbulators. The results showed that the Nusselt numbers were 131.8%, 169.5%, 187.7%, and 206.5% higher than those of a plain heat exchanger for basket turbulators and the combined basket-twisted tape inserts with y/w = 6, 3, and 2, respectively. On average, the maximum thermal efficiency of the elevated tubular heat exchanger was 1.63 times higher than that of the simple heat exchanger, which is due to a binary basket-shaped band for a twist ratio y/w equal to 2 at constant pump power. Finally, practical correlations for the Nusselt number and friction characteristics were developed and presented.
- The fourteenth article in this Special Issue dealt with the microstructural analysis and mechanical characteristics of hybrid nanocomposites of aluminium (Al), ferric oxide (Fe2O3), and silver (Ag). The aluminium alloys can be used in many fields such as electronics, aerospace, and automotive due to their properties such as lightness, strength, wear, and corrosion resistance. The authors (Salman, D.K.; Maliki, A.K.W.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq) aimed to specify the microstructure and investigate the mechanical properties of Al alloys, into which different amounts of Fe2O3 (3, 6, 9, 12, and 15 wt.%) were introduced with a constant Ag content of 1 wt.% [19]. The Al/Fe2O3 + Ag hybrid nanocomposite samples were synthesised via powder metallurgy, and the microstructure was visualised via field-emission scanning electron microscopy (FESEM) and X-ray diffraction (XRD) investigations. Furthermore, mechanical experiments, such as microhardness tests, compression tests, and wear tests, were performed to estimate the mechanical properties of the hybrid nanocomposites. The FESEM and XRD results showed that the Fe2O3 and Ag nanoparticles were uniformly distributed and dispersed in the Al matrix. Compared with pure Al, the mechanical test values increased with increasing weight percentages of the reinforcing nanoparticles up to 12 wt.% Fe2O3. Here, the microhardness was 34.19 HV, the compressive strength was 89.9 MPa, and the wear rate was about 1.8 × 10−8 g/cm for Al + 12 wt.% Fe2O3 + 1 wt.% Ag. The authors claimed that the results of this work demonstrated an improvement in the mechanical properties of aluminium alloys.
- The last article of this Special Issue was published by Salman, D.K.; Maliki, A.K.W.; Alobaid, F.; and Epple, B., from the Technical University of Darmstadt, Germany, and the University of Technology, Iraq [20]. Here, the authors synthesised a nickel–titanium—silver (Ni-Ti-Ag) shape memory alloy by casting to investigate its microstructure and mechanical properties. Ag (grain size 1 mm) was integrated into Ni-Ti alloys with different weight percentages (0, 1.5, 3, and 4.5 wt.% Ag) to prepare shape memory alloys using a vacuum arc remelting (VAR) furnace. The microstructural analysis was performed using field-emission scanning electron microscopy (FESEM) and X-ray diffraction (XRD), whereas the transformation temperatures of the Ni-Ti-Ag shape memory alloy were obtained via a differential scanning calorimetry (DSC) investigation. The results showed that Ag was homogeneously distributed in the Ni-Ti alloy. In addition, two primary phases (martensite phase and austenite phase) were formed with few impurities. The XRD investigation indicated that the number of Ag peaks showed an increase with the enhancement in Ag weight fraction. The DSC investigation revealed that the austenite transformation temperature began at −1.6 °C, and the transformation temperatures increased with increasing Ag content to 19.7 °C, 12.7 °C, and 12.3 °C, respectively. Increasing Ag content significantly affected the mechanical properties of the Ni-Ti base alloy in terms of its compressive strength and microhardness. The highest value of compressive strength was reached for the alloy with 3 wt.% Ag, whereas the microhardness tests showed a minor decrease with the increase in Ag weight fraction.
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alobaid, F.; Ströhle, J. Special issue “thermochemical conversion processes for solid fuels and renewable energies”. Appl. Sci. 2021, 11, 1907. [Google Scholar] [CrossRef]
- Schmid, M.; Hafner, S.; Scheffknecht, G. Experimental parameter study on synthesis gas production by steam-oxygen fluidized bed gasification of sewage sludge. Appl. Sci. 2021, 11, 579. [Google Scholar] [CrossRef]
- Kubonova, L.; Janakova, I.; Malikova, P.; Drabinova, S.; Dej, M.; Smelik, R.; Skalny, P.; Heviankova, S. Evaluation of waste blends with sewage sludge as a potential material input for pyrolysis. Appl. Sci. 2021, 11, 1610. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Onwudili, J.A.; Meng, A.; Zhang, Y.; Williams, P.T. Influence of process conditions on the formation of 2–4 ring polycyclic aromatic hydrocarbons from the pyrolysis of polyvinyl chloride. Fuel Process. Technol. 2016, 144, 299–304. [Google Scholar] [CrossRef]
- Al-Falahi, A.; Alobaid, F.; Epple, B. Thermo-Economic Comparisons of Environmentally Friendly Solar Assisted Absorption Air Conditioning Systems. Appl. Sci. 2021, 11, 2442. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Epple, B. Process simulation of steam gasification of torrefied woodchips in a bubbling fluidized bed reactor using aspen plus. Appl. Sci. 2021, 11, 2877. [Google Scholar] [CrossRef]
- Lucantonio, S.; Di Giuliano, A.; Gallucci, K. Influences of the Pretreatments of Residual Biomass on Gasification Processes: Experimental Devolatilizations Study in a Fluidized Bed. Appl. Sci. 2021, 11, 5722. [Google Scholar] [CrossRef]
- Dieringer, P.; Marx, F.; Alobaid, F.; Ströhle, J.; Epple, B. Process Control Strategies in Chemical Looping Gasification—A Novel Process for the Production of Biofuels Allowing for Net Negative CO2 Emissions. Appl. Sci. 2020, 10, 4271. [Google Scholar] [CrossRef]
- Al-Maliki, W.A.K.; Mahmoud, N.S.; Al-Khafaji, H.M.; Alobaid, F.; Epple, B. Design and Implementation of the Solar Field and Thermal Storage System Controllers for a Parabolic Trough Solar Power Plant. Appl. Sci. 2021, 11, 6155. [Google Scholar] [CrossRef]
- Al-Maliki, W.A.K.; Hadi, A.S.; Al-Khafaji, H.M.; Alobaid, F.; Epple, B. Dynamic Modelling and Advanced Process Control of Power Block for a Parabolic Trough Solar Power Plant. Energies 2021, 15, 129. [Google Scholar] [CrossRef]
- Alobaid, F.; Mertens, N.; Starkloff, R.; Lanz, T.; Heinze, C.; Epple, B. Progress in dynamic simulation of thermal power plants. Prog. Energy Combust. Sci. 2017, 59, 79–162. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Dieringer, P.; Epple, B. Biomass-based chemical looping gasification: Overview and recent developments. Appl. Sci. 2021, 11, 7069. [Google Scholar] [CrossRef]
- Al-Maliki, W.A.K.; Al-Hasnawi, A.G.T.; Abdul Wahhab, H.A.; Alobaid, F.; Epple, B. A Comparison Study on the Improved Operation Strategy for a Parabolic trough Solar Power Plant in Spain. Appl. Sci. 2021, 11, 9576. [Google Scholar] [CrossRef]
- Almoslh, A.; Alobaid, F.; Heinze, C.; Epple, B. Experimental Study of the Influence of Gas Flow Rate on Hydrodynamic Characteristics of Sieve Trays and Their Effect on CO2 Absorption. Appl. Sci. 2021, 11, 10708. [Google Scholar] [CrossRef]
- Al-Maliki, W.A.K.; Alobaid, F.; Keil, A.; Epple, B. Dynamic Process Simulation of a Molten-Salt Energy Storage System. Appl. Sci. 2021, 11, 11308. [Google Scholar] [CrossRef]
- Judran, H.K.; Al-Hasnawi, A.G.T.; Al Zubaidi, F.N.; Al-Maliki, W.A.K.; Alobaid, F.; Epple, B. A High Thermal Conductivity of MgO-H2O Nanofluid Prepared by Two-Step Technique. Appl. Sci. 2022, 12, 2655. [Google Scholar] [CrossRef]
- Aghel, B.; Janati, S.; Alobaid, F.; Almoslh, A.; Epple, B. Application of Nanofluids in CO2 Absorption: A Review. Appl. Sci. 2022, 12, 3200. [Google Scholar] [CrossRef]
- Khafaji, H.Q.; Abdul Wahhab, H.A.; Alsaedi, S.S.; Al-Maliki, W.A.K.; Alobaid, F.; Epple, B. Thermal Performance Evaluation of a Tubular Heat Exchanger Fitted with Combined Basket–Twisted Tape Inserts. Appl. Sci. 2022, 12, 4807. [Google Scholar] [CrossRef]
- Salman, K.D.; Al-Maliki, W.A.K.; Alobaid, F.; Epple, B. Microstructural Analysis and Mechanical Properties of a Hybrid Al/Fe2O3/Ag Nano-Composite. Appl. Sci. 2022, 12, 4730. [Google Scholar] [CrossRef]
- Salman, K.D.; Al-Maliki, W.A.K.; Alobaid, F.; Epple, B. Microstructural Analysis and Mechanical Characterization of Shape Memory Alloy Ni-Ti-Ag Synthesized by Casting Route. Appl. Sci. 2022, 12, 4639. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alobaid, F.; Ströhle, J.; Epple, B. Special Issue “Thermochemical Conversion Processes for Solid Fuels and Renewable Energies: Volume II”. Appl. Sci. 2022, 12, 7478. https://doi.org/10.3390/app12157478
Alobaid F, Ströhle J, Epple B. Special Issue “Thermochemical Conversion Processes for Solid Fuels and Renewable Energies: Volume II”. Applied Sciences. 2022; 12(15):7478. https://doi.org/10.3390/app12157478
Chicago/Turabian StyleAlobaid, Falah, Jochen Ströhle, and Bernd Epple. 2022. "Special Issue “Thermochemical Conversion Processes for Solid Fuels and Renewable Energies: Volume II”" Applied Sciences 12, no. 15: 7478. https://doi.org/10.3390/app12157478
APA StyleAlobaid, F., Ströhle, J., & Epple, B. (2022). Special Issue “Thermochemical Conversion Processes for Solid Fuels and Renewable Energies: Volume II”. Applied Sciences, 12(15), 7478. https://doi.org/10.3390/app12157478





