Response of Osteoblasts to Electric Field Line Patterns Emerging from Molecule Stripe Landscapes
Abstract
1. Introduction
2. Materials and Methods
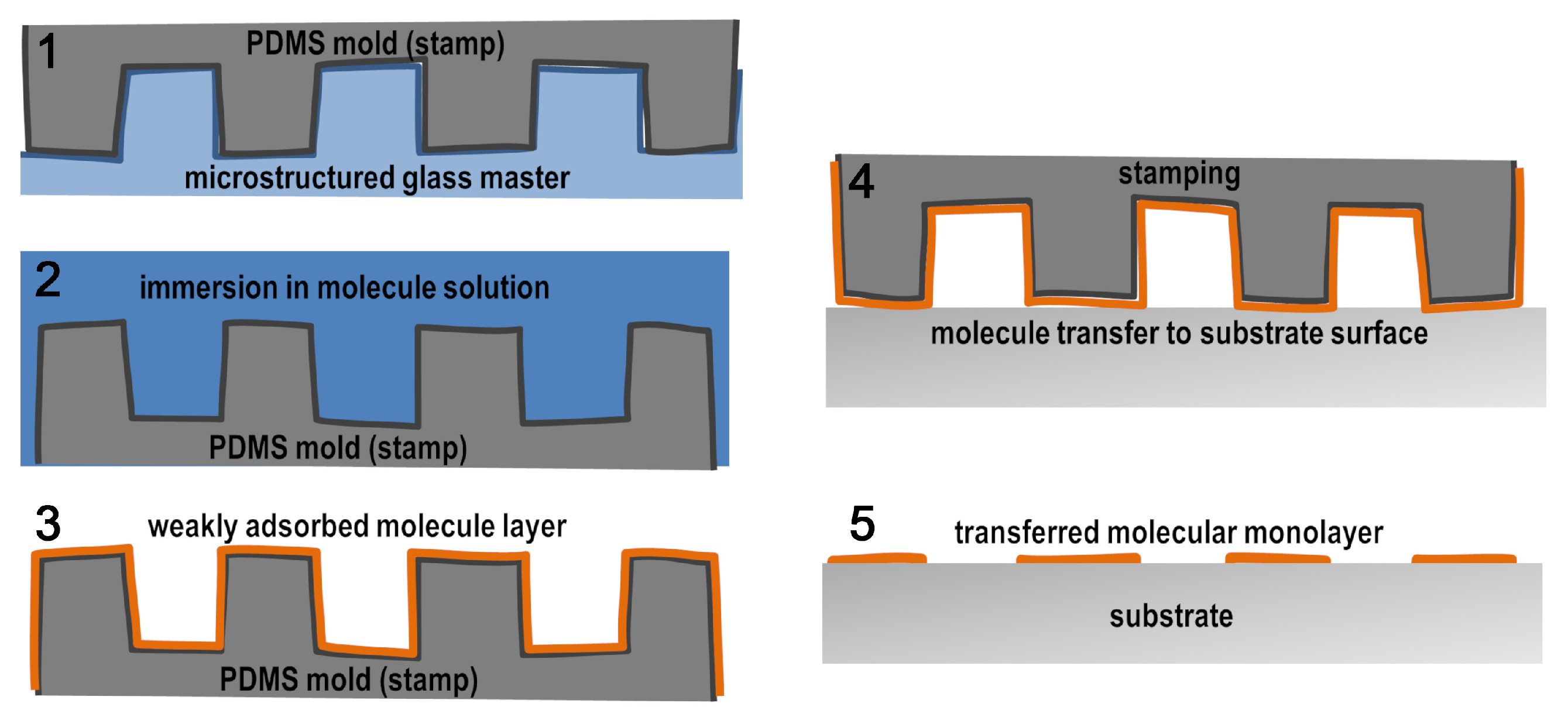
2.1. Preparation of PDMS Stamps
2.2. Microcontact Printing of Molecules on Glass
2.3. SICM & AFM Topography Measurements
2.4. Cell Culture
2.5. Fluorescence Microscopy
2.6. Statistics
3. Results and Discussion
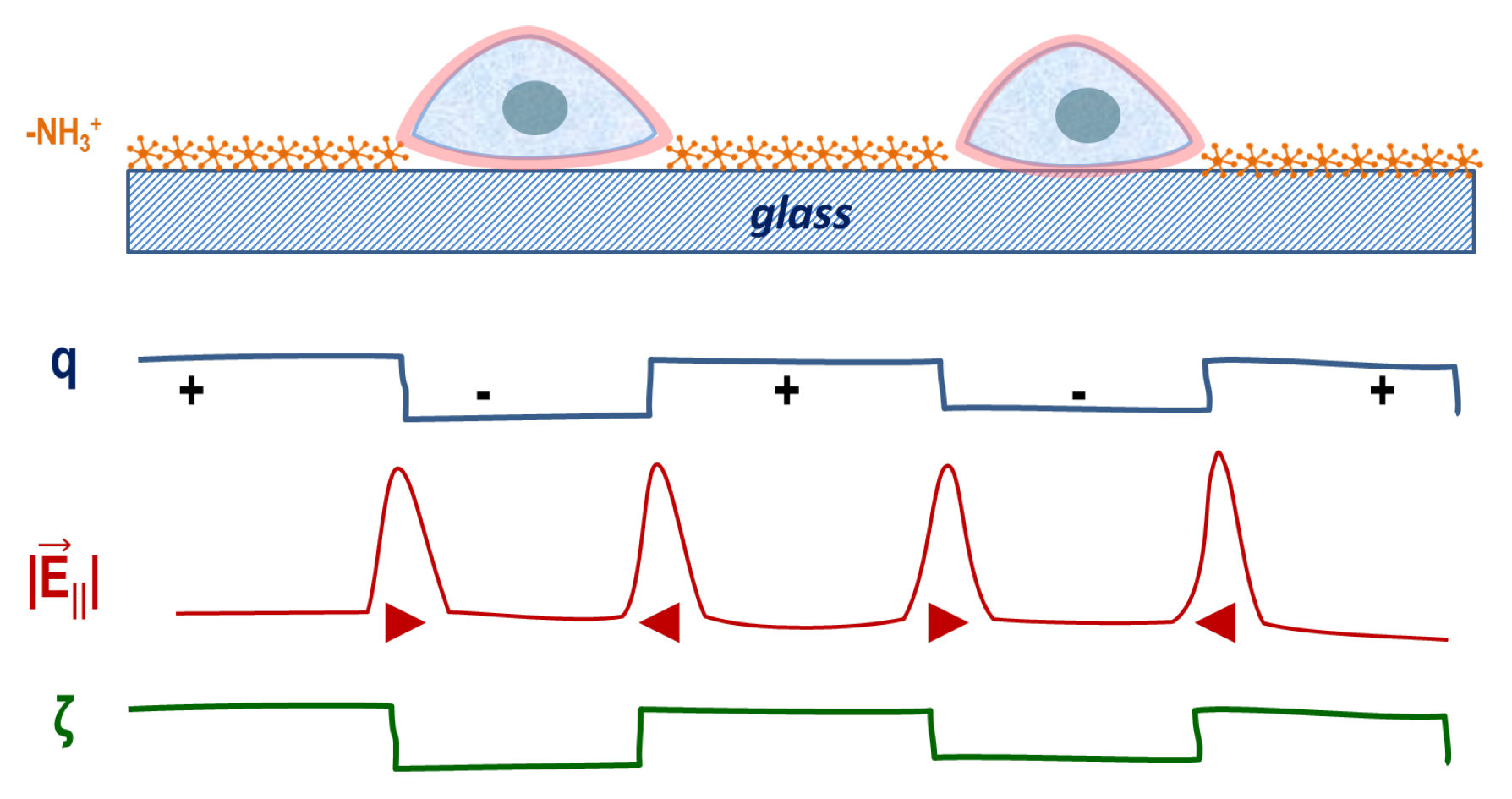
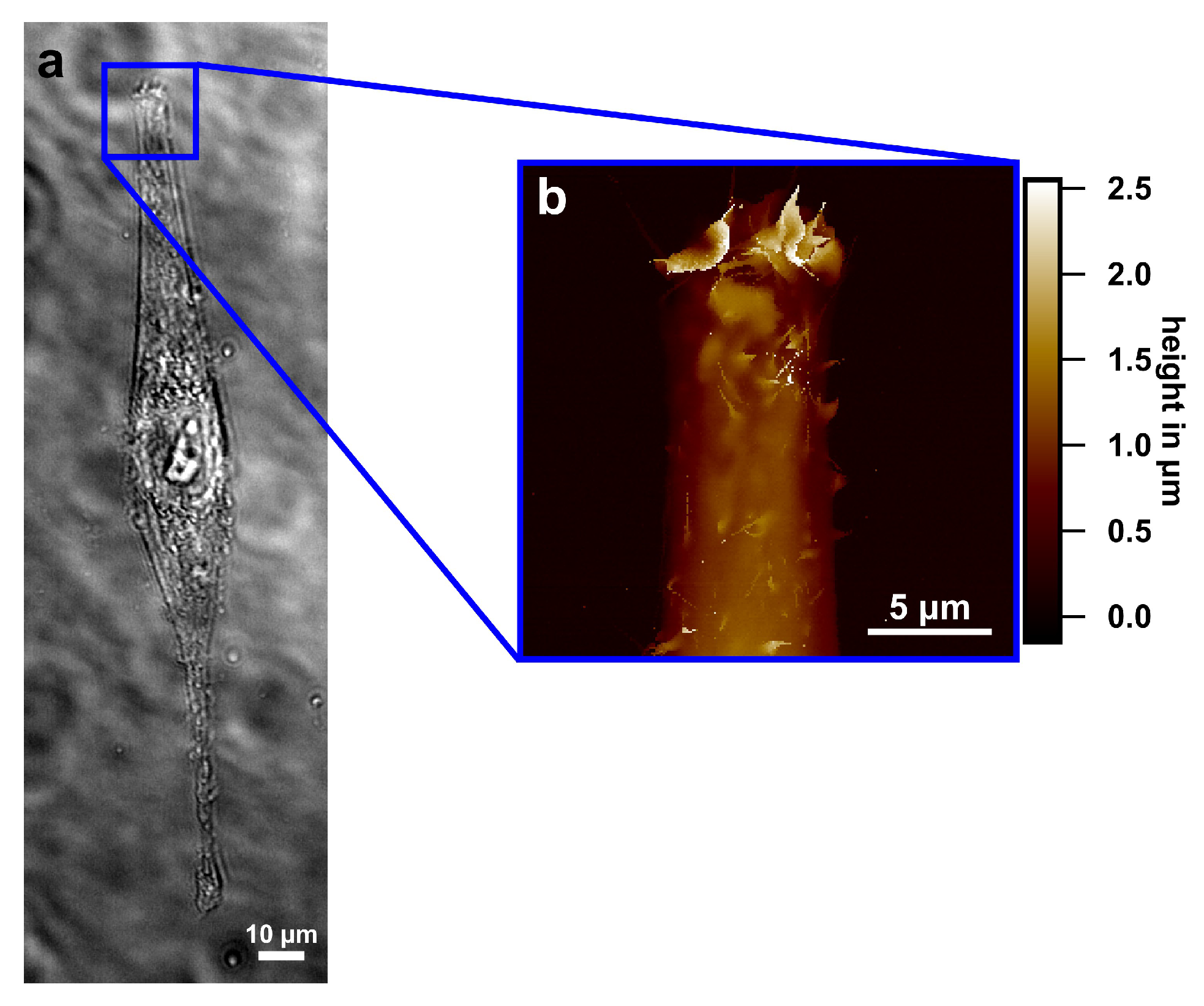
3.1. Characterization of Molecular Stripe Patterns
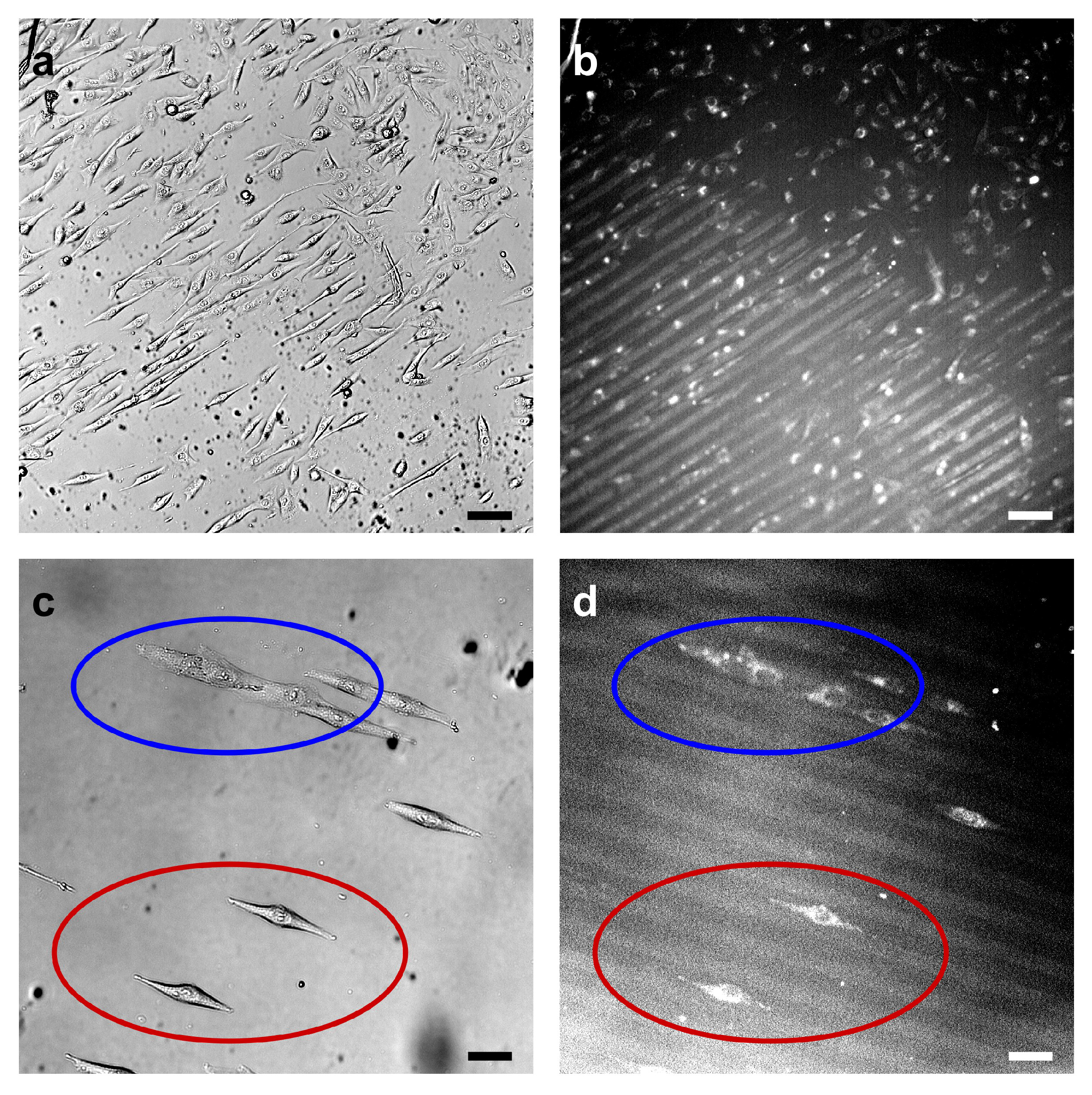
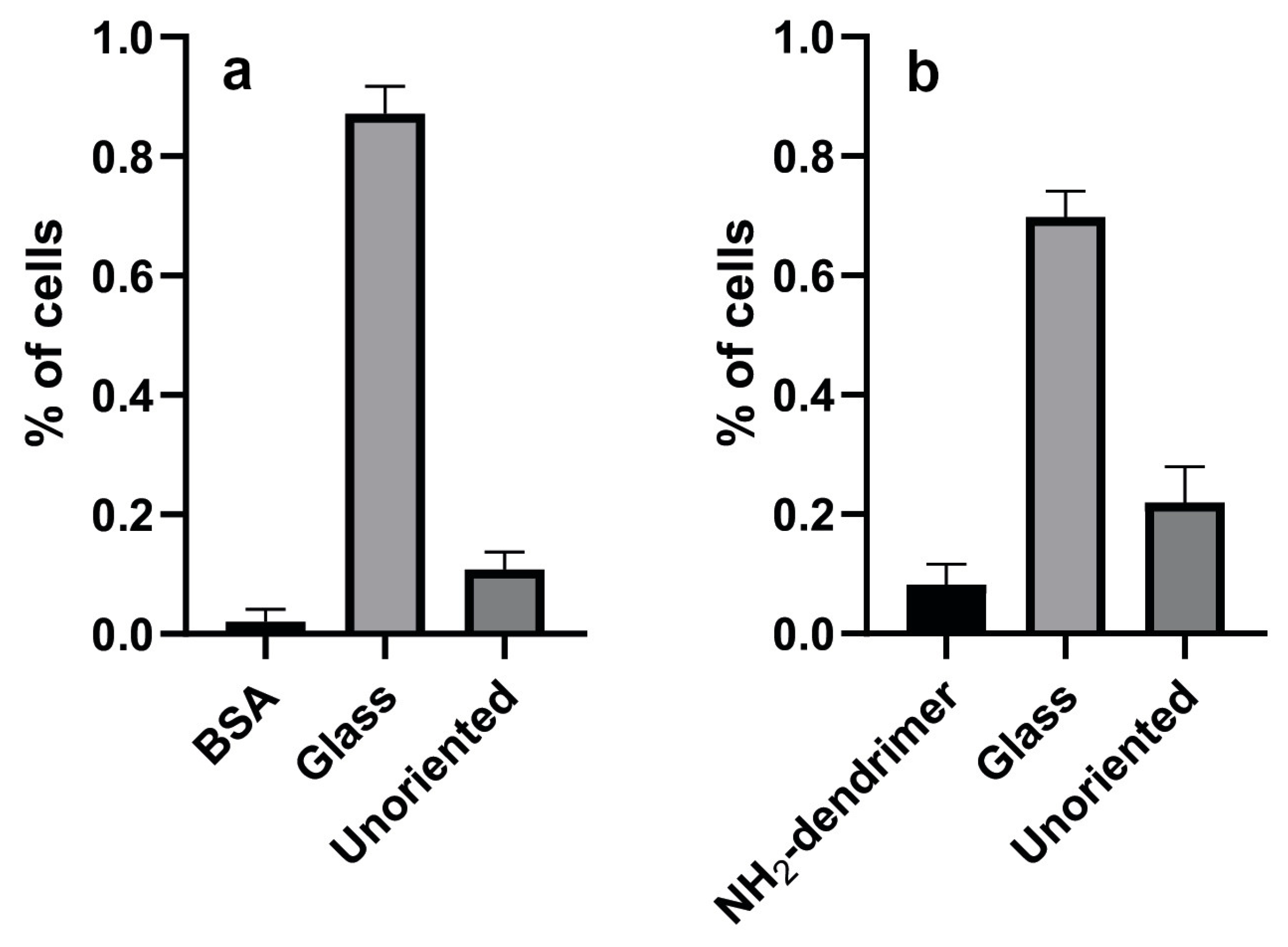
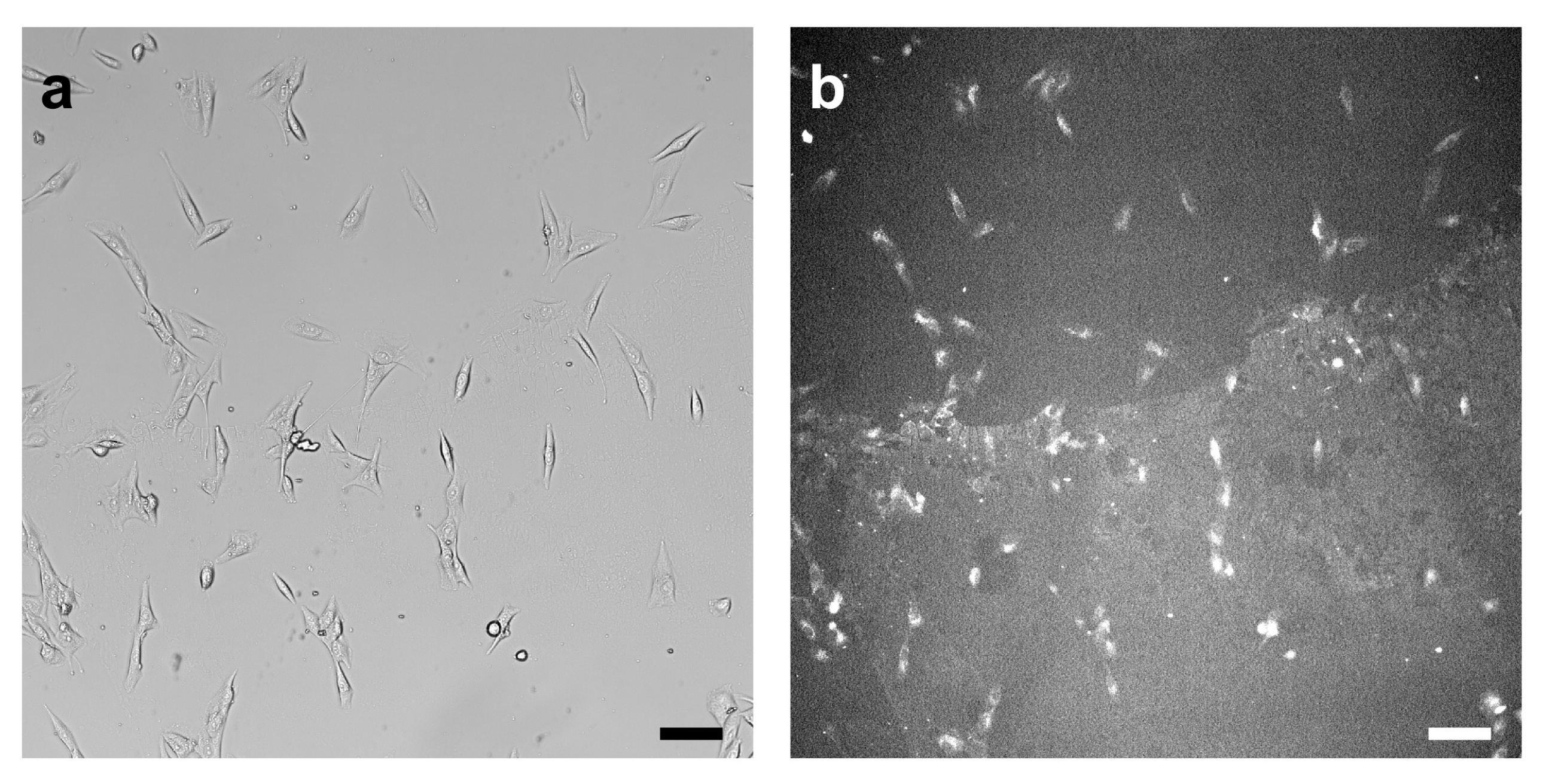
3.2. Cell Response to Molecular Landscapes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| APTES | (3-Aminopropyl)triethoxysilane |
| BSA | Bovine serum albumine |
| ECM | Extracellular matrix |
| GFP | Green fluorescent protein |
| HEPES | Isotonic 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer |
| CP | Microcontact printing |
| PAMAM | Polyamidoamine |
| PBS | Phosphate-buffered saline |
| PDADMA | Polydialyldimethylammonium |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethylene glycol |
| PSS | Polystyrene sulfonate |
| SICM | Scanning ion conductance microscopy |
| TMP | Trimethylol propane |
References
- Kam, N.W.S.; Jan, E.; Kotov, N.A. Electrical stimulation of neural stem cells mediated by humanized carbon nanotube composite made with extracellular matrix protein. Nano Lett. 2009, 9, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.M.; Do, A.T.; Wang, J.K.; Yeoh, C.L.; Yeo, K.S.; Choong, C. Development of a miniaturized stimulation device for electrical stimulation of cells. J. Biol. Eng. 2015, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Webster, T.J. Greater osteoblast proliferation on anodized nanotubular titanium upon electrical stimulation. Int. J. Nanomed. 2008, 3, 477–485. [Google Scholar] [CrossRef]
- Roessler, S.; Zimmermann, R.; Scharnweber, D.; Werner, C.; Worch, H. Characterization of oxide layers on Ti6Al4V and titanium by streaming potential and streaming current measurements. Colloids Surf. Biointerfaces 2002, 26, 387–395. [Google Scholar] [CrossRef]
- Luxbacher, T. The Zeta Potential for Solid Surface Analysis: A Practical Guide to Streaming Potential Measurement, 1st ed.; Anton Paar GmbH: Graz, Austria, 2014. [Google Scholar]
- Dupont-Gillain, C.C.; Nonckreman, C.J.; Adriaensen, Y.; Rouxhet, P.G. Fabrication of Surfaces with Bimodal Roughness Through Polyelectrolyte/Colloid Assembly. In Advances in Unconventional Lithography; Kostovski, G., Ed.; InTech: London, UK, 2011; p. 53. [Google Scholar] [CrossRef]
- Gruening, M.; Neuber, S.; Nestler, P.; Lehnfeld, J.; Dubs, M.; Fricke, K.; Schnabelrauch, M.; Helm, C.A.; Müller, R.; Staehlke, S.; et al. Enhancement of Intracellular Calcium Ion Mobilization by Moderately but Not Highly Positive Material Surface Charges. Front. Bioeng. Biotechnol. 2020, 8, 1016. [Google Scholar] [CrossRef]
- Curtze, S.; Dembo, M.; Miron, M.; Jones, D.B. Dynamic changes in traction forces with DC electric field in osteoblast-like cells. J. Cell Sci. 2004, 117, 2721–2729. [Google Scholar] [CrossRef]
- Delenda, B. Using Reaction-Diffusion Equations to Model and Simulate the Interaction of Bone Cells with Electrical Stimulation. Doctoral Dissertation, University Rostock, Rostock, Germany, 2020. [Google Scholar] [CrossRef]
- Rajnicek, A.M.; Foubister, L.E.; McCaig, C.D. Alignment of corneal and lens epithelial cells by co-operative effects of substratum topography and DC electric fields. Biomaterials 2008, 29, 2082–2095. [Google Scholar] [CrossRef]
- Loesberg, W.A.; te Riet, J.; van Delft, F.C.M.J.M.; Schön, P.; Figdor, C.G.; Speller, S.; van Loon, J.J.W.A.; Walboomers, X.F.; Jansen, J.A. The threshold at which substrate nanogroove dimensions may influence fibroblast alignment and adhesion. Biomaterials 2007, 28, 3944–3951. [Google Scholar] [CrossRef]
- Lamers, E.; Walboomers, X.F.; Domanski, M.; te Riet, J.; van Delft, F.C.M.J.M.; Luttge, R.; Winnubst, L.A.J.A.; Gardeniers, H.J.G.E.; Jansen, J.A. The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition. Biomaterials 2010, 31, 3307–3316. [Google Scholar] [CrossRef]
- Gates, B.D.; Xu, Q.; Stewart, M.; Ryan, D.; Willson, C.G.; Whitesides, G.M. New approaches to nanofabrication: Molding, printing, and other techniques. Chem. Rev. 2005, 105, 1171–1196. [Google Scholar] [CrossRef]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef]
- Koblinski, J.E.; Wu, M.; Demeler, B.; Jacob, K.; Kleinman, H.K. Matrix cell adhesion activation by non-adhesion proteins. J. Cell Sci. 2005, 118, 2965–2974. [Google Scholar] [CrossRef]
- Sousa, S.R.; Lamghari, M.; Sampaio, P.; Moradas-Ferreira, P.; Barbosa, M.A. Osteoblast adhesion and morphology on TiO2 depends on the competitive preadsorption of albumin and fibronectin. J. Biomed. Mater. Res. Part 2008, 84, 281–290. [Google Scholar] [CrossRef]
- Yang, Y.; Cavin, R.; Ong, J.L. Protein adsorption on titanium surfaces and their effect on osteoblast attachment. J. Biomed. Mater. Res. Part 2003, 67, 344–349. [Google Scholar] [CrossRef]
- Hindié, M.; Camand, E.; Agniel, R.; Carreiras, F.; Pauthe, E.; van Tassel, P. Effects of human fibronectin and human serum albumin sequential adsorption on preosteoblastic cell adhesion. Biointerphases 2014, 9, 029008. [Google Scholar] [CrossRef]
- Haag, S.L.; Schiele, N.R.; Bernards, M.T. Enhancement and mechanisms of MC3T3-E1 osteoblast-like cell adhesion to albumin through calcium exposure. Biotechnol. Appl. Biochem. 2022, 69, 492–502. [Google Scholar] [CrossRef]
- Fox, L.J.; Richardson, R.M.; Briscoe, W.H. PAMAM dendrimer—Cell membrane interactions. Adv. Colloid Interface Sci. 2018, 257, 1–18. [Google Scholar] [CrossRef]
- Buskermolen, A.B.C.; Ristori, T.; Mostert, D.; van Turnhout, M.C.; Shishvan, S.S.; Loerakker, S.; Kurniawan, N.A.; Deshpande, V.S.; Bouten, C.V.C. Cellular Contact Guidance Emerges from Gap Avoidance. Cell Rep. Phys. Sci. 2020, 1, 100055. [Google Scholar] [CrossRef]
- Alford, P.W.; Nesmith, A.P.; Seywerd, J.N.; Grosberg, A.; Parker, K.K. Vascular smooth muscle contractility depends on cell shape. Integr. Biol. Quant. Biosci. Nano Macro 2011, 3, 1063–1070. [Google Scholar] [CrossRef]
- Xia, N.; Thodeti, C.K.; Hunt, T.P.; Xu, Q.; Ho, M.; Whitesides, G.M.; Westervelt, R.; Ingber, D.E. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. FASEB J. 2008, 22, 1649–1659. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Moores, G.R. Cell guidance by micropatterned adhesiveness in vitro. J. Cell Sci. 1992, 103, 287–292. [Google Scholar] [CrossRef]
- Ramirez-San Juan, G.R.; Oakes, P.W.; Gardel, M.L. Contact guidance requires spatial control of leading-edge protrusion. Mol. Biol. Cell 2017, 28, 1043–1053. [Google Scholar] [CrossRef]
- Romsey, N.R.; Hou, Y.; Lara Rodriguez, L.; Schneider, I.C. The Number of Lines a Cell Contacts and Cell Contractility Drive the Efficiency of Contact Guidance. Cell. Mol. Bioeng. 2014, 7, 122–135. [Google Scholar] [CrossRef]
- Rossier, O.M.; Gauthier, N.; Biais, N.; Vonnegut, W.; Fardin, M.A.; Avigan, P.; Heller, E.R.; Mathur, A.; Ghassemi, S.; Koeckert, M.S.; et al. Force generated by actomyosin contraction builds bridges between adhesive contacts. EMBO J. 2010, 29, 1055–1068. [Google Scholar] [CrossRef]
- Altomare, L.; Riehle, M.; Gadegaard, N.; Tanzi, M.C.; Farè, S. Microcontact printing of fibronectin on a biodegradable polymeric surface for skeletal muscle cell orientation. Int. J. Artif. Organs 2010, 33, 535–543. [Google Scholar] [CrossRef]
- Zimerman, B.; Arnold, M.; Ulmer, J.; Blümmel, J.; Besser, A.; Spatz, J.P.; Geiger, B. Formation of focal adhesion-stress fibre complexes coordinated by adhesive and non-adhesive surface domains. IEE Proc. Nanobiotechnol. 2004, 151, 62–66. [Google Scholar] [CrossRef]
- Funk, R.H.W.; Monsees, T.K. Effects of electromagnetic fields on cells: Physiological and therapeutical approaches and molecular mechanisms of interaction. A review. Cells Tissues Organs 2006, 182, 59–78. [Google Scholar] [CrossRef]
- Glaser, R. Biophysics: An Introduction, 2nd ed; Springer: Berlin/Heidelberg, Germany, 2012; p. 407. [Google Scholar]
- Butt, H.J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; p. 461. [Google Scholar]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics dependent on defined microtopographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef]
- Wang, Q.; Dou, X.; Chen, X.; Zhao, Z.; Wang, S.; Wang, Y.; Sui, K.; Tan, Y.; Gong, Y.; Zhang, Y.; et al. Reevaluating Protein Photoluminescence: Remarkable Visible Luminescence upon Concentration and Insight into the Emission Mechanism. Angew. Chem. Int. Ed. 2019, 58, 12667–12673. [Google Scholar] [CrossRef]
- Rheinlaender, J.; Schäffer, T.E. Lateral Resolution and Image Formation in Scanning Ion Conductance Microscopy. Anal. Chem. 2015, 87, 7117–7124. [Google Scholar] [CrossRef]
- Gan, Y. Atomic and subnanometer resolution in ambient conditions by atomic force microscopy. Surf. Sci. Rep. 2009, 64, 99–121. [Google Scholar] [CrossRef]
- Wright, A.K.; Thompson, M.R. Hydrodynamic structure of bovine serum albumin determined by transient electric birefringence. Biophys. J. 1975, 15, 137–141. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Feliu, N.; Walter, M.V.; Montañez, M.I.; Kunzmann, A.; Hult, A.; Nyström, A.; Malkoch, M.; Fadeel, B. Stability and biocompatibility of a library of polyester dendrimers in comparison to polyamidoamine dendrimers. Biomaterials 2012, 33, 1970–1981. [Google Scholar] [CrossRef]
- Wong, J.E.; Rehfeldt, F.; Hänni, P.; Tanaka, M.; Klitzing, R.V. Swelling Behavior of Polyelectrolyte Multilayers in Saturated Water Vapor. Macromolecules 2004, 37, 7285–7289. [Google Scholar] [CrossRef]
- McKelvey, K.; Kinnear, S.L.; Perry, D.; Momotenko, D.; Unwin, P.R. Surface charge mapping with a nanopipette. J. Am. Chem. Soc. 2014, 136, 13735–13744. [Google Scholar] [CrossRef]
- Staehlke, S.; Lehnfeld, J.; Schneider, A.; Nebe, J.B.; Müller, R. Terminal chemical functions of polyamidoamine dendrimer surfaces and its impact on bone cell growth. Mater. Sci. Eng. Mater. Biol. Appl. 2019, 101, 190–203. [Google Scholar] [CrossRef]
- Ferrier, J.; Ross, S.M.; Kanehisa, J.; Aubin, J.E. Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J. Cell. Physiol. 1986, 129, 283–288. [Google Scholar] [CrossRef]
- Guo, A.; Song, B.; Reid, B.; Gu, Y.; Forrester, J.V.; Jahoda, C.A.B.; Zhao, M. Effects of physiological electric fields on migration of human dermal fibroblasts. J. Investig. Dermatol. 2010, 130, 2320–2327. [Google Scholar] [CrossRef]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling cell behavior electrically: Current views and future potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef]
- Gunja, N.J.; Dujari, D.; Chen, A.; Luengo, A.; Fong, J.V.; Hung, C.T. Migration responses of outer and inner meniscus cells to applied direct current electric fields. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2012, 30, 103–111. [Google Scholar] [CrossRef]
- Özkucur, N.; Monsees, T.K.; Perike, S.; Do, H.Q.; Funk, R.H.W. Local calcium elevation and cell elongation initiate guided motility in electrically stimulated osteoblast-like cells. PLoS ONE 2009, 4, e6131. [Google Scholar] [CrossRef]
- Rohde, M.; Ziebart, J.; Kirschstein, T.; Sellmann, T.; Porath, K.; Kühl, F.; Delenda, B.; Bahls, C.; van Rienen, U.; Bader, R.; et al. Human Osteoblast Migration in DC Electrical Fields Depends on Store Operated Ca2+-Release and Is Correlated to Upregulation of Stretch-Activated TRPM7 Channels. Front. Bioeng. Biotechnol. 2019, 7, 422. [Google Scholar] [CrossRef]
- Voelkner, C.; Wendt, M.; Lange, R.; Ulbrich, M.; Gruening, M.; Staehlke, S.; Nebe, B.; Barke, I.; Speller, S. The nanomorphology of cell surfaces of adhered osteoblasts. Beilstein J. Nanotechnol. 2021, 12, 242–256. [Google Scholar] [CrossRef]
- Mycielska, M.E.; Djamgoz, M.B.A. Cellular mechanisms of direct-current electric field effects: Galvanotaxis and metastatic disease. J. Cell Sci. 2004, 117, 1631–1639. [Google Scholar] [CrossRef]
- Allen, G.M.; Mogilner, A.; Theriot, J.A. Electrophoresis of cellular membrane components creates the directional cue guiding keratocyte galvanotaxis. Curr. Biol. 2013, 23, 560–568. [Google Scholar] [CrossRef]
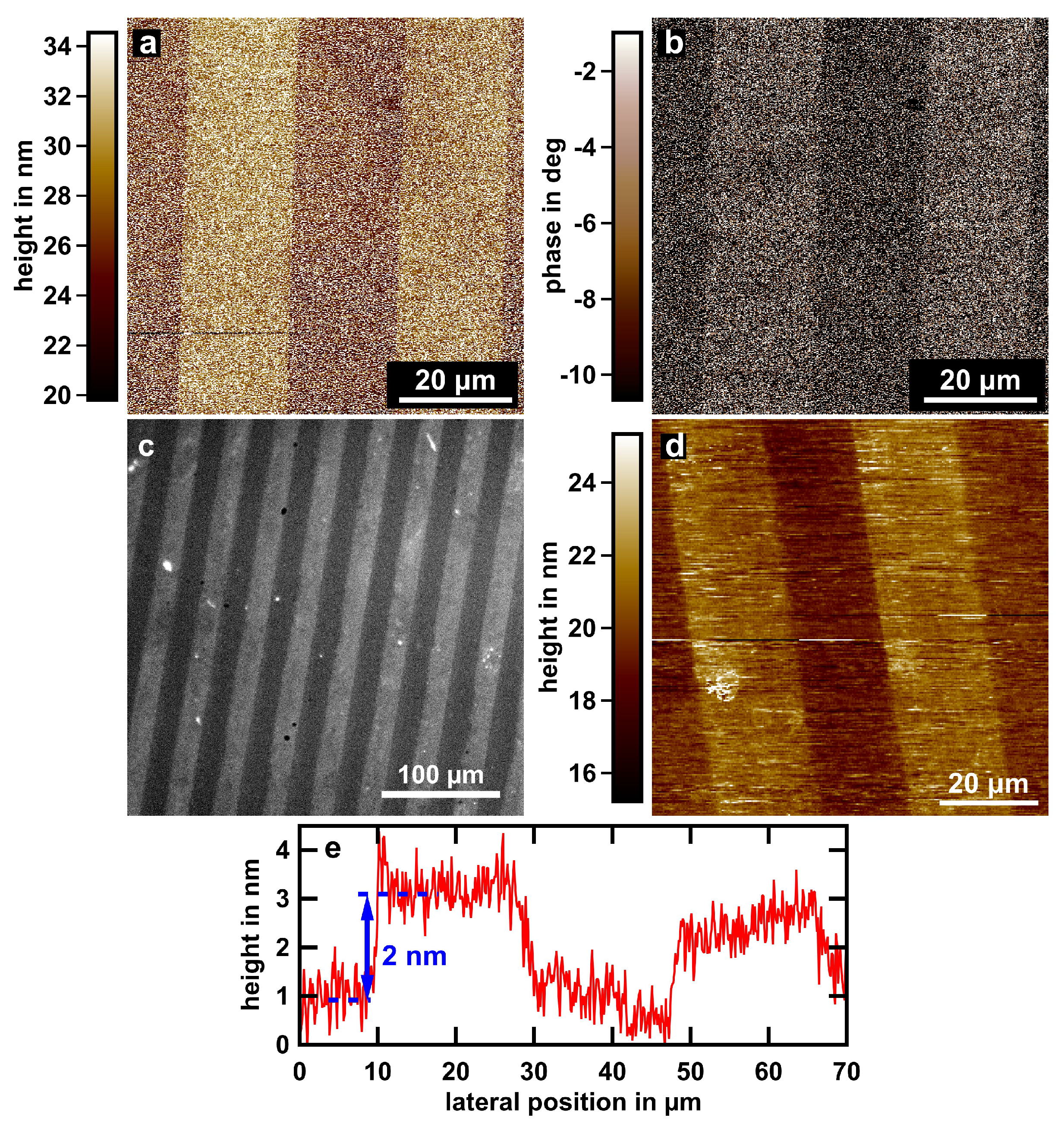
| AFM [nm] | SICM [nm] | |
|---|---|---|
| BSA | 4 | 2 |
| -terminated dendrimer (PAMAM, gen. 1) | 2 | 2 |
| COOH-terminated dendrimer | 1 | 2–3 |
| PDADMA | 1–3 | 0.5–1 |
| PSS | 1 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voelkner, C.; Assi, I.; Karberg, W.; Lange, R.; Neuber, S.; Helm, C.A.; Gruening, M.; Nebe, J.B.; Barke, I.; Speller, S. Response of Osteoblasts to Electric Field Line Patterns Emerging from Molecule Stripe Landscapes. Appl. Sci. 2022, 12, 7329. https://doi.org/10.3390/app12147329
Voelkner C, Assi I, Karberg W, Lange R, Neuber S, Helm CA, Gruening M, Nebe JB, Barke I, Speller S. Response of Osteoblasts to Electric Field Line Patterns Emerging from Molecule Stripe Landscapes. Applied Sciences. 2022; 12(14):7329. https://doi.org/10.3390/app12147329
Chicago/Turabian StyleVoelkner, Christian, Issam Assi, Willi Karberg, Regina Lange, Sven Neuber, Christiane A. Helm, Martina Gruening, J. Barbara Nebe, Ingo Barke, and Sylvia Speller. 2022. "Response of Osteoblasts to Electric Field Line Patterns Emerging from Molecule Stripe Landscapes" Applied Sciences 12, no. 14: 7329. https://doi.org/10.3390/app12147329
APA StyleVoelkner, C., Assi, I., Karberg, W., Lange, R., Neuber, S., Helm, C. A., Gruening, M., Nebe, J. B., Barke, I., & Speller, S. (2022). Response of Osteoblasts to Electric Field Line Patterns Emerging from Molecule Stripe Landscapes. Applied Sciences, 12(14), 7329. https://doi.org/10.3390/app12147329










