Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
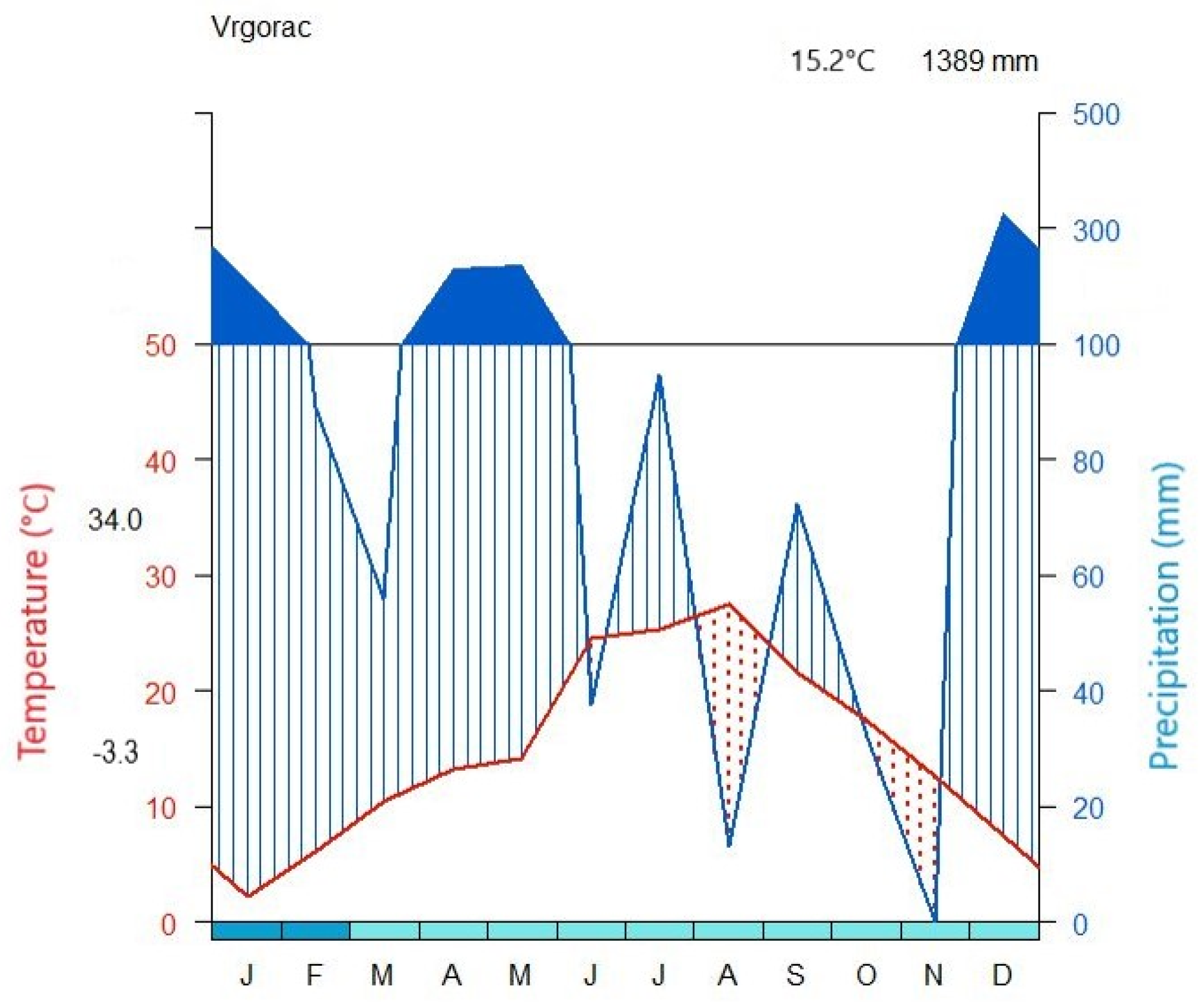
2.1. Vineyard Site and Climate Conditions
2.2. Experimental Setup
2.3. Analysis of Physicochemical Components of Grape Juice
2.4. Microvinifications
2.5. Analysis of Physiochemical Components of Wine
2.6. Analysis of Volatile Compounds Using GC-MS
2.7. Odor Activity Values of Volatile Aromas (OAV)
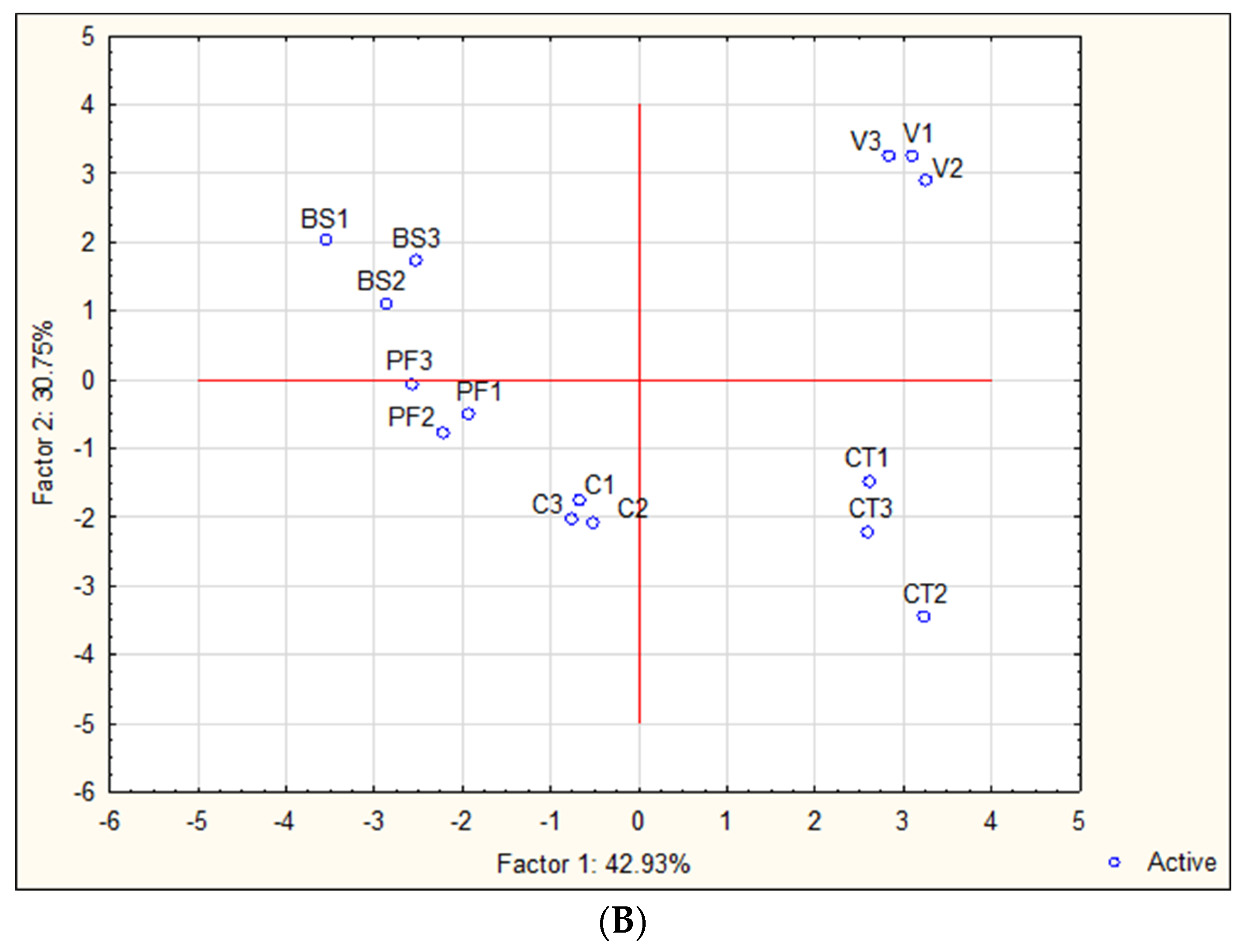
2.8. Statistical Analysis
3. Results
3.1. Yield Components and Physicochemical Composition of Maraština Musts and Wines
3.2. Aroma Composition of Maraština Wines
3.2.1. Varietal Volatile Composition of Maraština Wine
3.2.2. Fermentation Volatile Composition of Maraština Wine
3.3. Odor Activity Values of Volatile Compounds in Maraština Wine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pastore, C.; Santo, S.D.; Zenoni, S.; Movahed, N.; Allegro, G.; Valentini, G.; Filippetti, I.; Tornielli, G.B. Whole plant temperature manipulation affects flavonoid metabolism and the transcriptome of grapevine berries. Front. Plant Sci. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Young, P.R.; Eyeghe-Bickong, H.A.; du Plessis, K.; Alexandersson, E.; Jacobson, D.A.; Coetzee, Z.; Deloire, A.; Vivier, M.A. Grapevine plasticity in response to an altered microclimate: Sauvignon Blanc modulates specific metabolites in response to increased berry exposure. Plant Physiol. 2016, 170, 1235–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozina, B.; Karoglan, M.; Herjavec, S.; Jeromel, A.; Orlic, S. Influence of basal leaf removal on the chemical composition of Sauvignon Blanc and Riesling wines. J. Food Agric. Environ. 2008, 6, 28. [Google Scholar]
- Kok, D. Influences of pre- and post-veraison cluster thinning treatments on grape composition variables and monoterpene levels of Vitis vinifera L. cv. Sauvignon Blanc. J. Food Agric. Environ. 2011, 9, 22–26. [Google Scholar]
- Sivilotti, P.; Falchi, R.; Herrera, J.C.; Škvarč, B.; Butinar, L.; Lemut, M.S.; Bubola, M.; Sabbatini, P.; Lisjak, K.; Vanzo, A. Combined effects of early season leaf removal and climatic conditions on aroma precursors in Sauvignon Blanc grapes. J. Agric. Food Chem. 2017, 65, 8426–8434. [Google Scholar] [CrossRef]
- Yue, X.; Ma, X.; Tang, Y.; Wang, Y.; Wu, B.; Jiao, X.; Zhang, Z.; Ju, Y. Effect of cluster zone leaf removal on monoterpene profiles of Sauvignon Blanc grapes and wines. Food Res. Int. 2020, 131, 109028. [Google Scholar] [CrossRef]
- Šuklje, K.; Antalick, G.; Coetzee, Z.; Schmidtke, L.M.; Česnik, H.B.; Brandt, J.; du Toit, W.J.; Lisjak, K.; Deloire, A. Effect of leaf removal and ultraviolet radiation on the composition and sensory perception of Vitis vinifera L. cv. Sauvignon Blanc wine. Aust. J. Grape Wine Res. 2014, 20, 223–233. [Google Scholar] [CrossRef]
- Škrab, D.; Sivilotti, P.; Comuzzo, P.; Voce, S.; Degano, F.; Carlin, S.; Arapitsas, P.; Masuero, D.; Vrhovšek, U. Cluster thinning and vineyard site modulate the metabolomic profile of Ribolla Gialla base and sparkling wines. Metabolites 2021, 11, 331. [Google Scholar] [CrossRef]
- Bubola, M.; Rusjan, D.; Lukić, I. Crop level vs. leaf removal: Effects on Istrian Malvasia wine aroma and phenolic acids composition. Food Chem. 2020, 312, 126046. [Google Scholar] [CrossRef]
- Maletić, E.; Pejić, I.; Kontić, J.K.; Zdunić, G.; Preiner, D.; Šimon, S.; Andabaka, Ž.; Mihaljević, M.Ž.; Bubola, M.; Marković, Z.; et al. Ampelographic and genetic characterization of Croatian grapevine varieties. Vitis. J. Grapevine Res. 2015, 54, 93–98. [Google Scholar]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Office International de la Vigne et du Vin. Compendium of International Methods of Wine and Must Analysis—Volume I; Organisation Internationale de la Vigne et du Vin (OIV): Paris, France, 2015. [Google Scholar]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Korenika, A.M.J.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile profile characterization of Croatian commercial sparkling wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef]
- Maslov, L.; Tomaz, I.; Žulj, M.M.; Jeromel, A. Aroma characterization of predicate wines from Croatia. Eur. Food Res. Technol. 2017, 243, 263–274. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Data Science Workbench, Version 14. 2020. Available online: http://tibco.com (accessed on 2 March 2022).
- Flavornet. Available online: http://www.flavornet.org/flavornet.html (accessed on 28 February 2022).
- Condurso, C.; Cincotta, F.; Tripodi, G.; Sparacio, A.; Giglio, D.M.L.; Sparla, S.; Verzera, A. Effects of cluster thinning on wine quality of Syrah cultivar (Vitis vinifera L.). Eur. Food Res. Technol. 2016, 242, 1719–1726. [Google Scholar] [CrossRef]
- Palliotti, A.; Cartechini, A. Cluster thinning effects on yield and grape composition in different grapevine cultivars. Acta Hortic. 2000, 512, 111–120. [Google Scholar] [CrossRef]
- Keller, M.; Mills, L.J.; Wample, R.L.; Spayd, S.E. Cluster thinning effects on three deficit-irrigated Vitis vinifera cultivars. Am. J. Enol. Vitic. 2005, 56, 91–103. [Google Scholar]
- Poni, S.; Casalini, L.; Bernizzoni, F.; Civardi, S.; Intrieri, C. Effects of early defoliation on shoot photosynthesis, yield components, and grape composition. Am. J. Enol. Vitic. 2006, 57, 397–407. [Google Scholar]
- Hernandez-Orte, P.; Concejero, B.; Astrain, J.; Lacau, B.; Cacho, J.; Ferreira, V. Influence of viticulture practices on grape aroma precursors and their relation with wine aroma. J. Sci. Food Agric. 2015, 95, 688–701. [Google Scholar] [CrossRef]
- Bubola, M.; Lukić, I.; Radeka, S.; Sivilotti, P.; Grozić, K.; Vanzo, A.; Bavčar, D.; Lisjak, K. Enhancement of Istrian Malvasia wine aroma and hydroxycinnamate composition by hand and mechanical leaf removal. J. Sci. Food Agric. 2019, 99, 904–914. [Google Scholar] [CrossRef]
- Ferrari, V.; Disegna, E.; Dellacassa, E.; Coniberti, A. Influence of timing and intensity of fruit zone leaf removal and kaolin applications on bunch rot control and quality improvement of Sauvignon blanc grapes, and wines, in a temperate humid climate. Sci. Hortic. 2017, 223, 62–71. [Google Scholar] [CrossRef]
- Scafidi, P.; Pisciotta, A.; Patti, D.; Tamborra, P.; Di Lorenzo, R.; Barbagallo, M.G. Effect of artificial shading on the tannin accumulation and aromatic composition of the Grillo cultivar (Vitis vinifera L.). BMC Plant Biol. 2013, 13, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedel, M.; Frotscher, J.; Nitsch, M.; Hofmann, M.; Bogs, J.; Stoll, M.; Dietrich, H. Light promotes expression of monoterpene and flavonol metabolic genes and enhances flavour of winegrape berries (Vitis vinifera L. cv. Riesling). Aust. J. Grape Wine Res. 2016, 22, 409–421. [Google Scholar] [CrossRef]
- Baron, M.; Prusova, B.; Tomaskova, L.; Kumsta, M.; Sochor, J. Terpene content of wine from the aromatic grape variety “Irsai Oliver” (Vitis vinifera L.) depends on maceration time. Open Life Sci. 2017, 12, 42–50. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.C.; van Leeuwen, C.; Dubourdieu, D. Which impact for β-damascenone on red wines aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef]
- Escudero, A.; Asensio, E.; Cacho, J.; Ferreira, V. Sensory and chemical changes of young white wines stored under oxygen. An assessment of the role played by aldehydes and some other important odorants. Food Chem. 2002, 77, 325–331. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Humar, I.; Gajdoš Kljusurić, J.; Zdunić, G.; Zlatić, E. Free and bound volatile aroma compounds of ‘Maraština’ grapes as influenced by dehydration techniques. Appl. Sci. 2020, 10, 8928. [Google Scholar] [CrossRef]
- Plotto, A.; Margaría, C.A.; Goodner, K.L.; Goodrich, R.; Baldwin, E.A. Odour and flavour thresholds for key aroma components in an orange juice matrix: Terpenes and aldehydes. Flavour Fragr. J. 2004, 19, 491–498. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Boidron, J.N.; Terrier, A. Aroma of Muscat grape varieties. J. Agric. Food Chem. 1975, 23, 1042–1047. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Trujillo, M.; García Ruiz, A.; González Viñas, M.A. Aroma profile of Malbec red wines from La Mancha region: Chemical and sensory characterization. Food Res. Int. 2017, 100, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Slaghenaufi, D.; Luzzini, G.; Samaniego Solis, J.; Forte, F.; Ugliano, M. Two sides to one story—Aroma chemical and sensory signature of Lugana and Verdicchio wines. Molecules 2021, 26, 2127. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the aroma of grapes: A review. S. Afr. J. Enol. Vitic. 1983, 4, 49–60. [Google Scholar]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Francis, L.; Newton, J. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Pardo, E.; Rico, J.; Gil, J.V.; Orejas, M. De novo production of six key grape aroma monoterpenes by a geraniol synthase-engineered S. cerevisiae wine strain. Microb. Cell Fact. 2015, 14, 136. [Google Scholar] [CrossRef] [Green Version]
- Sacks, G.L.; Gates, M.J.; Ferry, F.X.; Lavin, E.H.; Kurtz, A.J.; Acree, T.E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical characterization of the aroma of Grenache rosé wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Cacho, J.F.; Ferreira, V.; Vicario, I.M.; Heredia, F.J. Volatile components of Zalema white wines. Food Chem. 2007, 100, 1464–1473. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Culleré, L.; Escudero, A.; Cacho, J.; Ferreira, V. Gas chromatography− olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004, 52, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Gammacurta, M.; Tempere, S.; Marchand, S.; Moine, V.; de Revel, G. Ethyl 2-hydroxy-3-methylbutanoate enantiomers: Quantitation and sensory evaluation in wine. OENO One 2018, 52, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Piombino, P.; Moio, L.; Genovese, A. Orthonasal vs. retronasal: Studying how volatiles’ hydrophobicity and matrix composition modulate the release of wine odorants in simulated conditions. Food Res. Int. 2019, 116, 548–558. [Google Scholar] [CrossRef]
- Azcarate, S.M.; Cantarelli, M.A.; Marchevsky, E.J.; Camiña, J.M. Evaluation of Geographic Origin of Torrontés Wines by Chemometrics. J. Food Res. 2013, 2, 48. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Buttery, R.G.; Okano, S. Odour thresholds of some organic compounds associated with food flavours. J. Sci. Food Agric. 1963, 14, 761–765. [Google Scholar] [CrossRef]
- Sonni, F.; Moore, E.G.; Chinnici, F.; Riponi, C.; Smyth, H.E. Characterisation of Australian Verdelho wines from the Queensland Granite Belt region. Food Chem. 2016, 196, 1163–1171. [Google Scholar] [CrossRef] [Green Version]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Escudero, A.; Gogorza, B.; Melús, M.A.; Ortín, N.; Cacho, J.; Ferreira, V. Characterization of the aroma of a wine from Maccabeo. Key role played by compounds with low odor activity values. J. Agric. Food Chem. 2004, 52, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Jarauta, I.; Ortega, L.; Cacho, J. Simple strategy for the optimization of solid-phase extraction procedures through the use of solid–liquid distribution coefficients: Application to the determination of aliphatic lactones in wine. J. Chromatogr. A 2004, 1025, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bao, M.L.; Chen, H.L.; Li, Q. Impact of sucrose addition on the physiochemical properties and volatile compounds of “Shuangyou” red wines. J. Food Qual. 2017, 2017, 2926041. [Google Scholar] [CrossRef] [Green Version]

| PF | BS | V | CT | C | |
|---|---|---|---|---|---|
| Yield components | |||||
| Number clusters/vine | 8.9 ± 3.2 a | 7.5 ± 2.9 ab | 7.5 ± 2.4 ab | 5.9 ± 1.7 b | 7.7 ± 2.5 ab |
| Yield/vine (kg) | 1.3 ± 0.8 ab | 1.2 ± 0.5 b | 1.4 ± 0.6 ab | 1.4 ± 0.7 ab | 1.7 ± 0.8 a |
| Cluster weight (g) | 165.6 ± 99.2 b | 161.4 ± 54.2 b | 183.0 ± 72.0 ab | 229.1 ± 84.2 a | 222.5 ± 57.3 a |
| Berries per cluster | 225 ± 50 a | 192 ± 50 a | 224 ± 53 a | 253 ± 44 a | 227 ± 79 a |
| Must composition | |||||
| TSS (°Brix) * | 18.9 ± 0.2 b | 18.7 ± 0.1 b | 18.9 ± 0.1 b | 19.6 ± 0.2 a | 19.5 ± 0.2 a |
| Total acidity ** | 4.7 ± 0.0 a | 4.3 ± 0.1 c | 4.6 ± 0.1 b | 4.5 ± 0.0 b | 4.7 ± 0.1 a |
| pH | 3.66 ± 0.01 b | 3.74 ± 0.01 a | 3.74 ± 0.01 a | 3.74 ± 0.01 a | 3.52 ± 0.01 c |
| Wine composition | |||||
| ASv (vol %) *** | 10.5 ± 0.1 b | 10.6 ± 0.1 b | 10.7 ± 0.2 b | 11.3 ± 0.1 a | 11.3 ± 0.1 a |
| Residual sugars (g L−1) | 1.0 ± 0.0 b | 1.0 ± 0.1 b | 1.1 ± 0.2 b | 1.6 ± 0.3 a | 1.2 ± 0.2 ab |
| Total extract (g L−1) | 16.5 ± 0.6 b | 16.9 ± 0.2 b | 17.8 ± 0.4 a | 18.5 ± 0.6 a | 19.0 ± 0.9 a |
| pH | 3.61 ± 0.02 c | 3.64 ± 0.04 bc | 3.76 ± 0.01 a | 3.69 ± 0.01 ab | 3.68 ± 0.05 bc |
| Total acidity (g L−1) | 4.8 ± 0.1 ab | 4.9 ± 0.1 a | 4.5 ± 0.1 b | 4.6 ± 0.1 b | 4.7 ± 0.1 ab |
| Volatile acidity (g L−1) **** | 0.5 ± 0.0 b | 0.5 ± 0.0 b | 0.7 ± 0.0 a | 0.6 ± 0.0 a | 0.6 ± 0.0 a |
| Compound (µg L−1) | PF | BS | V | CT | C |
|---|---|---|---|---|---|
| α-Pinene | 0.89 ± 0.07 b | 0.50 ± 0.06 c | 1.53 ± 0.07 a | 1.45 ± 0.15 a | 0.64 ± 0.04 c |
| Tetrahydrolinalool | 7.86 ± 0.45 ab | 5.53 ± 0.46 c | 5.75 ± 0.32 bc | 7.97 ± 1.23 a | 8.52 ± 1.08 a |
| Ethyl linalyl acetal | 0.05 ± 0.03 b | 0.08 ± 0.01 ab | 0.17 ± 0.20 ab | 0.09 ± 0.03 ab | 0.30 ± 0.01 a |
| cis-Linalool oxide, furan | 0.18 ± 0.03 b | 0.26 ± 0.04 b | 0.14 ± 0.02 b | 0.17 ± 0.05 b | 0.46 ± 0.07 a |
| Linalool | 14.98 ± 2.35 b | 12.27 ± 0.95 b | 12.35 ± 2.14 b | 20.40 ± 0.82 a | 15.29 ± 0.81 b |
| Terpinen-4-ol | 0.78 ± 0.24 c | 1.60 ± 0.41 b | 0.66 ± 0.30 c | 3.33 ± 0.28 a | 0.77 ± 0.06 c |
| Hotrienol | 2.74 ± 0.29 a | 0.80 ± 0.06 b | 2.39 ± 0.09 a | 2.67 ± 0.28 a | 0.78 ± 0.20 b |
| trans-β-Farnesene | 0.72 ± 0.17 c | 0.55 ± 0.05 c | 2.11 ± 0.31 b | 2.62 ± 0.22 a | 0.53 ± 0.01 c |
| cis-β-Farnesene | 0.95 ± 0.04 a | 1.00 ± 0.08 a | 1.00 ± 0.09 a | 0.92 ± 0.03 a | 1.07 ± 0.09 a |
| α-Farnesene | 0.89 ± 0.02 a | 0.94 ± 0.06 a | 0.93 ± 0.09 a | 0.91 ± 0.12 a | 1.08 ± 0.06 a |
| 2,6-dimethyl-7-octene-2,6-diol | 4.32 ± 0.56 a | 4.04 ± 0.41 a | 2.50 ± 0.12 b | 2.36 ± 0.12 b | 5.23 ± 0.83 a |
| α-Terpineol | 1.16 ± 0.24 a | 1.50 ± 0.36 a | 1.39 ± 0.29 a | 1.31 ± 0.16 a | 1.22 ± 0.10 a |
| Citronellol | 12.02 ± 0.62 b | 12.61 ± 1.74 b | 12.31 ± 2.12 b | 19.26 ± 3.05 a | 15.87 ± 2.17 ab |
| Nerol | 0.57 ± 0.07 a | 0.50 ± 0.13 a | 0.44 ± 0.06 a | 0.42 ± 0.03 a | 0.49 ± 0.08 a |
| ε-Fenchene | 0.92 ± 0.11 a | 0.90 ± 0.04 a | 1.10 ± 0.11 a | 1.03 ± 0.09 a | 1.03 ± 0.11 a |
| Geraniol | 3.56 ± 0.41 b | 3.25 ± 0.11 b | 3.59 ± 0.40 b | 4.53 ± 0.38 a | 3.61 ± 0.17 b |
| 6,7-dihydro-7-hydroxylinalool | 4.18 ± 0.01 b | 4.24 ± 0.14 b | 4.36 ± 0.25 b | 4.51 ± 0.33 b | 5.23 ± 0.28 a |
| Geranyl acetate | 2.64 ± 0.09 a | 2.55 ± 0.04 a | 2.68 ± 0.14 a | 2.42 ± 0.17 a | 2.78 ± 0.29 a |
| 8-Hidroxylinalool | 5.93 ± 0.88 a | 9.83 ± 0.45 ab | 13.89 ± 1.04 a | 12.60 ± 3.88 a | 7.21 ± 0.43 b |
| Farnesol | 3.61 ± 0.45 c | 6.52 ± 0.46 a | 5.07 ± 0.09 b | 4.53 ± 0.19 bc | 5.21 ± 0.65 b |
| Neric acid | 2.57 ± 0.25 a | 2.40 ± 0.09 a | 2.50 ± 0.04 a | 3.78 ± 1.66 a | 2.62 ± 0.26 a |
| 1,8-Terpin | 0.35 ± 0.04 d | 0.70 ± 0.08 bc | 0.90 ± 0.08 ab | 0.43 ± 0.11 cd | 1.13 ± 0.18 a |
| Neralidol | 0.66 ± 0.02 a | 0.70 ± 0.06 a | 0.82 ± 0.05 a | 0.73 ± 0.05 a | 0.70 ± 0.13 a |
| ∑Terpenes | 72.52 ± 3.41 b | 73.34 ± 2.73 b | 78.58 ± 4.63 b | 98.44 ± 8.52 a | 81.76 ± 2.59 b |
| TDN * | 0.72 ± 0.16 a | 0.63 ± 0.03 a | 0.61 ± 0.10 a | 0.74 ± 0.17 a | 0.65 ± 0.16 a |
| β-Damascenone | 3.61 ± 0.44 a | 2.38 ± 0.40 a | 2.43 ± 0.25 a | 3.10 ± 1.23 a | 3.46 ± 0.66 a |
| Dihydroactinidiolide | 0.83 ± 0.06 a | 1.16 ± 0.22 a | 0.82 ± 0.20 a | 0.93 ± 0.18 a | 1.02 ± 0.09 a |
| ∑C13-norisoprenoids | 5.15 ± 0.33 a | 4.17 ± 0.60 a | 3.86 ± 0.12 a | 4.77 ± 1.55 a | 5.13 ± 0.68 a |
| Compound (µg L−1) | PF | BS | V | CT | C |
|---|---|---|---|---|---|
| Isobutyl acetate | 22.18 ± 1.60 c | 16.62 ± 0.47 d | 24.30 ± 1.06 c | 27.89 ± 0.95 b | 31.37 ± 1.89 a |
| Ethyl butanoate | 165.94 ± 9.33 c | 145.79 ± 2.41 d | 210.23 ± 3.08 a | 221.89 ± 2.52 a | 187.91 ± 5.45 b |
| Ethyl 2-methylbutanoate | 4.48 ± 0.39 a | 3.39 ± 0.22 b | 2.09 ± 0.08 c | 4.51 ± 0.26 a | 4.43 ± 0.26 a |
| Ethyl 3-methylbutanoate | 9.55 ± 0.44 b | 7.78 ± 0.81 bc | 6.12 ± 0.64 c | 12.06 ± 0.89 a | 12.16 ± 0.57 a |
| Isoamyl acetate | 955.88 ± 37.78 b | 650.86 ± 20.22 c | 1373.66 ± 110.61 a | 1403.59 ± 4.97 a | 1031.45 ± 22.90 b |
| Ethyl hexanoate | 258.46 ± 24.48 c | 322.49 ± 8.91 bc | 440.27 ± 36.02 a | 364.94 ± 28.57 b | 351.05 ± 11.05 b |
| Hexyl acetate | 15.30 ± 1.12 c | 17.28 ± 0.26 c | 27.15 ± 1.91 a | 21.95 ± 1.33 b | 14.42 ± 1.09 c |
| Ethyl lactate | 586.12 ± 15.73 a | 463.50 ± 29.66 b | 358.71 ± 13.27 c | 561.73 ± 36.89 a | 439.51 ± 33.59 b |
| Ethyl 2-hydroxy-3-methylbutanoate | 95.68 ± 3.49 ab | 61.26 ± 1.12 d | 101.22 ± 1.31 a | 90.06 ± 1.97 b | 81.68 ± 2.55 c |
| Ethyl octanoate | 516.94 ± 20.99 ab | 362.08 ± 32.57 c | 547.21 ± 47.58 a | 444.28 ± 25.05 b | 438.25 ± 11.46 bc |
| Methyl 2-furoate | 149.72 ± 0.92 a | 150.84 ± 0.67 a | 149.91 ± 0.10 a | 149.74 ± 1.11 a | 150.38 ± 0.99 a |
| Ethyl furoate | 1.52 ± 0.19 a | 1.10 ± 0.08 abc | 0.80 ± 0.15 c | 1.35 ± 0.25 ab | 1.05 ± 0.05 bc |
| Ethyl decanoate | 110.80 ± 7.23 b | 82.87 ± 5.16 c | 150.04 ± 6.29 a | 145.90 ± 8.78 a | 97.01 ± 3.79 bc |
| Diethyl succinate | 267.11 ± 35.33 bc | 205.91 ± 4.96 d | 252.80 ± 21.43 cd | 412.96 ± 19.04 a | 319.80 ± 6.12 b |
| Methyl geranoate | 2.67 ± 0.25 ab | 2.37 ± 0.35 b | 3.40 ± 0.28 a | 2.52 ± 0.37 b | 2.56 ± 0.05 b |
| 2-Phenylethyl acetate | 6.55 ± 0.39 bc | 5.22 ± 0.30 c | 8.43 ± 1.14 a | 7.60 ± 0.37 ab | 7.04 ± 0.43 ab |
| Ethyl 3-hydroxyhexanoate | 1.68 ± 0.34 a | 0.83 ± 0.14 b | 1.62 ± 0.14 ab | 2.34 ± 0.42 a | 2.02 ± 0.35 a |
| Ethyl hexadecanoate | 0.26 ± 0.04 ab | 0.14 ± 0.04 c | 0.08 ± 0.02 c | 0.18 ± 0.01 bc | 0.29 ± 0.06 a |
| ∑Esters | 3,170.85 ± 79.85 c | 2,500.35 ± 80.45 d | 3,658.03 ± 48.58 b | 3,875.49 ± 28.09 a | 3,172.37 ± 42.68 c |
| Isobutanol | 2,555.41 ± 126.36 bc | 2,225.97 ± 18.98 c | 2,545.03 ± 121.52 bc | 3,235.33 ± 255.55 a | 2,752.73 ± 103.98 b |
| 1-Butanol | 36.84 ± 1.83 b | 39.20 ± 3.78 b | 40.86 ± 1.13 b | 58.69 ± 1.35 a | 41.10 ± 3.14 b |
| 2-Methyl-1-butanol | 9,752.26 ± 126.73 c | 7,997.82 ± 85.59 d | 10,115.13 ± 112.48 bc | 12,452.80 ± 466.25 a | 10,787.12 ± 381.73 b |
| Isoamyl alcohol | 2,397.85 ± 232.52 c | 1,609.71 ± 100.10 c | 9,411.80 ± 503.45 a | 7,487.32 ± 408.50 b | 8,482.01 ± 420.15 a |
| 1-Pentanol | 5.36 ± 0.46 c | 5.34 ± 0.03 c | 8.21 ± 0.26 b | 11.35 ± 2.12 a | 6.32 ± 0.50 bc |
| 3-Methyl-1-pentanol | 132.52 ± 3.26 ab | 124.94 ± 5.19 b | 103.70 ± 4.68 c | 146.56 ± 4.89 a | 100.66 ± 9.17 c |
| 1-Hexanol | 0.42 ± 0.03 b | 0.49 ± 0.09 b | 0.60 ± 0.08 b | 1.19 ± 0.23 a | 0.30 ± 0.03 b |
| trans-3-Hexen-1-ol | 22.64 ± 2.66 c | 24.79 ± 0.94 c | 32.00 ± 1.06 b | 44.17 ± 2.94 a | 28.59 ± 3.10 bc |
| 2-Ethyl-1-hexanol | 1.16 ± 0.16 a | 0.98 ± 0.07 a | 1.23 ± 0.14 a | 1.35 ± 0.21 a | 1.00 ± 0.12 a |
| 1-Octen-3-ol | 1.56 ± 0.27 b | 1.37 ± 0.24 b | 2.56 ± 0.18 a | 2.78 ± 0.25 a | 2.25 ± 0.19 a |
| 1-Heptanol | 11.62 ± 0.63 a | 8.96 ± 0.19 b | 4.86 ± 0.84 c | 6.34 ± 0.62 c | 11.69 ± 0.98 a |
| 1-Octanol | 7.88 ± 1.29 a | 7.97 ± 1.11 a | 10.57 ± 1.34 a | 10.44 ± 1.07 a | 11.08 ± 1.45 a |
| 1-Nonanol | 0.44 ± 0.14 bc | 0.14 ± 0.02 c | 0.75 ± 0.15 ab | 0.90 ± 0.09 a | 0.91 ± 0.15 a |
| 1-Decanol | 4.76 ± 0.17 a | 1.86 ± 0.55 c | 3.41 ± 0.44 b | 4.10 ± 0.62 ab | 3.76 ± 0.18 ab |
| Phenylethyl alcohol | 5,126.42 ± 154.61 a | 4,917.57 ± 69.47 a | 5,055.14 ± 225.33 a | 4,861.04 ± 94.37 a | 5,272.90 ± 184.53 a |
| ∑Higher alcohols | 20,057.14 ± 201.50 b | 16,967.12 ± 130.06 c | 27,335.85 ± 709.84 a | 28,324.36 ± 215.46 a | 27,502.42 ± 367.05 a |
| 1,2-Propanediol | 1.65 ± 0.21 b | 3.34 ± 0.38 a | 1.50 ± 0.21 b | 3.75 ± 0.44 a | 1.93 ± 0.22 b |
| 2,3-Butanediol | 31.07 ± 1.51 ab | 23.52 ± 2.63 c | 32.46 ± 2.61 ab | 33.72 ± 1.82 a | 27.01 ± 1.17 bc |
| Furfuryl alcohol | 2.40 ± 0.15 a | 2.59 ± 0.35 a | 2.68 ± 0.28 a | 2.66 ± 0.32 a | 2.46 ± 0.31 a |
| Benzyl alcohol | 8.03 ± 1.35 b | 7.81 ± 1.73 b | 10.65 ± 0.72 ab | 11.79 ± 1.61 a | 7.75 ± 0.57 b |
| 1,4-Butanediol | 1.31 ± 0.48 a | 1.25 ± 0.16 a | 0.60 ± 0.06 b | 1.01 ± 0.03 ab | 1.22 ± 0.13 ab |
| ∑Other alcohols | 44.45 ± 3.04 bc | 38.51 ± 4.06 c | 47.89 ± 3.13 ab | 52.93 ± 0.48 a | 40.37 ± 1.81 bc |
| 2-Octenal | 274.53 ± 19.69 b | 219.22 ± 8.76 c | 315.23 ± 6.75 a | 280.30 ± 8.58 b | 263.40 ± 12.70 b |
| 2,4-Heptadienal | 0.38 ± 0.07 ab | 0.32 ± 0.04 b | 0.45 ± 0.04 ab | 0.62 ± 0.20 a | 0.40 ± 0.03 ab |
| Furfural | 1.11 ± 0.11 c | 0.72 ± 0.04 d | 1.41 ± 0.03 c | 2.25 ± 0.21 a | 1.7 ± 0.16 b |
| Decanal | 1.45 ± 0.38 a | 1.30 ± 0.06 a | 1.37 ± 0.11 a | 1.54 ± 0.40 a | 1.61 ± 0.32 a |
| Benzaldehyde | 8.06 ± 1.79 b | 6.26 ± 0.38 b | 11.91 ± 0.33 a | 12.47 ± 0.70 a | 6.91 ± 0.27 b |
| Benzeneacetaldehyde | 7.42 ± 0.37 b | 8.55 ± 0.29 a | 6.21 ± 0.60 c | 5.77 ± 0.22 c | 8.48 ± 0.13 a |
| Neral | 8.84 ± 0.08 a | 7.13 ± 1.14 ab | 4.11 ± 0.36 c | 3.33 ± 0.83 c | 6.2 ± 0.86 b |
| 2,4-Decadienal | 0.20 ± 0.03 a | 0.15 ± 0.02 ab | 0.08 ± 0.03 bc | 0.05 ± 0.02 c | 0.19 ± 0.06 a |
| ∑Aldehydes | 301.99 ± 19.32 b | 243.65 ± 8.11 c | 340.78 ± 5.61 a | 306.33 ± 7.40 b | 289.02 ± 13.77 b |
| Propanoic acid | 3.01 ± 0.18 c | 4.88 ± 0.19 a | 3.84 ± 0.36 bc | 4.29 ± 0.42 ab | 4.03 ± 0.35 b |
| 2-Methylpropionic acid | 3.52 ± 0.40 b | 2.63 ± 0.23 c | 4.60 ± 0.45 a | 2.79 ± 0.17 bc | 3.19 ± 0.19 bc |
| Butanoic acid | 2.71 ± 0.36 c | 3.80 ± 0.11 a | 3.02 ± 0.09 bc | 3.17 ± 0.24 bc | 3.30 ± 0.18 ab |
| Isovaleric acid | 7.67 ± 1.37 a | 3.02 ± 0.41 b | 5.94 ± 1.17 ab | 5.29 ± 0.52 ab | 8.09 ± 1.58 a |
| Hexanoic acid | 1,112.94 ± 103.53 b | 1,315.46 ± 85.31 ab | 1,493.72 ± 176.20 a | 1,455.99 ± 127.64 a | 1,307.05 ± 95.62 ab |
| Heptanoic acid | 3.57 ± 0.33 b | 8.95 ± 0.85 a | 3.69 ± 0.20 b | 8.75 ± 0.33 a | 9.07 ± 0.83 a |
| Nonanoic acid | 8.24 ± 0.16 a | 7.50 ± 0.77 a | 8.21 ± 0.26 a | 8.45 ± 0.46 a | 8.24 ± 0.43 a |
| Decanoic acid | 864.94 ± 51.74 ab | 737.23 ± 83.28 bc | 863.64 ± 33.27 ab | 876.59 ± 22.24 a | 696.02 ± 43.03 c |
| ∑Fatty acids | 2,006.59 ± 75.06 b | 2,083.47 ± 151.65 ab | 2,386.66 ± 155.65 a | 2,365.30 ± 126.77 a | 2,038.99 ± 137.26 ab |
| Eugenol | 0.35 ± 0.03 ab | 0.14 ± 0.05 c | 0.22 ± 0.07 bc | 0.39 ± 0.04 a | 0.49 ± 0.09 a |
| 4-Ethylphenol | 0.04 ± 0.02 a | 0.06 ± 0.04 a | 0.06 ± 0.03 a | 0.07 ± 0.02 a | 0.05 ± 0.04 a |
| 4-Vinylguaiacol | 30.54 ± 6.03 b | 32.38 ± 3.01 b | 35.02 ± 1.81 b | 50.09 ± 0.80 a | 50.66 ± 4.77 a |
| Vanillin | 0.92 ± 0.09 bc | 1.26 ± 0.14 a | 0.80 ± 0.07 bc | 0.75 ± 0.15 c | 1.12 ± 0.16 ab |
| Homovanillyl alcohol | 30.90 ± 6.13 a | 30.91 ± 5.22 a | 37.64 ± 4.46 a | 37.15 ± 7.80 a | 33.29 ± 6.79 a |
| ∑Volatile phenols | 62.76 ± 12.16 b | 64.76 ± 4.53 b | 73.74 ± 3.41 ab | 88.45 ± 6.84 a | 85.61 ± 3.20 a |
| γ-Butyrolactone | 311.44 ± 30.80 c | 380.71 ± 34.38 bc | 392.35 ± 15.52 b | 562.58 ± 27.68 a | 358.31 ± 25.18 bc |
| γ–Hexalactone | 1.73 ± 0.15 b | 1.63 ± 0.43 b | 2.64 ± 0.32 a | 2.50 ± 0.29 ab | 2.34 ± 0.41 ab |
| γ-Octalactone | 0.33 ± 0.04 a | 0.36 ± 0.01 a | 0.40 ± 0.04 a | 0.41 ± 0.02 a | 0.37 ± 0.06 a |
| γ-Nonalactone | 5.52 ± 0.39 ab | 5.07 ± 0.33 b | 6.34 ± 0.65 ab | 6.57 ± 0.22 ab | 6.94 ± 1.02 a |
| γ–Decalactone | 1.54 ± 0.16 b | 1.59 ± 0.13 b | 1.79 ± 0.19 b | 2.24 ± 0.15 a | 1.89 ± 0.15 ab |
| δ-Decalactone | 2.11 ± 0.22 a | 2.03 ± 0.02 a | 1.96 ± 0.12 a | 2.17 ± 0.13 a | 2.17 ± 0.29 a |
| γ-Undecalactone | 0.58 ± 0.10 a | 0.62 ± 0.04 a | 0.56 ± 0.09 a | 0.66 ± 0.07 a | 0.58 ± 0.06 a |
| ∑Lactones | 323.24 ± 30.85 c | 392.00 ± 33.76 bc | 406.04 ± 16.59 b | 577.13 ± 28.07 a | 372.59 ± 26.69 bc |
| 2-Pentylfuran | 228.35 ± 11.34 a | 224.63 ± 2.79 a | 201.88 ± 14.39 a | 213.08 ± 17.82 a | 207.61 ± 18.96 a |
| 6-Methyl-5-hepten-2-one | 114.17 ± 4.70 a | 89.90 ± 5.40 b | 62.49 ± 4.16 c | 113.50 ± 14.41 a | 117.64 ± 4.10 a |
| Acetoin | 4.07 ± 0.22 c | 13.20 ± 1.64 a | 6.16 ± 0.75 bc | 7.04 ± 0.50 b | 5.03 ± 0.99 bc |
| Acetylfurane | 1.13 ± 0.20 a | 1.03 ± 0.14 a | 1.10 ± 0.14 a | 1.20 ± 0.21 a | 1.12 ± 0.06 a |
| ∑Other compounds | 110.92 ± 15.03 b | 107.11 ± 7.81 b | 124.90 ± 6.52 ab | 145.05 ± 6.58 a | 129.67 ± 4.89 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mucalo, A.; Lukšić, K.; Budić-Leto, I.; Zdunić, G. Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate. Appl. Sci. 2022, 12, 7327. https://doi.org/10.3390/app12147327
Mucalo A, Lukšić K, Budić-Leto I, Zdunić G. Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate. Applied Sciences. 2022; 12(14):7327. https://doi.org/10.3390/app12147327
Chicago/Turabian StyleMucalo, Ana, Katarina Lukšić, Irena Budić-Leto, and Goran Zdunić. 2022. "Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate" Applied Sciences 12, no. 14: 7327. https://doi.org/10.3390/app12147327
APA StyleMucalo, A., Lukšić, K., Budić-Leto, I., & Zdunić, G. (2022). Cluster Thinning Improves Aroma Complexity of White Maraština (Vitis vinifera L.) Wines Compared to Defoliation under Mediterranean Climate. Applied Sciences, 12(14), 7327. https://doi.org/10.3390/app12147327









