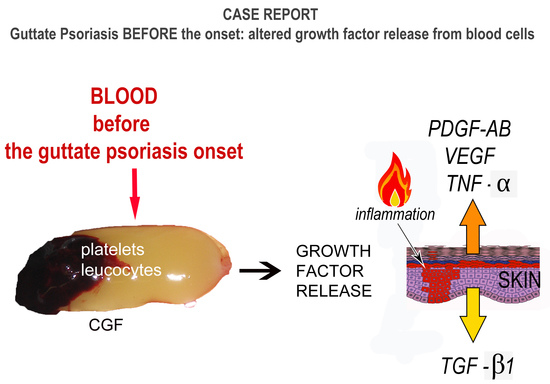
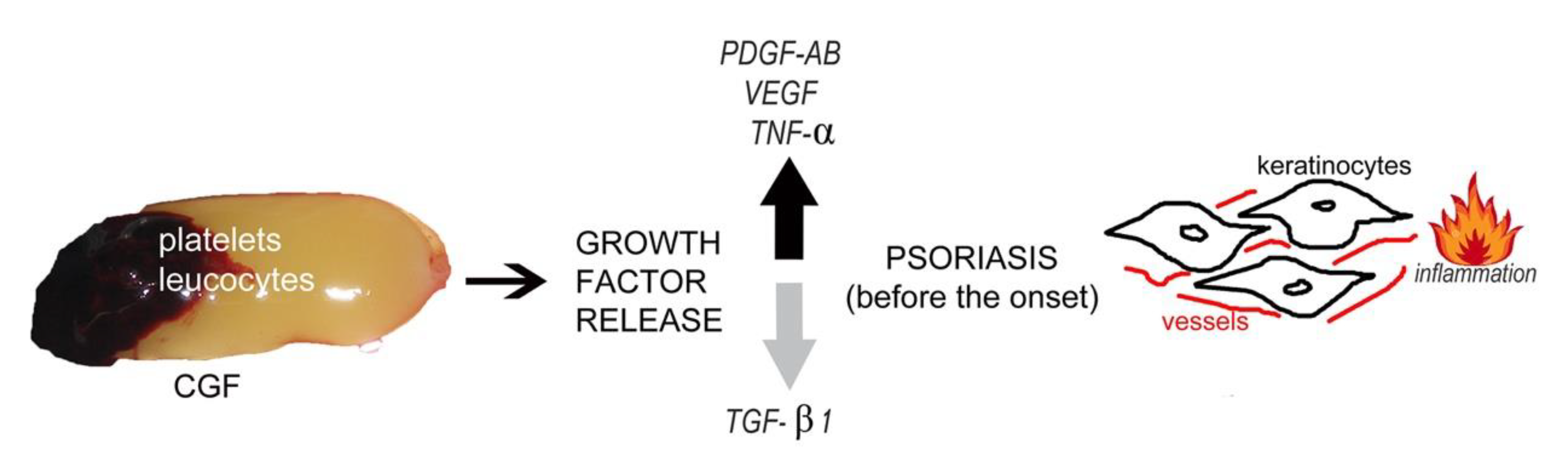
The Growth Factor Release from a Platelet-Rich Plasma Preparation Is Influenced by the Onset of Guttate Psoriasis: A Case Report
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Case Reports
2.3. Blood Collection
2.4. CGF Preparation
2.5. Cumulative Growth Factor Release
3. Results
3.1. Healthy Case
3.2. Case Report–Guttate Psoriasis before the Onset
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamagawa-Mineoka, R. Important roles of platelets as immune cells in the skin. J. Dermatol. Sci. 2015, 77, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.; Ashcroft, D.M. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and therapy of psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Dopytalska, K.; Ciechanowicz, P.; Wiszniewski, K.; Szymańska, E.; Walecka, I. The Role of Epigenetic Factors in Psoriasis. Int. J. Mol. Sci. 2021, 22, 9294. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa-Mineoka, R.; Katoh, N.; Kishimoto, S. Platelet activation in patients with psoriasis: Increased plasma levels of plate-let-derived microparticles and soluble P-selectin. J. Am. Acad. Dermatol. 2010, 62, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Herster, F.; Karbach, S.; Chatterjee, M.; Weber, A.N. Platelets: Underestimated Regulators of Autoinflammation in Psoriasis. J. Investig. Dermatol. 2021, 141, 1395–1403. [Google Scholar] [CrossRef]
- Senzel, L.; Gnatenko, D.V.; Bahou, W.F. The platelet proteome. Curr. Opin. Hematol. 2009, 16, 329–333. [Google Scholar] [CrossRef]
- Visser, M.J.E.; Venter, C.; Roberts, T.J.; Tarr, G.; Pretorius, E. Psoriatic disease is associated with systemic inflammation, endothelial activation, and altered haemostatic function. Sci. Rep. 2021, 11, 13043. [Google Scholar] [CrossRef]
- Korkmaz, S. Mean platelet volume and platelet distribution width levels in patients with mild psoriasis vulgaris with metabolic syndrome. Adv. Dermatol. Allergol. 2018, 35, 367–371. [Google Scholar] [CrossRef]
- Alkhateeb, A.; Fain, P.R.; Thody, A.; Bennett, D.; Spritz, R.A. Epidemiology of Vitiligo and Associated Autoimmune Diseases in Caucasian Probands and Their Families. Pigment Cell Res. 2003, 16, 208–214. [Google Scholar] [CrossRef]
- Jansen, E.E.; Braun, A.; Jansen, P.; Hartmann, M. Platelet-Therapeutics to Improve Tissue Regeneration and Wound Healing—Physiological Background and Methods of Preparation. Biomedicines 2021, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sánchez, M.; Orive, G.; Andia, I. Delivering growth factors for therapeutics. Trends Pharmacol. Sci. 2008, 29, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barbero, J.E.; Galindo-Moreno, P.; Avila-Ortiz, G.; Caba, O.; Sánchez-Fernández, E.; Wang, H.-L. Flow cytometric and morphological characterization of platelet-rich plasma gel. Clin. Oral Implant. Res. 2006, 17, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Cocchi, M.A.; Castrezzati, S.; Scari’, G.; Baldi, F.; Pandini, S.; Licenziati, S.; Parolini, S.; et al. Biological Characterization and In Vitro Effects of Human Concentrated Growth Factor Preparation: An Innovative Approach to Tissue Regeneration. Biol. Med. 2015, 7, 1–11. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scarì, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.M.; Prado, R.; Alkhraisat, M.H.; Orive, G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: Evaluation of the effect of leukocyte inclusion. J. Biomed. Mater. Res. Part A 2015, 103, 1011–1020. [Google Scholar] [CrossRef]
- Rätsep, R.; Kingo, K.; Karelson, M.; Reimann, E.; Raud, K.; Silm, H.; Vasar, E.; Kõks, S. Gene expression study of IL10 family genes in vitiligo skin biopsies, peripheral blood mononuclear cells and sera. Br. J. Dermatol. 2008, 159, 1275–1281. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.M.; Alkhraisat, M.H.; Orive, G. Release kinetics of platelet-derived and plasma-derived growth factors from autologous plasma rich in growth factors. Ann. Anat.-Anat. Anz. 2013, 195, 461–466. [Google Scholar] [CrossRef]
- Bonazza, V.; Borsani, E.; Buffoli, B.; Castrezzati, S.; Rezzani, R.; Rodella, L.F. How the different material and shape of the blood collection tube influences the Concentrated Growth Factors production. Microsc. Res. Tech. 2016, 79, 1173–1178. [Google Scholar] [CrossRef]
- Takeshita, J.; Mohler, E.R.; Krishnamoorthy, P.; Moore, J.; Rogers, W.T.; Zhang, L.; Gelfand, J.M.; Mehta, N.N. Endothelial cell-, platelet-, and monocyte/macrophage-derived microparticles are elevated in psoriasis beyond cardiometabolic risk factors. J. Am. Heart Assoc. 2014, 3, e000507. [Google Scholar] [CrossRef]
- Tseng, J.C.; Chang, Y.C.; Huang, C.M.; Hsu, L.C.; Chuang, T.H. Therapeutic Development Based on the Immunopathogenic Mechanisms of Psoriasis. Pharmaceutics 2021, 13, 1064. [Google Scholar] [CrossRef] [PubMed]
- Aghamajidi, A.; Raoufi, E.; Parsamanesh, G.; Jalili, A.; Salehi-Shadkami, M.; Mehrali, M.; Mohsenzadegan, M. The attentive focus on T cell-mediated autoimmune pathogenesis of psoriasis, lichen planus and vitiligo. Scand. J. Immunol. 2021, 93, e13000. [Google Scholar] [CrossRef]
- Gisondi, P.; Girolomoni, G. Psoriasis and Atherothrombotic Diseases: Disease-Specific and Non–Disease-Specific Risk Factors. Semin. Thromb. Hemost. 2009, 35, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.; Carton, J.; Wakelin, S. Subacute cutaneous lupus erythematosus: Is clopidogrel a trigger? Clin. Exp. Dermatol. 2018, 43, 600–601. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.A.; Chandratreya, A.; Lee, P.Y.F. A Systematic Review on the Use of Aspirin in the Prevention of Deep Vein Thrombosis in Major Elective Lower Limb Orthopedic Surgery: An Update from the Past 3 Years. Surg. J. 2017, 3, e191–e196. [Google Scholar] [CrossRef] [PubMed]
- Habets, K.L.; Huizinga, T.W.; Toes, R.E. Platelets and autoimmunity. Eur. J. Clin. Investig. 2013, 43, 746–757. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Psoriasis. Int. J. Mol. Sci. 2020, 21, 5405. [Google Scholar] [CrossRef]
- Grine, L.; Dejager, L.; Libert, C.; Vandenbroucke, R.E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015, 26, 25–33. [Google Scholar] [CrossRef]
- Papp, K.A.; Poulin, Y.; Bissonnette, R.; Bourcier, M.; Toth, D.; Rosoph, L.; Poulin-Costello, M.; Setterfield, M.; Syrotuik, J. Assessment of the long-term safety and effectiveness of etanercept for the treatment of psoriasis in an adult population. J. Am. Acad. Dermatol. 2012, 66, e33–e45. [Google Scholar] [CrossRef]
- Swindell, W.R.; Xing, X.; Stuart, P.E.; Chen, C.S.; Aphale, A.; Nair, R.P.; Voorhees, J.J.; Elder, J.T.; Johnston, A.; Gudjonsson, J.E. Heterogeneity of Inflammatory and Cytokine Networks in Chronic Plaque Psoriasis. PLoS ONE 2012, 7, e34594. [Google Scholar] [CrossRef]
- Tsoi, L.C.; Spain, S.L.; Knight, J.; Ellinghaus, E.; Stuart, P.E.; Capon, F.; Ding, J.; Li, Y.; Tejasvi, T.; Gudjonsson, J.E.; et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nat. Genet. 2012, 44, 1341–1348. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular endothelial growth factor and angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef] [PubMed]
- Meki, A.-R.M.A.; Al-Shobaili, H. Serum Vascular Endothelial Growth Factor, Transforming Growth Factor β1, and Nitric Oxide Levels in Patients with Psoriasis Vulgaris: Their Correlation to Disease Severity. J. Clin. Lab. Anal. 2014, 28, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.E., Jr.; Harrison, R.G. Demonstration and characterization of an epidermal angiogenic factor. J. Investig. Dermatol. 1973, 61, 130–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhushan, M.; McLaughlin, B.; Weiss, J.B.; Griffiths, C.E. Levels of endothelial cell stimulating angiogenesis factor and vascular endothelial growth factor are elevated in psoriasis. Br. J. Dermatol. 1999, 141, 1054–1060. [Google Scholar] [CrossRef]
- Creamer, D.; Allen, M.H.; Sousa, A.; Poston, R.; Barker, J.N. Localization of endothelial proliferation and microvascular expansion in active plaque psoriasis. Br. J. Dermatol. 1997, 136, 859–865. [Google Scholar] [CrossRef]
- Detmar, M.; Brown, L.F.; Claffey, K.P.; Yeo, K.T.; Kocher, O.; Jackman, R.W.; Berse, B.; Dvorak, H.F. Overexpression of vascular permeability factor/vascular endothelial growth factor and its receptors in psoriasis. J. Exp. Med. 1994, 180, 1141–1146. [Google Scholar] [CrossRef]
- Andrys, C.; Borska, L.; Pohl, D.; Fiala, Z.; Hamakova, K.; Krejsek, J. Angiogenic activity in patients with psoriasis is significantly decreased by Goeckerman’s therapy. Arch. Dermatol. Res. 2007, 298, 479–483. [Google Scholar] [CrossRef]
- Young, H.S.; Bhushan, M.; Griffiths, C.E.; Summers, A.M.; Brenchley, P. Single-Nucleotide Polymorphisms of Vascular Endothelial Growth Factor in Psoriasis of Early Onset. J. Investig. Dermatol. 2004, 122, 209–215. [Google Scholar] [CrossRef]
- Elder, J.T.; Ellingsworth, L.R.; Fisher, G.J.; Voorhees, J.J. Transforming growth factor-beta in psoriasis. Pathogenesis and therapy. Ann. N. Y. Acad. Sci. 1990, 593, 218–230. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef]
- Li, A.G.; Wang, D.; Feng, X.H.; Wang, X.J. Latent TGFbeta1 overexpression in keratinocytes results in a severe psoriasis-like skin disorder. EMBO J. 2004, 23, 1770–1781. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.-M.; Huang, X.-R.; Wang, X.-J.; Yang, L.; Lan, H.Y. Transforming growth factor-β1 mediates psoriasis-like lesions via a Smad3-dependent mechanism in mice. Clin. Exp. Pharmacol. Physiol. 2014, 41, 921–932. [Google Scholar] [CrossRef]
- Hosgood, G. Wound healing. The role of platelet-derived growth factor and transforming growth factor beta. Vet. Surg. 1993, 22, 490–495. [Google Scholar] [CrossRef]
- Gu, X.-F.; Raynaud, F.; Evain-Brion, D. Increased Chemotactic and Mitogenic Response of Psoriatic Fibroblasts to Platelet-derived Growth Factor. J. Investig. Dermatol. 1988, 91, 599–602. [Google Scholar] [CrossRef]
- Krane, J.F.; Murphy, D.P.; Gottlieb, A.B.; Carter, D.M.; Hart, C.E.; Krueger, J.G. Increased Dermal Expression of Platelet-Derived Growth Factor Receptors in Growth-Activated Skin Wounds and Psoriasis. J. Investig. Dermatol. 1991, 96, 983–986. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borsani, E.; Buffoli, B.; Bonomini, F.; Rezzani, R. The Growth Factor Release from a Platelet-Rich Plasma Preparation Is Influenced by the Onset of Guttate Psoriasis: A Case Report. Appl. Sci. 2022, 12, 7250. https://doi.org/10.3390/app12147250
Borsani E, Buffoli B, Bonomini F, Rezzani R. The Growth Factor Release from a Platelet-Rich Plasma Preparation Is Influenced by the Onset of Guttate Psoriasis: A Case Report. Applied Sciences. 2022; 12(14):7250. https://doi.org/10.3390/app12147250
Chicago/Turabian StyleBorsani, Elisa, Barbara Buffoli, Francesca Bonomini, and Rita Rezzani. 2022. "The Growth Factor Release from a Platelet-Rich Plasma Preparation Is Influenced by the Onset of Guttate Psoriasis: A Case Report" Applied Sciences 12, no. 14: 7250. https://doi.org/10.3390/app12147250
APA StyleBorsani, E., Buffoli, B., Bonomini, F., & Rezzani, R. (2022). The Growth Factor Release from a Platelet-Rich Plasma Preparation Is Influenced by the Onset of Guttate Psoriasis: A Case Report. Applied Sciences, 12(14), 7250. https://doi.org/10.3390/app12147250