Abstract
A lateral periodontal cyst (LPC) is a rare finding. Patients diagnosed with LPC younger than 40 years of age are not that common. Mostly asymptomatic LPCs can be discovered on routine radiographs as an oval radiolucency between two mandibular premolar teeth roots and can vary in shape and size. Most cases are treated with enucleation, bone curettage, or a combination of both, greatly depending on the surgeon’s preference. Because most LPCs are quite small, lesions do not require any regenerative procedures. In the presented case, because of cortical expansion of the lesion and loss of vital bone bridge surrounding two adjacent maxillary teeth, a guided bone regenerative procedure (GBR) with xenograft bone substitute was used. Despite that small lesions can heal on their own, nowadays the approach of full-mouth therapy (FMT), including direct soft or hard tissue reconstruction in the oral cavity, seems to be a wise treatment. The presented paper presents and describes an unusual LPC case with cortical bone expansion in a very rare maxillary canine–premolar region.
1. Introduction
Cysts and tumors of odontogenic and non-odontogenic origins are common findings in the oral cavity. In order to fully differentiate and recognize a selected cyst or tumor, a detailed clinical, radiological, and pathomorphological knowledge is important. A lateral periodontal cyst (LPC) is a rare finding in the jaw bones. It is an unusual odontogenic cyst of benign potential, with occurrence estimation of 0.4–1% [1,2,3,4]. Due to its rare occurrence, LPC has some characteristic features of an odontogenic cyst of developmental origin mostly found in adults > 40 years of age. No sex-predilection is notable. Re-occurrence rate is very low [1,2,3].
The first LPC literature description was reported by Standish and Shafer in 1958. In 1987, Gardner et al. described glandular odontogenic cyst (GOC) variation from LPC. Later, Altini and Shear, in 1992, evaluated and described three LPS forms: monocystic, multicystic, and botryoid type [4,5,6]. Most commonly, LPC is presented as a unilocular lesion on radiological evaluation.
Some hypotheses of their origins are known. The first of them describes its origin as a dentigerous cyst arising from expansion of the dental follicle along its lateral root aspect. The second one explains its origins from the remnants of Malassez cells during odontogenesis [3,4,5,6,7,8].
Most commonly, LPC is found in the mandible canine and premolar region, and less commonly in anterior maxilla, while other locations remain extremely rare. Clinically it presents without any pain or inflammation, and rarely tenderness or teeth mobility. Most often it is described as a non-keratinized, noninflammatory related lesion present along mostly lateral surface of a fully developed, erupted, and healthy vital tooth. Pain might be present if a vital tooth is involved in the lesion or is displaced [1,2,3,4,5,6,7].
Cases of LPC inflammation or abscess formation are rare. Larger cysts can cause bone perforations, fenestrations, and be painful. Sometimes an LPC appears as a small blue cyst when outside of the bone. Potential local aggressiveness should be established during clinical examination, and presence of such factors as teeth mobility, radiolucency with irregular borders, and teeth/root resorption with or without any gingival lesions might confirm this [5,6,7,8]. In most cases, a presented lesion is found accidentally on routine dental radiographs, or is perhaps visible in the oral cavity as an atypical swelling between two dental roots. Quite often vital teeth do not give any features such as local inflammation, pain, abscess formation, or other. Most commonly, LPCs are small, oval/round lesions between heathy dental roots with various sizes, without teeth resorption or their displacement. Unilateral cystic lesion with clear visible borders rarely cause tooth displacement, and never their resorption [1,3,5,6,7,8,9].
Histologic evaluation mostly reveals a thin, nonkeratinizing layer of some squamous or even cuboidal/flattened epithelium. In most cases, 1–3 layers are present, similar to a reduced enamel epithelium or even dental lamina (Serres remnants). Furthermore, some collagen fibers, vascular spaces, glycogen-rich clear cells, or rarely other cells can be found in the cystic lining, also presented sometimes with focal plaques of epithelial thickenings looking similar to a fusiform or spindle-shaped pattern [4,5,6,7]. Parts of odontogenic epithelium within cyst walls are also present. So far, no malignant LPC form or transformation has been noted [8,9].
Over time, the World Health Organization (WHO) odontogenic tumor classification and nomenclature has changed. LPCs are known also for their two other rare types, namely, botryoid odontogenic cysts (BOCs) and glandular odontogenic cysts (GOCs). GOC is the most aggressive form, it is 70% common in mandible and very rarely <1%, with high re-occurrence rate even at 22%. On the other hand, BOC is the less common form, with expansive growth pattern, common in mandible, high reoccurrence rate, >30% after treatment, and is characteristic for its botryoid-like small oval-shaped features microscopically [1,2,3,4,5]. Differential diagnosis of LPC should include other dental cysts, unicystic ameloblastoma, odontogenic keratocyst, follicular cyst, residual cysts, lateral radicular cyst, ossifying fibroma (OF), BOC and GOC types, gingival cyst of adults (GCA), or others [7,8,9]. Atypical findings might also include other cyst-like pathologies adjacent to one, or present between two fully developed roots [5,7,8,9]. After either of the mentioned pathologies removal, presence of various bone defects can be treated with known bone regenerative techniques or can heal by their own by spontaneous healing. The indication for those are greatly case-related, dependent on future perspectives, such as dental implant placement and presence of bone ridge, prosthesis usage, visual and esthetic aspects, and others.
Based on the following, the scope of each surgery can be very easily planned on any CBCT when a detailed examination is focused on: radiolucent or radiopaque areas with or without irregular borders; expansion between roots or extending far beyond their apexes; localized in the superior/inferior part of the alveolar ridge; cortical bone expansion, loss of vital bone ridge; close relation to vital structures such as mandibular canal, maxillary sinus, naso-palatal canal, mental foramen, and nasal cavity.
The aim of this study is to present a very rare case of lateral maxillary LPC with buccal cortical plane expansion towards gingival soft tissues along with a follow-up period of one year. Furthermore, surgical curettage with ostectomy and guided bone regeneration (GBR) repair with xenograft injectable platelet rich fibrin (iPRF) and resorbable collagen membrane and later outcomes will be presented.
2. Case Report Presentation
A 35-year-old female patient presented with a small, well-defined lesion on the attached gingival area, approximately 5/6 mm in diameter between roots of teeth 23–24, reported to the Privat Dental Clinic. The patient’s main concern was focused on gingival asymmetry and a tumor-like mass, painless and sometimes tender on tooth brushing. Clinical anamnesis revealed the occurrence of this asymptomatic mass for over 6 months. Because of swelling and atypical cortical expansion, the patient was referred for consultation and treatment. The patient was generally healthy without any chronic illness or important medical and dental past. A routine panoramic radiograph revealed a well-defined, radiolucent, left maxillary lesion located between the roots of the canine and first maxillary premolar (Figure 1 and Figure 2).
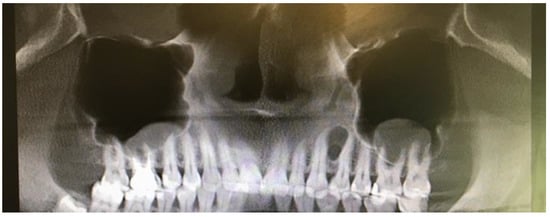
Figure 1.
Panoramic radiograph slice from CBCT evaluation of LPC.
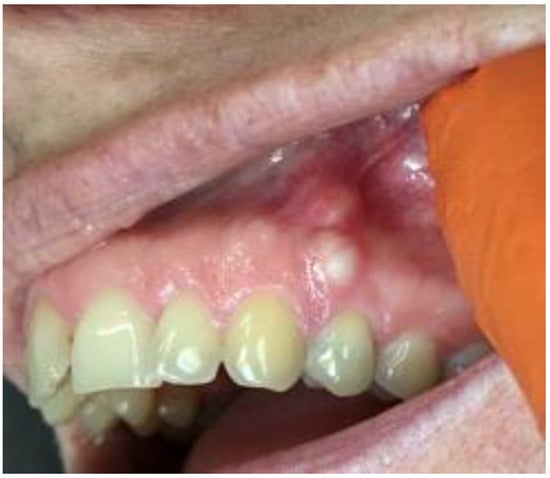
Figure 2.
LPC expanding towards attached gingiva with buccal cortical plate resorption.
Clinically, teeth remain vital, non-displaced, stably situated in the alveolar socket with visible asymptomatic solitary swelling on the buccal gingiva with visible perforation of buccal cortical plate, not fluctuant on palpation. No ulceration or perforation through the attached gingiva was present. Presented cystic lesion was unilateral, painless in examination, and well related with adjacent expanded gingiva. Neither inflammation nor abscess formation was present. Both premolars responded well to cold stimulus. Surrounding teeth dental and periodontal status was good, all vital signs normal, and there was no periodontal disease without inflammated sockets or any other pathologies in surrounding bone and periodontal structures.
Cone beam computed tomography (CBCT) evaluation revealed the perforation of the buccal cortical bone plate, with expansion towards soft tissues, measuring about 6 mm at longest diameter (Figure 3 and Figure 4). Palatal bone plate remained without any lesions. Because of close proximity to the apex of first premolar and scheduled enucleation with bone curettage/drilling, a decision of endodontic treatment 24 was scheduled. Secondly, such treatment was also indicated because of (1) the loss of cortical plane (possible local aggressiveness of lesion), (2) possible GOC/BOC or other dental-related cyst/tumor occurrence; (3) possible teeth pulp necrosis/inflammation after extensive curettage/bone drilling used for local radicalization protocol; (4) minimizing the time needed for secondary surgery if such would be necessary in case of other pathology. On CBCT further evaluation, a slight sclerotic border close to both tooth apex/roots was visible. Teeth structure remained preserved, with only the buccal cortical bone perforation. On the floor of the left maxillary sinus, a retention mucous cyst was seen; however, due to the clinical irrelevance, only a laryngologist consultation was scheduled.
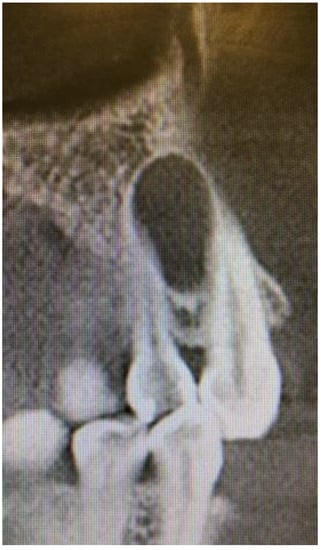
Figure 3.
CBCT sagittal view of LPC.
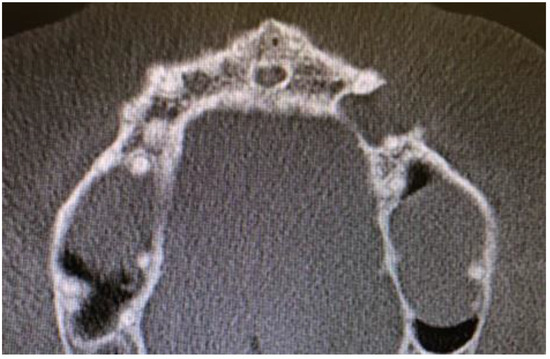
Figure 4.
CBCT axial view of LPC, note 23 teeth close to apex relation with lesion.
The decision was made firstly to prepare the first left maxillary premolar for endodontic treatment, then schedule dental hygienization as preparation for surgery. The surgical plan consisted of cyst removal, followed by bone curettage with ostectomy if needed and guided bone regeneration (GBR) under local anesthesia. Intra-oral premedication 60 minutes before procedure consisted of 2 g Amoxicillin (Sandoz Poland, Novartis Division-Basel, Switzerland) and 200 mg Nimesil (Nimesulidum, Laboratori Guidotti, Pisa, Italy). Oral cavity was rinsed with 0.1% CHX (chlorhexidine gluconate) (Eludril, Pierre-Fabre Oral Care, Paris, France) solution for 30 seconds. Additionally, lips and skin surrounding the operating field were scrubbed with alcohol—ethanol 96% solution–AHD 1000 solution (MediLab, Lysoform, Wuerzburg, Germany). Protective sterile Vaseline (Unilever, Leatherhead, England) cream was applied to lips. Two ampules of local anesthetic (1.7 mL each), Ubistesin forte 4% (artycaine with epinephrine, 40 mg + 0.012 mg; 3M Espe Oral Care, St. Paul, MN, USA), was administered.
Some special concerns should be mentioned regarding the drug Nimesil (Nimesulidum), one of the known non-steroidal anti-inflammatory drugs (NSAIDs). The drug presented herein drug is prohibited in some countries because of a few known cases of liver failures, mentioned and presented worldwide rare fatal cases; however, in Polish Drug Agency guidelines, its usage is not prohibited. Therefore, the authors would like to address this issue and point out that other NSAIDs drugs can be used instead with good pain control and fewer possible complications [10,11].
A modified gingival crevicular incision with lateral counter incisions extending from 22–25 was used. Lateral incisions were supposed to help, to rotate and advance the flap in case of flap perforation because of the buccal swelling and very thin gingival layer. Secondly, if flap perforation would be hard to close, a layer of long resorbable membrane +iPRF was supposed to help with granulation process on used collagen membrane.
Mucoperiosteal flap elevation helped gain full visibility of the cyst, which had been perforating the buccal cortical bone and ingrowing from inside onto the released flap. Blunt dissection helped to divide the cyst from the thin flap without any perforation. Cyst integrity remain untouched and flap had been carefully elevated further downwards from the bony defect. Macroscopically, there was a cystic cavity with some fluid accumulation, soft in palpation, without any tumor-mass-like structures inside.
A surgical removal consisted of cyst enucleation, followed by bone curettage, which resulted in removal of the entire cyst. Later on, because of cortical bone expansion and occupation of the adjacent apex of the teeth, a high-speed surgical drill ostectomy of adjacent bone walls and endodontically treated apex was performed in order to maximize the radical surgery protocol.
After cyst removal and bone cavity radicalization, saline irrigation helped to estimated bone boundaries. Adjacent teeth remained stable, without any pathological movement. Palatal cortical bone remained unchanged. Because of a significant hard tissue loss and bone defect, a decision was planned for GBR procedure—guided bone regeneration with xenograft bone (1 g The Graft 0.25–1 mm, Manufacturer Purgo Biologics Inc., Seongnam-si, Gyeonggi-do, Korea) and resorbable collagen membrane (20 × 30 mm OsseoGuard Membrane-Zimmer Biomet, Collagen Matrix Inc., Oakland, CA, USA) was performed. Xenograft material was mixed with two iPRF tubes. A centrifuge (Model: Minifuge, AMD Company, New Delhi, India) was used at 2660 rpm for 12 minutes. Suturing was made with 5.0 interrupted sutures (Dafilon, B.Braun, Melsungen/Ag, Germany) and 6.0 nylon vertical mattress sutures (Figure 5).
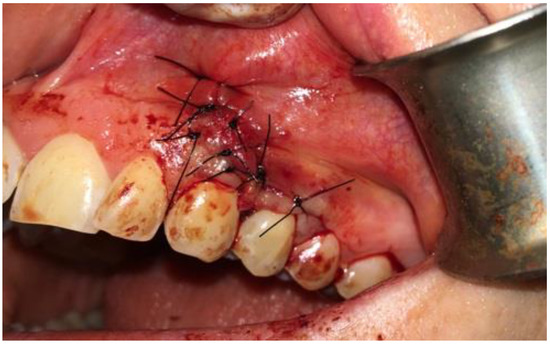
Figure 5.
Intraoperative photograph with sutured collagen membrane onto xenograft bone with iPRF.
Control CBCT revealed good, complete bone filling with bone and stable position of the material (Figure 6 and Figure 7). The patient was discharged with Amoxicillin solutab solution 1 g 2 times per day with Nimesil 100 mg 2 times per day and additional ad hoc paracetamol 1 g 3 times per day if necessary.
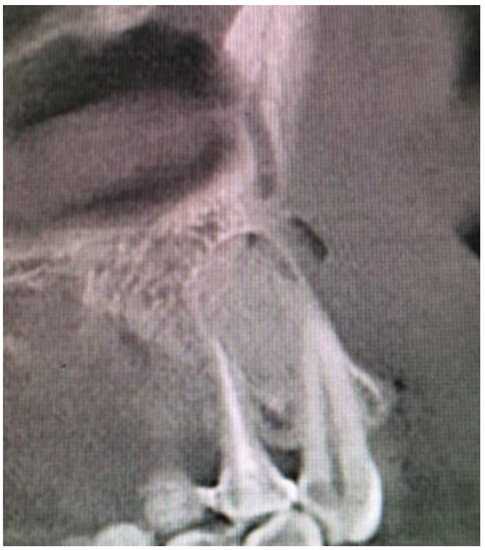
Figure 6.
CBCT sagittal view after surgery.
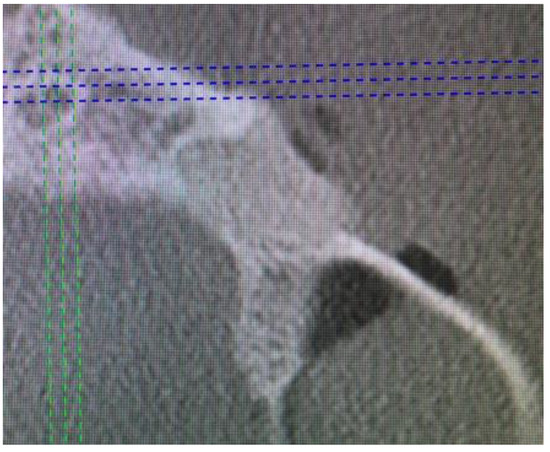
Figure 7.
CBCT axial view directly after surgery to evaluate buccal bone plate restoration.
After-surgical wound care included 3 times per day rinsing with 0.1% CHX solution-Eludril 0.20% (Pierre-Fabre Oral Care); 3 times per day H2O2 hydrogen peroxide 3% (HascoLek, Wrocław, Poland) and appliance for sleep CHX gel solution on the wound. After local wound dehiscence, additional Solcoseryl dental adhesive paste (Meda Pharma, Solna, Sweden) was used to improve wound granulation (Figure 8). Wound hygiene was maintained for 14 days.
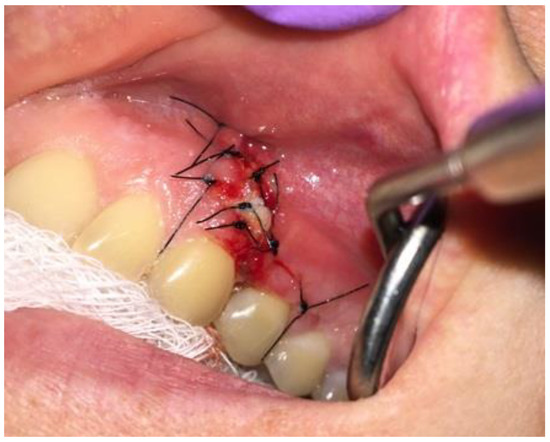
Figure 8.
Intraoral photograph 3 days after surgery.
Thin attached gingiva did not heal as well as planned, therefore collagen membrane and iPRF helped the granulation process to fill and heal the gap in the flap (Figure 9 and Figure 10). The surgical period was uneventful, no inflammation or other complications were present during healing. Good healing of xenograft bone and gingival contour was achieved. Routine follow-ups took place every week for six week, then every month till six months. After 18 months post-op, an overall good final surgical outcome was achieved, and control CBCT evaluation underlined a good condition of the healed bone (Figure 11, Figure 12 and Figure 13). Histopathological evaluation confirmed the diagnosis of LPC, without any inflammation, granulation, or other tumor-related features.
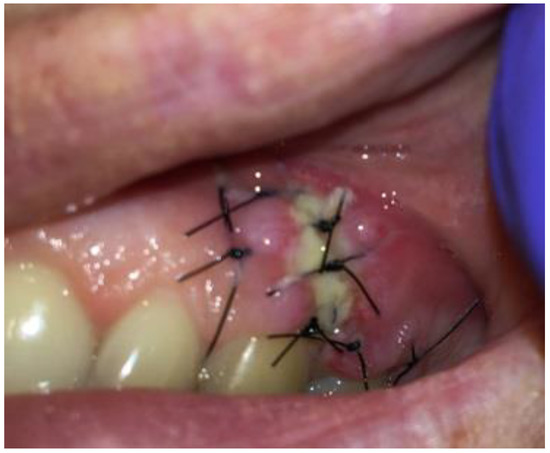
Figure 9.
Intraoral photograph 7 days after surgery.
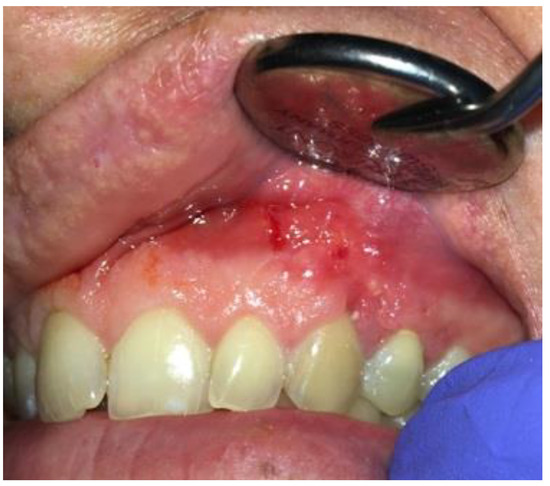
Figure 10.
Intraoral photograph 14 days after surgery.
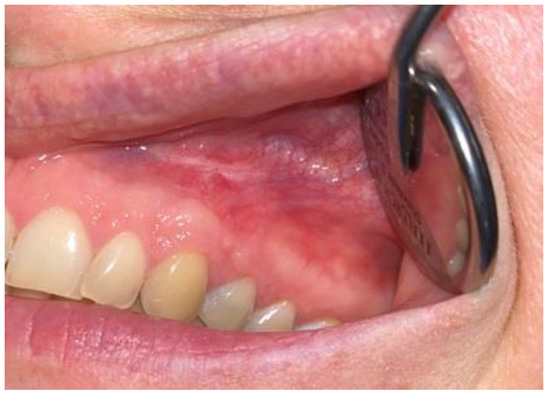
Figure 11.
Intraoral photograph 15 months after surgery.
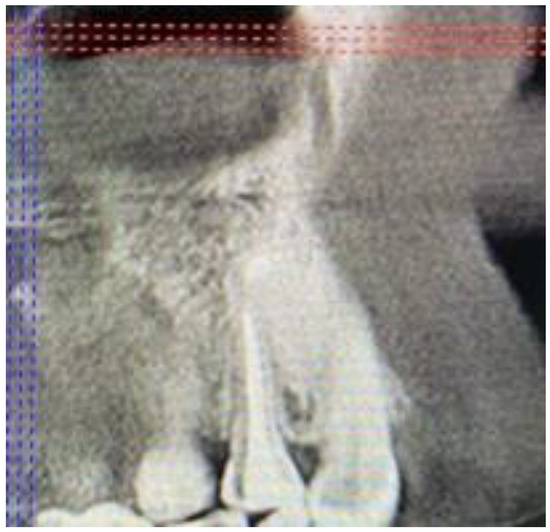
Figure 12.
CBCT sagittal view after 15 months.
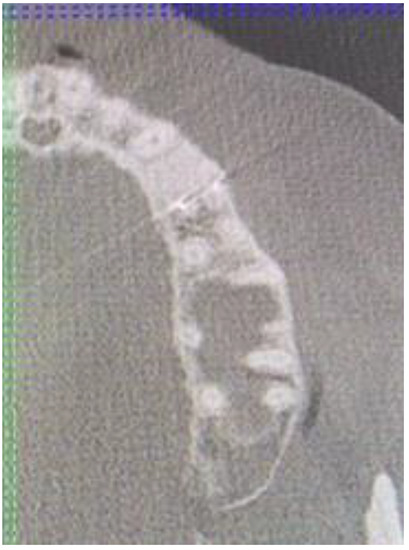
Figure 13.
CBCT axial view after 15 months after surgery.
The result of the surgery was good, without any re-occurrence of the LPC. Surgical enucleation seems to be the treatment of choice; however, additional curretage or bone ostectomy remain individually derived for a case; such as cortical expansion, large defects, large cysts, or a surgeon’s own preferences.
Clinical consideration should include the following: (1) dental status of adjacent teeth and periodontal tissues; (2) positive vital test for opposite teeth; (3) condition of palatal/buccal cortical walls; (4) careful planning of type of bone regeneration based on tissue defect; (5) local bone defect curretage or burr ostectomy for surgical radicalization; (6) in case of flap dehiscence, plan a secondary approach: collagen membrane and/or rotational full flap surgery to cover the defect; (7) maintain sutures for 2–3 weeks; (8) improve local granulation in case of flap necrosis/dehiscence; (9) maintain patient oral hygiene for healing 2–4 weeks (0.1% CHX, or other).
3. Discussion
Any type of oral cavity cysts or tumors should be fully visualized and evaluated on a CBCT. Detailed patient anamnesis should include any clinical symptoms occurring with the lesion, co-existing morbidities, and time of occurrence. Most tumors and cysts occur in the presence of a retained tooth, mostly in the mandibular posterior region.
LPC can be easily diagnosed based on radiological and clinical symptoms [1,2,3,4,5]. Most often, its location between anterior/premolar mandibular tooth roots is found as a typical sign of this cyst. Some authors hypothesize that because of an LPC’s close relation with root surface, a possible origin from the cell rests of Malassez could be important [3,5]. Rarely, its atypical features, such as cortical expansion or tooth displacement, occur. An additional CBCT is mandatory to establish close features of the lesion [5,6,7,8]. According to Altini et al. and Shear et al., cortical plate expansions should always be carefully examined, especially in any case of GOC/BOC or any other appearances of irregular lesions in classic radiographs [3,5]. On the other hand, to fully establish the degree of bone loss and direct proximity of vital teeth, maxillary sinus or nerve/artery branches, in any cystic lesion, a CBCT is nowadays a gold standard of diagnostics, which is also confirmed by Chrcanovic et al. [8]. In cases of tooth resorption and irregular bone lesions, other odontogenic or non-odontogenic cysts or tumors can be suspected [7,9]. Usually, LPC is diagnosed accidentally during routine control radiograph, which is well described in most cases [12,13,14,15]. Rarely, atypical situations might occur, including its inflammation, huge size, and atypical location between crowns or opposite to the buccal side. Secondary infection in LPC is rare, and might be related with as surrounding teeth remain non-vital because of damage to the apex vital structures [5,6,7,8,9,10,11,12].
Since LPC has a low re-occurrence rate that requires in most cases simple cyst removal, with or without additional curettage, no other special approaches are needed [7,16,17]. Curettage itself is used to avoid remaining any cyst remnants and improve surgical quality [17,18,19,20,21]. On the other hand, cases of bigger, expansive LPC forms, along with GOC and BOC forms of LPS, which are rare, require a more radical approach because of a big possible re-occurrence rate around 22–35% in total [18,19,22]. Shah et al. underlined the necessity of more extensive surgeries in cases of bigger pathologies; nevertheless, involvement of two or more teeth, propagation towards nasal cavity, or maxillary sinus or palate destruction in maxillary lesions are considered factors of more difficult surgery [17,18,19,22].
The more aggressive form, BOC is named by many authors as a polymorphous odontogenic cyst [7,15]. Microscopic evaluation of the cyst wall can describe the form of the lesion, either a classic LPC or a more aggressive multicystic or botryoid form. In any case of microcyst formation in the microscopically evaluated cyst wall, this finding should arouse suspicion about a possible more aggressive form of the lesion [3,7,9,15,16,22].
Most pathologies are located in the posterior mandible, mostly associated with some type of impacted teeth, especially third molars. In most cases, odontogenic keratocysts (ODC), dentigerous cysts, variants of ameloblastoma, or others, including BOC and GOC, in the anterior mandible/maxillary region should be differentiated from [1,2,3,4,5,6,7].
Since their bone aggression is bigger and more rapid in time, more radical surgery is discussable. A detailed histopathological specimen biopsy is needed to establish the nature and aggressiveness of the lesion [5,9,14,15,16]. Therefore, authors in the presented study, because of cortical expansion, additionally combined bone curettage with local bone cavity ostectomy. In this place, we hypothesize that the scope and degree of surgery is established after evaluation shape and size of the cyst (<1 cm; 1–2 cm, >2 cm), condition of the adjacent bone (cortical expansion in one wall, two walls, entire defect), and teeth (displacement, resorption). Rarely, teeth need to be extracted or treated endodontically. This situation is greatly dependent on the condition of the teeth, close proximity of the cyst, and the vitality of teeth.
A great GOC aggressive potential in some cases might even result in local bone resection with simultaneously performed reconstruction [5,6,7]. Bone grafting, microsurgery, bone/skeletal scaffolds, or others also can be used with or without collagen resorbable or synthetic non-resorbable membranes to protect and enhance healing in the grafted bone [15,16,22].
The degree of bone reconstruction depends mostly on the degree of bone loss and type of damage caused by the cyst: bone resorption, lack of cortical plate, and teeth resorption or displacement. Standard protocol in small defects consists of simultaneously performed GBR procedure with xeno-bone graft. Great interest in iPRF usage is still growing. Ramalingam et al.’s study suggests that GBR, xenograft/iPRF, and collagen membrane usage should be more common [18]. Used herein, collagen membrane resorption profile lasts 6–9 months, which is considered as a long period and is (1) adequate for bone/GBR healing procedures; (2) enables soft tissue granulation on its surface; (3) can be shaped onto desirable position; (4) is easily sewn; (5) grants adequate passive stable position if sutured to adjacent tissues; (6) enhances bone healing. Collagen membrane should help in maintaining stable xenograft bone position [17,18,19]. In some cases of bigger cysts, especially in older peoples with chronic illness or possibilities of pathological bone fractures, or children, a two-step surgery consisting of Partsch I with histological evaluation and silicone or rubber obturator can be used [17,19].
If GBR procedures are not used, a spontaneous healing method can also be used. The mentioned method should be indicated for small lesions, inflammated tissues, presence of good adequate volume of cortical bone and adequate bony ridge height and shape, and others [5,8,14,21,22].
In most cases, a fully erupted tooth is related to LPC, with typical location in the anterior mandible, incisor, or premolar area [20]. An atypical case of LPC associated with impacted teeth was described by Buchholzer et al. [21]. Since most case are unilocular, rarely multiple or multifocal LPC cyst presentation can also occur, similar to in Siponen et al.’s case [23]. Other atypical locations, depending on the localization between dental roots, crown, situated more coronally or laterally in the erupted teeth, are also known [24].
After surgical removal of the cyst, each clinician should remember to achieve a good final result. Propper planning of soft and bony tissue regeneration is necessary to avoid any bone asymmetry, gum recession, or others related with surgery. Especially, the anterior maxillary region requires special attention in maintaining esthetical and functional outcome, especially resulting in no pathological teeth mobility or their displacement because of lacking bone support after cyst removal.
4. Conclusions
LPC is a slowly growing lesion. The unicystic form, without any tooth or bone resorption, can be successfully treated with cyst enucleation. Some studies have shown that additional bone curettage might greatly decrease potential re-occurrence of the lesion, while others indicate that bony defect reconstruction is a treatment of choice nowadays, if LPC is confirmed. Because a lot of clinical and radiological symptoms of LPC are typical, only rarely can its true nature be revealed after a microscopical evaluation of the specimen. A more radical approach should include the BOC and GOC forms. Each surgeon should plan for a second surgery and radicalize the approach or use a wait-and-see approach if needed after microscopic evaluation of the excised specimen. In this case, a classic LPC was successfully treated with enucleation with bone curettage and high-speed bone ostectomy and GBR procedure with xenograft, iPRF, and collagen membrane usage. After 15 months of observation, the used herein xenograft material healed and no cyst reoccurrence was found. It was adequate enough to reconstruct the bony defect, and healing period can be easily evaluated during routine check-ups. Each case of LPC should be carefully monitored after surgery and each surgeon should always remember the two aggressive forms, namely, BOC and GOC.
Author Contributions
Conceptualization, K.N.; methodology, K.N. and R.J.; software, M.J.; validation, K.N., E.P. and S.B.; formal analysis, K.N. and M.M.-K.; investigation, K.N.; resources, R.J. and S.B.; data curation, M.J. and M.D.; writing—original draft preparation, K.N. and R.J.; writing—review and editing, S.B. and M.M.-K.; visualization, K.N.; supervision, R.J.; project administration, M.J., M.D. and E.P.; funding acquisition, M.J. and M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed by a subsidy from Wroclaw Medical University, number SUBZ.B180.22.091.
Institutional Review Board Statement
This manuscript is a case report in which the patient cannot be identified, and therefore the requirement for obtaining informed consent from the patient was waived. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from the patient involved in the study.
Data Availability Statement
Availability of supporting data—the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Andrade, M.; Silva, A.P.; de Moraes Ramos-Perez, F.M.; Silva-Sousa, Y.T.; da Cruz Perez, D.E. Lateral periodontal cyst: Report of case and review of the literature. Oral Maxillofac. Surg. 2012, 16, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, E.; Angelopoulos, A.P. Lateral periodontal cyst. Review of the literature and report of a case. J. Periodontol. 1990, 61, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Kerezoudis, N.P.; Donta-Bakoyianni, C.; Siskos, G. The lateral periodontal cyst: Aetiology, clinical significance and diagnosis. Endod. Dent. Traumatol. 2000, 16, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shear, M.; Pindborg, J.J. Microscopic features of the lateral periodontal cyst. Scand. J. Dent. Res. 1975, 2013, 103–110. [Google Scholar] [CrossRef]
- Altini, M.; Shear, M. The lateral periodontal cyst: An update. J. Oral Pathol. Med. 1992, 21, 245–250. [Google Scholar] [CrossRef]
- Vidaković, B.; Uljanić, I.; Grgurević, J.; Perić, B.; Manojlović, S. Botryoid Cyst, a Rare Type of Odontogenic Cyst. Acta Clin. Croat. 2016, 55, 510–514. [Google Scholar] [CrossRef]
- Carter, L.C.; Carney, Y.L.; Perez-Pudlewski, D. Lateral periodontal cyst. Multifactorial analysis of a previously unreported series. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 210–216. [Google Scholar] [CrossRef]
- Chrcanovic, B.R.; Gomez, R.S. Gingival cyst of the adult, lateral periodontal cyst, and botryoid odontogenic cyst: An updated systematic review. Oral Dis. 2019, 25, 26–33. [Google Scholar] [CrossRef]
- Bilodeau, E.A.; Collins, B.M. Odontogenic Cysts and Neoplasms. Surg. Pathol. Clin. 2017, 10, 177–222. [Google Scholar] [CrossRef]
- Available online: https://www.ti.ubc.ca/2008/02/04/nimesulide-must-be-withdrawn-worldwide-due-serious-liver-damage/ (accessed on 17 June 2022).
- Available online: http://chpl.com.pl/#detail=655996!66442544 (accessed on 17 June 2022).
- Ramalingam, S.; Alrayyes, Y.F.; Almutairi, K.; Bello, I.O. Lateral Periodontal Cyst Treated with Enucleation and Guided Bone Regeneration: A Report of a Case and a Review of Pertinent Literature. Hindawi Case Rep. Dent. 2019, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Matarese, G.; Lo Giudice, G.; Briguglio, F.; Alibrandi, A.; Crupi, A. A New Approach for the Treatment of Lateral Periodontal Cysts with an 810-nm Diode Laser. Int. J. Periodontics Restor. Dent. 2017, 37, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Sivolella, S.; Perin, C.; Capecchi, M.; Buongiorno, V.; Valente, M. Guided Bone Regeneration in the Treatment of a Lateral Periodontal Cyst: 2-Year Clinical and Radiologic Follow-up. Int. J. Periodontics Restor. Dent. 2018, 38, 747–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noonan, V.; Ollerhead, T.; Kabani, S. Lateral periodontal cyst. J Mass Dent Soc. 2010, 59, 47. [Google Scholar] [PubMed]
- Nikitakis, N.G.; Brooks, J.K.; Melakopoulos, I. Lateral periodontal cysts arising in periapical sites: A report of two cases. J. Endod. 2010, 2013, 1707–1711. [Google Scholar] [CrossRef]
- Friedrich, R.E.; Scheuer, H.A.; Zustin, J. Lateral Periodontal Cyst. In Vivo 2014, 28, 595–598. [Google Scholar]
- Ramesh, R.; Sadasivan, A. Lateral Periodontal Cyst—A diagnostic dilemma: Report of a rare case with CBCT and histological findings. Int. J. Surg. Case Rep. 2020, 75, 454–457. [Google Scholar] [CrossRef]
- Babu, S.; Sudhakar, S.; Rajesh, E.; Anitha, N. Lateral Periodontal Cyst—A Case Report. Biomed. Pharmacol. J. 2017, 10, 435–437. [Google Scholar] [CrossRef]
- de Carvalho, L.F.; Lima, C.F.; Cabral, L.A.; Brandão, A.A.; Almeida, J.D. Lateral Periodontal Cyst: A Case Report and Literature Review. J. Oral Maxillofac. Res. 2010, 1, e5. [Google Scholar]
- Buchholzer, S.; Bornert, F.; Di Donna, D. Atypical presentation of lateral periodontal cyst associated with impacted teeth: Two case reports. BMC Oral Health 2021, 21, 178. [Google Scholar] [CrossRef]
- Shah, A.A.; Sangle, A.; Bussari, S.; Koshy, A.V. Glandular odontogenic cyst: A diagnostic dilemma. Indian J. Dent. 2016, 7, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Siponen, M.; Neville, B.W.; Damm, D.D.; Allen, C.M. Multifocal lateral periodontal cysts: A report of 4 cases and review of the literature. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Byatnal, A.R.; Parvathidevi, M.K.; Rukmangada, T.; Koppal, S. An unfamiliar presentation of a lateral periodontal cyst. BMJ Case Rep. 2013, 2013, bcr2013200852. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).