Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates
2.2. Analytical Methods
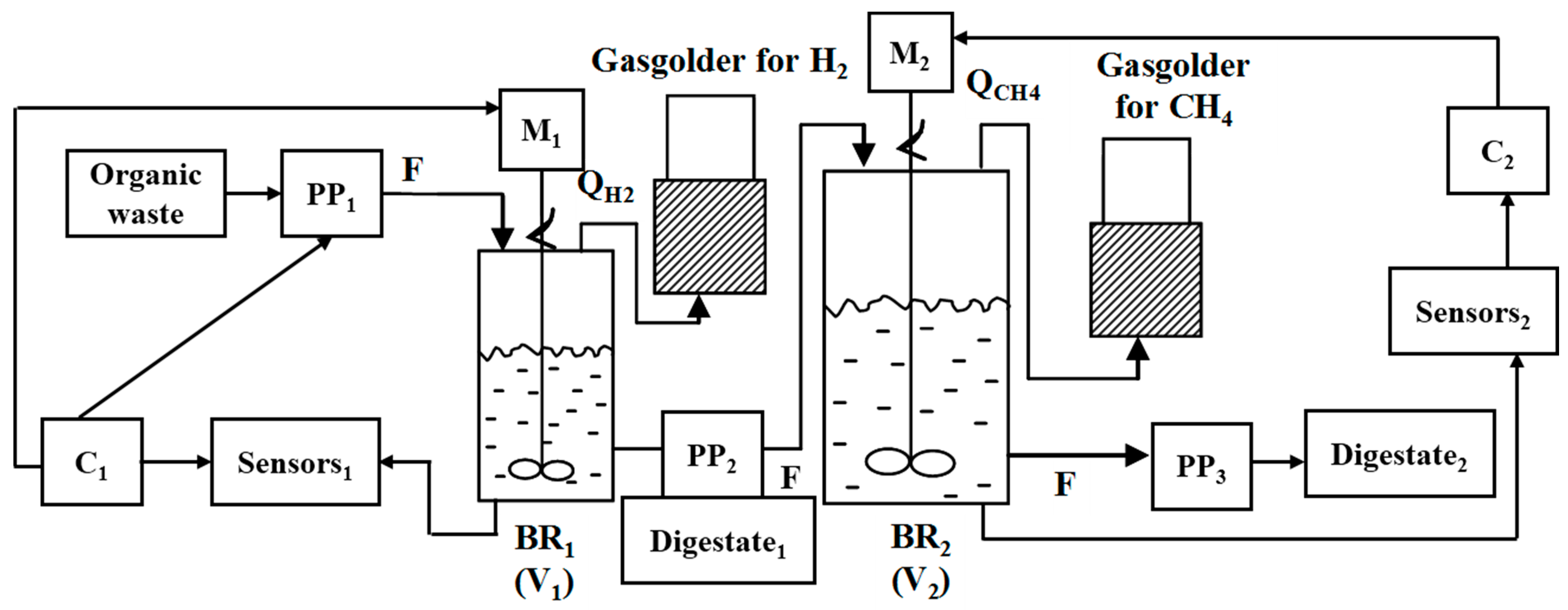
2.3. Experimental Setup
2.3.1. BR1 (Hydrogen) Control System
2.3.2. BR2 (Methane) Control System
- The temperature in the bioreactor (in this case, mesophilic = 35 °C);
- Feeding the influent of BR1 into the bioreactor with PP2;
- Measurement of CH4 and CO2 in biogas;
- Measurement of the biogas flow rate;
- Control of the pressure in the BR2 and at its inlet (against clogging of the pipelines);
- Stirring control in the bioreactor.
2.4. Inoculum
3. Results and Discussion
3.1. Experimental Studies in Laboratory Bioreactors
- Glucose—batch and continuous mode of operation with organic loads of 2, 3, 4, and 6 g/L);
- Corn steep liquor.
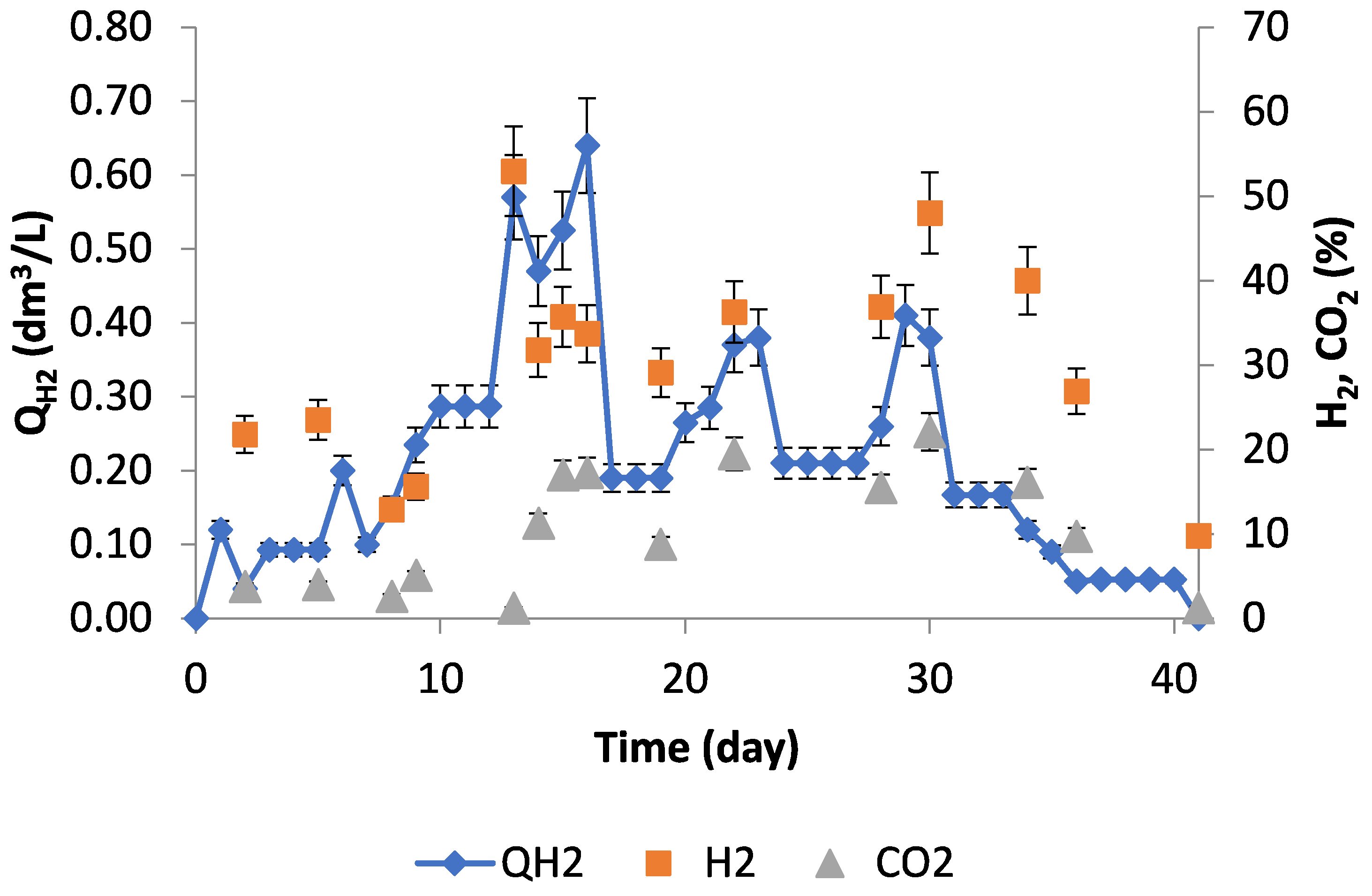
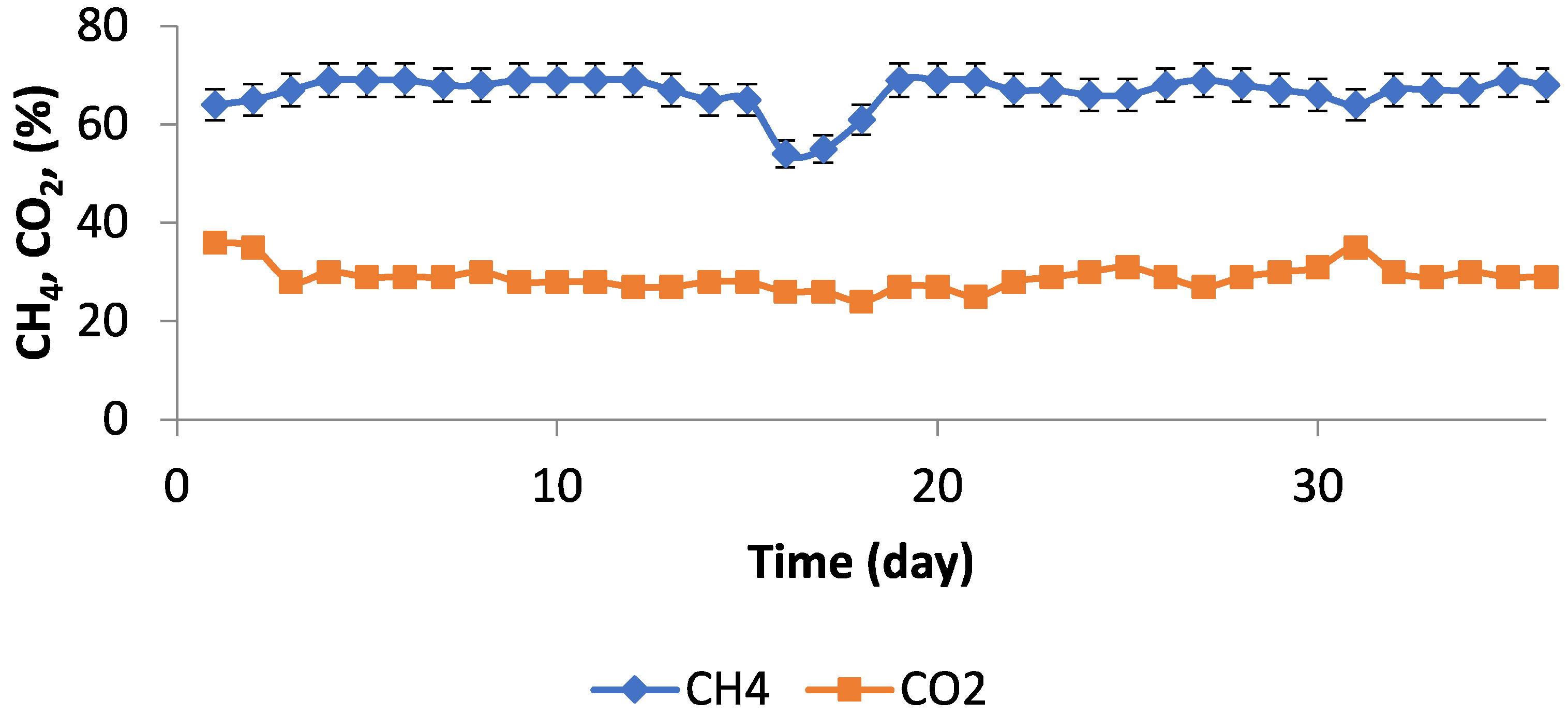
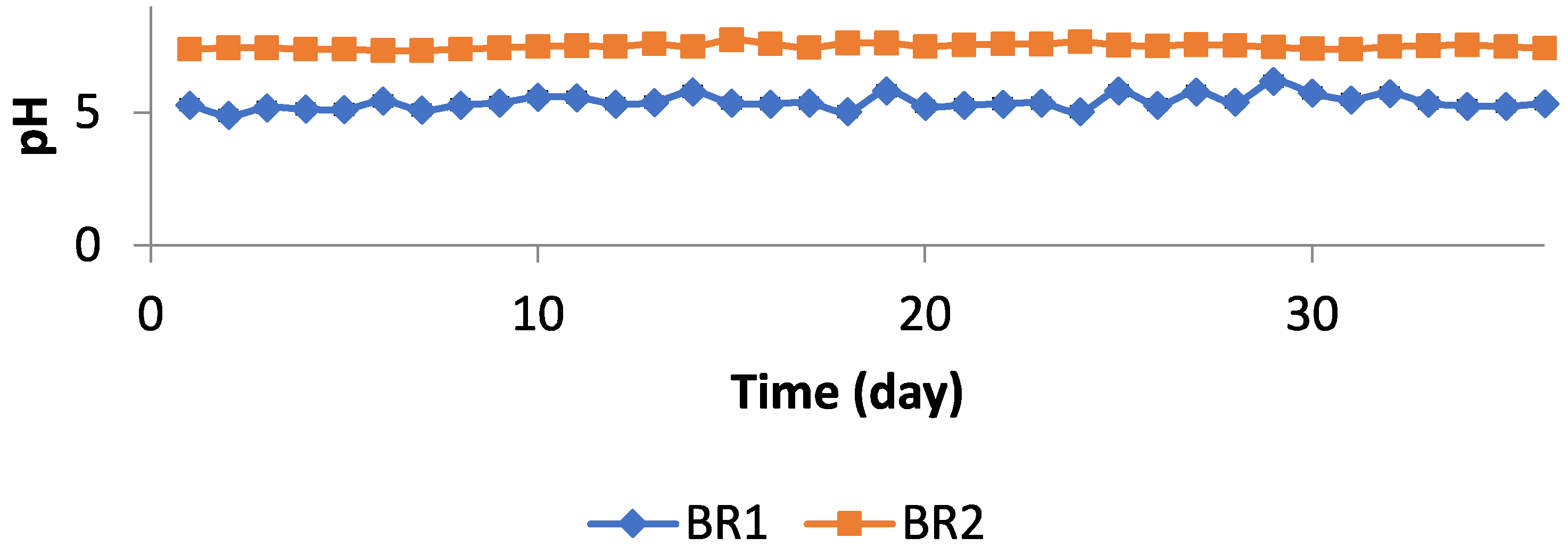
3.2. Experimental Studies in Pilot Scale Biogas Plant
3.3. Discussion
4. Energetical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources; John Wiley & Sons: Weinheim, Germany, 2008. [Google Scholar]
- Buivydas, E.; Navickas, K.; Venslauskas, K.; Žalys, B.; Župerka, V.; Rubežius, M. Biogas Production Enhancement through Chicken Manure Co-Digestion with Pig Fat. Appl. Sci. 2022, 12, 4652. [Google Scholar] [CrossRef]
- Iglesias, R.; Muñoz, R.; Polanco, M.; Díaz, I.; Susmozas, A.; Moreno, A.D.; Guirado, M.; Carrerasand, N.; Ballesteros, M. Biogas from Anaerobic Digestion as an Energy Vector: CurrentUpgrading Development. Energies 2021, 14, 2742. [Google Scholar] [CrossRef]
- Ruggeri, B.; Tommasi, T.; Sanfilippo, S. BioH2&BioCH4 through Anaerobic Digestion (From Research to Full-Scale Applications); Springer: Berlin/Heidelberg, Germany, 2015; 215p. [Google Scholar]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saady, N.M.C. Homoacetogenesis during hydrogen production by mixed cultures dark fermentation: Unresolved challenge. Int. J. Hydrogen Energy 2013, 38, 13172–13191. [Google Scholar] [CrossRef]
- Schievano, A.; Tenca, A.; Lonati, S.; Manzini, E.; Adani, F. Can two-stage instead one-stage anaerobic digestion really increase energy recovery from biomass? Appl. Energy 2014, 124, 335–342. [Google Scholar] [CrossRef]
- Xiao, K.; Maspolim, Y.; Zhou, Y.; Guo, C.; Ng, W.J. Effect of Sodium on Methanogens in a Two-Stage Anaerobic System. Appl. Sci. 2022, 12, 956. [Google Scholar] [CrossRef]
- Markphan, W.; Mamimin, C.; Suksong, W.; Prasertsan, P.; Sompong, O. Comparative assessment of single-stage and two-stage anaerobic digestion for biogas production from high moisture municipal solid waste. PeerJ 2020, 8, e9693. [Google Scholar] [CrossRef]
- Chatterjee, B.; Mazumder, D. Performance evaluation of three-stage AD for stabilization of fruit and vegetable waste. J. Indian Chem. Soc. 2018, 95, 65–80. [Google Scholar]
- Simeonov, I. Laboratory studies and mathematical modeling of the anaerobic digestion in a cascade of two bioreactors. Ecol. Eng. Environ. Prot. 2005, 2, 51–57. (In Bulgarian) [Google Scholar]
- Martinez-Burgos, W.J.; Sydney, E.B.; Rodrigues de Paula, D.; Medeirosa, A.B.P.; Cesar de Carvalho, J.; Molina, D.; Soccol, C.R. Hydrogen production by dark fermentation using a new low-cost culture medium composed of corn steep liquor and cassava processing water: Process optimization and scale-up. Bioresour. Technol. 2021, 320, 124370. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, Q.; Rouse, J.D.; Qiao, S.; Furukawa, K. Treatment of high-strength corn steep liquor using cultivated Polyvinyl alcohol gelbeads in an anaerobic fluidized-bed reactor. J. Biosci. Bioeng. 2009, 107, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, W.; Yang, J.; Zhao, H.; Xin, H.; Zhang, Y. Effect of different levels of corn steep liquor addition on fermentation characteristics and aerobic stability of fresh rice straw silage. Anim. Nutr. 2016, 2, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for Examination of Water and Waste Water, 23rd ed.; American Public Health Association (APHA): Washington, DC, USA, 2003. [Google Scholar]
- Simeonov, I.; Kalchev, B.; Mihaylova, S.; Hubenov, V.; Valevski, G.; Aleksandrov, A.; Georgiev, R. Pilot-scale biogas plant for research and development of new technologies. Ecol. Eng. Environ. Prot. 2012, 2, 63–71. (In Bulgarian) [Google Scholar]
- Simeonov, I.; Chorukova, E.; Diop, S.; Christov, N. Pilot scale biogas reactor with computer system for monitoring and control. In Proceedings of the Septième édition du Colloque Francophone en Energie, Environnement, Economie et Thermodynamique—COFRET’14, Paris, France, 23–25 April 2014; pp. 711–723. [Google Scholar]
- Simeonov, I.; Chorukova, E.; Akivanov, V.; Lakov, V.; Mihailova, S. Experimental pilot scale biogas plant with computer system for monitoring and control. In Proceedings of the International Conference Automatics and Informatics, Sofia, Bulgaria, 4–6 October 2017; pp. 239–242. [Google Scholar]
- Denchev, D.; Hubenov, V.; Simeonov, I.; Kabaivanova, L. Biohydrogen production from lignocellulosic waste with anaerobic bacteria. In Proceedings of the 4th International Conference on Water, Energy and Environment (ICWEE), Burgas, Bulgaria, 1–3 June 2016; pp. 7–12. [Google Scholar]
- Diop, S.; Chorukova, E.; Simeonov, I. Modeling and Specific Growth Rates Estimation of a Two-Stage Anaerobic Digestion Process for Hydrogen and Methane Production. IFAC PapersOnLine 2017, 50, 12641–12646. [Google Scholar] [CrossRef]
- Salomoni, C.; Caputo, A.; Bonoli, M.; Francioso, O.; Rodriguez-Estrada, M.T.; Palenzona, D. Enhanced methane production in a two-phase anaerobic digestion plant, after CO2 capture and addition to organic wastes. Bioresour. Technol. 2011, 102, 6443–6448. [Google Scholar] [CrossRef]
- Nsair, A.; Cinar, S.O.; Alassali, A.; Qdais, H.A.; Kuchta, K. Operational Parameters of Biogas Plants: A Review and Evaluation Study. Energies 2020, 13, 3761. [Google Scholar] [CrossRef]
- Sevillano, C.A.; Pesantes, A.A.; Carpio, E.P.; Martínez, E.J.; Gómez, X. Anaerobic Digestion for Producing Renewable Energy—The Evolution of This Technology in a New Uncertain Scenario. Entropy 2021, 23, 145. [Google Scholar] [CrossRef]
- Weinrich, S.; Nelles, M. Basics of Anaerobic Digestion—Biochemical Conversion and Process Modelling; DBFZ: Leipzig, Germany, 2021; Volume VIII, pp. 9–76. ISBN 978-3-946629-72-6. [Google Scholar]
- Chorukova, E.; Simeonov, I. Mathematical modeling of the anaerobic digestion in two-stage system with production of hydrogen and methane including three intermediate products. Int. J. Hydrogen Energy 2020, 45, 11550–11558. [Google Scholar] [CrossRef]
- Borisov, M.; Dimitrova, N.; Simeonov, I. Mathematical Modelling and Stability Analysis of a Two-phase Biosystem. Processes 2020, 8, 791. [Google Scholar] [CrossRef]
- Sompong, O.; Mamimin, C.; Prasertsan, P. Biohythane production from organic wastes by two-stage anaerobic fermentation technology. In Advances in Biofuels and Bioenergy; Intech Open: London, UK, 2018; p. 83. [Google Scholar]
- Liu, Z.; Si, B.; Li, J.; He, J.; Zhang, C.; Lu, Y.; Zhang, Y.; Xing, X.H. Bioprocess engineering for biohythane production from low-grade waste biomass: Technical challenges towards scale up. Curr. Opin. Biotechnol. 2018, 50, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Cremonez, P.A.; Teleken, J.G.; Meier, T.R.W.; Alves, H.J. Two-Stage anaerobic digestion in agroindustrial waste treatment: A review. J. Environ. Manag. 2021, 281, 111854. [Google Scholar] [CrossRef]






| Lot | pH * | TS *, % | VS *, % | Proteins, g/L | Reducing Sugars, g/L | Cellulose, g/L |
|---|---|---|---|---|---|---|
| Amilum, 2010 | 4.2 | 50.5 | 91 | 10.2 ± 0.5 | 282.4 ± 10.1 | 2.46 ± 0.1 |
| ADM, 2018 | 4.2 | 50.5 | 91 | 48.0 ± 0.6 | 110.0 ± 5.7 | N/A |
| Substrate and Organic Loading (g/L) | Experiment Duration (day) | Cumulative Biogas Yield from 1 L of Working Volume (dm3) | Maximal Value of the H2 Content in the Biogas (%) |
|---|---|---|---|
| Glucose (2 g/L) | 3 | 1.42 ± 0.10 | 42.3 ± 0.02 |
| Glucose (6 g/L) | 3 | 1.05 ± 0.14 | 46.7 ± 0.02 |
| CSL (10 mL/L) | 3 | 0.39 ± 0.12 | 4.7 ± 0.21 |
| CSL (15 mL/L) | 5 | 0.80 ± 0.18 | 21.0 ± 0.05 |
| Type of Process, Substrate and Organic Loading (g/L) | Experiment Duration (Day) | Cumulative Biogas Yield from 1 L of Working Volume (dm3) | Maximal Value of the H2 Content in the Biogas (%) | Maximal Value of the H2S (ppm) |
|---|---|---|---|---|
| Semi-continuous process; 4 g glucose (D = 0.1 day−1) | 16 | 6.769 ± 0.02 | 5.7 ± 0.11 | 4 ± 1 |
| Batch process; 15 g glucose | 4 | 1.719 ± 0.08 | 17.1 ± 0.34 | N/A |
| Batch process; 18 mL CSL | 15 | 2.751 ± 0.05 | 5.9 ± 0.12 | 705 ± 35 |
| Batch process; 300 mL CSL | 10 | 4.428 ± 0.03 | 14.5 ± 0.29 | >1000 |
| Semi-continuous process; 50 mL CSL (D = 0.1 day−1) | 10 | 1.319 ± 0.11 | 4.2 ± 0.08 | >1000 |
| Day | Q1 (dm3/L Working Volume) | H2 [%] | CO2 [%] | pH (F1out) | Q2 (dm3/L Working Volume) |
|---|---|---|---|---|---|
| 1 | 0.00 | N/A | N/A | 5.36 | 0.00 |
| 2 | 0.40 | 27.5 | 23.8 | 5.05 | 0.26 |
| 3 | 0.29 | N/A | N/A | N/A | 0.14 |
| 4 | 0.29 | N/A | N/A | N/A | 0.14 |
| 5 | 0.29 | 38.63 | 42 | 4.72 | 0.14 |
| 6 | 0.12 | N/A | N/A | 4.53 | 0.14 |
| 7 | 0.09 | 21.65 | 37.61 | 4.52 | 0.18 |
| 8 | 0.04 | N/A | N/A | 4.49 | 0.00 |
| 9 | 0.04 | N/A | N/A | 4.37 | 0.45 |
| 10 | 0.02 | N/A | 36.05 | N/A | 0.00 |
| 11 | 0.02 | N/A | N/A | 4.36 | 0.00 |
| 12 | 0.02 | N/A | N/A | N/A | 0.00 |
| 13 | 0.00 | N/A | N/A | 4.32 | 0.01 |
| 14 | 0.00 | N/A | N/A | 4.62 | 0.00 |
| Hydraulic retention time for H2 reactor (days) | 2.5 |
| Dilution rate D1 (days−1) | 0.4 |
| Hydraulic retention time for CH4 reactor (days) | 25 |
| Dilution rate D2 (days−1) | 0.04 |
| Hydrogen production (dm3 L−1day−1) | 0.241 |
| Methane production (dm3 L−1day−1) | 0.405 |
| Total energy production (kWh/day) | 0.005028 |
| Hydraulic retention time for H2 reactor (days) | 0.25 | 1 | 1.5 |
| Dilution rate D1 (days−1) | 4 | 1 | 0.667 |
| Hydraulic retention time for CH4 reactor (days) | 2.5 | 10 | 15 |
| Dilution rate D2 (days−1) | 0.4 | 0.1 | 0.0667 |
| Hydrogen production (NL L−1day−1) | 1.67 | 0.49 | 0.19 |
| Methane production (NL L−1day−1) | 4.52 | 1.31 | 0.99 |
| Total energy production (kWh/day) | 0.0004997 | 0.14472 | 0.108 |
| Efficiency η (%) | 49 | 56 | 63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorukova, E.; Hubenov, V.; Gocheva, Y.; Simeonov, I. Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production. Appl. Sci. 2022, 12, 6274. https://doi.org/10.3390/app12126274
Chorukova E, Hubenov V, Gocheva Y, Simeonov I. Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production. Applied Sciences. 2022; 12(12):6274. https://doi.org/10.3390/app12126274
Chicago/Turabian StyleChorukova, Elena, Venelin Hubenov, Yana Gocheva, and Ivan Simeonov. 2022. "Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production" Applied Sciences 12, no. 12: 6274. https://doi.org/10.3390/app12126274
APA StyleChorukova, E., Hubenov, V., Gocheva, Y., & Simeonov, I. (2022). Two-Phase Anaerobic Digestion of Corn Steep Liquor in Pilot Scale Biogas Plant with Automatic Control System with Simultaneous Hydrogen and Methane Production. Applied Sciences, 12(12), 6274. https://doi.org/10.3390/app12126274






