Arsenic: A Review on a Great Health Issue Worldwide
Abstract
:1. Introduction
2. Chemical Forms and Properties of Arsenic
3. Uses of Arsenic
4. Arsenic Sources and Exposure
5. Metabolic Pathways and Toxicity of Arsenic
6. Effects of Arsenic in Mitochondrial Dysfunction
7. Arsenic Epigenetic Modifications
8. Detoxification from Arsenic
9. Arsenic Phytoremediation
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Juhasz, A.; Weber, J.; Naidu, R. Arsenic uptake and speciation in rice plants grown under greenhouse conditions with arsenic contaminated irrigation water. Sci. Total Environ. 2008, 392, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular mechanism of arsenic-induced neurotoxicity including neuronal dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Hatakeyama, T.; Sugawara, K. Release behavior of arsenic, chromium, and copper during heat treatments of CCA-treated wood. J. Mater. Cycles Waste Manag. 2021, 23, 1636–1645. [Google Scholar] [CrossRef]
- Ozturk, M.; Metin, M.; Altay, V.; Baht, R.A.; Ejaz, M.; Gul, A.; Unal, B.T.; Hassanuzzaman, M.; Nibir, L.; Nahar, K.; et al. Arsenic and human health: Genotoxicity, epigenomic effects, and cancer signaling. Biol. Trace Elem. Res. 2022, 200, 988–1001. [Google Scholar] [CrossRef]
- Saintilnord, W.N.; Fondufe-Mittendorf, Y. Arsenic-induced epigenetic changes in cancer development. Semin. Cancer Biol. 2021, 76, 195–205. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Y.; Fang, M.; Jehan, S.; Zhou, W. Current advances of nanomedicines delivering arsenic trioxide for enhanced tumor therapy. Pharmaceutics 2022, 14, 743. [Google Scholar] [CrossRef]
- Kaya, G.; Turkoglu, S. Bioaccumulation of heavy metals in various tissues of some fish species and green tiger shrimp (Penaeus semisulcatus) from İskenderun Bay, Turkey, and risk assessment for human health. Biol. Trace Elem. Res. 2017, 180, 314–326. [Google Scholar] [CrossRef]
- Soultani, G.; Sinanoglu, V.J.; Stathopoulu, E.; Rasmussen, R.R.; Lacobsen, C.; Komaitis, M.; Sloth, J.J. Evaluation of lead, mercury, cadmium and arsenic accumulation, and fatty acids’ profile in muscle and cephalothorax of Parapenaeus longirostris (Mediterranean shrimp) and of Pandalus borealis (northern shrimp). Int. Food Res. J. 2019, 26, 175–185. [Google Scholar]
- Zhang, W.; Miao, A.J.; Wang, N.X.; Li, C.; Sha, J.; Jia, J.; Alessi, D.S.; Yan, B.; Ok, Y.S. Arsenic bioaccumulation and biotransformation in aquatic organisms. Environ. Intern. 2022, 163, 107221. [Google Scholar] [CrossRef]
- Maher, W.; Waring, J.; Krikowa, F.; Duncan, E.; Foster, S. Ecological factors affecting the accumulation and speciation of arsenic in twelve Australian coastal bivalve molluscs. Environ. Chem. 2018, 15, 46–57. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Monographs on the Evaluation. 2012. Available online: monographs.iarc.fr/ENG/Monographs/vol100C/mono100C.pdf (accessed on 15 January 2020).
- Andrewes, P.; DeMarini, D.M.; Funasaka, K.; Wallace, K.; Lai, V.W.; Sun, H.; Cullen, W.R.; Kitchin, K.T. Do arsenosugars pose a risk to human health? The comparative toxicities of a trivalent and pentavalent arsenosugar. Environ. Sci. Technol. 2004, 38, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Lunde, G. Occurrence and transformation of arsenic in the marine environment. Environ. Health Perspect. 1977, 19, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, C.; Raab, A.; Williams, P.N.; Deacon, C.; Haris, P.I.; Meharg, A.A.; Feldmann, J. Accumulation or production of arsenobetaine in humans? J. Environ. Monit. 2010, 12, 832–837. [Google Scholar] [CrossRef]
- Hackethal, C.; Kopp, J.F.; Sarvan, I.; Schwerdtle, T.; Lindtner, O. Total arsenic and water-soluble arsenic species in foods of the first German total diet study (BfR MEAL Study). Food Chem. 2021, 346, 128913. [Google Scholar] [CrossRef]
- Prato, E.; Biandolino, F.; Parlapiano, I.; Giandomenico, S.; Denti, G.; Calò, M.; Spada, L.; Di Leo, A. Proximate, fatty acids and metals in edible marine bivalves from Italian market: Beneficial and risk for consumers health. Sci. Total Environ. 2019, 648, 153–163. [Google Scholar] [CrossRef]
- Barbosa, I.D.S.; Brito, G.B.; Dos Santos, G.L.; Santos, L.N.; Teixeira, L.S.; Araujo, R.G.; Korn, M.G.A. Multivariate data analysis of trace elements in bivalve molluscs: Characterization and food safety evaluation. Food Chem. 2019, 273, 64–70. [Google Scholar] [CrossRef]
- Kato, L.S.; Ferrari, R.G.; Leite, J.V.M.; Conte-Junior, C.A. Arsenic in shellfish: A systematic review of its dynamics and potential health risks. Mar. Pollut. Bull. 2020, 161 Pt A, 111693. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Sahira Joshi, S.; Sharma, M.; Kumari, A.; Surendra Shrestha, S.; Shrestha, B. Arsenic removal from water by adsorption onto iron oxide/nano-porous carbon magnetic composite. Appl. Sci. 2019, 9, 3732. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Lv, X.; Zhang, Z.; Yu, J.; Wang, T. Arsenic removal from water by photocatalytic functional Fe2O3–TiO2 porous ceramic. J. Porous Mater. 2017, 24, 1227–1235. [Google Scholar] [CrossRef]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for arsenic removal: Challenges, recent developments, and perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Hesami, F.; Bina, B.; Ebrahimi, A.; Amin, M.M. Arsenic removal by coagulation using ferric chloride and chitosan from water. Int. J. Environ. Health Eng. 2013, 2, 17. [Google Scholar]
- Bissen, M.; Frimmel, F.H. Arsenic-a review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Srivastava, S.; Shukla, A.; Rajput, V.D.; Kumar, K.; Minkina, T.; Mandzhieva, S.; Shmaraeva, A.; Suprasanna, P. Arsenic remediation through sustainable phytoremediation approaches. Minerals 2021, 11, 936. [Google Scholar] [CrossRef]
- Meghana, K.M.; Sayantan, D. Critical review on arsenic: Its occurrence, contamination and remediation from water and soil. J. Appl. Nat. Sci. 2021, 13, 861–879. [Google Scholar] [CrossRef]
- Matzen, S.L.; Lobo, G.P.; Fakra, S.C.; Kakouridis, A.; Nico, P.S.; Pallud, C.E. Arsenic hyperaccumulator Pteris vittata shows reduced biomass in soils with high arsenic and low nutrient availability, leading to increased arsenic leaching from soil. Sci. Total Environ. 2022, 818, 151803. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Upadhyay, S.K.; Kumari, A.; Ranjan, A.; Mandzhieva, S.; Sushkova, S.; Singh, R.K.; Verma, K.K. Nanotechnology in the restoration of polluted soil. Nanomaterials 2022, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Garje, S.S.; Jain, V.K. The chemistry of organo-arsenic, antimony and bismuth compounds: An overview. Main Group Met. Chem. 1999, 22, 45–58. [Google Scholar] [CrossRef]
- Rimondi, V.; Costagliola, P.; Lattanzi, P.; Catelani, T.; Fornasaro, S.; Medas, D.; Morelli, G.; Paolieri, M. Bioaccessible arsenic in soil of thermal areas of Viterbo, Central Italy: Implications for human health risk. Environ. Geochem. Health 2022, 44, 465–485. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for Drinking Water Quality, Recommendation; WHO: Geneva, Switzerland, 1993; pp. 1–11.
- Yang, Y.; Chi, L.; Lai, Y.; Hsiao, Y.C.; Ru, H.; Lu, K. The gut microbiome and arsenic-induced disease-iAs metabolism in mice. Curr. Environ. Health Rep. 2021, 8, 89–97. [Google Scholar] [CrossRef] [PubMed]
- European Union (Drinking Water) Regulations, S.I. No. 122/2014. In WHO Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; WHO Press: Geneva, Switzerland, 2014.
- Rahaman, S.; Sinha, A.C.; Pati, R.; Mukhopadhyay, D. Arsenic contamination: A potential hazard to the affected areas of West Bengal, India. Environ. Geochem. Health 2013, 35, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Somanna, Y.; Kim, H.J.I.J. Source, distribution, toxicity and remediation of arsenic in the environment—A review. Int. J. Appl. Environ. Sci. 2016, 11, 559–581. [Google Scholar]
- Sabatini, B.J.; Cziegler, A.; Mödlinger, M. Casting simulations of arsenical copper: New insights into prehistoric metal production and materials. JOM 2020, 72, 3269–3278. [Google Scholar] [CrossRef]
- Holton, E.C. Insecticides and fungicides. Ind. Eng. Chem. 1926, 18, 931–933. [Google Scholar] [CrossRef]
- Buscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human African trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Jennewein, M.; Lewis, M.A.; Zhao, D.; Tsyganov, E.; Slavine, N.; He, J.; Watkins, L.; Kodibagkar, V.D.; O’Kelly, S.; Kulkarni, P.; et al. Vascular imaging of solid tumors in rats with a radioactive arsenic- labeled antibody that binds exposed phosphatidylserine. Clin. Cancer Res. 2008, 14, 1377–1385. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A. Toxicity of indium arsenide, gallium arsenide, and aluminium gallium arsenide. Toxicol. Appl. Pharmacol. 2004, 198, 405–411. [Google Scholar] [CrossRef]
- Olson, K.R.; Cihacek, L. The fate of Agent Blue, the arsenic based herbicide, used in South Vietnam during the Vietnam War. Open J. Soil Sci. 2020, 10, 518–577. [Google Scholar] [CrossRef]
- Subastri, A.; Arun, V.; Sharma, P.; Preedia Babu, E.; Suyavaran, A.; Nithyananthan, S.; Alshammari, G.M.; Aristatile, B.; Dharuman, V.; Thirunavukkarasu, C. Synthesis and characterisation of arsenic nanoparticles and its interaction with DNA and cytotoxic potential on breast cancer cells. Chem. Biol. Interact. 2018, 295, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Rahamanab, M.S.; Rahman, M.; Mise, N.; Sikderd, T.; Ichihara, G.; Uddin, K.; Kurasaki, M.; Ichihara, S. Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management. Environ. Pollut. 2021, 289, 117940. [Google Scholar] [CrossRef] [PubMed]
- Shaji, E.; Santosh, M.; Sarath, K.; Prakash, P.; Deepchand, V.; Divya, B. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Santra, S.C.; Samal, A.C.; Bhattacharya, P.; Banerjee, S.; Biswas, A.; Majumdar, J. Arsenic in foodchain and community health risk: A study in Gangetic West Bengal. Procedia Environ. Sci. 2013, 18, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Varol, M.; Gündüz, K.; Sünbül, M.R.; Aytop, H. Arsenic and trace metal concentrations in different vegetable types and assessment of health risks from their consumption. Environ. Res. 2021, 206, 112252. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Djordjevic, A.B.; Crisponi, G.; Alexander, J.; Bjørklund, G.; Aaseth, J. Arsenic toxicity: Molecular targets and therapeutic agents. Biomolecules 2020, 10, 235. [Google Scholar] [CrossRef] [Green Version]
- Francesconi, K.A. Arsenic species in seafood: Origin and human health implications. Pure Appl. Chem. 2010, 82, 373–381. [Google Scholar] [CrossRef]
- Itoh, T.; Zhang, Y.F.; Murai, S.; Saito, H.; Nagahama, H.; Miyate, H.; Saito, Y.; Abe, E. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol. Lett. 1990, 54, 345–353. [Google Scholar]
- Escudero-Lourdes, C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. NeuroToxicology 2016, 53, 223–235. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 8th ed.; Macmillan Learning; W.H. Freeman: New York, NY, USA, 2021; ISBN 101319228003. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J.; Stryer, L. Biochemistry, 9th ed.; Macmillan Learning; W.H. Freeman: New York, NY, USA, 2019; ISBN 101319114652. [Google Scholar]
- Tseng, C.H.; Huang, Y.K.; Huang, Y.L.; Chung, C.J.; Yang, M.H.; Chen, C.J.; Hsueh, Y.M. Arsenic exposure, urinary arsenic speciation, and peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Toxicol. Appl. Pharmacol. 2005, 206, 299–308. [Google Scholar] [CrossRef]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef] [Green Version]
- Prakash, C.; Soni, M.; Kumar, V. Biochemical and molecular alterations following arsenic-induced oxidative stress and mitochondrial dysfunction in rat brain. Biol. Trace Elem. Res. 2015, 167, 121–129. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Garcia-Esquinas, E.; Pollán, M.; Umans, J.G.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Howard, B.V.; Yeh, J.; Best, L.; Navas-Acien, A. Arsenic exposure and cancer mortality in a US-based prospective cohort: The Strong Heart Study. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1944–1953. [Google Scholar] [CrossRef] [Green Version]
- Celik, I.; Gallicchio, L.; Boyd, K.; Lam, T.K.; Matanoski, G.; Tao, X.; Alberg, A.J. Arsenic in drinking water and lung cancer: A systematic review. Environ. Res. 2008, 108, 48–55. [Google Scholar] [CrossRef]
- Baris, D.; Waddell, R.; Beane Freeman, L.E.; Schwenn, M.; Colt, J.S.; Ayotte, J.D.; Ward, M.H.; Nuckols, J.; Schned, A.; Jackson, B.; et al. Elevated bladder cancer in northern new england: The role of drinking water and arsenic. J. Natl. Cancer Inst. 2016, 108, 1–9. [Google Scholar] [CrossRef]
- Wallace, D.R.; Taalab, Y.M.; Heinze, S.; Lovakovi´c, B.T.; Pizent, A.; Renieri, E.; Tsatsakis, A.; Farooqi, A.A.; Javorac, D.; Andjelkovic, M.; et al. Toxic-metal-induced alteration in miRNA expression profile as a proposed mechanism for disease development. Cells 2020, 9, 901. [Google Scholar] [CrossRef] [Green Version]
- Bustaffa, E.; Stoccoro, A.; Bianchi, F.; Migliore, L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch. Toxicol. 2014, 88, 1043–1067. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Shia, W.J.; Pattenden, S.G.; Workman, J.L. Histone H4 lysine 16 acetylation breaks the genome’s silence. Genome Biol. 2006, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.J.; Ren, X.; Chu, F.; Aleshin, M.; Wintz, H.; Burlingame, A.; Smith, M.T.; Vulpe, C.D.; Zhang, L. Acetylated H4K16 by MYST1 protects UROtsa cells from arsenic toxicity and is decreased following chronic arsenic exposure. Toxicol. Appl. Pharmacol. 2009, 241, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Sun, H.; Ellen, T.P.; Chen, H.; Costa, M. Arsenite alters global histone H3 methylation. Carcinogenesis 2008, 29, 1831–1836. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Q.; Arita, A.; Sun, H.; Costa, M. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol. Appl. Pharmacol. 2009, 236, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Meng, X.-Z.; Zheng, T.-S.; Chen, X.; Wang, J.-B.; Zhang, W.-H.; Pan, S.-H.; Jiang, H.-C.; Liu, L.-X. microRNA expression alteration after arsenic trioxide treatment in HepG-2 cells. J. Gastroenterol. Hepatol. 2010, 26, 186–193. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, P.; Wen, W.; Liu, L. Relative miRNA and mRNA expression involved in arsenic methylation. PLoS ONE 2018, 13, e0209014. [Google Scholar] [CrossRef]
- Esteller, M. CPG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene 2002, 21, 5427–5440. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Aaseth, J.; Chirumbolo, S.; Urbina, M.A.; Uddin, R. Effects of arsenic toxicity beyond epigenetic modifications. Environ. Geochem. Health 2018, 40, 955–965. [Google Scholar] [CrossRef]
- Reichard, J.F.; Puga, A. Effects of arsenic exposure on DNA methylation and epigenetic gene regulation. Epigenomics 2010, 2, 87–104. [Google Scholar] [CrossRef] [Green Version]
- Koestler, D.C.; Avissar-Whiting, M.; Houseman, E.A.; Karagas, M.R.; Marsit, C.J. Differential DNA methylation in umbilical cord blood of infants exposed to low levels of arsenic in utero. Environ. Health Perspect. 2013, 121, 971–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilsner, J.R.; Hall, M.N.; Liu, X.; Ilievski, V.; Slavkovich, V.; Levy, D.; Factor-Litvak, P.; Yunus, M.; Rahman, M.; Graziano, J.H.; et al. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PLoS ONE 2012, 7, e37147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedzwiecki, M.M.; Liu, X.; Hall, M.N.; Thomas, T.; Slavkovich, V.; Ilievski, V.; Levy, D.; Alam, S.; Siddique, A.B.; Parvez, F.; et al. Sex-Specific Associations of Arsenic Exposure with Global DNA Methylation and hydroxymethylation in leukocytes: Results from two studies in Bangladesh. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1748–1757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosnett, M.J. The role of chelation in the treatment of arsenic and mercury poisoning. J. Med. Toxicol. 2013, 9, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weerasundara, L.; Ok, Y.S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2021, 268, 115668. [Google Scholar] [CrossRef]
- Sanyal, T.; Bhattacharjee, P.; Paul, S.; Bhattacharjee, P. Recent advances in arsenic research: Significance of differential susceptibility and sustainable strategies for mitigation. Front. Public Health 2020, 8, 464. [Google Scholar] [CrossRef]
- Spanu, A.; Daga, L.; Orlandoni, A.M.; Sanna, G. The role of irrigation techniques in arsenic bioaccumulation in rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 46, 8333–8340. [Google Scholar] [CrossRef]
- Suwannaporn, P.; Linnemann, A. Rice-eating quality among consumers in different rice grain preference countries. J. Sens. Studies 2008, 23, 1–13. [Google Scholar] [CrossRef]
- Javed, A.; Farooqi, A.; Baig, Z.U.; Ellis, T.; van Geen, A. Soil arsenic but not rice arsenic increasing with arsenic in irrigation water in the Punjab plains of Pakistan. Plant Soil 2020, 450, 601–611. [Google Scholar] [CrossRef]
- Baloch, M.Y.J.; Talpur, S.A.; Talpur, H.A.; Iqbal, J.; Mangi, S.H.; Memon, S. Effects of arsenic toxicity on the environment and its remediation techniques: A review. J. Water Environ. Technol. 2020, 18, 275–289. [Google Scholar] [CrossRef]
- Xu, X.Y.; McGrath, S.P.; Meharg, A.A.; Zhao, F.J. Growing rice aerobically markedly decreases arsenic accumulation. Environ. Sci. Technol. 2008, 42, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E. Simple cooking methods flush arsenic out of rice. Nature 2015. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.K.; Dey, A.; Mukherjee, S.; Pradhan, N.K. The effect of coadministration of α-tocopherol and ascorbic acid on arsenic trioxide-induced testicular toxicity in adult rats. J. Bas. Clin. Physiol. Pharmacol. 2013, 24, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Zhen, J.; Du, Y.; Zhou, J.K.; Peng, A.; Vaziri, N.D.; Mohan, C.; Xu, Y.; Zhou, X.J. Green tea polyphenol (−)-epigallocatechin-3-gallate restores Nrf2 activity and ameliorates crescentic glomerulonephritis. PLoS ONE 2015, 10, e0119543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S. Medicinal plants and natural products in amelioration of arsenic toxicity: A short review. Pharm. Biol. 2017, 55, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Zhang, J.; Xue, X.; Zhao, Y.; Lu, L.; Cui, M.; Miao, W.; Fan, S. Theaflavin ameliorates ionizing radiation-induced hematopoietic injury via the NRF2 pathway. Free Radic. Biol. Med. 2017, 113, 59–70. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. The double face of metals: The intriguing case of chromium. Appl. Sci. 2021, 11, 638. [Google Scholar] [CrossRef]
- Genchi, G.; Carocci, A.; Lauria, G.; Catalano, A.; Sinicropi, M.S. Thallium use, toxicity, and detoxification therapy: An overview. Appl. Sci. 2021, 11, 8322. [Google Scholar] [CrossRef]
- Ahmad, F.D.; Ahmad, N.; Masood, K.R.; Hussain, M.; Malik, M.F. Qayyum, A. Phytoremediation of arsenic-contaminated soils by Eucalyptus camaldulensis, Terminalia arjuna and Salix tetrasperma. J. Appl. Bot. Food Qual. 2018, 91, 8–13. [Google Scholar]
- Wei, C.Y.; Chen, T.B. Arsenic accumulation by two brake ferns growing on an arsenic mine and their potential in phytoremediation. Chemosphere 2006, 63, 1048–1053. [Google Scholar] [CrossRef]
- Cantamessa, S.; Massa, N.; Gamalero, E.; Berta, G. Phytoremediation of a highly arsenic polluted site, using Pteris vittata L. and Arbuscular Mycorrhizal Fungi. Plants 2020, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Roy, A.; Bharadvaja, N. Remediation of heavy metals using nanophytoremediation. In Advanced Oxidation Processes for Effluent Treatment Plants; Elsevier: Amsterdam, The Netherlands, 2021; pp. 273–296. [Google Scholar]
- Ashraf, S.; Siddiqa, A.; Shahida, S.; Qaisar, S. Titanium-based nanocomposite materials for arsenic removal from water: A review. Heliyon 2019, 5, e01577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Hu, J.; Wu, F.; Zhang, X.; Wang, B.; Yang, Y.; Shen, G.; Liu, J.; Tao, S.; Wang, X. Application of TiO2 nanoparticles to reduce bioaccumulation of arsenic in rice seedlings (Oryza sativa L.): A mechanistic study. J. Hazard. Mater. 2021, 405, 124047. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Wu, F.; Zhou, S.; Yang, J.; Tang, X.; Ye, W. Zinc oxide nanoparticles alleviate the arsenic toxicity and decrease the accumulation of arsenic in rice (Oryza sativa L.). BMC Plant Biol. 2021, 21, 150. [Google Scholar] [CrossRef] [PubMed]

| Atomic number | 33 |
| Atomic weight | 74.92 u |
| Electronic configuration | [Ar] 3d104s24p3 |
| Melting point | 817 °C |
| Boiling point (sublimation) * | 615 °C |
| Density at 20 °C | 5.73 g/cm3 |
| Covalent radius | 119 ± 4 pm |
| Van der Waals radius | 185 pm |
| Heat of fusion (grey) | 24.44 KJ/mol |
| Heat of vaporization | 34.76 KJ/mol |
| Pauling electronegativity number | 2.18 |
| First ionization energy | 947.0 KJ/mol |
| Second ionization energy | 1798.0 KJ/mol |
| Third ionization energy | 2735.0 KJ/mol |
| Standard potential | −0.3 V (As3+/As) |
| Allotropes | Grey, Yellow, Black |
| Structure | Compound |
|---|---|
 | Methylarsonic acid (MMA) |
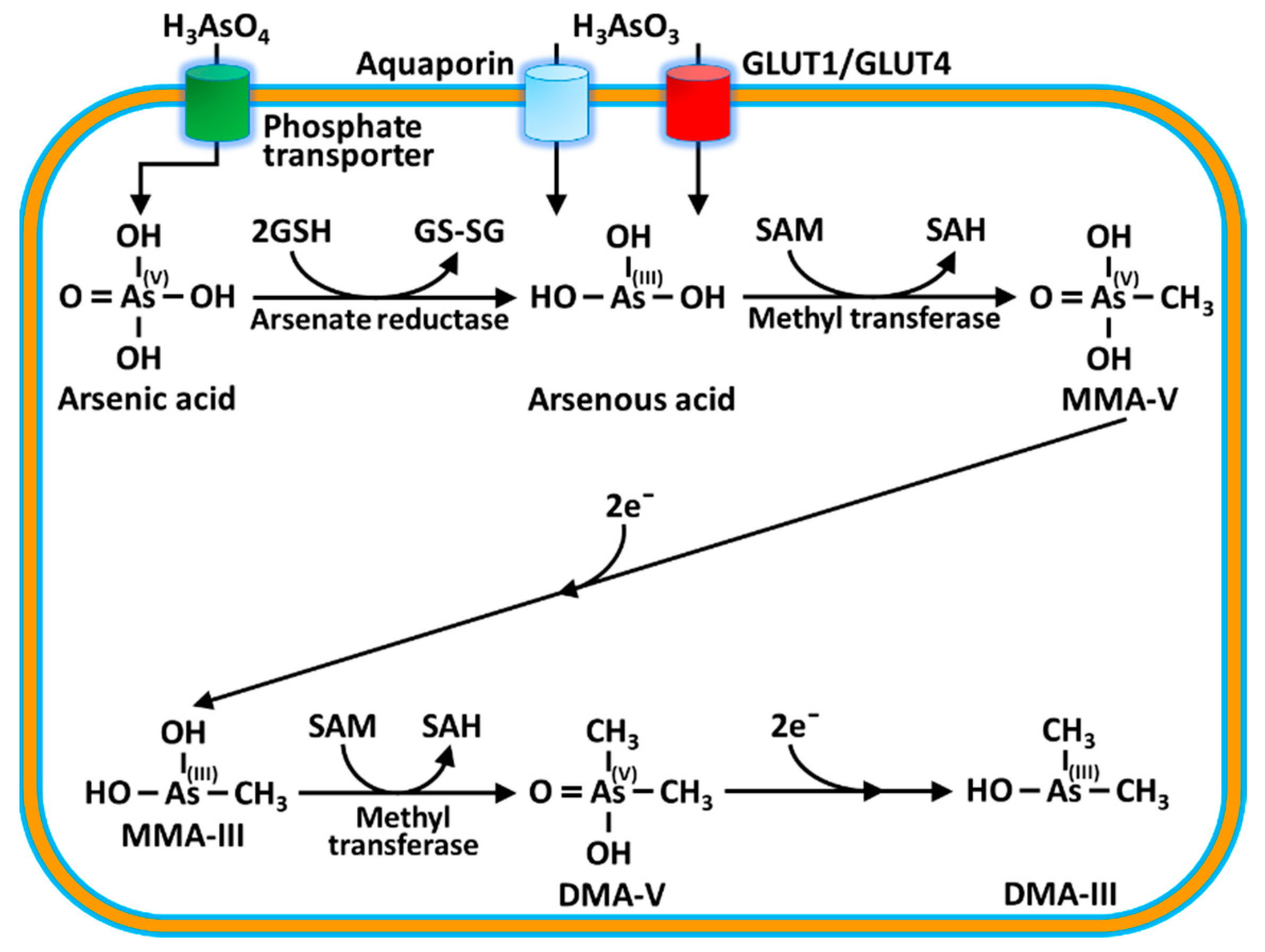
 | Cacodylic acid or DMA (dimethyl arsinic acid) |
 | Arsenobetaine (trimethyl arseniumyl acetate) |
 | Paris green (copper acetate triarsenite) |
 | Scheele green (copper arsenite) |
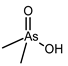
 | Arsphenamine (also known as Salvarsan) |
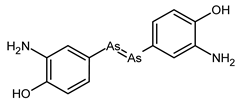
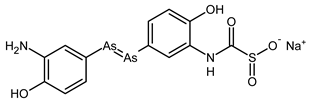 | Neosalvarsan |
 | Melarsoprol |
 | Lewisite (2-chloro vinyl dichloro arsine) |
 | Adamsite (diphenylaminechlorarsine). |
| Structure | Name |
|---|---|
 | BAL (British Anti-Lewisite; 2,3-dimercapto propanol, dimercaprol) |
 | DMPS (2,3-dimercapto-1-propanesulfonic acid) |
 | DMSA (dimercaptosuccinic acid) |
 | DMPA (N-(2,3-dimercaptopropyl)-phthalamidic acid) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A Review on a Great Health Issue Worldwide. Appl. Sci. 2022, 12, 6184. https://doi.org/10.3390/app12126184
Genchi G, Lauria G, Catalano A, Carocci A, Sinicropi MS. Arsenic: A Review on a Great Health Issue Worldwide. Applied Sciences. 2022; 12(12):6184. https://doi.org/10.3390/app12126184
Chicago/Turabian StyleGenchi, Giuseppe, Graziantonio Lauria, Alessia Catalano, Alessia Carocci, and Maria Stefania Sinicropi. 2022. "Arsenic: A Review on a Great Health Issue Worldwide" Applied Sciences 12, no. 12: 6184. https://doi.org/10.3390/app12126184
APA StyleGenchi, G., Lauria, G., Catalano, A., Carocci, A., & Sinicropi, M. S. (2022). Arsenic: A Review on a Great Health Issue Worldwide. Applied Sciences, 12(12), 6184. https://doi.org/10.3390/app12126184








