Effectiveness and Safety of ANTI SARS-CoV-2 Vaccination in Transplant Patients Treated with Immunosuppressants: A Real-World Pilot Study with a 1-Year Follow-Up
Abstract
:1. Introduction
- Antivirals: Nirmatrelvir/Ritonavir, Remdesivir, Molnupiravir;
- Monoclonal antibodies: Bamlanivimab/Etesevimab, Casirivimab/Imdevimab, Sotrovimab, Tixagevimab/Cilgavimab;
- Vaccines: mRNA vaccines, BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna) and viral vector vaccine Ad26.COV2.S (Johnson & Johnson/Janssen) [2], ChAdOx1-S (Astrazeneca).
2. Materials and Methods
2.1. Study Design
2.2. Experimental Protocol
2.3. Inclusion and Exclusion Criteria
2.4. End Points
2.5. Statistical Analysis
3. Results
3.1. Therapeutic Drug Monitoring (TDM)
3.2. IgG Anti SARS-CoV-2
3.3. ADRs Related to Vaccine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and distributions of SARS-CoV-2 spike proteins on intact virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19); NIH: Bethesda, MD, USA, 2022; Volume 2019, pp. 1–243.
- Negahdaripour, M.; Shafiekhani, M.; Moezzi Iman, S.M.; Amiri, S.; Rasekh, S.; Bagheri, A.; Mosaddeghi, P.; Vazin, A. Administration of COVID-19 vaccines in immunocompromised patients. Int. Immunopharmacol. 2021, 99, 108021. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Ram, E.; Lavee, J.; Sternik, L.; Segev, A.; Wieder-Finesod, A.; Mandelboim, M.; Indenbaum, V.; Levy, I.; Raanani, E.; et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J. Heart Lung Transpl. 2021, 40, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.-L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol 2021, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transpl. 2021, 40, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Danthu, C.; Hantz, S.; Dahlem, A.; Duval, M.; Ba, B.; Guibbert, M.; Ouafi, Z.E.; Ponsard, S.; Berrahal, I.; Achard, J.-M.; et al. Humoral Response after SARS-CoV-2 mRNA Vaccination in a Cohort of Hemodialysis Patients and Kidney Transplant Recipients. J. Am. Soc. Nephrol. 2021, 32, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Buffone, G.; Gallelli, L.; De Franciscis, S. The effects of minocycline on extracellular matrix in patients with chronic venous leg ulcers. Acta Phlebol. 2013, 14, 99–107. [Google Scholar]
- Gareri, P.; Fazio, P.D.; Gallelli, L.; Fazio, S.D.; Davoli, A.; Seminara, G.; Cotroneo, A.; Sarro, G. De Venlafaxine—Propafenone Interaction Resulting in Hallucinations and Psychomotor Agitation. Ann. Pharmacother. 2008, 42, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, Z.; Krueger, K.; Thomas, L.D.; Overton, E.T.; Ison, M.; Halasa, N. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am. J. Transpl. 2021, 21, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Vnučák, M.; Graňák, K.; Beliančinová, M.; Jeseňák, M.; Macháleková, K.K.; Benko, J.; Samoš, M.; Dedinská, I. Acute kidney rejection after anti-SARS-CoV-2 virus-vectored vaccine—Case report. NPJ Vaccines 2022, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Negrea, L.; Rovin, B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy Weak anti—SARS-CoV-2 antibody response after the fi rst injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021, 99, 1487. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Ruddy, J.A.; Connolly, C.M.; Ou, M.T.; Werbel, W.A.; Garonzik-Wang, J.M.; Segev, D.L.; Paik, J.J. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1098–1099. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Clarke, C.; Edwards, H.; Mcintyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Palich, R.; Veyri, M.; Marot, S.; Vozy, A.; Gligorov, J.; Maingon, P.; Marcelin, A.-G.; Spano, J.-P. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann. Oncol. 2021, 32, 1051–1053. [Google Scholar] [CrossRef] [PubMed]
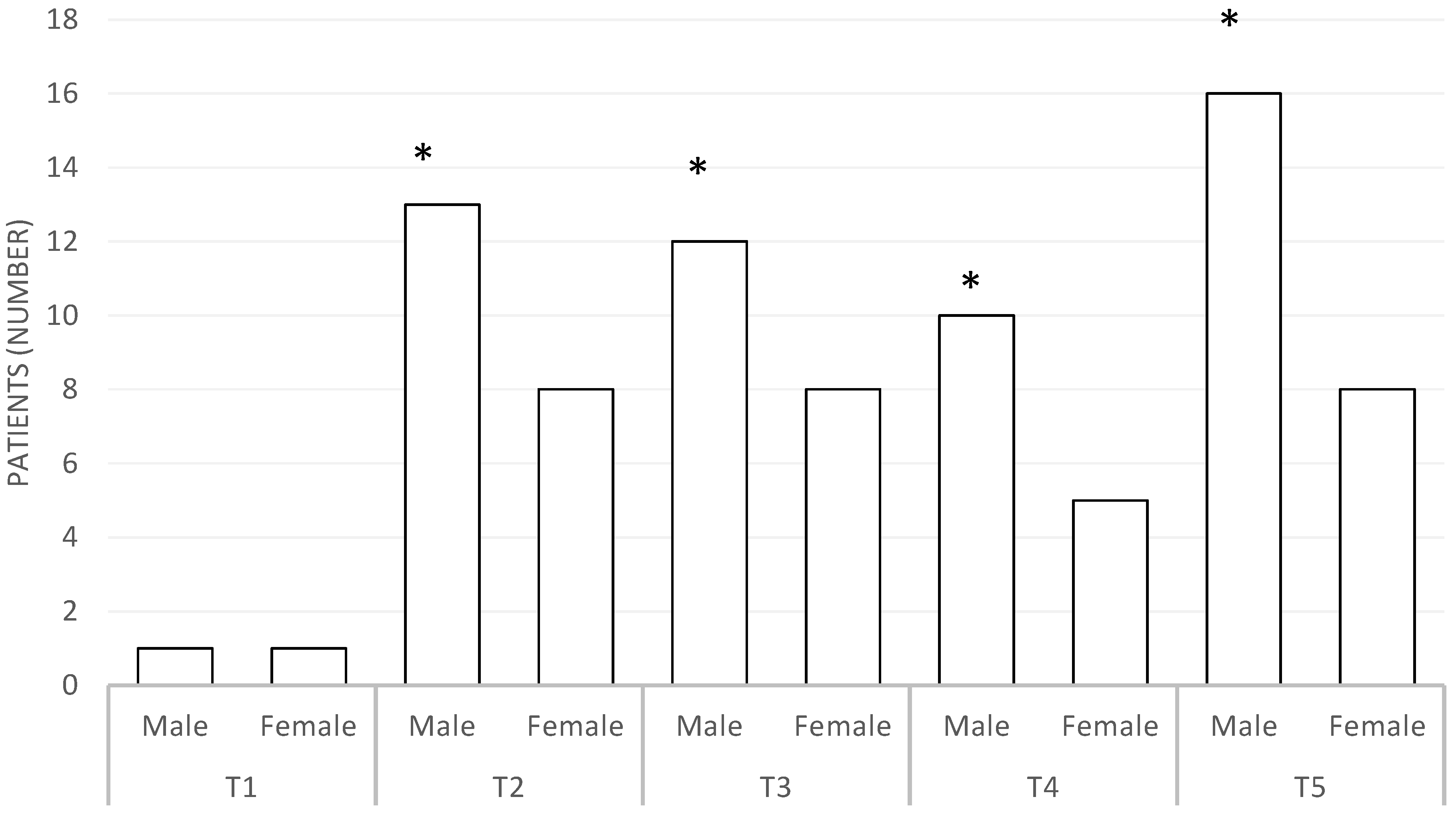
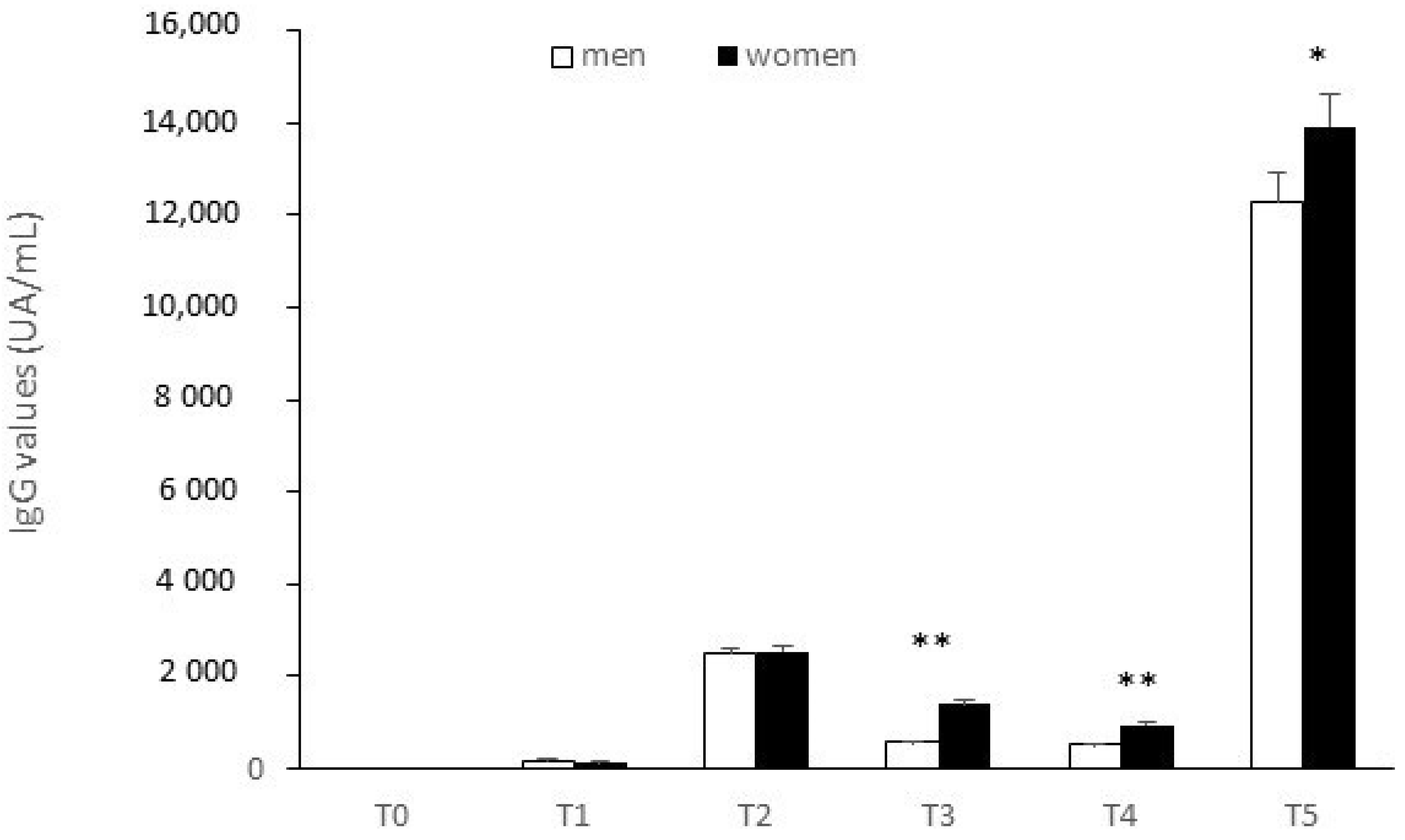
| Characteristics | Data |
|---|---|
| Patients (number) | 33 |
| Men (number) | 19 |
| Women (number) | 14 |
| Age (mean ± standard deviation) | 55.4 ± 12.8 |
| Tacrolimus (number) | 25 |
| Everolimus (number) | 1 |
| Cyclosporine (number) | 7 |
| Mycophenolate (number) | 26 |
| Sirolimus (number) | 1 |
| Prednisone (number) | 24 |
| Dexamethasone (number) | 1 |
| Azathioprine (number) | 1 |
| Transplant from | |
| Cadaver (number) | 30 |
| Living (number) | 3 |
| IgG-SARS-CoV-2 (AU/mL; mean ± standard deviation) | |
| T0 | 0 |
| T1 | 23.83 ± 52.14 |
| T2 | 1606.32 ± 3476.97 |
| T3 | 599.18 ± 1025.64 |
| T4 | 325.46 ± 568.15 |
| T5 | 9646.33 ± 14,176.99 |
| Drug | Normal Range ng/mL | P-p | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|---|---|
| Tacrolimus | 5–15 | 6.2 ± 1.4 | 6.5 ± 1.6 | 6.3 ± 1.5 | 6.6 ± 1.8 | 6.4 ± 1.5 | 6.2 ± 1.4 | 6.3 ± 1.6 |
| Everolimus | 3–8 | 5.2 | 5.6 | 5.4 | 5.6 | 5.7 | 5.5 | 5.6 |
| Cyclosporine | 300–600 | 86 ± 21.5 | 92 ± 25.3 | 90 ± 23.8 | 88 ± 23.4 | 87 ± 24.6 | 89 ± 25.4 | 87 ± 23.8 |
| Sirolimus | 8.4 | 7.8 | 8.1 | 8.3 | 7.9 | 8.2 | 8.3 | |
| Mycophenolate | 2.8 ± 0.7 | 2.6 ± 0.9 | 2.9 ± 1.2 | 2.8 ± 1.1 | 3.1 ± 1.2 | 3.2 ± 1.1 | 3.1 ± 1.1 |
| Patients | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| All patients | 0.22 | 23.83 | 1606.32 | 599.18 | 316.60 | 9024.51 |
| Unresponsive to vaccine | 0.02 | 8.72 | 8.42 | 7.27 | 15.23 | 15.24 |
| Responsive to vaccine | 0.22 | 174.93 * | 2519.40 * | 895.13 * | 658.15 * | 12,710.12 * |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Sex | Man | Man | Man | Man | Woman | Woman |
| Age | 45 | 71 | 49 | 35 | 72 | 61 |
| IgG (AU/mL) | 5682.3 | 5248.2 | 3985.2 | 2773.8 | 184.4 | 0.8 |
| Immunosuppressant | Cyc + myc | Cyc + Myc + Methyl | Tac + Myc + Methyl | Tac + Myc + Methyl | Tac + Myc + Methyl | Tac + Myc + Methyl |
| Organ transplant | Cadaver | Cadaver | Cadaver | Living | Cadaver | Cadaver |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caglioti, A.; Rania, V.; Vocca, C.; Marcianò, G.; Arcidiacono, V.; Catarisano, L.; Casarella, A.; Basile, E.; Colosimo, M.; Palleria, C.; et al. Effectiveness and Safety of ANTI SARS-CoV-2 Vaccination in Transplant Patients Treated with Immunosuppressants: A Real-World Pilot Study with a 1-Year Follow-Up. Appl. Sci. 2022, 12, 6103. https://doi.org/10.3390/app12126103
Caglioti A, Rania V, Vocca C, Marcianò G, Arcidiacono V, Catarisano L, Casarella A, Basile E, Colosimo M, Palleria C, et al. Effectiveness and Safety of ANTI SARS-CoV-2 Vaccination in Transplant Patients Treated with Immunosuppressants: A Real-World Pilot Study with a 1-Year Follow-Up. Applied Sciences. 2022; 12(12):6103. https://doi.org/10.3390/app12126103
Chicago/Turabian StyleCaglioti, Alfredo, Vincenzo Rania, Cristina Vocca, Gianmarco Marcianò, Valentina Arcidiacono, Luca Catarisano, Alessandro Casarella, Emanuele Basile, Manuela Colosimo, Caterina Palleria, and et al. 2022. "Effectiveness and Safety of ANTI SARS-CoV-2 Vaccination in Transplant Patients Treated with Immunosuppressants: A Real-World Pilot Study with a 1-Year Follow-Up" Applied Sciences 12, no. 12: 6103. https://doi.org/10.3390/app12126103
APA StyleCaglioti, A., Rania, V., Vocca, C., Marcianò, G., Arcidiacono, V., Catarisano, L., Casarella, A., Basile, E., Colosimo, M., Palleria, C., Mazzuca, D., Di Mizio, G., De Sarro, C., Pileggi, C., De Sarro, G., & Gallelli, L. (2022). Effectiveness and Safety of ANTI SARS-CoV-2 Vaccination in Transplant Patients Treated with Immunosuppressants: A Real-World Pilot Study with a 1-Year Follow-Up. Applied Sciences, 12(12), 6103. https://doi.org/10.3390/app12126103








