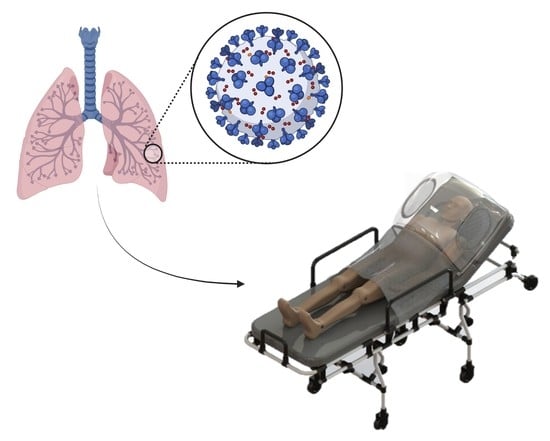
A Brief Analysis of a New Device to Prevent Early Intubation in Hypoxemic Patients: An Observational Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
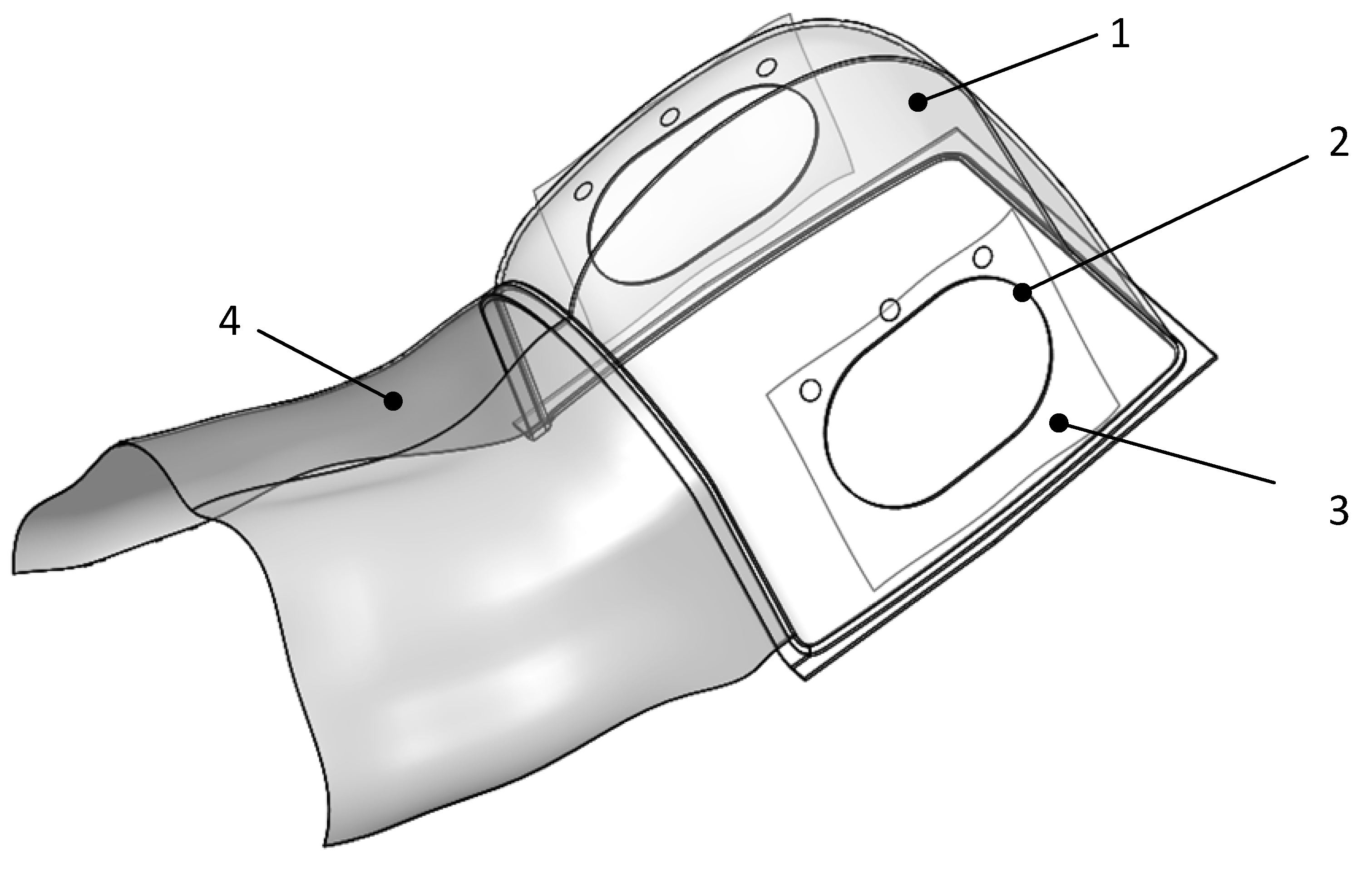
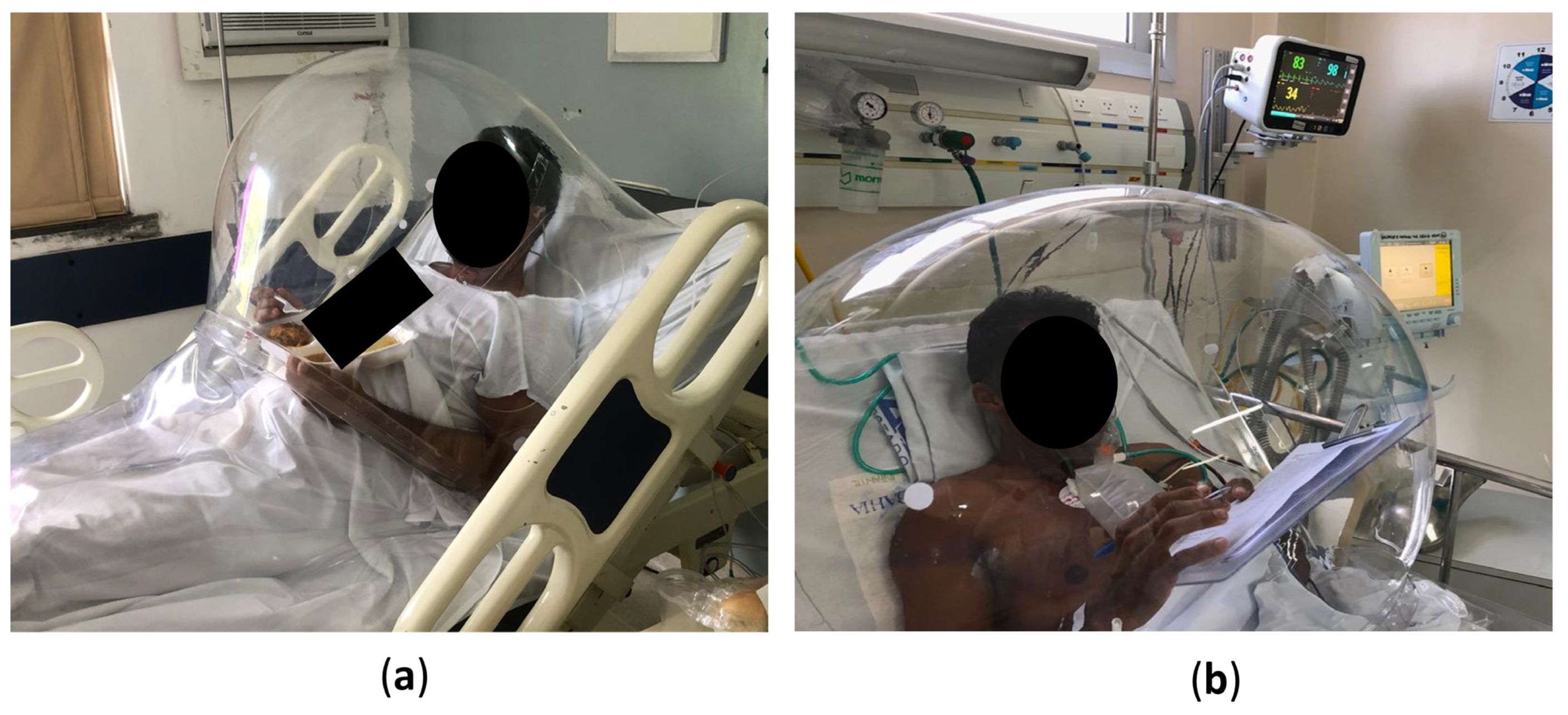
2.1. The Device
2.2. Study Design and Ethical Considerations
2.3. Study Site and Team
2.4. Study Population
2.5. Data Collection: Clinical Monitoring
2.6. Data Collection: Patients’ and Professionals’ Perception of the Device
2.7. Outcomes
2.8. Statistical Analysis
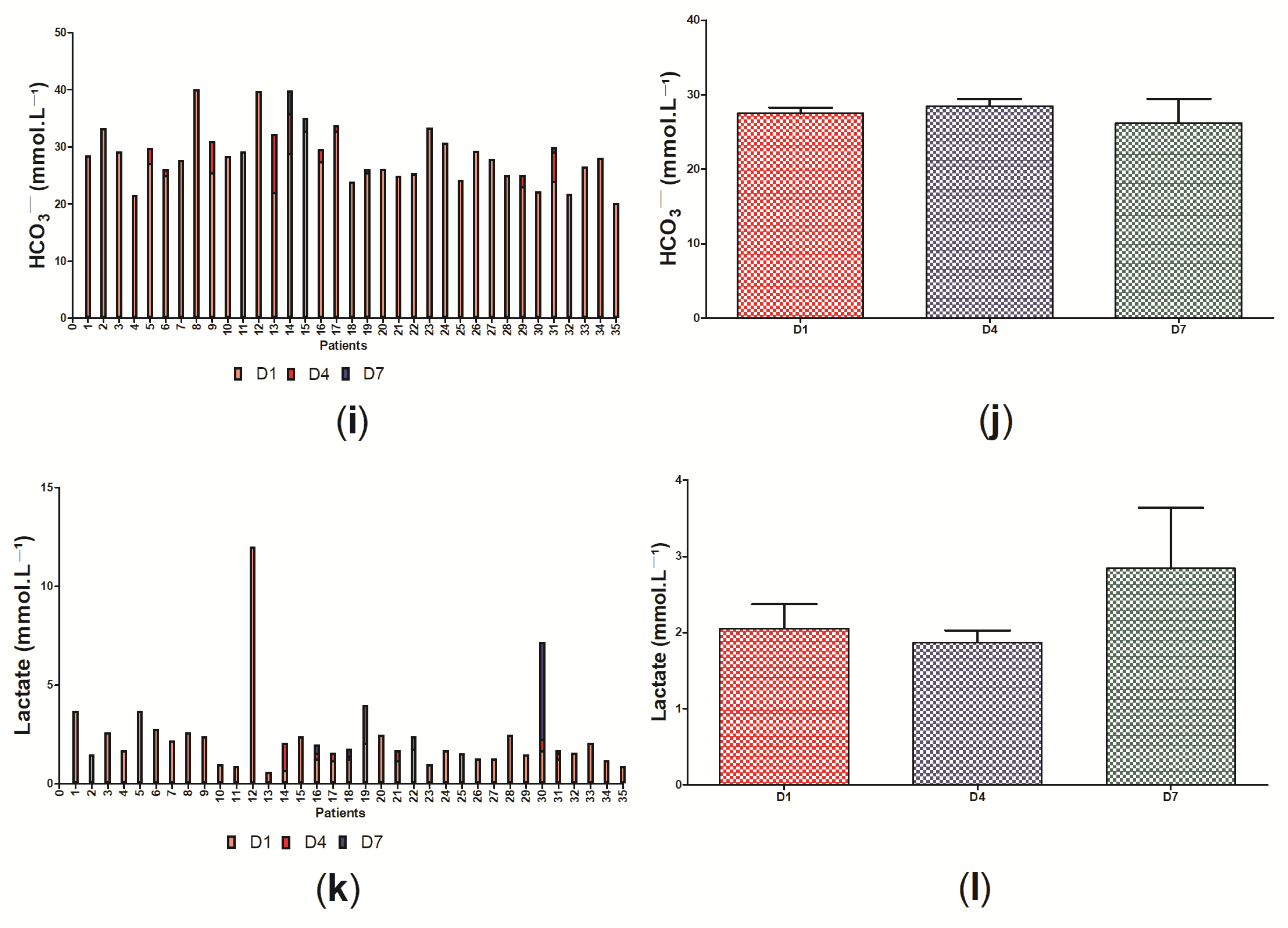
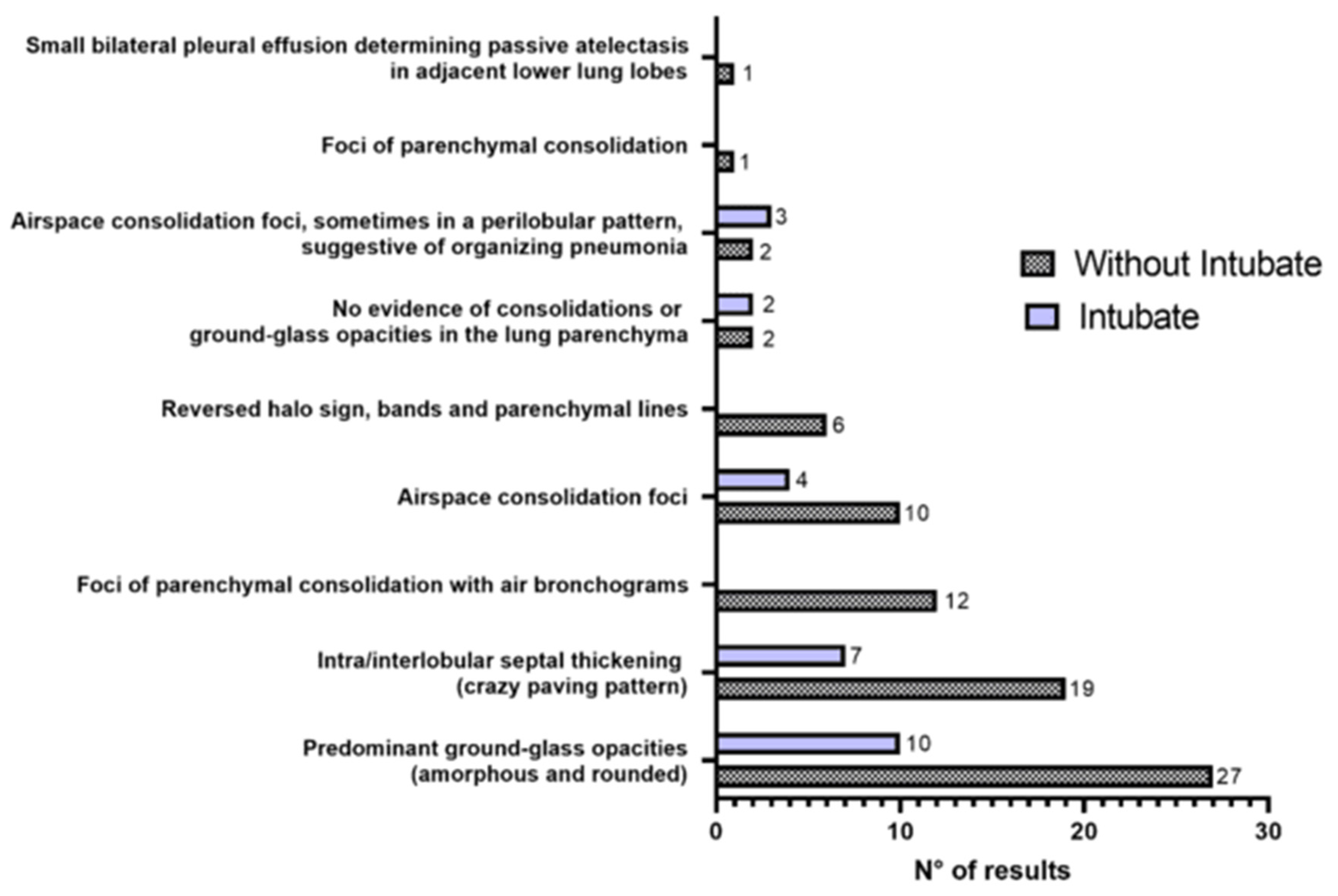
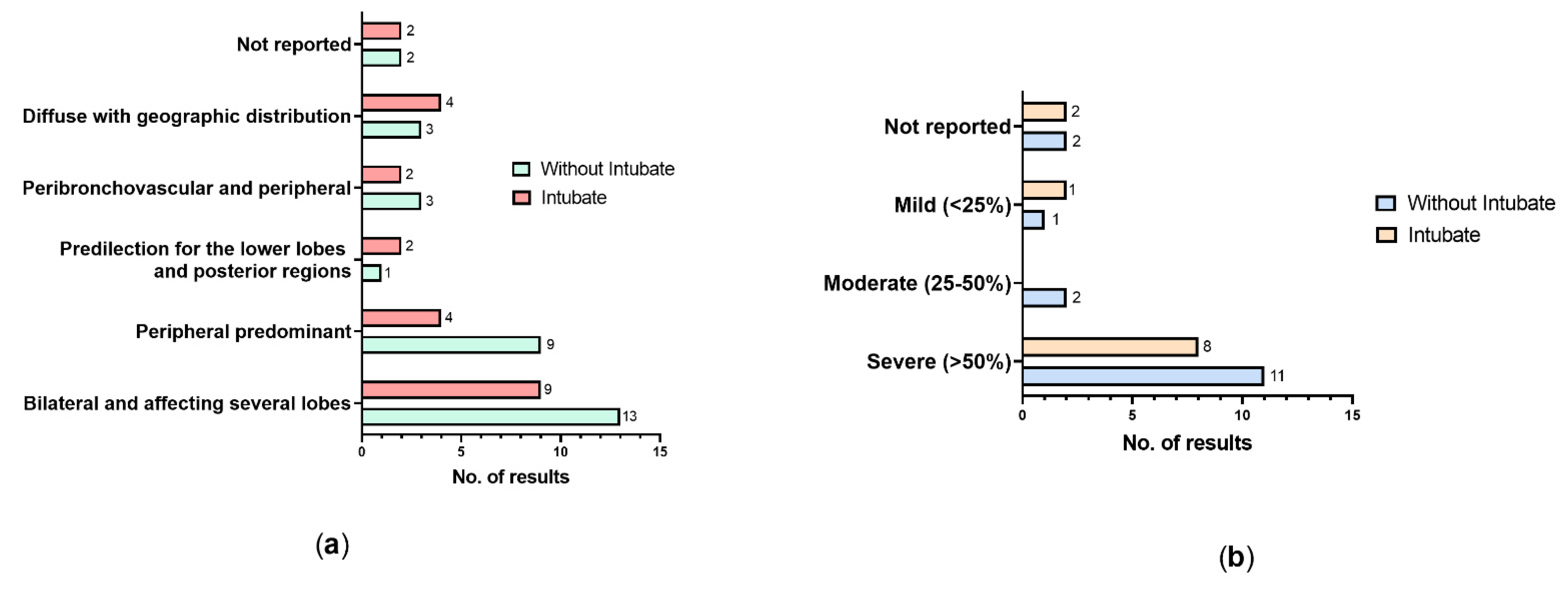
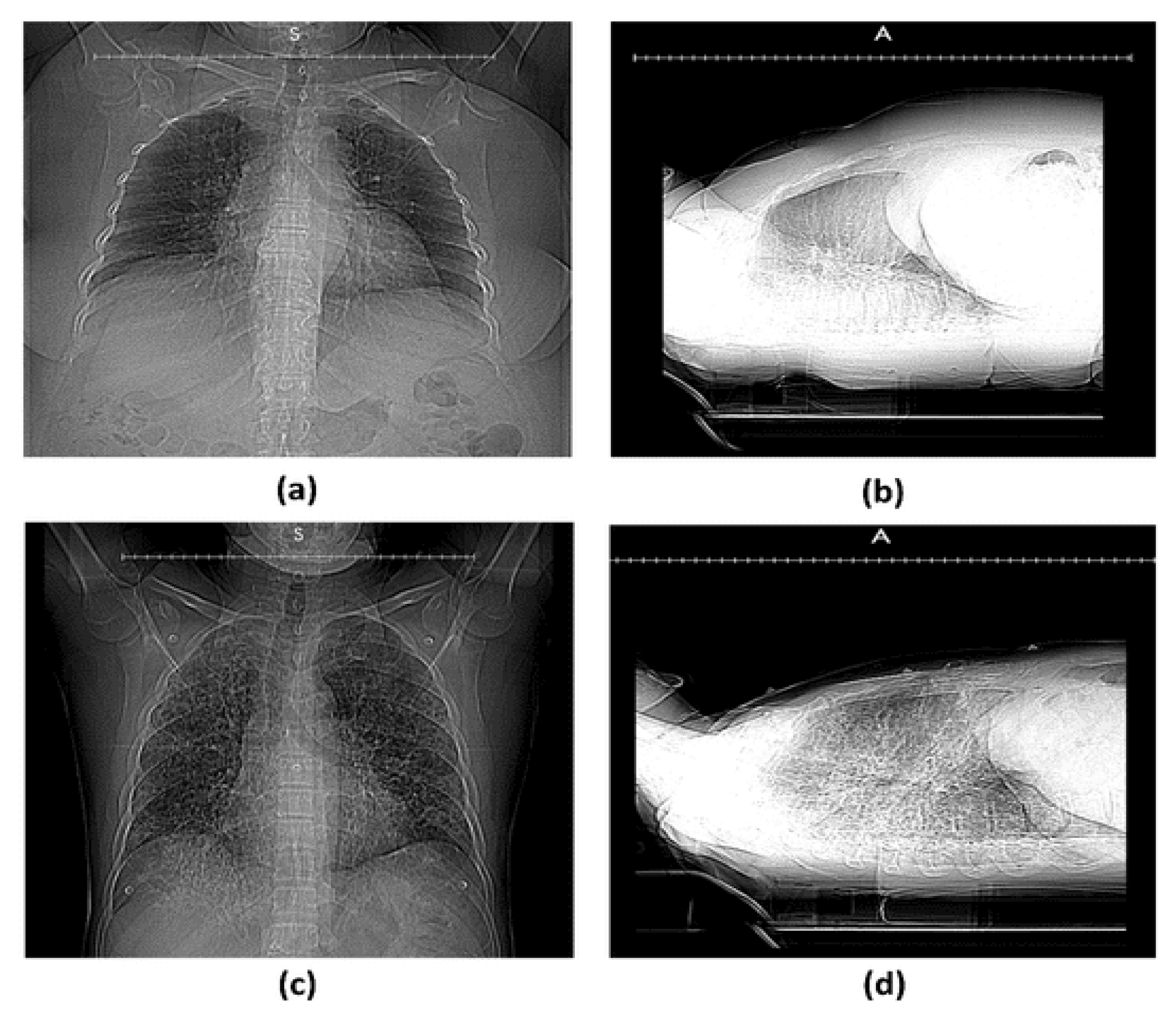
3. Results
3.1. Baseline Data of Participants
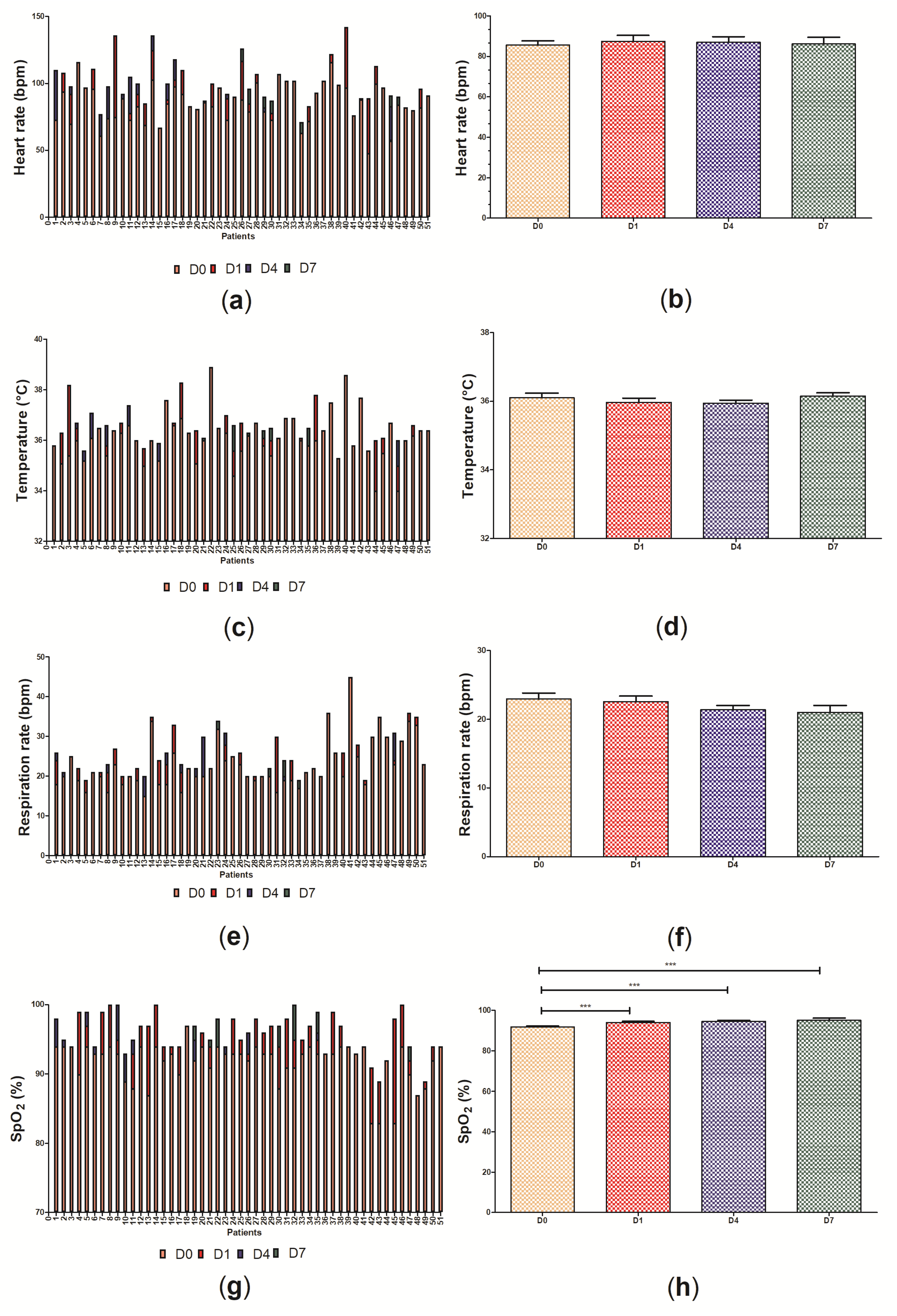
3.2. Clinical Data of the Participants
3.3. Independent Predictors of the Primary Outcome: A Multivariate Analysis
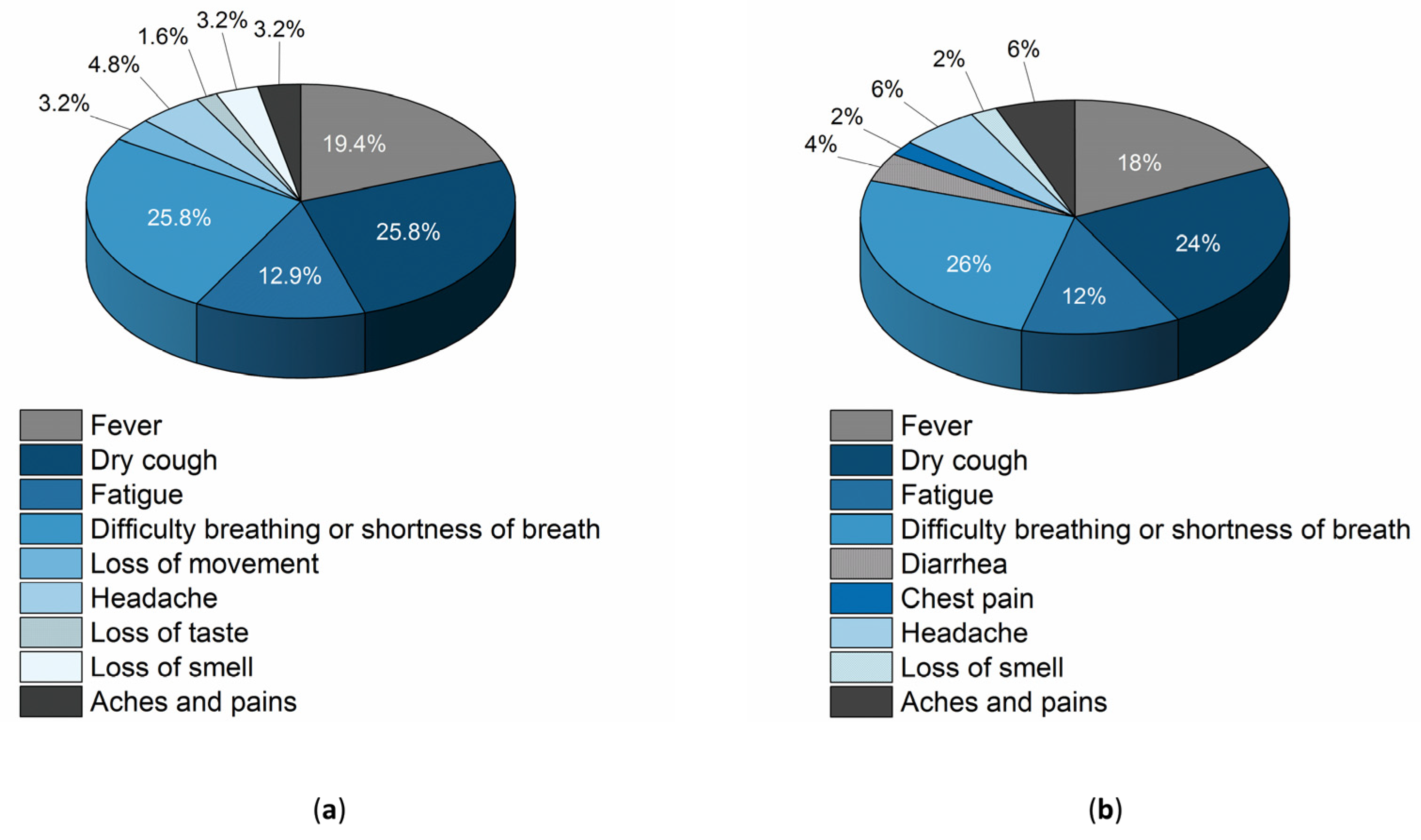
3.4. Perception of Patients and Professionals Regarding the Use of the Device
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Gruson, D.; Kabamba, B.; Dahma, H.; Van den Wijngaert, S.; Reza, S.; Carbone, V.; Vandenberg, O.; Gulbis, B.; Wolff, F.; et al. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J. Clin. Virol. 2020, 128, 104413. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.-Y.; Chuang, Y.-C.; Liu, T.-J.; Chien, C.-W.; Tung, T.-H. Insights from the comparisons of SARS-CoV and COVID-19 outbreaks. Medicine 2021, 100, e24650. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Sacco, O.; Mancino, E.; Cristiani, L.; Midulla, F. Differences and similarities between SARS-CoV and SARS-CoV-2: Spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection 2020, 48, 665–669. [Google Scholar] [CrossRef]
- Lu, H.; Stratton, C.W.; Tang, Y. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef] [Green Version]
- WHO WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 12 May 2022).
- Bigoni, A.; Malik, A.M.; Tasca, R.; Carrera, M.B.M.; Schiesari, L.M.C.; Gambardella, D.D.; Massuda, A. Brazil’s health system functionality amidst of the COVID-19 pandemic: An analysis of resilience. Lancet Reg. Health Am. 2022, 10, 100222. [Google Scholar] [CrossRef]
- Ferigato, S.; Fernandez, M.; Amorim, M.; Ambrogi, I.; Fernandes, L.M.M.; Pacheco, R. The Brazilian Government’s mistakes in responding to the COVID-19 pandemic. Lancet 2020, 396, 1636. [Google Scholar] [CrossRef]
- Akhtar, Z.; Sharma, S.; Elbatran, A.I.; Leung, L.W.M.; Kontogiannis, C.; Spartalis, M.; Roberts, A.; Bajpai, A.; Zuberi, Z.; Gallagher, M.M. Medium-term outcomes in COVID-19. J. Clin. Med. 2022, 11, 2033. [Google Scholar] [CrossRef]
- Rothe, C.; Schunk, M.; Sothmann, P.; Bretzel, G.; Froeschl, G.; Wallrauch, C.; Zimmer, T.; Thiel, V.; Janke, C.; Guggemos, W.; et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020, 382, 970–971. [Google Scholar] [CrossRef] [Green Version]
- Milovančev, A.; Petrović, M.; Popadić, V.; Miljković, T.; Klašnja, S.; Djuran, P.; Ilić, A.; Kovačević, M.; Stojšić Milosavljević, A.; Brajković, M.; et al. Characteristics and outcomes of patients with acute coronary syndrome and COVID-19. J. Clin. Med. 2022, 11, 1791. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Yelin, D.; Margalit, I.; Nehme, M.; Bordas-Martínez, J.; Pistelli, F.; Yahav, D.; Guessous, I.; Durà-Miralles, X.; Carrozzi, L.; Shapira-Lichter, I.; et al. Patterns of long COVID symptoms: A multi-center cross sectional study. J. Clin. Med. 2022, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Liang, W.; Zhao, Y.; Liang, H.; Chen, Z.; Li, Y.; Liu, X.; Chen, R.; Tang, C.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, P.J.; de Araújo Nobre, M.; Elias, C.; Feteira-Santos, R.; Martinho, A.C.-V.; Camarinha, C.; Bacelar-Nicolau, L.; Costa, A.S.; Furtado, C.; Morais, L.; et al. Multimorbidity profile of COVID-19 deaths in Portugal during 2020. J. Clin. Med. 2022, 11, 1898. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, V.; Tun, N.L.; Haque, W.Z.; Majella, M.G.; Sivakumar, R.K.; Kumar, A.; Hsu, A.T.-W.; Ishak, I.A.; Nur, A.A.; Ayeh, S.K.; et al. Factors associated with disease severity and mortality among patients with COVID-19: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241541. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E.; Sanguine, V.; et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Roedl, K.; Jarczak, D.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Nierhaus, A.; Kluge, S.; Wichmann, D. Chronic critical illness in patients with COVID-19: Characteristics and outcome of prolonged intensive care therapy. J. Clin. Med. 2022, 11, 1049. [Google Scholar] [CrossRef]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Mori, V.; Bates, J.H.T.; Suki, B. Modeling lung perfusion abnormalities to explain early COVID-19 hypoxemia. Nat. Commun. 2020, 11, 4883. [Google Scholar] [CrossRef] [PubMed]
- Castro, V.M.; McCoy, T.H.; Perlis, R.H. Laboratory findings associated with severe illness and mortality among hospitalized individuals with coronavirus disease 2019 in eastern Massachusetts. JAMA Netw. Open 2020, 3, e2023934. [Google Scholar] [CrossRef] [PubMed]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Gutiérrez-Zarate, D.; Rosas-Sánchez, K.; Flores-Carrillo, J.C.; Medrano-Ahumada, S.; Martínez-Franco, M. Early management of critically ill patients with COVID-19. J. Am. Coll. Emerg. Physicians Open 2020, 1, 1418–1426. [Google Scholar] [CrossRef]
- Brown, C.A.; Mosier, J.M.; Carlson, J.N.; Gibbs, M.A. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J. Am. Coll. Emerg. Physicians Open 2020, 1, 80–84. [Google Scholar] [CrossRef]
- Zuo, M.; Huang, Y.; Ma, W.; Xue, Z.; Zhang, J.; Gong, Y.; Che, L. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chinese Med. Sci. J. 2020, 35, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Cook, T.M.; El-Boghdadly, K.; McGuire, B.; McNarry, A.F.; Patel, A.; Higgs, A. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia 2020, 75, 785–799. [Google Scholar] [CrossRef]
- Siempos, I.I.; Xourgia, E.; Ntaidou, T.K.; Zervakis, D.; Magira, E.E.; Kotanidou, A.; Routsi, C.; Zakynthinos, S.G. Effect of early vs. delayed or no intubation on clinical outcomes of patients With COVID-19: An observational study. Front. Med. 2020, 7, 614152. [Google Scholar] [CrossRef]
- Hajjar, L.A.; da Silva Costa, I.B.S.; Rizk, S.I.; Biselli, B.; Gomes, B.R.; Bittar, C.S.; de Oliveira, G.Q.; de Almeida, J.P.; de Oliveira Bello, M.V.; Garzillo, C.; et al. Intensive care management of patients with COVID-19: A practical approach. Ann. Intensive Care 2021, 11, 36. [Google Scholar] [CrossRef]
- Windisch, W.; Weber-Carstens, S.; Kluge, S.; Rossaint, R.; Welte, T.; Karagiannidis, C. Invasive and non-invasive ventilation in patients with COVID-19. Dtsch. Aerzteblatt Online 2020, 117, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Gregson, F.K.A.; Shrimpton, A.; Cook, T.M.; Bzdek, B.R.; Reid, J.P.; Pickering, A.E. A quantitative evaluation of aerosol generation during tracheal intubation and extubation. Anaesthesia 2021, 76, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Raoof, S.; Nava, S.; Carpati, C.; Hill, N.S. High-Flow, Noninvasive ventilation and awake (Nonintubation) proning in patients with coronavirus disease 2019 with respiratory failure. Chest 2020, 158, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Kress, J.P.; Hall, J.B. Alternatives to invasive ventilation in the COVID-19 pandemic. JAMA 2020, 324, 43. [Google Scholar] [CrossRef]
- Kotfis, K.; Williams Roberson, S.; Wilson, J.E.; Dabrowski, W.; Pun, B.T.; Ely, E.W. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit. Care 2020, 24, 176. [Google Scholar] [CrossRef]
- Bastos, L.S.; Ranzani, O.T.; Souza, T.M.L.; Hamacher, S.; Bozza, F.A. COVID-19 hospital admissions: Brazil’s first and second waves compared. Lancet Respir. Med. 2021, 9, e82–e83. [Google Scholar] [CrossRef]
- Ranzani, O.T.; Bastos, L.S.L.; Gelli, J.G.M.; Marchesi, J.F.; Baião, F.; Hamacher, S.; Bozza, F.A. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: A retrospective analysis of nationwide data. Lancet Respir. Med. 2021, 9, 407–418. [Google Scholar] [CrossRef]
- WHO Clinical Management Clinical Management Living Guidance COVID-19; WHO: Geneva, Switzerland, 2021.
- Ministry of Health. Orientações Sobre a Otimização Do Uso de Oxigênio e Suporte Ventilatório em Pacientes Graves com COVID-19; Ministry of Health: Brasília, Brazil, 2021.
- OPS. Flowchart for the Management of COVID-19 Infection at the First Level of Care and in Remote Areas; OPS: Washington, DC, USA, 2020. [Google Scholar]
- NIH Oxygenation and Ventilation. Available online: https://www.covid19treatmentguidelines.nih.gov/management/critical-care/oxygenation-and-ventilation/ (accessed on 14 March 2022).
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Cns Resolução no 466, de 12 de Dezembro de 2012. Available online: https://conselho.saude.gov.br/resolucoes/2012/Reso466.pdf (accessed on 30 August 2020).
- Falavigna, M.; Colpani, V.; Stein, C.; Azevedo, L.C.P.; Bagattini, A.M.; de Brito, G.V.; Chatkin, J.M.; Cimerman, S.; de Corradi, M.F.D.B.; da Cunha, C.A.; et al. Guidelines for the pharmacological treatment of COVID-19. The task force/consensus guideline of the Brazilian association of intensive care medicine, the Brazilian society of infectious diseases and the Brazilian society of pulmonology and tisiology. Rev. Bras. Ter. Intensiv. 2020, 32, 166. [Google Scholar] [CrossRef]
- Moser, C.A.; Kalton, G. Survey Methods in Social Investigation; Routledge: Oxfordshire, UK, 2017; ISBN 9781351896726. [Google Scholar]
- SMS Indicadores–SMS–COVID-19–Transparência. Available online: http://www.saude.salvador.ba.gov.br/covid/indicadorescovid/ (accessed on 23 August 2021).
- IBGE Salvador (BA)|Cidades e Estados. Available online: https://www.ibge.gov.br/cidades-e-estados/ba/salvador.html (accessed on 23 August 2021).
- Pisano, A.; Yavorovskiy, A.; Verniero, L.; Landoni, G. Indications for tracheal intubation in patients with coronavirus disease 2019 (COVID-19). J. Cardiothorac. Vasc. Anesth. 2021, 35, 1276–1280. [Google Scholar] [CrossRef]
- Hernandez-Romieu, A.C.; Adelman, M.W.; Hockstein, M.A.; Robichaux, C.J.; Edwards, J.A.; Fazio, J.C.; Blum, J.M.; Jabaley, C.S.; Caridi-Scheible, M.; Martin, G.S.; et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: A single-center cohort study. Crit. Care Med. 2020, 48, e1045–e1053. [Google Scholar] [CrossRef] [PubMed]
- Lanza, E.; Muglia, R.; Bolengo, I.; Santonocito, O.G.; Lisi, C.; Angelotti, G.; Morandini, P.; Savevski, V.; Politi, L.S.; Balzarini, L. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur. Radiol. 2020, 30, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Boerma, E.C.; Bethlehem, C.; Stellingwerf, F.; de Lange, F.; Streng, K.W.; Koetsier, P.M.; Bootsma, I.T. Hemodynamic characteristics of mechanically ventilated COVID-19 patients: A cohort analysis. Crit. Care Res. Pract. 2021, 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Parikh, A.; Lopez-Ruiz, A.; Carrilo, M.; Goldberg, J.; Cearras, M.; Fernainy, K.; Andersen, S.; Mercado, L.; Guan, J.; et al. ICU outcomes and survival in patients with severe COVID-19 in the largest health care system in central Florida. PLoS ONE 2021, 16, e0249038. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Cattaneo, E.; Florio, G.; Ippolito, M.; Zanella, A.; Cortegiani, A.; Huang, J.; Pesenti, A.; Einav, S. Mechanical ventilation parameters in critically ill COVID-19 patients: A scoping review. Crit. Care 2021, 25, 115. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020, 323, 2052. [Google Scholar] [CrossRef]
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R.; Nahid, M.; Ringel, J.B.; et al. Clinical characteristics of Covid-19 in New York city. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef]
- de Souza, F.S.H.; Hojo-Souza, N.S.; de Oliveira Batista, B.D.; da Silva, C.M.; Guidoni, D.L. On the analysis of mortality risk factors for hospitalized COVID-19 patients: A data-driven study using the major Brazilian database. PLoS ONE 2021, 16, e0248580. [Google Scholar] [CrossRef]
- Mukhtar, A.; Lotfy, A.; Hasanin, A.; El-Hefnawy, I.; El Adawy, A. Outcome of non-invasive ventilation in COVID-19 critically ill patients: A retrospective observational study. Anaesth. Crit. Care Pain Med. 2020, 39, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Esquinas, A.; Leon, M.; Gonzalez, G.; Alarcon, A.; Torres, A. Noninvasive ventilation in severe hypoxemic respiratory failure. Am. J. Respir. Crit. Care Med. 2003, 168, 1438–1444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.; Lu, Z.; Zhang, X.; Wang, Y.; Wang, J.; Cai, Y.; Tian, K.; Xiong, Z.; Zhong, Q.; Ran, X.; et al. Clinical characteristics and risk factors of fatal patients with COVID-19: A retrospective cohort study in Wuhan, China. BMC Infect. Dis. 2021, 21, 951. [Google Scholar] [CrossRef] [PubMed]
- Farzan, N.; Vahabi, S.; Hashemi Madani, S.S.; Farzan, B. Evaluating characteristics associated with the mortality among invasive ventilation COIVD-19 patients. Ann. Med. Surg. 2021, 69, 102832. [Google Scholar] [CrossRef]
- Coppadoro, A.; Benini, A.; Fruscio, R.; Verga, L.; Mazzola, P.; Bellelli, G.; Carbone, M.; Mulinacci, G.; Soria, A.; Noè, B.; et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: An observational study during the COVID-19 outbreak. Crit. Care 2021, 25, 80. [Google Scholar] [CrossRef]
- Alghamdi, S. Clinical characteristics and treatment outcomes of severe (ICU) COVID-19 patients in Saudi Arabia: A single centre study. Saudi Pharm. J. 2021, 29, 1096–1101. [Google Scholar] [CrossRef]
- Sahin, S.; Sezer, H.; Cicek, E.; Yagız Ozogul, Y.; Yildirim, M.; Icli, T.B.; Polat Korkmaz, O.; Durcan, E.; Sulu, C.; Somay, K.; et al. The role of obesity in predicting the clinical outcomes of COVID-19. Obes. Facts 2021, 14, 481–489. [Google Scholar] [CrossRef]
- Suliman, L.A.; Abdelgawad, T.T.; Farrag, N.S.; Abdelwahab, H.W. Validity of ROX index in prediction of risk of intubation in patients with COVID-19 pneumonia. Adv. Respir. Med. 2021, 89, 1–7. [Google Scholar] [CrossRef]
- Alhazzani, W.; Hylander Møller, M.; Arabi, Y.M.; Loeb, M.; Ng Gong, M.; Fan, E.; Oczkowski, S.; Levy, M.M.; Maitland, K.; Alshamsi, F.; et al. Surviving sepsis campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020, 5, 854–887. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, Y.; Yang, Z.; Wu, H.; Liang, J.; Liang, H.; Lin, H.; Chen, R.; Ou, Y.; Wang, F.; et al. The use of non-invasive ventilation in COVID-19: A systematic review. Int. J. Infect. Dis. 2021, 106, 254–261. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID- 19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54. [Google Scholar] [CrossRef] [PubMed]
- Oranger, M.; Gonzalez-Bermejo, J.; Dacosta-Noble, P.; Llontop, C.; Guerder, A.; Trosini-Desert, V.; Faure, M.; Raux, M.; Decavele, M.; Demoule, A.; et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: A two-period retrospective case-control study. Eur. Respir. J. 2020, 56, 2001692. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, É.; Kouatchet, A.; Jaber, S.; Lambert, J.; Meziani, F.; Schmidt, M.; Schnell, D.; Mortaza, S.; Conseil, M.; Tchenio, X.; et al. Noninvasive mechanical ventilation in patients having declined tracheal intubation. Intensive Care Med. 2013, 39, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pesenti, A.; Matthay, M. Understanding blood gas analysis. Intensive Care Med. 2018, 44, 91–93. [Google Scholar] [CrossRef]
- Amati, F.; Aliberti, S.; Misuraca, S.; Simonetta, E.; Bindo, F.; Vigni, A.; Bassi, L.; Mazzucco, A.; Cara, A.; Blasi, F. Lung recruitability of COVID-19 pneumonia in patients undergoing helmet CPAP. Arch. Bronconeumol. 2021, 57, 92–94. [Google Scholar] [CrossRef]
- Larkin, B.G.; Zimmanck, R.J. Interpreting arterial blood gases successfully. AORN J. 2015, 102, 343–357. [Google Scholar] [CrossRef]
- Mejía, F.; Medina, C.; Cornejo, E.; Morello, E.; Vásquez, S.; Alave, J.; Schwalb, A.; Málaga, G. Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PLoS ONE 2020, 15, e0244171. [Google Scholar] [CrossRef]
- Wilson-Baig, N.; McDonnell, T.; Bentley, A. Discrepancy between SpO2 and SaO2 in patients with COVID-19. Anaesthesia 2021, 76, 6–7. [Google Scholar] [CrossRef]
- Philip, K.E.J.; Bennett, B.; Fuller, S.; Lonergan, B.; McFadyen, C.; Burns, J.; Tidswell, R.; Vlachou, A. Working accuracy of pulse oximetry in COVID-19 patients stepping down from intensive care: A clinical evaluation. BMJ Open Respir. Res. 2020, 7, e000778. [Google Scholar] [CrossRef]
- Katayama, S.; Shima, J.; Tonai, K.; Koyama, K.; Nunomiya, S. Accuracy of two pulse-oximetry measurements for INTELLiVENT-ASV in mechanically ventilated patients: A prospective observational study. Sci. Rep. 2021, 11, 9001. [Google Scholar] [CrossRef]
- Thijssen, M.; Janssen, L.; le Noble, J.; Foudraine, N. Facing SpO2 and SaO2 discrepancies in ICU patients: Is the perfusion index helpful? J. Clin. Monit. Comput. 2020, 34, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Zhan, X.; Zhou, Z.; Li, Y.; Xie, P.; Zhang, S.; Li, X.; Yu, Y.; Zhou, C.; Zhang, L.; et al. AI-based analysis of CT images for rapid triage of COVID-19 patients. NPJ Digit. Med. 2021, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Satu, M.S.; Howlader, K.C.; Mahmud, M.; Kaiser, M.S.; Shariful Islam, S.M.; Quinn, J.M.W.; Alyami, S.A.; Moni, M.A. Short-term prediction of COVID-19 cases using machine learning models. Appl. Sci. 2021, 11, 4266. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Kaufman, A.E.; Naidu, S.; Ramachandran, S.; Kaufman, D.S.; Fayad, Z.A.; Mani, V. Review of radiographic findings in COVID-19. World J. Radiol. 2020, 12, 142–155. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; La Penna, A.; Crocamo, A.; Sguazzo, F.; Viceconti, R.; Barlotti, V.; Gambardella, M. Heterogeneity of clinical and radiological findings of COVID-19. Postgrad. Med. J. 2020, 97, 268–269. [Google Scholar] [CrossRef]
- Gresser, E.; Rueckel, J.; Puhr-Westerheide, D.; Schwarze, V.; Fink, N.; Kunz, W.G.; Wassilowsky, D.; Irlbeck, M.; Ricke, J.; Ingrisch, M.; et al. Prognostic value of admission chest CT findings for invasive ventilation therapy in COVID-19 pneumonia. Diagnostics 2020, 10, 1108. [Google Scholar] [CrossRef]
- Leoni, M.L.G.; Lombardelli, L.; Colombi, D.; Bignami, E.G.; Pergolotti, B.; Repetti, F.; Villani, M.; Bellini, V.; Rossi, T.; Halasz, G.; et al. Prediction of 28-day mortality in critically ill patients with COVID-19: Development and internal validation of a clinical prediction model. PLoS ONE 2021, 16, e0254550. [Google Scholar] [CrossRef]
- Bellos, I.; Tavernaraki, K.; Stefanidis, K.; Michalopoulou, O.; Lourida, G.; Korompoki, E.; Thanou, I.; Thanos, L.; Pefanis, A.; Argyraki, A. Chest CT severity score and radiological patterns as predictors of disease severity, ICU admission, and viral positivity in COVID-19 patients. Respir. Investig. 2021, 59, 436–445. [Google Scholar] [CrossRef]
- Spagnoli, L.; Morrone, M.F.; Giampieri, E.; Paolani, G.; Santoro, M.; Curti, N.; Coppola, F.; Ciccarese, F.; Vara, G.; Brandi, N.; et al. Outcome prediction for SARS-CoV-2 patients using machine learning modeling of clinical, radiological, and radiomic features derived from chest CT images. Appl. Sci. 2022, 12, 4493. [Google Scholar] [CrossRef]
- Ruch, Y.; Kaeuffer, C.; Ohana, M.; Labani, A.; Fabacher, T.; Bilbault, P.; Kepka, S.; Solis, M.; Greigert, V.; Lefebvre, N.; et al. CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin. Microbiol. Infect. 2020, 26, 1417.e5–1417.e8. [Google Scholar] [CrossRef] [PubMed]


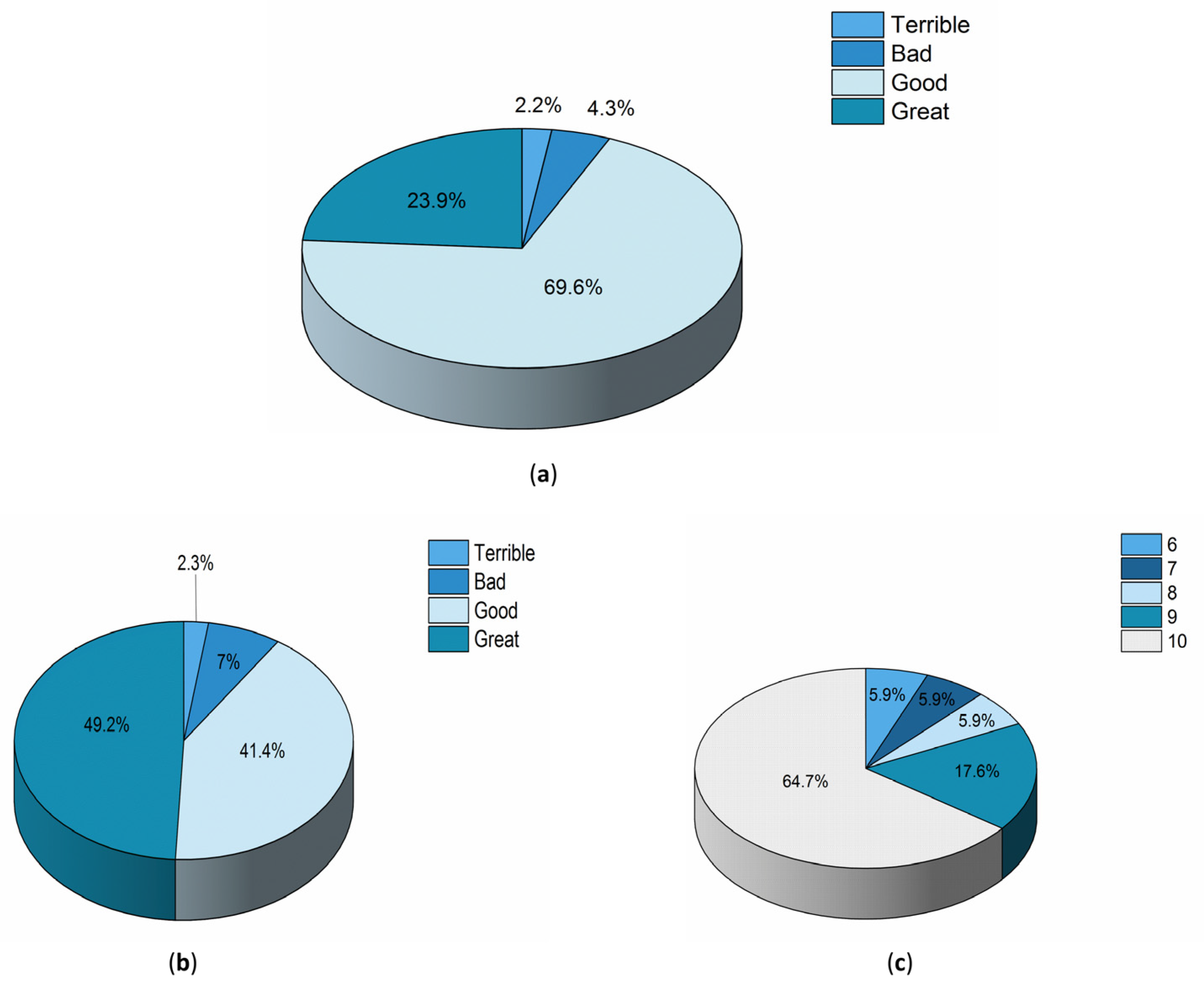
| Variables | All Participants n = 51 | Primary Outcome | p Value | |
|---|---|---|---|---|
| Patients Who Did Not Need Invasive Ventilation n = 35 | Patients Who Needed Invasive Ventilation n = 16 | |||
| Female, n (%) | 23 | 12 (52.17) | 11 (47.83) | 0.0951 (>0.05) |
| Male, n (%) | 28 | 23 (82.14) | 5 (17.86) | 0.0169 (<0.05) |
| Age, average (interval) | 66 | 65 [IQR 53–81] (41–97) | 65 [IQR 55–74.5] (26–86) | 0.4871 (>0.05) |
| Type of NIV, Nasal catheter, n (%) | 29 | 25 (86.21) | 4 (13.79) | 0.1296 (<0.05) |
| NIV Type, Non-Reinhalant Mask, n (%) | 22 | 10 (45.45) | 12 (54.55) | 0.3679 (>0.05) |
| Factor | p-Value | │z│ | Odds Ratio [95% C.I.] |
|---|---|---|---|
| Age (years) | 0.1540 | 1.425 | 0.9870 to 1.145 |
| Male (n) | 0.0145 | 2.444 | 1.858 to 79.24 |
| Absence of comorbidity | 0.6165 | 0.5008 | 0.01984 to 9.036 |
| SpO2 (%) | 0.0131 | 2.482 | 0.01543 to 0.5497 |
| NIV Type: Non-Rebreathing Mask | 0.0477 | 1.980 | 1.047 to 2.028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascarenhas, L.A.B.; Machado, B.A.S.; Beal, V.E.; Hodel, K.V.S.; Nogueira, L.M.; Barreto, T.; de Oliveira Jezler, S.F.; De Azevedo, L.R.L.; da Silva, U.F.T.; da Cruz, L.L.; et al. A Brief Analysis of a New Device to Prevent Early Intubation in Hypoxemic Patients: An Observational Study. Appl. Sci. 2022, 12, 6052. https://doi.org/10.3390/app12126052
Mascarenhas LAB, Machado BAS, Beal VE, Hodel KVS, Nogueira LM, Barreto T, de Oliveira Jezler SF, De Azevedo LRL, da Silva UFT, da Cruz LL, et al. A Brief Analysis of a New Device to Prevent Early Intubation in Hypoxemic Patients: An Observational Study. Applied Sciences. 2022; 12(12):6052. https://doi.org/10.3390/app12126052
Chicago/Turabian StyleMascarenhas, Luís Alberto Brêda, Bruna Aparecida Souza Machado, Valter Estevão Beal, Katharine Valéria Saraiva Hodel, Luciana Moreira Nogueira, Thayse Barreto, Sérgio Fernandes de Oliveira Jezler, Leonardo Redig Lisboa De Azevedo, Uener Franklyn Teixeira da Silva, Laiane Lopes da Cruz, and et al. 2022. "A Brief Analysis of a New Device to Prevent Early Intubation in Hypoxemic Patients: An Observational Study" Applied Sciences 12, no. 12: 6052. https://doi.org/10.3390/app12126052
APA StyleMascarenhas, L. A. B., Machado, B. A. S., Beal, V. E., Hodel, K. V. S., Nogueira, L. M., Barreto, T., de Oliveira Jezler, S. F., De Azevedo, L. R. L., da Silva, U. F. T., da Cruz, L. L., de Oliveira Júnior, L. C., Oliveira, V. S., & Badaró, R. (2022). A Brief Analysis of a New Device to Prevent Early Intubation in Hypoxemic Patients: An Observational Study. Applied Sciences, 12(12), 6052. https://doi.org/10.3390/app12126052