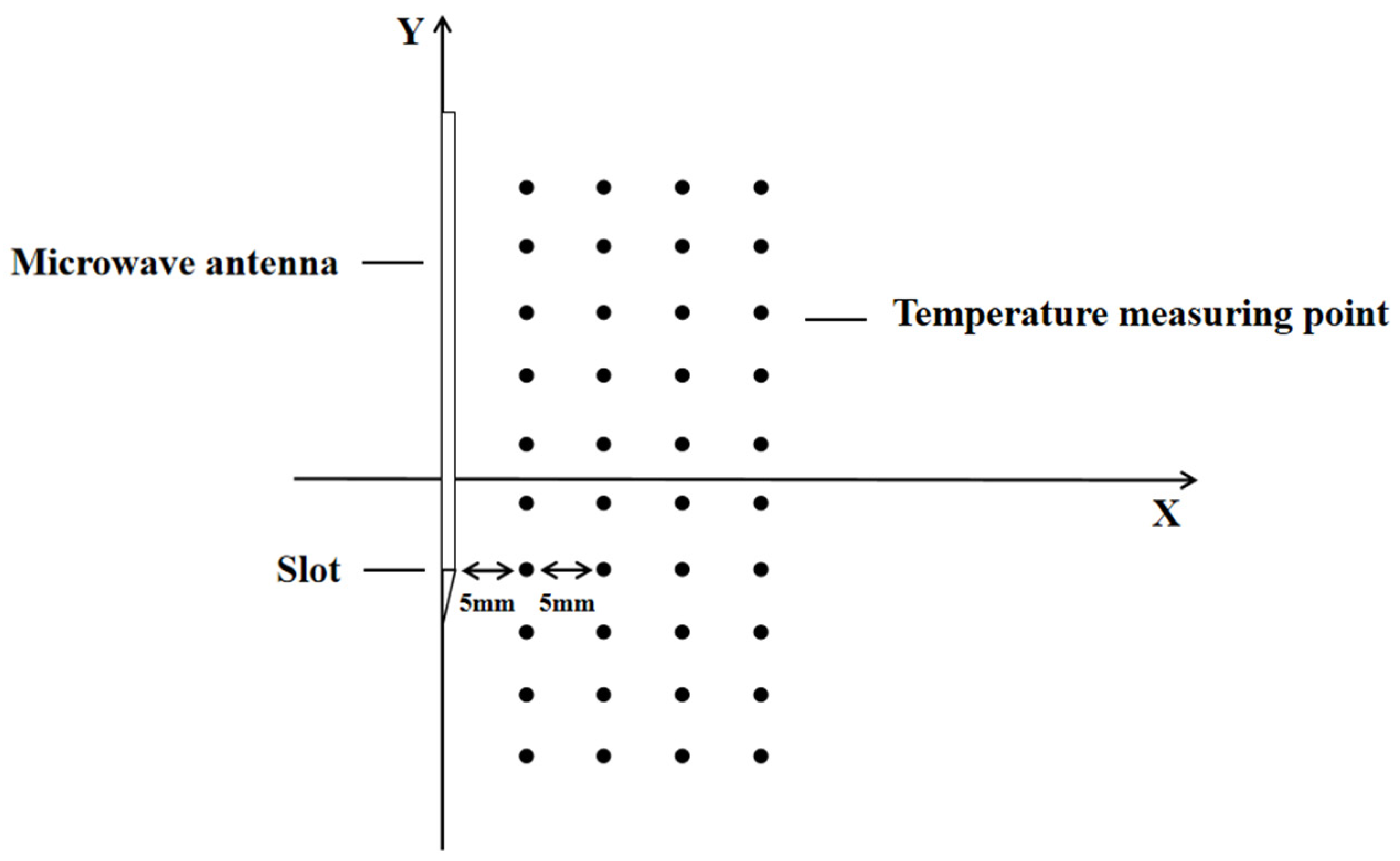
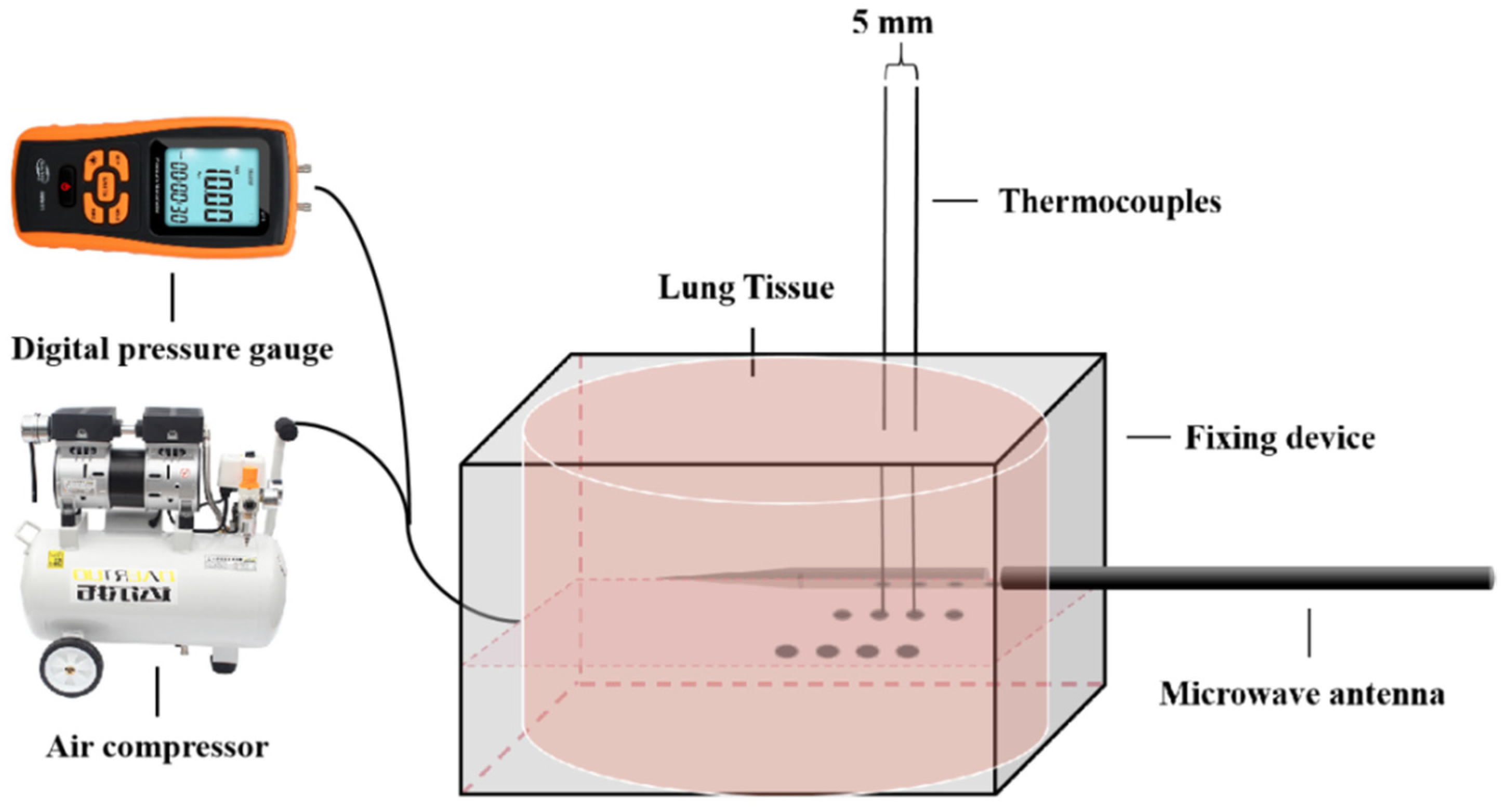
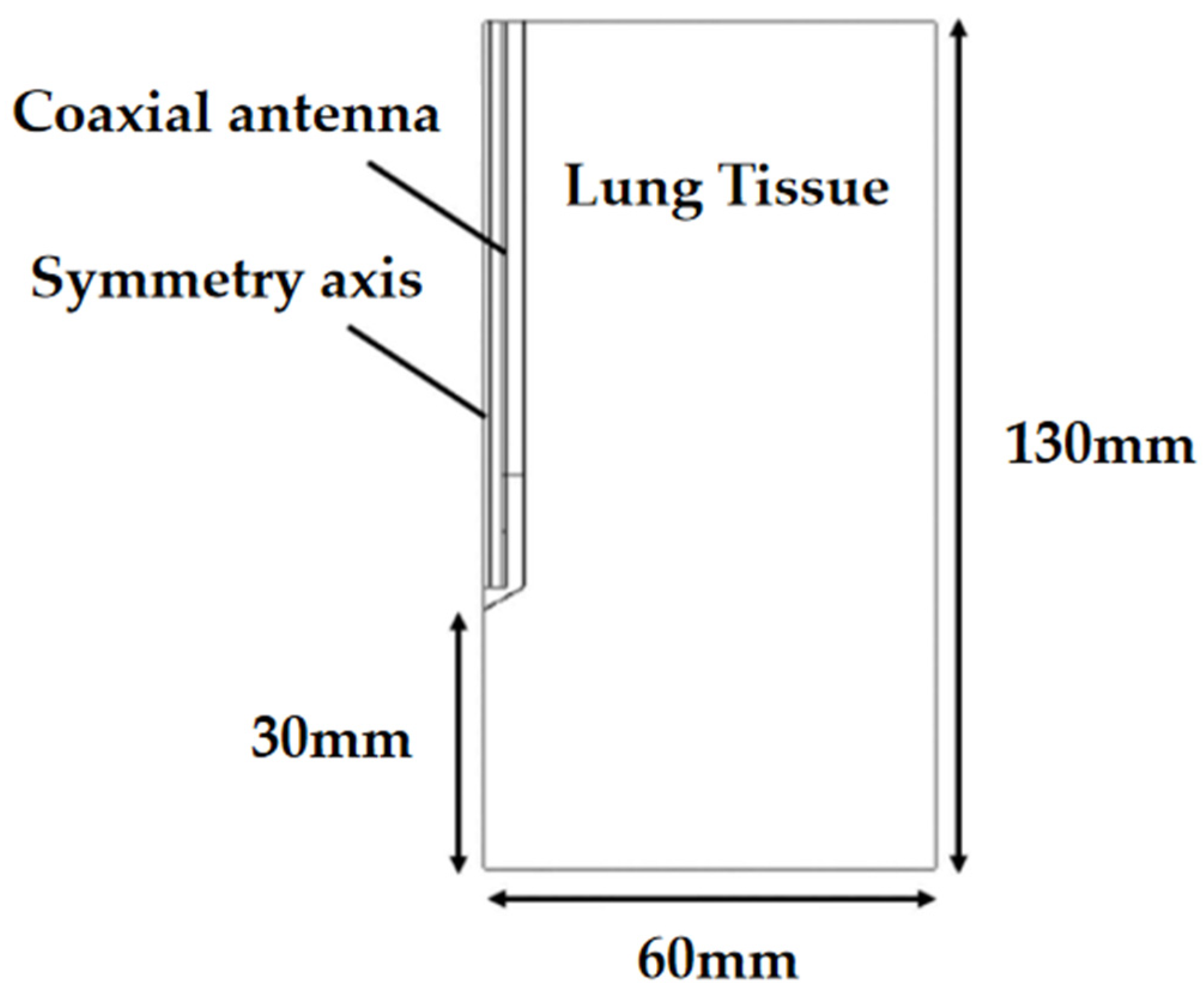
1. Introduction
Lung cancer is one of the greatest threats to human health and life. About 2.1 million of the total diagnosed lung cancer patients worldwide are diagnosed annually, and the highest incidence rate of malignancies in the world [
1]. Surgical resection is the main treatment for primary lung cancer, but only one-third of patients are suitable for surgery. For non-surgical lung cancer patients, thermal ablation has become an important and effective treatment option [
2,
3,
4,
5].
In recent years, thermal ablation has become one of the treatment methods for unresectable primary and secondary tumors [
6]. According to different ablation principles, thermal ablation is mainly divided into microwave ablation (MWA), radio frequency ablation (RFA), high intensity focused ultrasound (HIFU) and laser ablation (LA) [
7]. As a local treatment, thermal ablation has a highly effective killing effect on primary and metastatic lung cancer, and has the advantages of less damage to surrounding normal tissues and rapid postoperative recovery [
8,
9].
At present, RFA is the most commonly used thermal ablation therapy for cancer. The advantage of RFA is its rich experience in clinical application. Since Dupuy et al. [
10] reported three cases of lung cancer in 2000, this technique has been applied to lung cancer for about 20 years. However, this treatment method is easily affected by the heat sink effect of peripheral blood vessels and tissue carbonization, resulting in incomplete ablation. Therefore, it cannot achieve ideal therapeutic effect in the treatment of lung cancer [
11,
12].
MWA is one of the local minimally invasive treatment techniques for lung tumors, which has been developed in recent years. At present, microwave ablation has been widely used in parts of Western countries, mainly for the ablation of solid tumors of liver, spleen, lung, adrenal and other organs [
13]. Due to its effectiveness in the treatment of solid organ tumors, the use of MWA in patients with primary or metastatic lung tumors is gradually increasing [
14]. Compared with RFA, microwave ablation is not easy to be affected by heat sink effect, and can produce higher temperature and larger ablation area in a shorter time. Therefore, MWA is more suitable for the treatment of lung tumors, and has received more and more attention in clinical applications [
15,
16,
17].
However, the current clinical microwave ablation lacks the best imaging guidance mode, and cannot monitor the size of the ablation area in real time. Therefore, microwave ablation may not be effective for some large or irregularly shaped tumors. Gao et al. [
5] have carried out some in vitro experiments and simulation studies on microwave ablation of porcine lungs and established prediction models, but did not consider the effect of air in the lungs on the microwave ablation. In contrast with other solid organs, the lungs are filled with hot and humid air, which makes the lung tissue have a natural high impedance [
18,
19,
20], which will affect the temperature distribution of the microwave ablation area and cause great difficulties in accurately predicting and controlling the microwave ablation area.
In this paper, in vitro experiments and simulations of microwave ablation of porcine lung were designed, and the two were verified by each other to determine the effect of microwave ablation in the inflated lung, and compared with the deflated lung.
4. Conclusions
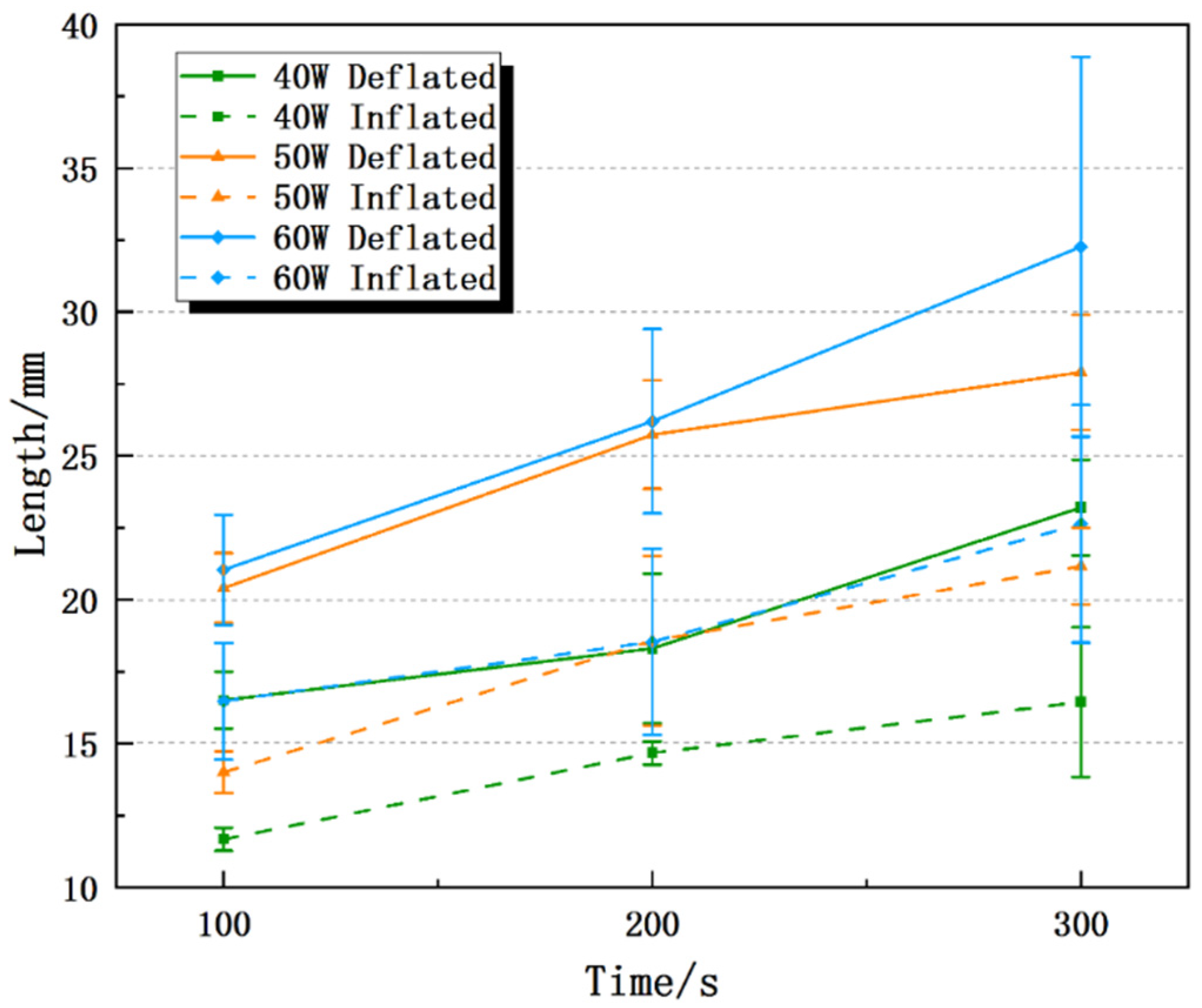
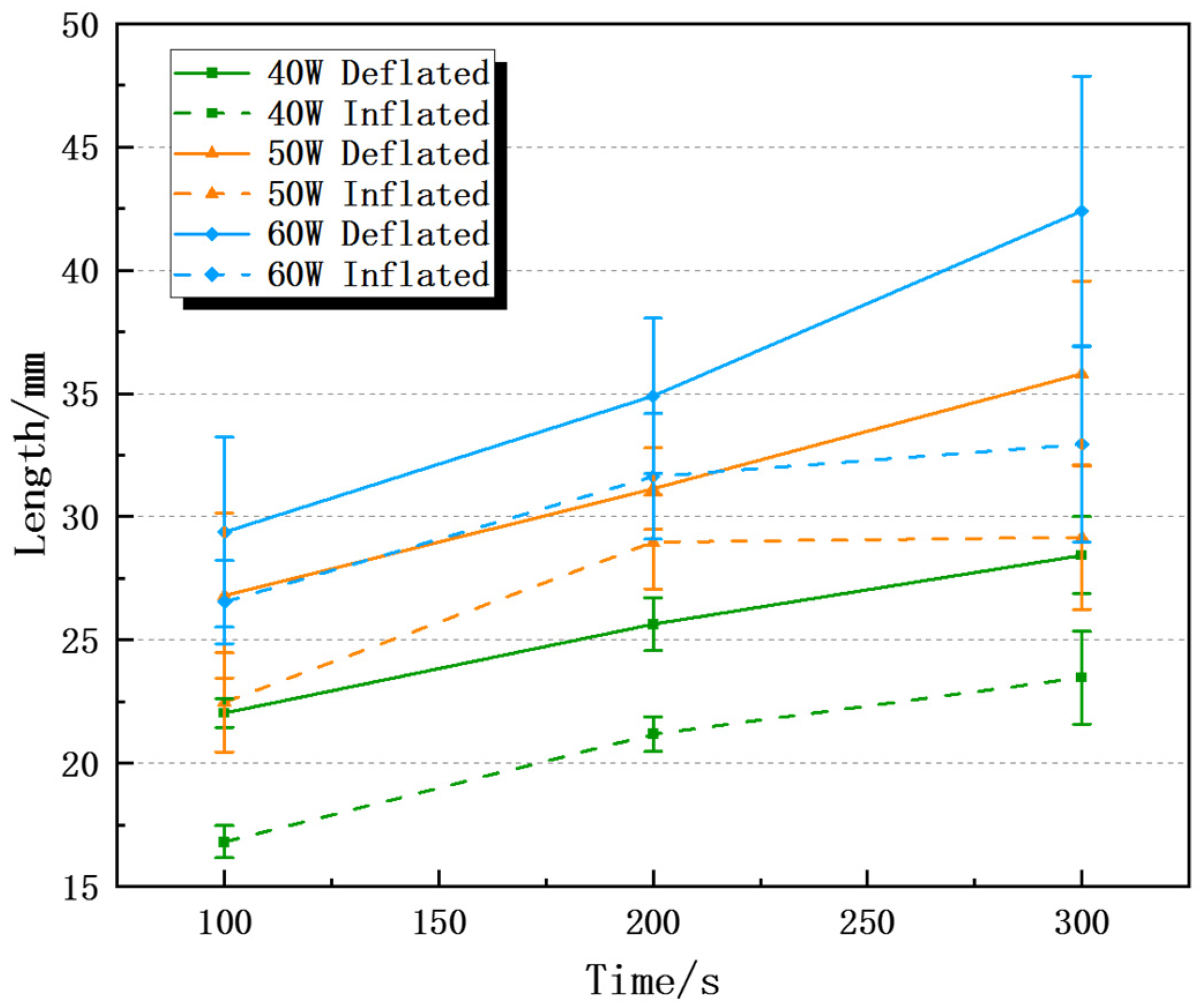
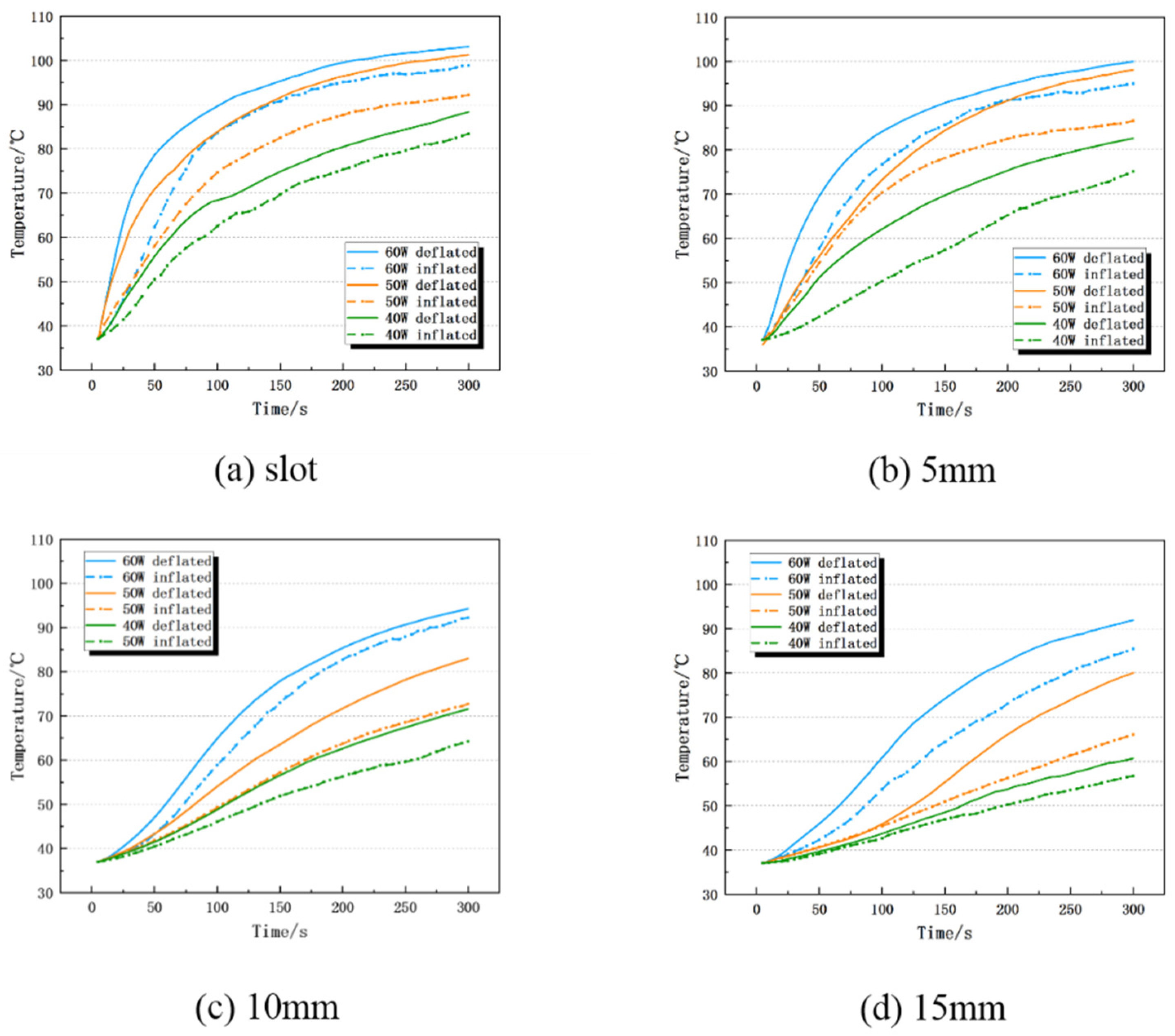
The lung tissue contains a lot of hot and humid air, and the microwave energy is not easily transmitted in the tissue, which makes the prediction and control of the ablation area very difficult for MWA in clinical application [
35]. This paper designed an ex-vivo experiment and a simulation experiment to explore the influence of lung inflation on the effect of MWA. The results of the in vitro experiments show that the inflated lung weakens the microwave ablation effect, which is due to the inflatable reduces the relative permittivity, electrical conductivity, thermal conductivity and other physics parameters of the lung tissue, which affects the transmission and accumulation of microwave energy in the tissue [
36]. Under the same ablation power and time, Dl and Ds of the inflated lung were 7.6–30.9% and 24.5–45.7% smaller than those of the deflated lung, respectively, and the isoperimetric ratio was 0.011–0.097 smaller than deflated group. The maximum temperature and heating rate at different positions of the ablation antenna were also lower than deflated group. In addition, we used the COMSOL Multiphysics software to simulate the microwave ablation of the inflated lung, and obtained similar results to the in vitro experiments, which verified the correctness of the in vitro experiments. Specifically, Dl, Ds and the isoperimetric ratio of the inflated lung are, respectively, 0–7.3%, 15.5–28.0% and 0.059–0.072 smaller than deflated group. This is consistent with the simulation experiment conclusion that inflated lung weakens the microwave ablation effect reported in the literature, which further verifies the correctness of the experiment in this paper [
37,
38,
39].
Compared with RFA, MWA has the advantage of activating multiple microwave antennas, and the synergistic effect of multiple antennas in ablation can effectively expand the volume of the ablation area, solve the ablation deficiency in the treatment of large tumors, and reduce the recurrence rate after ablation [
40]. Planché et al. [
41] used multi-antenna to ablate pig lungs in vivo experiments. When the ablation power was 60 W, the ablation time was 10 min, and three antennas were used for ablation, the average longitudinal diameter was 43.0 mm ± 7.7 mm, and the transverse diameter was 54.8 mm ± 8.5 mm, and the isoperimetric ratio was 0.70 ± 0.10. Compared with single-antenna ablation under the same conditions, the average longitudinal diameter is increased by about 20%, the average transverse diameter is increased by about 67.5%, and the isoperimetric ratio is increased by about 9%. It can be seen that multi-antenna ablation can produce a larger volume of ablation and a higher isoperimetric ratio. However, when three-antenna ablation was used, and the power and ablation time were increased to 75 W and 15 min, two animals died of heart failure due to carbonization of lung tissue and myocardial burn, so the use of multi-antenna ablation in clinical treatment must comprehensively consider ablation power and time. Cazzato et al. [
42] studied the shape and volume of the ablation area obtained by multi-antenna microwave ablation of liver tumors. For tumors larger than 3 cm in diameter, three-antenna ablation was used, and tumors with a diameter of 2–3 cm were ablated with dual antennas. 65 W, ablation time 10 min, antenna spacing 2 cm. The results showed that the average ablation volume after three-antenna ablation was 3 times that of the two-antenna ablation, and the three-antenna ablation had higher sphericity, it was more suitable for treating liver tumors larger than 3 cm in diameter. In conclusion, under the conditions of reasonable ablation power and ablation time, compared with single-antenna ablation, multi-antenna ablation can produce a larger ablation area, and the ablation area have higher sphericity, so it is more suitable for treating large tumor. However, there is currently less experience using multi-antenna ablation in clinical treatment, and further scientific research is still needed.
There are certain differences between in vivo and in vitro. Lung is a porous tissue consisting of millions of tiny alveoli and air sacs, and air inhalation and exhalation occur all the time. To obtain more accurate simulation results, it is necessary to consider the distribution of alveoli and capillaries and other factors, this will be a daunting challenge in finite element simulation experiments. The simulation model in this paper simply treats lung tissue as a homogeneous material, which has certain limitations. These questions need to be further investigated through complementary experiments and more theoretical studies.
In this study, microwave ablation in vitro experiments and simulation experiments were established to study the effect of lung tissue inflated on the ablation area, although there are some differences between the in vitro experiments and simulation results, the effect of inflated lung on the ablation area is still similar, which is expected to provide reference for future clinical medicine and simulation experiments.