Biomaterials for Ophthalmic Applications
Abstract
:1. Introduction
2. Biomaterials in Ophthalmology
2.1. Contact Lenses
2.2. Intraocular Lenses
2.2.1. Intraocular Acrylic Lenses
2.2.2. Intraocular Elastomer Lenses
2.3. Artificial Tears
2.3.1. Lacrimal Film Composition
2.3.2. Formulation of Lacrimal Supplements and Tear Substitutes
2.4. Inlays
2.5. Vitreous Substitutes
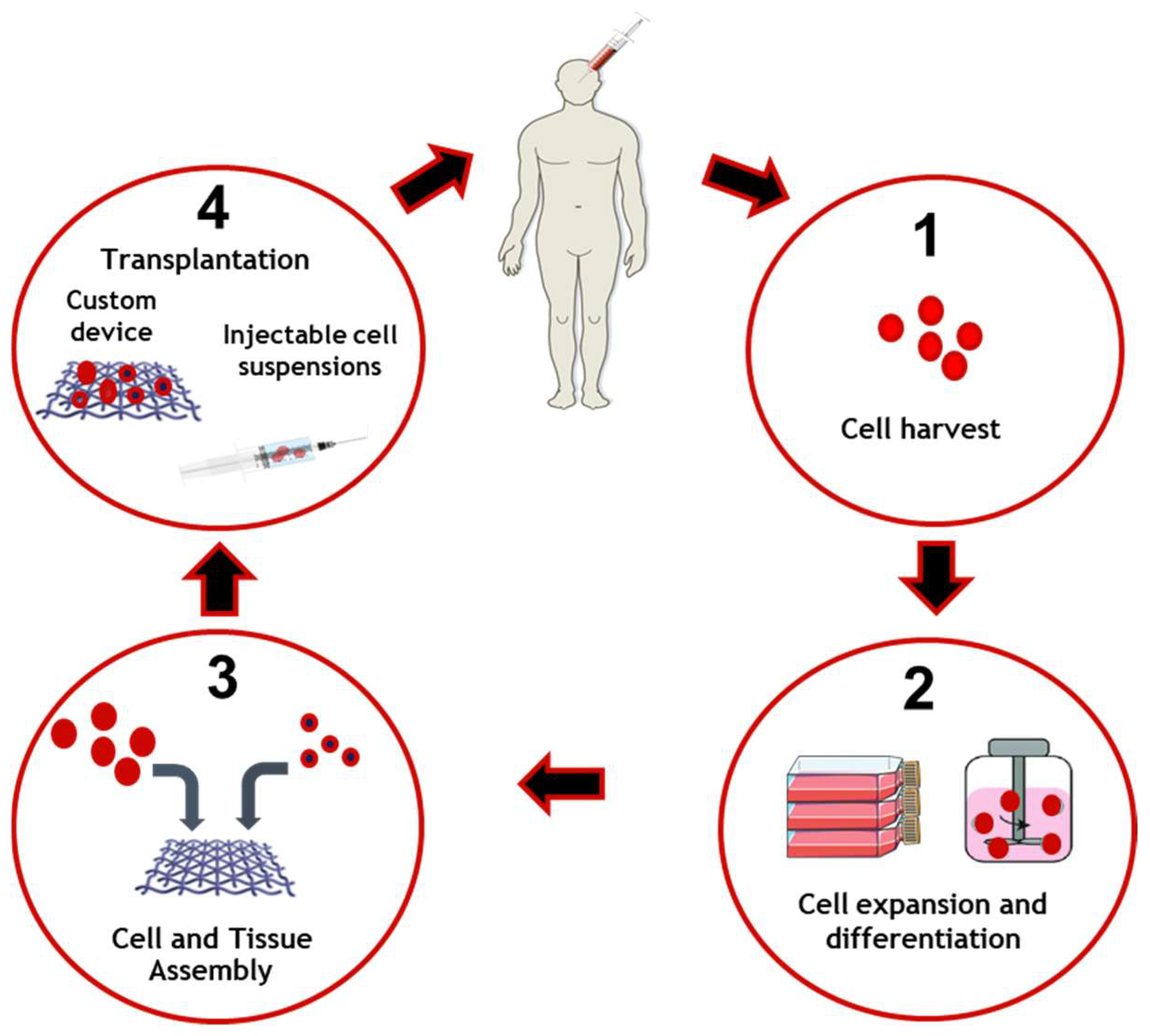
2.6. Tissue Engineering
3. Concluding Remarks and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chirila, T.; Harkin, D.; ScienceDirect. Biomaterials and Regenerative Medicine in Ophthalmology; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Amato, S.; Amato, S.F.; Ezzell, R.M.; Amato, S.F. ProQuest. In Regulatory Affairs for Biomaterials and Medical Devices, 1st ed.; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Friedman, N.J.; Kaiser, P.K. Essentials of Ophthalmology, 1st ed.; Saunders Elsevier: Philadelphia, PA, USA, 2007; 294p. [Google Scholar]
- Basak, S.K. Essentials of Ophthalmology, 7th ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2019; 606p. [Google Scholar]
- Decker, S. Essentials of Ophthalmology; Foster Academics: New York, NY, USA, 2021; 432p. [Google Scholar]
- Winterton, L.C.; Lally, J.M.; Sentell, K.B.; Chapoy, L.L. The elution of poly (vinyl alcohol) from a contact lens: The realization of a time release moisturizing agent/artificial tear. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.H.; Lally, J.M.; Hoong, L.D.; Huth, S.W. Lipophilic versus hydrodynamic modes of uptake and release by contact lenses of active entities used in multipurpose solutions. Contact Lens Anterior Eye 2010, 33, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, G.A.; Hopkins, G.; Pearson, R.M.; ScienceDirect. Ophthalmic Drugs: Diagnostic and Therapeutic Uses, 5th ed.; Butterworth-Heinemann Elsevier: Edinburgh, UK, 2007. [Google Scholar]
- Moarefi, M.A.; Bafna, S.; Wiley, W. A Review of Presbyopia Treatment with Corneal Inlays. Ophthalmol. Ther. 2017, 6, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhof, S.; Goepferich, A.M.; Brandl, F.P. Hydrogels in ophthalmic applications. Eur. J. Pharm. Biopharm. 2015, 95, 227–238. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [Green Version]
- Donati, S.; Caprani, S.M.; Airaghi, G.; Vinciguerra, R.; Bartalena, L.; Testa, F.; Mariotti, C.; Porta, G.; Simonelli, F.; Azzolini, C. Vitreous substitutes: The present and the future. Biomed. Res. Int. 2014, 2014, 351804. [Google Scholar] [CrossRef]
- Baino, F. Towards an ideal biomaterial for vitreous replacement: Historical overview and future trends. Acta Biomater. 2011, 7, 921–935. [Google Scholar] [CrossRef] [Green Version]
- Yadav, I.; Purohit, S.D.; Singh, H.; Bhushan, S.; Yadav, M.K.; Velpandian, T.; Chawla, R.; Hazra, S.; Mishra, N.C. Vitreous substitutes: An overview of the properties, importance, and development. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1156–1176. [Google Scholar] [CrossRef]
- Santos, L.; Ferraz, M.P.; Shirosaki, Y.; Lopes, M.A.; Fernandes, M.H.; Osaka, A.; Santos, J.D. Degradation studies and biological behavior on an artificial cornea material. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4274–4281. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Williams, D.F. Definitions of Biomaterials for the Twenty-First Century: Proceedings of a Consensus Conference Held in Chengdu, People’s Republic of China, June 11th and 12th 2018, Organized under the Auspices of the International Union of Societies for Biomaterials Science & Engineering; Hosted and Supported by Sichuan University, Chengdu and the Chinese Society for Biomaterials, China; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Williams, D.F. Essential Biomaterials Science; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Morgan, P.B.; Efron, N. Global contact lens prescribing 2000–2020. Clin. Exp. Optom. 2022, 105, 298–312. [Google Scholar] [CrossRef]
- Nicolson, P.C.; Vogt, J. Soft contact lens polymers: An evolution. Biomaterials 2001, 22, 3273–3283. [Google Scholar] [CrossRef]
- Findl, O.; Leydolt, C. Meta-analysis of accommodating intraocular lenses. J. Cataract Refract. Surg. 2007, 33, 522–527. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Das, M.; Jain, S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin. Drug Deliv. 2012, 9, 383–402. [Google Scholar] [CrossRef]
- Teixeira, S.; Fernandes, M.H.; Ferraz, M.P.; Monteiro, F.J. Proliferation and mineralization of bone marrow cells cultured on macroporous hydroxyapatite scaffolds functionalized with collagen type I for bone tissue regeneration. J. Biomed. Mater. Res. A 2010, 95, 1–8. [Google Scholar] [CrossRef]
- Teixeira, S.; Yang, L.; Dijkstra, P.J.; Ferraz, M.P.; Monteiro, F.J. Heparinized hydroxyapatite/collagen three-dimensional scaffolds for tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 2385–2392. [Google Scholar] [CrossRef]
- Mozafari, M.; Mozafari, M.; Sefat, F.; Atala, A.; ScienceDirect. Handbook of Tissue Engineering Scaffolds; Woodhead Publishing: Duxford, UK, 2019. [Google Scholar]
- Lanza, R.P.; Langer, R.; Vacanti, J.; Atala, A.; Science, D. Principles of Tissue Engineering, 5th ed.; Academic Press: London, UK, 2020. [Google Scholar]
- Sun, M.T.; O’Connor, A.J.; Wood, J.; Casson, R.; Selva, D. Tissue Engineering in Ophthalmology: Implications for Eyelid Reconstruction. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 157–162. [Google Scholar] [CrossRef]
- Efron, N. Contact Lens Practice; Elsevier: Edinburgh, UK, 2018. [Google Scholar]
- Hom, M.M.; Bruce, A.S. Manual of Contact Lens Prescribing and Fitting with CD-ROM; Butterworth Heinemann: Oxford, UK, 2006. [Google Scholar]
- Guillon, M.; Dumbleton, K.; Patel, T.; Patel, K.; Gupta, R.; Maissa, C.A. Corrigendum to “Quantification of contact lens wettability after prolonged visual device use under low humidity conditions” [Cont. Lens Anterior Eye 42 (2019) 386–391]. Contact Lens Anterior Eye 2020, 43, 91. [Google Scholar] [CrossRef] [Green Version]
- Morgan, P.B.; McCullough, S.J.; Saunders, K.J. Corrigendum to “Estimation of ocular axial length from conventional optometric measures” [Cont. Lens Anterior Eye 43 (1) (2020) 18–20]. Contact Lens Anterior Eye 2020, 43, 413. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Pillay, R.; Hansraj, R.; Rampersad, N. Historical Development, Applications and Advances in Materials Used in Spectacle Lenses and Contact Lenses. Clin. Optom. 2020, 12, 201–202. [Google Scholar] [CrossRef]
- Millis, E.A.W. Medical Contact Lens Practice; Elsevier Butterwoth Heinemann: Edinburgh, UK; New York, NY, USA, 2005. [Google Scholar]
- Bennett, E.S.; Bennett, E.S.; Henry, V.A. Clinical Manual of Contact Lenses, 5th ed.; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Stapleton, F.; Stretton, S.; Papas, E.; Skotnitsky, C.; Sweeney, D.F. Silicone hydrogel contact lenses and the ocular surface. Ocul. Surf. 2006, 4, 24–43. [Google Scholar] [CrossRef]
- Seo, E.; Kumar, S.; Lee, J.; Jang, J.; Park, J.H.; Chang, M.C.; Kwon, I.; Lee, J.S.; Huh, Y.I. Modified Hydrogels Based on Poly(2-hydroxyethyl methacrylate) (pHEMA) with Higher Surface Wettability and Mechanical Properties. Macromol. Res. 2017, 25, 704–711. [Google Scholar] [CrossRef]
- Ozgen, O.; Hasirci, N. Modification of Poly(methyl methacrylate) Surfaces with Oxygen, Nitrogen and Argon Plasma. J. Biomater. Tiss. Eng. 2014, 4, 479–487. [Google Scholar] [CrossRef]
- Peterson, R.C.; Wolffsohn, J.S.; Nick, J.; Winterton, L.; Lally, J. Clinical performance of daily disposable soft contact lenses using sustained release technology. Contact Lens Anterior Eye 2006, 29, 127–134. [Google Scholar] [CrossRef]
- Goda, T.; Ishihara, K. Soft contact lens biomaterials from bioinspired phospholipid polymers. Expert Rev. Med. Devices 2006, 3, 167–174. [Google Scholar] [CrossRef]
- Xinming, L.; Yingde, C.; Lloyd, A.W.; Mikhalovsky, S.V.; Sandeman, S.R.; Howel, C.A.; Liewen, L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Contact Lens Anterior Eye 2008, 31, 57–64. [Google Scholar] [CrossRef]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained drug release by contact lenses for glaucoma treatment-a review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef]
- Cardona, G.; Alonso, S.; Yela, S. Compliance versus Risk Awareness with Contact Lens Storage Case Hygiene and Replacement. Optom. Vis. Sci. 2022, 99, 449–454. [Google Scholar] [CrossRef]
- Barros, J.; Grenho, L.; Fontenente, S.; Manuel, C.M.; Nunes, O.C.; Melo, L.F.; Monteiro, F.J.; Ferraz, M.P. Staphylococcus aureus and Escherichia coli dual-species biofilms on nanohydroxyapatite loaded with CHX or ZnO nanoparticles. J. Biomed. Mater. Res. A 2017, 105, 491–497. [Google Scholar] [CrossRef]
- Gasson, A.; Morris, J.; Askews. The Contact Lens Manual: A Practical Guide to Fitting, 4th ed.; Butterworth/Heinemann: Edinburgh, UK, 2010. [Google Scholar]
- Nichols, J.J.; Chalmers, R.L.; Dumbleton, K.; Jones, L.; Lievens, C.W.; Merchea, M.M.; Szczotka-Flynn, L. The Case for Using Hydrogen Peroxide Contact Lens Care Solutions: A Review. Eye Contact Lens 2019, 45, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Willcox, M.; Zhu, H.; Stapleton, F. Contact lens hygiene compliance and lens case contamination: A review. Contact Lens Anterior Eye 2015, 38, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.S.; Sicks, L.A.; Pucker, A.D. Common Ophthalmic Preservatives in Soft Contact Lens Care Products: Benefits, Complications, and a Comparison to Non-Preserved Solutions. Clin. Optom. 2021, 13, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J. Therapeutic Contact Lenses with Polymeric Vehicles for Ocular Drug Delivery: A Review. Materials 2018, 11, 1125. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Xue, Y.; Hu, G.; Lin, T.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A comprehensive review on contact lens for ophthalmic drug delivery. J. Control. Release 2018, 281, 97–118. [Google Scholar] [CrossRef]
- Cheng, R.; Meng, F.; Deng, C.; Klok, H.A.; Zhong, Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials 2013, 34, 3647–3657. [Google Scholar] [CrossRef]
- Behl, G.; Iqbal, J.; O’Reilly, N.J.; McLoughlin, P.; Fitzhenry, L. Synthesis and Characterization of Poly(2-hydroxyethylmethacrylate) Contact Lenses Containing Chitosan Nanoparticles as an Ocular Delivery System for Dexamethasone Sodium Phosphate. Pharm. Res. 2016, 33, 1638–1648. [Google Scholar] [CrossRef]
- ElShaer, A.; Mustafa, S.; Kasar, M.; Thapa, S.; Ghatora, B.; Alany, R.G. Nanoparticle-Laden Contact Lens for Controlled Ocular Delivery of Prednisolone: Formulation Optimization Using Statistical Experimental Design. Pharmaceutics 2016, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Control. Release 2016, 226, 47–56. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Alvarez-Lorenzo, C.; Silva, M.; Balsa, L.; Couceiro, J.; Torres-Labandeira, J.J.; Concheiro, A. Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery. Biomaterials 2009, 30, 1348–1355. [Google Scholar] [CrossRef]
- Phan, C.M.; Subbaraman, L.N.; Jones, L. In vitro drug release of natamycin from beta-cyclodextrin and 2-hydroxypropyl-beta-cyclodextrin-functionalized contact lens materials. J. Biomater. Sci. Polym. Ed. 2014, 25, 1907–1919. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Yanez, F.; Barreiro-Iglesias, R.; Concheiro, A. Imprinted soft contact lenses as norfloxacin delivery systems. J. Control. Release 2006, 113, 236–244. [Google Scholar] [CrossRef]
- Malaekeh-Nikouei, B.; Vahabzadeh, S.A.; Mohajeri, S.A. Preparation of a molecularly imprinted soft contact lens as a new ocular drug delivery system for dorzolamide. Curr. Drug Deliv. 2013, 10, 279–285. [Google Scholar] [CrossRef]
- Lesher, G.A.; Gunderson, G.G. Continuous drug delivery through the use of disposable contact lenses. Optom. Vis. Sci. 1993, 70, 1012–1018. [Google Scholar] [CrossRef]
- Schrader, S.; Wedel, T.; Moll, R.; Geerling, G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2006, 244, 1345–1349. [Google Scholar] [CrossRef]
- Lee, D.; Cho, S.; Park, H.S.; Kwon, I. Ocular Drug Delivery through pHEMA-Hydrogel Contact Lenses Co-Loaded with Lipophilic Vitamins. Sci. Rep. 2016, 6, 34194. [Google Scholar] [CrossRef]
- Khandelwal, S.S.; Jun, J.J.; Mak, S.; Booth, M.S.; Shekelle, P.G. Effectiveness of multifocal and monofocal intraocular lenses for cataract surgery and lens replacement: A systematic review and meta-analysis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 863–875. [Google Scholar] [CrossRef] [Green Version]
- Güell, J.L. Cataract; Karger: Basel, Switzerland, 2013; 153p. [Google Scholar]
- Shekelle, P.G.; Khandelwal, S.; Jun, J. United StatesDepartment of Veterans AffairsHealth Services, RDevelopment, S.Quality Enhancement Research, I.West Los Angeles, V.A.M.C.E.-B.S.P.CComparative Effectiveness of Multifocal, Accommodative, and Monofocal Intraocular Lenses for Cataract Surgery and Lens Replacement; Department of Veterans Affairs, Veterans Health Administration, Quality Enhancement Research Initiative, Health Services Research & Development Service: Washington, DC, USA, 2018. [Google Scholar]
- Buratto, L.; Brint, S.F.; Boccuzzi, D.; Ebook Central Academic Complete. Cataract Surgery and Intraocular Lenses; Slack Incorporated: Thorofare, NJ, USA, 2014. [Google Scholar]
- Traxler, L.; Bayer, N.; Reutterer, B.; Drauschke, A. Improvement of Optics, Mechanics and the Usability of a Mechanical Eye Model for Vision Quality Evaluation of IOLs. IFAC-PapersOnLine 2015, 48, 1–18. [Google Scholar] [CrossRef]
- Leung, T.G.; Lindsley, K.; Kuo, I.C. Types of intraocular lenses for cataract surgery in eyes with uveitis. Cochrane Database Syst. Rev. 2014, 3, CD007284. [Google Scholar] [CrossRef] [Green Version]
- Wang, X. Intraocular Lens; IntechOpen: London, UK, 2020. [Google Scholar]
- Nguyen, J.; Werner, L. Intraocular Lenses for Cataract Surgery. In Webvision: The Organization of the Retina and Visual System; Kolb, H., Fernandez, E., Nelson, R., Eds.; University of Utah Health Sciences Center: Salt Lake City, UT, USA, 1995. [Google Scholar]
- Werner, L. Intraocular Lenses: Overview of Designs, Materials, and Pathophysiologic Features. Ophthalmology 2021, 128, e74–e93. [Google Scholar] [CrossRef]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Yao, W.; Davidson, R.S.; Durairaj, V.D.; Gelston, C.D. Dry eye syndrome: An update in office management. Am. J. Med. 2011, 124, 1016–1018. [Google Scholar] [CrossRef]
- Ocular surface, d.; Holland, E.J.; Mannis, M.J.; Lee, W.B.; ScienceDirect. Ocular Surface Disease: Cornea, Conjunctiva and Tear Film; Elsevier/Saunders: London, UK; New York, NY, USA, 2013. [Google Scholar]
- Marshall, L.L.; Roach, J.M. Treatment of Dry Eye Disease. Consult. Pharm. 2016, 31, 96–106. [Google Scholar] [CrossRef]
- Bron, A.J.; Tomlinson, A.; Foulks, G.N.; Pepose, J.S.; Baudouin, C.; Geerling, G.; Nichols, K.K.; Lemp, M.A. Rethinking dry eye disease: A perspective on clinical implications. Ocul. Surf. 2014, 12, S1–S31. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, D.F.; Millar, T.J.; Raju, S.R. Tear film stability: A review. Exp. Eye Res. 2013, 117, 28–38. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-Del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II Management and Therapy Report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Petznick, A.; Lee, S.; Tan, J. Choice of artificial tear formulation for patients with dry eye: Where do we start? Cornea 2012, 31 (Suppl. 1), S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; Geerling, G.; Kinoshita, S.; Lemp, M.A.; McCulley, J.P.; Nelson, D.; Novack, G.N.; Shimazaki, J.; Wilson, C. Management and therapy of dry eye disease: Report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 163–178. [Google Scholar] [CrossRef]
- Velpandian, T.; SpringerLink. Pharmacology of Ocular Therapeutics, 1st ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Klenkler, B.; Sheardown, H.; Jones, L. Growth factors in the tear film: Role in tissue maintenance, wound healing, and ocular pathology. Ocul. Surf. 2007, 5, 228–239. [Google Scholar] [CrossRef]
- Lorentz, H.; Jones, L. Lipid deposition on hydrogel contact lenses: How history can help us today. Optom. Vis. Sci. 2007, 84, 286–295. [Google Scholar] [CrossRef]
- Alovisi, C.; Panico, C.; de Sanctis, U.; Eandi, C.M. Vitreous Substitutes: Old and New Materials in Vitreoretinal Surgery. J. Ophthalmol. 2017, 2017, 3172138. [Google Scholar] [CrossRef] [Green Version]
- Binder, P.S. Intracorneal Inlays for the Correction of Presbyopia. Eye Contact Lens 2017, 43, 267–275. [Google Scholar] [CrossRef]
- Fenner, B.J.; Moriyama, A.S.; Mehta, J.S. Inlays and the cornea. Exp. Eye Res. 2021, 205, 108474. [Google Scholar] [CrossRef]
- Kleinberg, T.T.; Tzekov, R.T.; Stein, L.; Ravi, N.; Kaushal, S. Vitreous substitutes: A comprehensive review. Surv. Ophthalmol. 2011, 56, 300–323. [Google Scholar] [CrossRef]
- Gao, Q.Y.; Fu, Y.; Hui, Y.N. Vitreous substitutes: Challenges and directions. Int. J. Ophthalmol. 2015, 8, 437–440. [Google Scholar] [CrossRef]
- Barros, J.A.R.; Melo, L.D.R.; Silva, R.; Ferraz, M.P.; Azeredo, J.; Pinheiro, V.M.C.; Colaco, B.J.A.; Fernandes, M.H.R.; Gomes, P.S.; Monteiro, F.J. Encapsulated bacteriophages in alginate-nanohydroxyapatite hydrogel as a novel delivery system to prevent orthopedic implant-associated infections. Nanomedicine 2020, 24, 102145. [Google Scholar] [CrossRef] [Green Version]
- Leone, G.; Consumi, M.; Aggravi, M.; Donati, A.; Lamponi, S.; Magnani, A. PVA/STMP based hydrogels as potential substitutes of human vitreous. J. Mater. Sci. Mater. Med. 2010, 21, 2491–2500. [Google Scholar] [CrossRef]
- Swindle-Reilly, K.E.; Shah, M.; Hamilton, P.D.; Eskin, T.A.; Kaushal, S.; Ravi, N. Rabbit study of an in situ forming hydrogel vitreous substitute. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4840–4846. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Tong, X.; Zhang, Y.; Lai, J.; Huang, Y.; Jiang, Y.R.; Guo, B.H. Evaluation of an in situ chemically crosslinked hydrogel as a long-term vitreous substitute material. Acta Biomater. 2013, 9, 5022–5030. [Google Scholar] [CrossRef] [PubMed]
- Schramm, C.; Spitzer, M.S.; Henke-Fahle, S.; Steinmetz, G.; Januschowski, K.; Heiduschka, P.; Geis-Gerstorfer, J.; Biedermann, T.; Bartz-Schmidt, K.U.; Szurman, P. The cross-linked biopolymer hyaluronic acid as an artificial vitreous substitute. Investig. Ophthalmol. Vis. Sci. 2012, 53, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Su, W.Y.; Chen, K.H.; Chen, Y.C.; Lee, Y.H.; Tseng, C.L.; Lin, F.H. An injectable oxidated hyaluronic acid/adipic acid dihydrazide hydrogel as a vitreous substitute. J. Biomater. Sci. Polym. Ed. 2011, 22, 1777–1797. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Nishitsuka, K.; Takamura, H.; Yamamoto, T.; Yamashita, H. Cloning and characterization of human vitreous tissue-derived cells. Acta Ophthalmol. 2011, 89, 538–543. [Google Scholar] [CrossRef]
- McMahon, T.T.; Zadnik, K. Twenty-five years of contact lenses: The impact on the cornea and ophthalmic practice. Cornea 2000, 19, 730–740. [Google Scholar] [CrossRef]
- Alio, J.L.; Belda, J.I.; Artola, A.; Garcia-Lledo, M.; Osman, A. Contact lens fitting to correct irregular astigmatism after corneal refractive surgery. J. Cataract Refract. Surg. 2002, 28, 1750–1757. [Google Scholar] [CrossRef]
- Wang, J.; Fonn, D.; Simpson, T.L. Topographical thickness of the epithelium and total cornea after hydrogel and PMMA contact lens wear with eye closure. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1070–1074. [Google Scholar] [CrossRef] [Green Version]
- Thean, J.H.; McNab, A.A. Blepharoptosis in RGP and PMMA hard contact lens wearers. Clin. Exp. Optom. 2004, 87, 11–14. [Google Scholar] [CrossRef]
- Subbaraman, L.N.; Glasier, M.A.; Senchyna, M.; Sheardown, H.; Jones, L. Kinetics of in vitro lysozyme deposition on silicone hydrogel, PMMA, and FDA groups I, II, and IV contact lens materials. Curr. Eye Res. 2006, 31, 787–796. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, S.Y.; Sung, A.Y. Poly (Ethylene Glycol) Methyl Ether Methacrylate-Based Hydrogel and Cerium(IV) Oxide Nanoparticles as Ophthalmic Lens Material. Micromachines 2021, 12, 1111. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Zhang, X.; Sheng, R.; Lin, Q.; Song, W.; Hao, L. Novel Contact Lenses Embedded with Drug-Loaded Zwitterionic Nanogels for Extended Ophthalmic Drug Delivery. Nanomaterials 2021, 11, 2328. [Google Scholar] [CrossRef]
- Zare, M.; Bigham, A.; Zare, M.; Luo, H.; Rezvani Ghomi, E.; Ramakrishna, S. pHEMA: An Overview for Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 6376. [Google Scholar] [CrossRef]
- Maissa, C.; Guillon, M.; Cockshott, N.; Garofalo, R.J.; Lemp, J.M.; Boclair, J.W. Contact Lens Lipid Spoliation of Hydrogel and Silicone Hydrogel Lenses. Optom. Vis. Sci. 2014, 91, 1071–1083. [Google Scholar] [CrossRef]
- Dogru, M.; Ward, S.K.; Wakamatsu, T.; Ibrahim, O.; Schnider, C.; Kojima, T.; Matsumoto, Y.; Ogawa, J.; Shimazaki, J.; Tsubota, K. The effects of 2 week senofilcon-A silicone hydrogel contact lens daily wear on tear functions and ocular surface health status. Contact Lens Anterior Eye 2011, 34, 77–82. [Google Scholar] [CrossRef]
- Chalmers, R. Overview of factors that affect comfort with modern soft contact lenses. Contact Lens Anterior Eye 2014, 37, 65–76. [Google Scholar] [CrossRef]
- Sulley, A.; Young, G.; Hunt, C. Factors in the success of new contact lens wearers. Contact Lens Anterior Eye 2017, 40, 15–24. [Google Scholar] [CrossRef]
- Tasci, Y.Y.; Gurdal, C.; Sarac, O.; Onusever, A. Evaluation of the Tear Function Tests and the Ocular Surface in First-Time Users of Silicone Hydrogel Contact Lenses. Curr. Eye Res. 2017, 42, 976–981. [Google Scholar] [CrossRef]
- Hyon, S.H.; Cha, W.I.; Ikada, Y.; Kita, M.; Ogura, Y.; Honda, Y. Poly(vinyl alcohol) hydrogels as soft contact lens material. J. Biomater. Sci. Polym. Ed. 1994, 5, 397–406. [Google Scholar] [CrossRef]
- Phan, C.M.; Walther, H.; Riederer, D.; Lau, C.; Lorenz, K.O.; Subbaraman, L.N.; Jones, L. Analysis of polyvinyl alcohol release from commercially available daily disposable contact lenses using an in vitro eye model. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Daza, J.H.U.; Righetto, G.M.; Chaud, M.V.; da Conceicao Amaro Martins, V.; Lopes Baratella da Cunha Camargo, I.; Maria de Guzzi Plepis, A. PVA/anionic collagen membranes as drug carriers of ciprofloxacin hydrochloride with sustained antibacterial activity and potential use in the treatment of ulcerative keratitis. J. Biomater. Appl. 2020, 35, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.J.; Zhang, S.T.; Pu, J.H.; Zhao, X.; Ke, K.; Bao, R.Y.; Bai, L.; Liu, Z.Y.; Yang, M.B.; Yang, W. Nanofibrillar Poly(vinyl alcohol) Ionic Organohydrogels for Smart Contact Lens and Human-Interactive Sensing. ACS Appl. Mater. Interfaces 2020, 12, 23514–23522. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Chauhan, A.; Rajshekar, K.; Vashist, P.; Gupta, P.; Mathur, U.; Gupta, N.; Gupta, V.; Dutta, P.; Gauba, V.K. Generic and vision related quality of life associated with different types of cataract surgeries and different types of intraocular lens implantation. PLoS ONE 2020, 15, e0240036. [Google Scholar] [CrossRef] [PubMed]
- Maedel, S.; Evans, J.R.; Harrer-Seely, A.; Findl, O. Intraocular lens optic edge design for the prevention of posterior capsule opacification after cataract surgery. Cochrane Database Syst. Rev. 2021, 8, CD012516. [Google Scholar] [CrossRef]
- Shiraki, A.; Sakimoto, S.; Oie, Y.; Soma, T.; Miki, A.; Usui, S.; Sato, S.; Matsushita, K.; Sakaguchi, H.; Nishida, K. Inferior Removal of Dislocated Polymethyl Methacrylate Intraocular Lens and Scleral Refixation in Glaucomatous Eyes. Ophthalmol. Ther. 2022, 11, 881–886. [Google Scholar] [CrossRef]
- Tetz, M.; Jorgensen, M.R. New Hydrophobic IOL Materials and Understanding the Science of Glistenings. Curr. Eye Res. 2015, 40, 969–981. [Google Scholar] [CrossRef]
- Huang, J.F.; Zhong, J.; Chen, G.P.; Lin, Z.T.; Deng, Y.; Liu, Y.L.; Cao, P.Y.; Wang, B.; Wei, Y.; Wu, T.; et al. A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Serro, A.P.; Colaco, R.; Chauhan, A. Optimization of intraocular lens hydrogels for dual drug release: Experimentation and modelling. Eur. J. Pharm. Biopharm. 2019, 141, 51–57. [Google Scholar] [CrossRef]
- Jaitli, A.; Roy, J.; Chatila, A.; Liao, J.; Tang, L. Effect of time and temperature-dependent changes of IOL material properties on IOL: Lens capsule interactions. Exp. Eye Res. 2021, 211, 108726. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xie, C.; Wang, Y.; Shen, Y. Full-vision maintenance in extra-high myopia from implantable collamer lens to trifocal intraocular lens implantation. Int. J. Ophthalmol. 2018, 11, 1239–1242. [Google Scholar] [CrossRef]
- Tychsen, L.; Reynolds, M.; Hoekel, J. Reply: Safety of phakic intraocular collamer lens implantation in 95 highly myopic special-needs children. J. Cataract Refract. Surg. 2021, 47, 1606–1607. [Google Scholar] [CrossRef]
- Pinto, C.; Monteiro, T.; Franqueira, N.; Faria-Correia, F.; Mendes, J.; Vaz, F. Posterior chamber collamer phakic intraocular lens implantation: Comparison of efficacy and safety for low and moderate-to-high myopia. Eur. J. Ophthalmol. 2022, 32, 894–901.e1451. [Google Scholar] [CrossRef]
- Mulet, M.E.; Alio, J.L.; Knorz, M.C. Hydrogel intracorneal inlays for the correction of hyperopia: Outcomes and complications after 5 years of follow-up. Ophthalmology 2009, 116, 1455–1460. [Google Scholar] [CrossRef]
- Casini, G.; Loiudice, P.; De Cilla, S.; Radice, P.; Nardi, M. Sulfur hexafluoride (SF6) versus perfluoropropane (C3F8) tamponade and short term face-down position for macular hole repair: A randomized prospective study. Int. J. Retin. Vitr. 2016, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Liu, K.; Su, L.; Xia, X.; Xu, X. Perfluorocarbon liquid: Its application in vitreoretinal surgery and related ocular inflammation. Biomed. Res. Int. 2014, 2014, 250323. [Google Scholar] [CrossRef] [Green Version]
- Thacker, M.; Tseng, C.L.; Lin, F.H. Substitutes and Colloidal System for Vitreous Replacement and Drug Delivery: Recent Progress and Future Prospective. Polymers 2020, 13, 121. [Google Scholar] [CrossRef]
- Pruett, R.C.; Schepens, C.L.; Swann, D.A. Hyaluronic-Acid Vitreous Substitute—6-Year Clinical-Evaluation. Arch. Ophthalmol. 1979, 97, 2325–2330. [Google Scholar] [CrossRef]
- Barth, H.; Crafoord, S.W.; Vinchon, C.; Andreasson, S.; Ghosh, F.K. A cross-linked hyaluronic acid hydrogel (Healaflow (R)) as a potential vitreous substitute. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2338. [Google Scholar]
- Barth, H.; Crafoord, S.; Andreasson, S.; Ghosh, F. A cross-linked hyaluronic acid hydrogel (Healaflow((R))) as a novel vitreous substitute. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 697–703. [Google Scholar] [CrossRef]
- Schulz, A.; Rickmann, A.; Wahl, S.; Germann, A.; Stanzel, B.V.; Januschowski, K.; Szurman, P. Alginate- and Hyaluronic Acid-Based Hydrogels as Vitreous Substitutes: An In Vitro Evaluation. Transl. Vis. Sci. Technol. 2020, 9, 34. [Google Scholar] [CrossRef]
- Wang, S.; Chi, J.H.; Jiang, Z.W.; Hu, H.W.; Yang, C.Z.; Liu, W.S.; Han, B.Q. A self-healing and injectable hydrogel based on water-soluble chitosan and hyaluronic acid for vitreous substitute. Carbohydr. Polym. 2021, 256, 117519. [Google Scholar] [CrossRef]
- Yu, S.Q.; Wang, S.; Xia, L.X.; Hu, H.W.; Zou, M.Y.; Jiang, Z.W.; Chi, J.H.; Zhang, Y.J.; Li, H.J.; Yang, C.Z.; et al. Injectable self-crosslinking hydrogels based on hyaluronic acid as vitreous substitutes. Int. J. Biol. Macromol. 2022, 208, 159–171. [Google Scholar] [CrossRef]
- Greco, G.; Lamponi, S.; Leone, G.; Consumi, M.; Magnani, A. In Vitro Biocompatibility of New Hydrogels as Vitreous Body Substitutes. Eur. J. Ophthalmol. 2017, 27, E97. [Google Scholar]
- Lin, K.T.; Wang, A.; Nguyen, A.B.; Iyer, J.; Tran, S.D. Recent Advances in Hydrogels: Ophthalmic Applications in Cell Delivery, Vitreous Substitutes, and Ocular Adhesives. Biomedicines 2021, 9, 1203. [Google Scholar] [CrossRef]
- Stern, J.H.; Tian, Y.; Funderburgh, J.; Pellegrini, G.; Zhang, K.; Goldberg, J.L.; Ali, R.R.; Young, M.; Xie, Y.; Temple, S. Regenerating Eye Tissues to Preserve and Restore Vision. Cell Stem Cell 2018, 23, 453. [Google Scholar] [CrossRef] [PubMed]
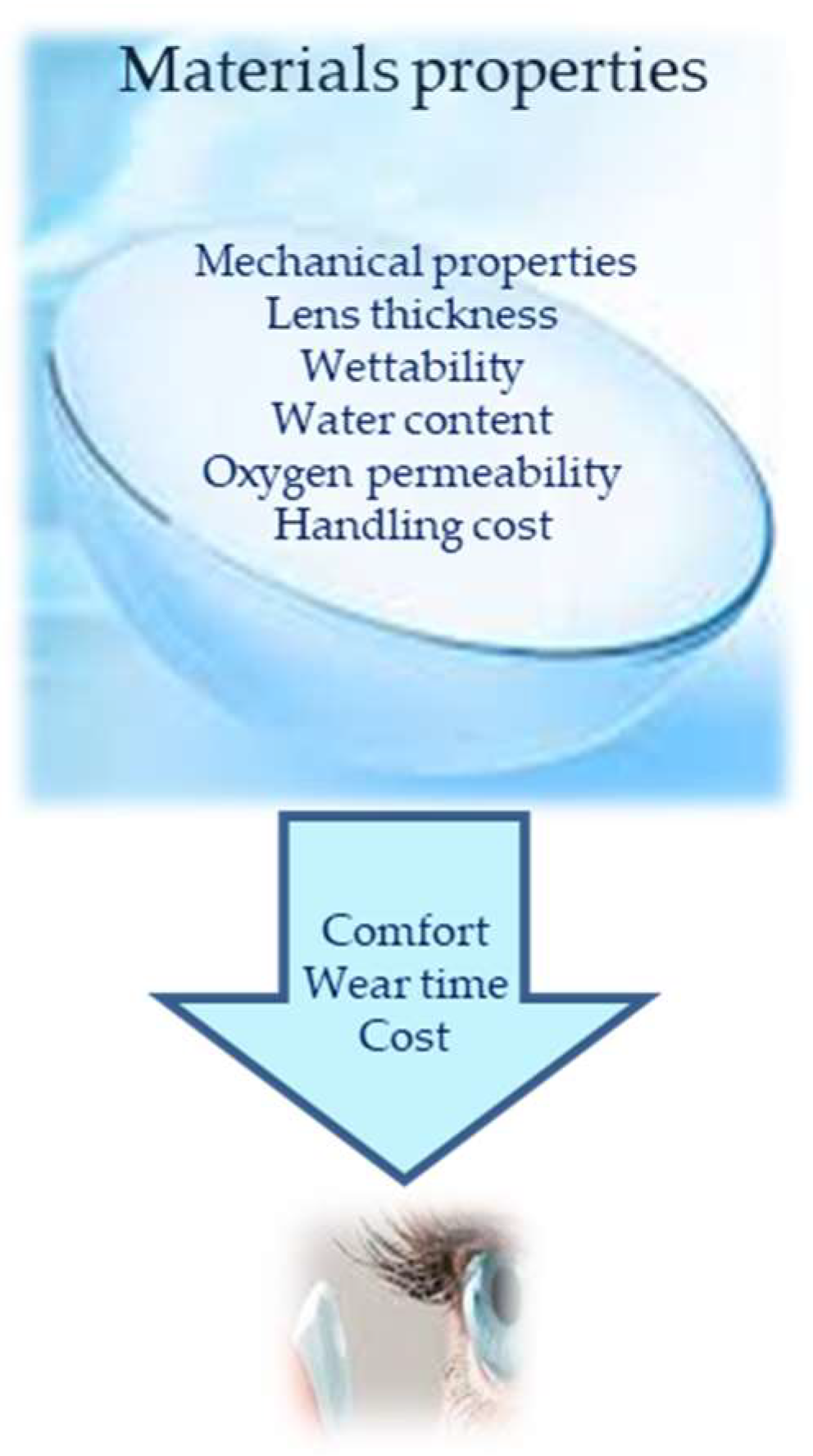
| Biomaterial | Disadvantages | Advantages |
|---|---|---|
| Poly(vinyl alcohol) (PVA) | Low permeability to oxygen; fixed water contact | Low cost; biocompatible; easy manufacturing |
| Silicon hydrogel | Expensive; abrasive behavior | High permeability to oxygen; high durability |
| Hydroxy ethyl methacrylate (HEMA) hydrogel | Low permeability to oxygen; protein deposition problems | Low cost; biocompatible; several copolymer possibilities |
| Polymethyl methacrylate (PMMA) | Impermeable to oxygen; not flexible in the eyes; abrasive behavior | Low cost; well-studied polymer |
| Component | Main Function | References |
|---|---|---|
| Viscosity-improving agents (Carbomer 940, Carboxymethyl cellulose (CMC), Hyaluronic acid (HA), Hydroxypropylme-thylcellulose (HPMC), polyvinylpyrrolidone (PVP)) | Increase the time of permanence in the eye due to their mucoadhesive properties | [81,86] |
| Osmoprotectors | Maintaining normal cellular metabolism, even under extreme osmotic stress | [71,79] |
| Trehalose | Bioprotection and osmoprotection | [71,72,77,79] |
| Quercetin, epigallocatechin gallate, n-propyl gallate and gallic acid | Protecting the corneal epithelium from oxidative damage | [75,79] |
| Benzalkonium chloride (BAC), Sodium perborate, sodium chlorite, Polyquaternium-1 | Preservatives | [75,79] |
| Clinical Application | Material | References |
|---|---|---|
| Contact lenses | PMMA | [98,99,100,101,102] |
| HEMA hydrogel | [37,103,104,105] | |
| Silicone hydrogel | [106,107,108,109,110] | |
| PVA | [111,112,113,114] | |
| Intraocular lenses | PMMA | [115,116,117] |
| Hydrophobic acrylate polymers | [70,118] | |
| Hydrophilic acrylate polymers | [119,120,121] | |
| Siloxanes | [70,104,122] | |
| Collamer | [123,124,125] | |
| Inlays | Hydrophilic hydrogels | [126] |
| Vitreous substitutes | Gas (air, sulfur hexafluoride (SF6) and perfluoropropane (C3F8) | [14,127] |
| Liquid (physiological solution, perfluorocarbon fluids (PFCL), semi-fluorinated alkanes (SFA) | [128,129] | |
| Natural and semi-synthetic polymers (hyaluronic acid and chitosan, silicone oil) | [130,131,132,133,134,135] | |
| Hydrogels | [135,136,137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, M.P. Biomaterials for Ophthalmic Applications. Appl. Sci. 2022, 12, 5886. https://doi.org/10.3390/app12125886
Ferraz MP. Biomaterials for Ophthalmic Applications. Applied Sciences. 2022; 12(12):5886. https://doi.org/10.3390/app12125886
Chicago/Turabian StyleFerraz, Maria Pia. 2022. "Biomaterials for Ophthalmic Applications" Applied Sciences 12, no. 12: 5886. https://doi.org/10.3390/app12125886
APA StyleFerraz, M. P. (2022). Biomaterials for Ophthalmic Applications. Applied Sciences, 12(12), 5886. https://doi.org/10.3390/app12125886






