A Molecular View on Biomaterials and Dental Stem Cells Interactions: Literature Review
Abstract
1. Introduction
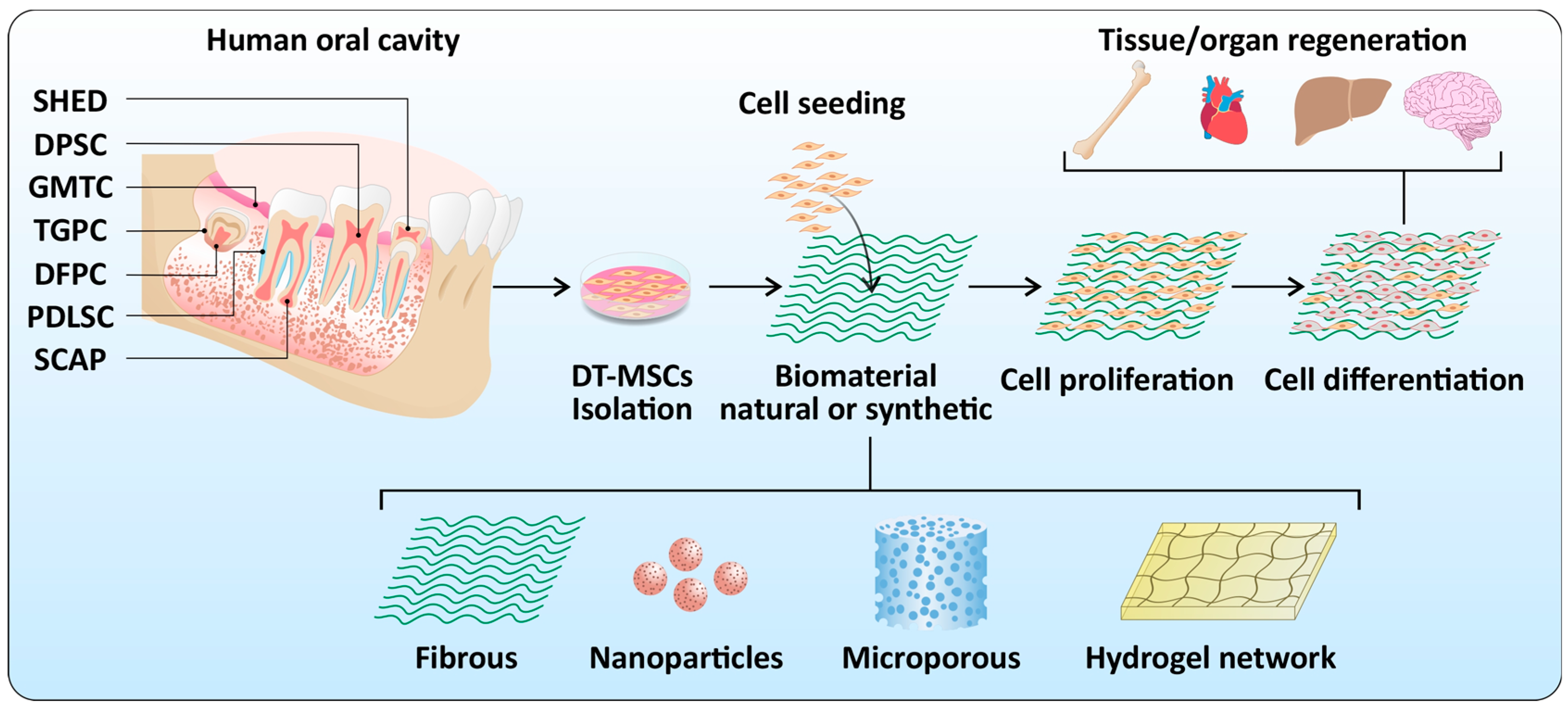
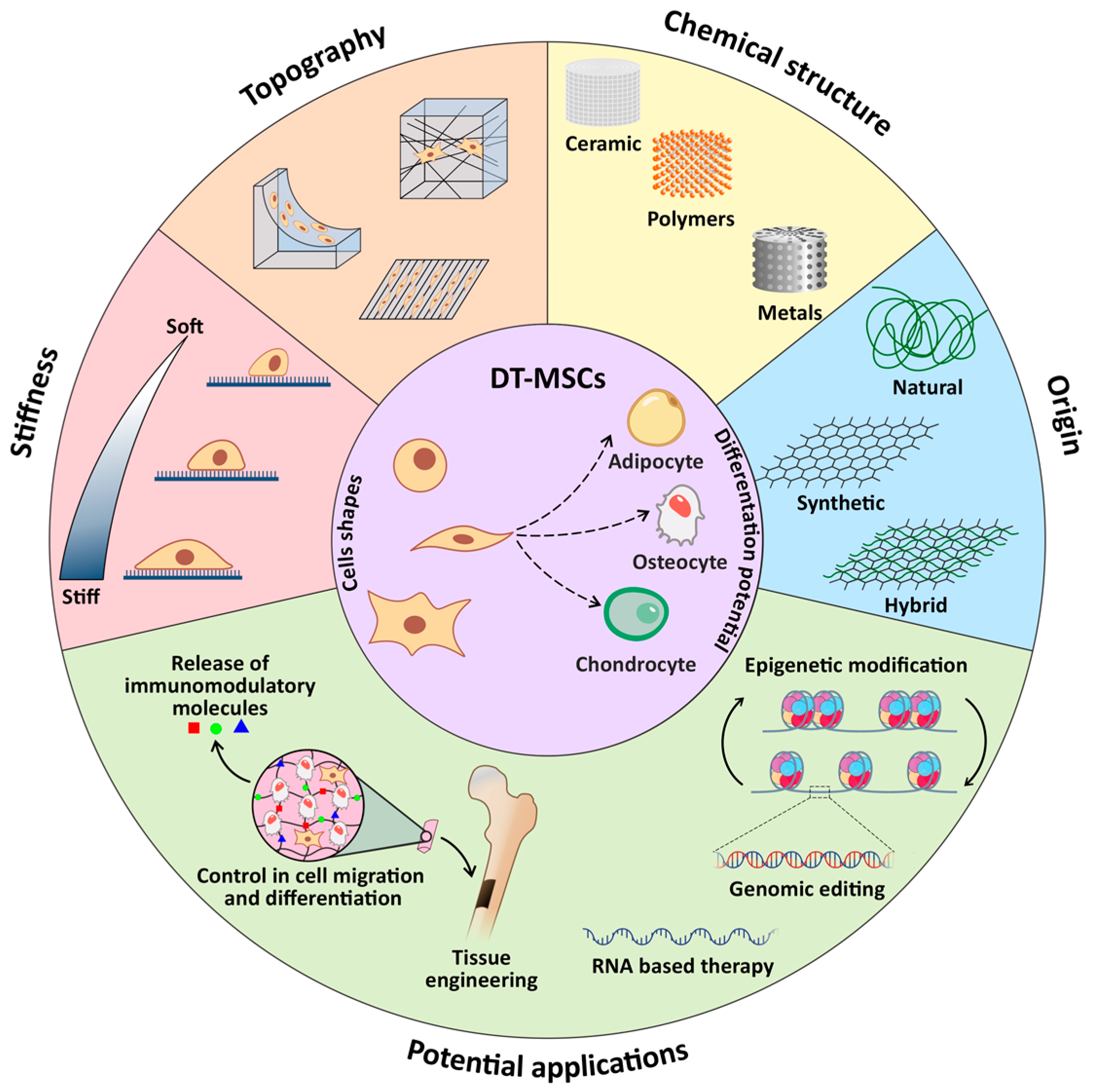
2. Dental Stem Cells
3. Biomaterials
3.1. Natural Biomaterials
3.2. Synthetic Biomaterials
4. Dental Stem Cells and Biomaterial Interactions
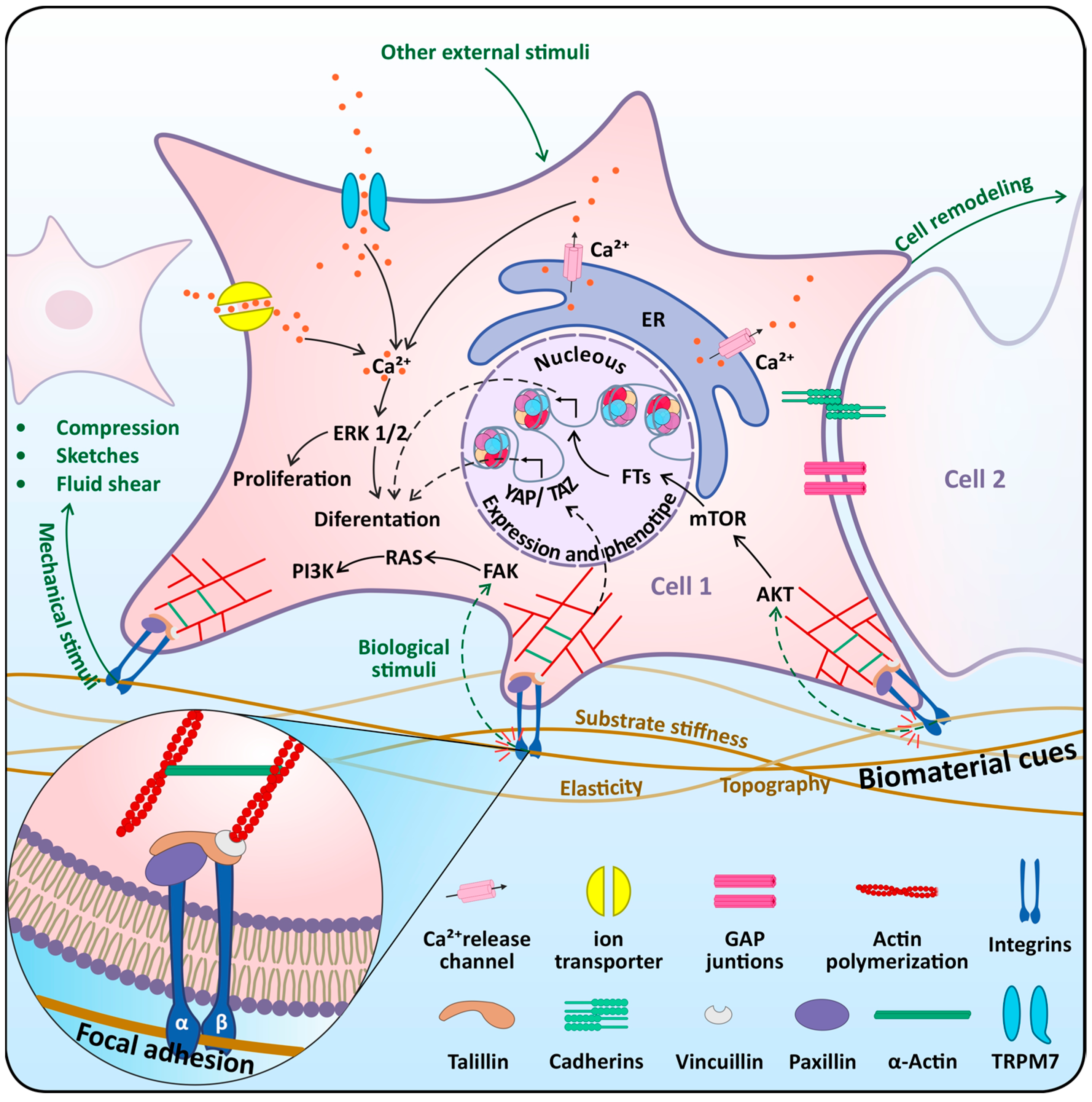
4.1. Mechasensors in Dental Stem Cells
4.2. Mechanotransduction Pathways in Dental Stem Cells
5. Conclusions
- Design and develop of smart biomaterials that favor the proliferation and differentiation of DT-MSCs on a large scale.
- Integrate multi-omic tools would allow a global perspective of the interactions between cells and biomaterials at the genomic, proteomic, and metabolomic levels.
- Delivery into the mechanogenomic field to facilitate the design of highly functionalized biomaterials and the epigenetic manipulation that can be performed to control the fate of DT-MSCs for their application in regenerative medicine.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodas-Junco, B.A.; Canul-Chan, M.; Rojas-Herrera, R.A.; De-la-Peña, C.; Nic-Can, G.I. Stem cells from dental pulp: What epigenetics can do with your tooth. Front. Physiol. 2017, 8, 999. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Meta-biomaterials. Biomater. Sci. 2020, 8, 18–38. [Google Scholar] [CrossRef] [PubMed]
- Granz, C.L.; Gorji, A. Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies. World J. Stem Cells 2020, 12, 897. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, W.; Dobrzyński, M.; Szymonowicz, M.; Rybak, Z. Stem cells: Past, present, and future. Stem Cell Res. Ther. 2019, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.G. Pluripotent and multipotent stem cells and current therapeutic applications. Stem Cells Cloning Adv. Appl. 2021, 14, 3. [Google Scholar] [CrossRef]
- Sharpe, P.T. Dental mesenchymal stem cells. Development 2016, 143, 2273–2280. [Google Scholar] [CrossRef]
- Hussein, A.M.; Darwish, Z.E.; Raslan, H.S.; Attia, M.A.; Abdel-Hamid, H.M. Dental stem cells (concepts and applications). Alex. Dent. J. 2021, 46, 66–71. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Bueno, C.; Insausti, C.L.; Meseguer, L.; Ramirez, M.C.; Blanquer, M.; Marin, N.; Martínez, S.; Moraleda, J.M. Mesenchymal stem cells derived from dental tissues. Int. Endod. J. 2011, 44, 800–806. [Google Scholar] [CrossRef]
- Lan, X.; Sun, Z.; Chu, C.; Boltze, J.; Li, S. Dental pulp stem cells: An attractive alternative for cell therapy in ischemic stroke. Front. Neurol. 2019, 10, 824. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry–part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef]
- Mercado-Rubio, M.D.; Pérez-Argueta, E.; Zepeda-Pedreguera, A.; Aguilar-Ayala, F.J.; Peñaloza-Cuevas, R.; Kú-González, A.; Rojas-Herrera, R.A.; Rodas-Junco, B.A.; Nic-Can, G.I. Similar Features, Different Behaviors: A Comparative In Vitro Study of the Adipogenic Potential of Stem Cells from Human Follicle, Dental Pulp, and Periodontal Ligament. J. Pers. Med. 2021, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Kumar, A.; Priyadharshini, A.; Oggu, G.S.; Bhatnagar, I.; Srivastava, A.; Chandra, P. Chitosan: An undisputed bio-fabrication material for tissue engineering and bio-sensing applications. Int. J. Biol. Macromol. 2018, 110, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Zalduendo, M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018, 20, 479–498. [Google Scholar] [CrossRef]
- Martino, S.; D’Angelo, F.; Armentano, I.; Kenny, J.M.; Orlacchio, A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012, 30, 338–351. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef]
- Lambrichts, I.; Driesen, R.B.; Dillen, Y.; Gervois, P.; Ratajczak, J.; Vangansewinkel, T.; Wolfs, E.; Bronckaers, A.; Hilkens, P. Dental Pulp Stem Cells: Their Potential in Reinnervation and Angiogenesis by Using Scaffolds. J. Endod. 2017, 43, S12–S16. [Google Scholar] [CrossRef]
- Asghari Sana, F.; Çapkın Yurtsever, M.; Kaynak Bayrak, G.; Tunçay, E.Ö.; Kiremitçi, A.S.; Gümüşderelioğlu, M. Spreading, proliferation and differentiation of human dental pulp stem cells on chitosan scaffolds immobilized with RGD or fibronectin. Cytotechnology 2017, 69, 617–630. [Google Scholar] [CrossRef]
- Pecci-Lloret, M.P.; Vera-Sánchez, M.; Aznar-Cervantes, S.; García-Bernal, D.; Sánchez, R.O.; Pecci-Lloret, M.R.; Moraleda, J.M.; Cenis, J.L.; Rodríguez-Lozano, F.J. Analysis of the Adherence of Dental Pulp Stem Cells on Two-Dimensional and Three-Dimensional Silk Fibroin-Based Biomaterials. J. Craniofac. Surg. 2017, 28, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Toldrà, R.; Martínez-Sarrà, E.; Gil-Recio, C.; Carrasco, M.Á.; Al Madhoun, A.; Montori, S.; Atari, M. Dental pulp pluripotent-like stem cells (DPPSC), a new stem cell population with chromosomal stability and osteogenic capacity for biomaterials evaluation. BMC Cell Biol. 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Flynn, N. Dental pulp stem cell-derived chondrogenic cells demonstrate differential cell motility in type I and type II collagen hydrogels. Spine J. 2018, 18, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Louvrier, A.; Euvrard, E.; Nicod, L.; Rolin, G.; Gindraux, F.; Pazart, L.; Houdayer, C.; Risold, P.Y.; Meyer, F.; Meyer, C. Odontoblastic differentiation of dental pulp stem cells from healthy and carious teeth on an original PCL-based 3D scaffold. Int. Endod. J. 2018, 51 (Suppl. S4), e252–e263. [Google Scholar] [CrossRef]
- Soancă, A.; Lupse, M.; Moldovan, M.; Pall, E.; Cenariu, M.; Roman, A.; Tudoran, O.; Surlin, P.; Șorițău, O. Applications of inflammation-derived gingival stem cells for testing the biocompatibility of dental restorative biomaterials. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2018, 218, 28–39. [Google Scholar] [CrossRef]
- Saberi, E.; Farhad-Mollashahi, N.; Sargolzaei Aval, F.; Saberi, M. Proliferation, odontogenic/osteogenic differentiation, and cytokine production by human stem cells of the apical papilla induced by biomaterials: A comparative study. Clin. Cosmet. Investig. Dent. 2019, 11, 181–193. [Google Scholar] [CrossRef]
- Parthiban, S.P.; He, W.; Monteiro, N.; Athirasala, A.; França, C.M.; Bertassoni, L.E. Engineering pericyte-supported microvascular capillaries in cell-laden hydrogels using stem cells from the bone marrow, dental pulp and dental apical papilla. Sci. Rep. 2020, 10, 21579. [Google Scholar] [CrossRef]
- Mansouri, N.; Al-Sarawi, S.; Losic, D.; Mazumdar, J.; Clark, J.; Gronthos, S.; O’Hare Doig, R. Biodegradable and biocompatible graphene-based scaffolds for functional neural tissue engineering: A strategy approach using dental pulp stem cells and biomaterials. Biotechnol. Bioeng. 2021, 118, 4217–4230. [Google Scholar] [CrossRef]
- Birant, S.; Gokalp, M.; Duran, Y.; Koruyucu, M.; Akkoc, T.; Seymen, F. Cytotoxicity of NeoMTA Plus, ProRoot MTA and Biodentine on human dental pulp stem cells. J. Dent. Sci. 2021, 16, 971–979. [Google Scholar] [CrossRef]
- Gu, Y.; Xie, X.; Zhuang, R.; Weir, M.D.; Oates, T.W.; Bai, Y.; Zhao, L.; Xu, H.H.K. A Biphasic Calcium Phosphate Cement Enhances Dentin Regeneration by Dental Pulp Stem Cells and Promotes Macrophages M2 Phenotype In Vitro. Tissue Eng. Part A 2021, 27, 1113–1127. [Google Scholar] [CrossRef]
- Vagropoulou, G.; Trentsiou, M.; Georgopoulou, A.; Papachristou, E.; Prymak, O.; Kritis, A.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A.; Koidis, P. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. 2021, 37, e23–e36. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.N.; Yazid, F.; Luchman, N.A.; Ariffin, S.H.Z.; Wahab, R.M.A. Comparative evaluation of osteogenic differentiation potential of stem cells derived from dental pulp and exfoliated deciduous teeth cultured over granular hydroxyapatite based scaffold. BMC Oral Health 2021, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Gendviliene, I.; Simoliunas, E.; Alksne, M.; Dibart, S.; Jasiuniene, E.; Cicenas, V.; Jacobs, R.; Bukelskiene, V.; Rutkunas, V. Effect of extracellular matrix and dental pulp stem cells on bone regeneration with 3D printed PLA/HA composite scaffolds. Eur. Cell. Mater. 2021, 41, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, C.-J.; Zhang, S.; Ma, X.-C.; Xiao, R.; Liu, H.-C. Effects of rhBMP-2 on Bone Formation Capacity of Rat Dental Stem/Progenitor Cells from Dental Follicle and Alveolar Bone Marrow. Stem Cells Dev. 2021, 30, 441–457. [Google Scholar] [CrossRef]
- Atila, D.; Hasirci, V.; Tezcaner, A. Coaxial electrospinning of composite mats comprised of core/shell poly(methyl methacrylate)/silk fibroin fibers for tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2022, 128, 105105. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, X.; Zhang, L.; Wang, H.; Zhang, T.; Bai, R.; Sun, Q.; Wang, X.; Yu, T.; Wu, D.; et al. Facile fabrication of a biocompatible composite gel with sustained release of aspirin for bone regeneration. Bioact. Mater. 2022, 11, 130–139. [Google Scholar] [CrossRef]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.-C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Alam, M.; Yazdanian, M.; Tebyanian, H.; Yazdanian, A.; Seifalian, A.; Mosaddad, S.A. Current biocompatible materials in oral regeneration: A comprehensive overview of composite materials. J. Mater. Res. Technol. 2020, 9, 11731–11755. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Fan, M. BioAggregate and iR oot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int. Endod. J. 2013, 46, 923–929. [Google Scholar] [CrossRef]
- Khan, R.; Khan, M.H. Use of collagen as a biomaterial: An update. J. Indian Soc. Periodontol. 2013, 17, 539–542. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Prescott, R.S.; Alsanea, R.; Fayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; John, A.S.; George, A. In vivo generation of dental pulp-like tissue by using dental pulp stem cells, a collagen scaffold, and dentin matrix protein 1 after subcutaneous transplantation in mice. J. Endod. 2008, 34, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Almushayt, A.; Narayanan, K.; Zaki, A.E.; George, A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006, 13, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chen, Q.; Shi, C.; Chen, M.; Ma, K.; Wan, J.; Liu, R. Dealing with the Foreign-Body Response to Implanted Biomaterials: Strategies and Applications of New Materials. Adv. Funct. Mater. 2021, 31, 2007226. [Google Scholar] [CrossRef]
- Husain, S.; Al-Samadani, K.H.; Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Qasim, S.B. Chitosan biomaterials for current and potential dental applications. Materials 2017, 10, 602. [Google Scholar] [CrossRef]
- Kamal, A.; Khalil, E. Assessment of Human Dental Pulp Stem Cells with Chitosan Scaffold Versus Xenografts in Implant Osseointegration. An Experimental Study in a Rabbit Model. Egypt. Dent. J. 2018, 64, 3499–3509. [Google Scholar] [CrossRef]
- Park, C.H.; Woo, K.M. Fibrin-Based Biomaterial Applications in Tissue Engineering and Regenerative Medicine. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Noh, I., Ed.; Springer: Singapore, 2018; Volume 1064, pp. 253–261. ISBN 978-981-13-0445-3. [Google Scholar]
- Ducret, M.; Costantini, A.; Gobert, S.; Farges, J.C.; Bekhouche, M. Fibrin-based scaffolds for dental pulp regeneration: From biology to nanotherapeutics. Eur. Cells Mater. 2021, 41, 1–14. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.C.; Verron, E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed]
- Bolhari, B.; Meraji, N.; Ghorbanzadeh, A.; Sarraf, P.; Moayeri, R. Applications of fibrin-based products in endodontics: A literature review. Dent. Hypotheses 2019, 10, 85. [Google Scholar]
- Ahmed, T.A.E.; Dare, E.V.; Hincke, M. Fibrin: A versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008, 14, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; El Moshy, S.; Radwan, I.A.; Rady, D.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Hydrogels and dentin–pulp complex regeneration: From the benchtop to clinical translation. Polymers 2020, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Shah, N.N.; Smith, C.P.; Rameshwar, P. 3D bioprinting and stem cells. In Somatic Stem Cells; Springer: Berlin/Heidelberg, Germany, 2018; pp. 93–103. [Google Scholar]
- Skeldon, G.; Lucendo-Villarin, B.; Shu, W. Three-dimensional bioprinting of stem-cell derived tissues for human regenerative medicine. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170224. [Google Scholar] [CrossRef]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.R.; Kang, H.W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [CrossRef]
- Enukashvily, N.I.; Dombrovskaya, J.A.; Kotova, A.V.; Semenova, N.; Karabak, I.; Banashkov, R.E.; Baram, D.; Paderina, T.; Bilyk, S.S.; Grimm, W.-D. Fibrin Glue Implants Seeded with Dental Pulp and Periodontal Ligament Stem Cells for the Repair of Periodontal Bone Defects: A Preclinical Study. Bioengineering 2021, 8, 75. [Google Scholar] [CrossRef]
- Singh, A.; Elisseeff, J. Biomaterials for stem cell differentiation. J. Mater. Chem. 2010, 20, 8832–8847. [Google Scholar] [CrossRef]
- Samavedi, S.; Poindexter, L.K.; Van Dyke, M.; Goldstein, A.S. Synthetic Biomaterials for Regenerative Medicine Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2014; ISBN 9780123985231. [Google Scholar]
- Kim, J.K.; Kim, H.J.; Chung, J.Y.; Lee, J.H.; Young, S.B.; Kim, Y.H. Natural and synthetic biomaterials for controlled drug delivery. Arch. Pharm. Res. 2014, 37, 60–68. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Aguilar-Pérez, F.J.; Vargas-Coronado, R.F.; Cervantes-Uc, J.M.; Cauich-Rodríguez, J.V.; Rosales-Ibañez, R.; Rodríguez-Ortiz, J.A.; Torres-Hernández, Y. Titanium—Castor oil based polyurethane composite foams for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1415–1432. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.K.; Shinaishin, S.F.; Eldeen, G.N.; Aly, R.M. Nano hydroxyapatite & mineral trioxide aggregate efficiently promote odontogenic differentiation of dental pulp stem cells. Open Access Maced. J. Med. Sci. 2018, 6, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, I.R.; Kim, Y.; Kim, D.H.; Park, S.B.; Park, B.S.; Bae, M.K.; Kim, Y. Il The effect of mesoporous bioactive glass nanoparticles/graphene oxide composites on the differentiation and mineralization of human dental pulp stem cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef]
- Corral Nunez, C.; Altamirano Gaete, D.; Maureira, M.; Martin, J.; Covarrubias, C. Nanoparticles of bioactive glass enhance biodentine bioactivity on dental pulp stem cells. Materials 2021, 14, 2684. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dang, M.; Zhang, Z.; Hu, J.; Eyster, T.W.; Ni, L.; Ma, P.X. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016, 36, 63–72. [Google Scholar] [CrossRef]
- Jamal, M.; Greish, Y.; Chogle, S.; Goodis, H.; Karam, S.M. Growth and Differentiation of Dental Stem Cells of Apical Papilla on Polycaprolactone Scaffolds. Adv. Exp. Med. Biol. 2018, 1077, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Alipour, M.; Aghazadeh, M.; Akbarzadeh, A.; Vafajoo, Z.; Aghazadeh, Z.; Raeisdasteh Hokmabad, V. Towards osteogenic differentiation of human dental pulp stem cells on PCL-PEG-PCL/zeolite nanofibrous scaffolds. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3431–3437. [Google Scholar] [CrossRef]
- Luo, L.; He, Y.; Jin, L.; Zhang, Y.; Guastaldi, F.P.; Albashari, A.A.; Hu, F.; Wang, X.; Wang, L.; Xiao, J.; et al. Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries. Bioact. Mater. 2021, 6, 638–654. [Google Scholar] [CrossRef]
- Jang, J.Y.; Park, S.H.; Park, J.H.; Lee, B.K.; Yun, J.H.; Lee, B.; Kim, J.H.; Min, B.H.; Kim, M.S. In Vivo Osteogenic Differentiation of Human Dental Pulp Stem Cells Embedded in an Injectable In Vivo-Forming Hydrogel. Macromol. Biosci. 2016, 16, 1158–1169. [Google Scholar] [CrossRef]
- McMurray, R.J.; Dalby, M.J.; Tsimbouri, P.M. Using biomaterials to study stem cell mechanotransduction, growth and differentiation. J. Tissue Eng. Regen. Med. 2015, 9, 528–539. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; Cusella De Angelis, M.G.; Lupi, S.M.; Rodriguez, Y.; Baena, R. Emerging Perspectives in Scaffold for Tissue Engineering in Oral Surgery. Stem Cells Int. 2017, 2017, 4585401. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.M.; McNamara, L.M. Stem cell mechanobiology and the role of biomaterials in governing mechanotransduction and matrix production for tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef] [PubMed]
- Argentati, C.; Morena, F.; Tortorella, I.; Bazzucchi, M.; Porcellati, S.; Emiliani, C.; Martino, S. Insight into mechanobiology: How stem cells feel mechanical forces and orchestrate biological functions. Int. J. Mol. Sci. 2019, 20, 5337. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Tiribuzi, R.; Armentano, I.; Kenny, J.M.; Martino, S.; Orlacchio, A. Mechanotransduction: Tuning Stem Cells Fate. J. Funct. Biomater. 2011, 2, 67–87. [Google Scholar] [CrossRef]
- Xiao, E.; Chen, C.; Zhang, Y. The mechanosensor of mesenchymal stem cells: Mechanosensitive channel or cytoskeleton? Stem Cell Res. Ther. 2016, 7, 7–10. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, B. The mechanosensitive Piezo1 channel: Structural features and molecular bases underlying its ion permeation and mechanotransduction. J. Physiol. 2018, 596, 969–978. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- He, L.; Ahmad, M.; Perrimon, N. Mechanosensitive channels and their functions in stem cell differentiation. Exp. Cell Res. 2019, 374, 259–265. [Google Scholar] [CrossRef]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental pulp stem cell mechanoresponsiveness: Effects of mechanical stimuli on dental pulp stem cell behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Wang, Y.; Ye, R.; Feng, X.; Jing, Z.; Zhao, Z. Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod. 2015, 85, 87–94. [Google Scholar] [CrossRef]
- Mousawi, F.; Peng, H.; Li, J.; Ponnambalam, S.; Roger, S.; Zhao, H.; Yang, X.; Jiang, L.H. Chemical activation of the Piezo1 channel drives mesenchymal stem cell migration via inducing ATP release and activation of P2 receptor purinergic signaling. Stem Cells 2020, 38, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Fels, B.; Bulk, E.; Pethő, Z.; Schwab, A. The role of TRP channels in the metastatic cascade. Pharmaceuticals 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.; Tran, T.D.N.; Zhang, H.; Zolochevska, O.; Figueiredo, M.; Feng, J.M.; Gutierrez, D.L.; Xiao, R.; Yao, S.; Penn, A.; et al. Transient receptor potential melastatin 4 channel controls calcium signals and dental follicle stem cell differentiation. Stem Cells 2013, 31, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, S.M.; Ma, D.D.; Wu, B.L. The effect of TRPM7 suppression on the proliferation, migration and osteogenic differentiation of human dental pulp stem cells. Int. Endod. J. 2014, 47, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Xiao, E.; Yang, H.Q.; Gan, Y.-H.H.; Duan, D.-H.H.; He, L.-H.H.; Guo, Y.; Wang, S.Q.; Zhang, Y. TRPM7 senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells 2015, 33, 615–621. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Liu, Y.-A.; Huang, C.-J.; Yen, M.-H.; Tseng, C.-T.; Chien, S.; Lee, O.K. Mechanosensitive TRPM7 mediates shear stress and modulates osteogenic differentiation of mesenchymal stromal cells through Osterix pathway. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Kim, G.Y.; Lee, C.H. Antimicrobial susceptibility and pathogenic genes of Staphylococcus aureus isolated from the oral cavity of patients with periodontitis. J. Periodontal Implant. Sci. 2015, 45, 223–228. [Google Scholar] [CrossRef]
- Yourek, G.; Hussain, M.A.; Mao, J.J. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO J. 2007, 53, 219–228. [Google Scholar] [CrossRef]
- Du, Y.; Montoya, C.; Orrego, S.; Wei, X.; Ling, J.; Lelkes, P.I.; Yang, M. Topographic cues of a novel bilayered scaffold modulate dental pulp stem cells differentiation by regulating YAP signalling through cytoskeleton adjustments. Cell Prolif. 2019, 52, e12676. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.Y.; Panayotov, I.; Secret, E.; Cunin, F.; Gergely, C.; Cuisinier, F.; Martin, M. Initial stem cell adhesion on porous silicon surface: Molecular architecture of actin cytoskeleton and filopodial growth. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Conserva, E.; Consolo, U.; Bellini, P. Adhesion and proliferation of human dental pulp stem cells on a laser microtextured implant surface: An in vitro study. Oral Health Care 2018, 3, 1–6. [Google Scholar] [CrossRef]
- Bachhuka, A.; Delalat, B.; Ghaemi, S.R.; Gronthos, S.; Voelcker, N.H.; Vasilev, K. Nanotopography mediated osteogenic differentiation of human dental pulp derived stem cells. Nanoscale 2017, 9, 14248–14258. [Google Scholar] [CrossRef] [PubMed]
- Marconi, G.D.; Fonticoli, L.; Della Rocca, Y.; Rajan, T.S.; Piattelli, A.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Human periodontal ligament stem cells response to titanium implant surface: Extracellular matrix deposition. Biology 2021, 10, 931. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, O.; Ermis, M.; Demirci, U.; Hasirci, N.; Hasirci, V. Square prism micropillars on poly (methyl methacrylate) surfaces modulate the morphology and differentiation of human dental pulp mesenchymal stem cells. Colloids Surf. B Biointerfaces 2019, 178, 44–55. [Google Scholar] [CrossRef]
- Ravindran, S.; Huang, C.C.; George, A. Extracellular matrix of dental pulp stem cells: Applications in pulp tissue engineering using somatic MSCs. Front. Physiol. 2014, 4, 395. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.J.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef]
- Heng, B.C.; Zhu, S.; Xu, J.; Yuan, C.; Gong, T.; Zhang, C. Effects of decellularized matrices derived from periodontal ligament stem cells and SHED on the adhesion, proliferation and osteogenic differentiation of human dental pulp stem cells in vitro. Tissue Cell 2016, 48, 133–143. [Google Scholar] [CrossRef]
- Bačáková, L.; Filová, E.; Rypáček, F.; Švorčík, V.; Starý, V. Cell Adhesion on Artificial Materials for Tissue Engineering. Physiol. Res. 2004, 53, S35–S45. [Google Scholar]
- Lee, J.S.; Yi, J.K.; An, S.Y.; Heo, J.S. Increased osteogenic differentiation of periodontal ligament stem cells on polydopamine film occurs via activation of integrin and PI3K signaling pathways. Cell. Physiol. Biochem. 2014, 34, 1824–1834. [Google Scholar] [CrossRef]
- Hung, C.J.; Hsu, H.I.; Lin, C.C.; Huang, T.H.; Wu, B.C.; Kao, C.T.; Shie, M.Y. The role of integrin αv in proliferation and differentiation of human dental pulp cell response to calcium silicate cement. J. Endod. 2014, 40, 1802–1809. [Google Scholar] [CrossRef]
- Liu, J.; Jin, T.C.; Chang, S.; Czajka-Jakubowska, A.; Clarkson, B.H. Adhesion and growth of dental pulp stem cells (DPSCs) on enamel-like fluorapatite (FA) surfaces. J. Biomed. Mater. Res. A 2011, 96, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Lee, E.S.; Kim, M.J.; Kim, J.J.; Lee, J.H.; Lee, H.H.; Park, K.R.; Yi, J.K.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffold-induced stimulation of migration and odontogenesis of human dental pulp cells through integrin signaling pathways. PLoS ONE 2015, 10, e0138614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Zhou, Y.; Tan, J.; Che, H.; Ning, F.; Zhang, X.; Xun, W.; Huo, N.; Tang, L.; et al. Natural mineralized scaffolds promote the dentinogenic potential of dental pulp stem cells via the mitogen-activated protein kinase signaling pathway. Tissue Eng.—Part A 2012, 18, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Cao, G.; Li, Y.; Zhang, Z.; Nör, J.E.; Clarkson, B.H.; Liu, J. Signals in Stem Cell Differentiation on Fluorapatite-Modified Scaffolds. J. Dent. Res. 2018, 97, 1331–1338. [Google Scholar] [CrossRef]
- Lim, H.C.; Nam, O.H.; Kim, M.j.; El-Fiqi, A.; Yun, H.M.; Lee, Y.M.; Jin, G.Z.; Lee, H.H.; Kim, H.W.; Kim, E.C. Delivery of dexamethasone from bioactive nanofiber matrices stimulates odontogenesis of human dental pulp cells through integrin/BMP/mTOR signaling pathways. Int. J. Nanomed. 2016, 11, 2557–2567. [Google Scholar] [CrossRef][Green Version]
- Zhou, M.; Liu, N.X.; Shi, S.R.; Li, Y.; Zhang, Q.; Ma, Q.Q.; Tian, T.R.; Ma, W.J.; Cai, X.; Lin, Y.F. Effect of tetrahedral DNA nanostructures on proliferation and osteo/odontogenic differentiation of dental pulp stem cells via activation of the notch signaling pathway. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1227–1236. [Google Scholar] [CrossRef]
- Crowder, S.W.; Leonardo, V.; Whittaker, T.; Papathanasiou, P.; Stevens, M.M. Material cues as potent regulators of epigenetics and stem cell function. Cell Stem Cell 2016, 18, 39–52. [Google Scholar] [CrossRef]
- Long Wei, L.V.; Yun Song, L.I.U.; Ping Zhang, M.G.U.; Xiang Song, B.A.I.; Xiong, C.Y.; Zhou, Y.S. Transcriptomics and functional analysis of graphene-guided osteogenic differentiation of mesenchymal stem cells. Chin. J. Dent. Res. 2018, 21, 101–111. [Google Scholar]
| Biomaterial | Cells Type | Findings | Reference |
|---|---|---|---|
| 3D-printed hydroxyapatite scaffolds containing peptide hydrogels | DPSCs | (Mice) blood vessel ingrowth, pulp-like tissue formation, and osteodentin deposition, suggesting osteogenic/odontogenic differentiation of hDPSCs | Lambrichts et al. (2017) [19] |
| Chitosan scaffolds with or without arginine-glycine-aspartic acid or fibronectin | DPSCs | Fibronectin-immobilized chitosan scaffolds may serve as suitable three-dimensional substrates for dental pulp stem cell attachment and proliferation | Asghari Sana et al. (2017) [20] |
| Silk fibroin-based 2D films and 3D scaffolds | DPSCs | Good in vitro biocompatibility of silk fibroin-based biomaterials, mainly when 3D scaffolds rather than 2D films are used. | Pecci-Lloret et al. (2017) [21] |
| Collagen and titanium | DPSCs | Compared with human sarcoma osteogenic cell line, DPPSC showed higher initial adhesion levels and similar osteogenic differentiation. These results promote the use of DPPSC as a new pluripotent-like cell model to evaluate the biocompatibility and the differentiation capacity of biomaterials used in bone regeneration | Núñez-Toldrà et al. (2017) [22] |
| Crosslinked type I and type II collagen hydrogels | DPSCs | Cells can potentially migrate from the hydrogels and migrate into the nucleus pulposus tissue | Yao and Flynn (2018) [23] |
| Polycaprolactone cone in an odontoblastic differentiation medium | DPSCs | Cells isolated from both carious and healthy mature teeth were able to colonize and proliferate and could be differentiated into functional odontoblast-like cells. | Louvrier et al. (2018) [24] |
| Commercial dental composite resins | GMSCs | Inflamed GMSCs retain their stem cell properties and could be used as a valuable cell line for testing dental biomaterials | Soancă et al. (2018) [25] |
| Calcium enriched mixture (CEM) cement, Biodentine, mineral trioxide aggregate (MTA), octacalcium phosphate (OCP), and Atlantik | SCAPs | Tested biomaterials could induce odontogenic/osteogenic differentiation in SCAPs. MTA had a more significant potential for induction of differentiation of SCAPs to odontoblast-like cells, while OCP had a higher potential to induce differentiation of SCAPs to osteoblast-like cells | Saberi et al. (2019) [26] |
| gelatin methacrylate (GelMA) hydrogel | BMSC, DPSCs, and SCAP | Among stem cells from different craniofacial regions, BMSCs appear more suitable for engineering mature vascularized networks than DPSCs or SCAPs | Parthiban et al. (2020) [27] |
| Three-dimensional (3D) graphene oxide (GO)/sodium alginate (GOSA) and reduced GOSA (RGOSA) scaffolds | DPSCs | The cytotoxicity of GO-based scaffolds showed that DPSCs could be seeded in serum-free media without cytotoxic effects. This is critical for human translation as cellular transplants are typically serum-free. | Mansouri et al. (2021) [28] |
| NeoMTA Plus, ProRoot MTA and Biodentine | DPSCs | Materials are not cytotoxic and do not induce apoptosis | Birant et al. (2021) [29] |
| Calcium phosphate cement | DPSCs | CPC is promising for dental pulp-capping, base, and liner applications to promote dentin regeneration | Gu et al. (2021) [30] |
| Chitosan/gelatin/nanohydroxyapatite scaffolds | DPSCs | Scaffolds support the viability and proliferation of DPSCs, and provide a biomimetic microenvironment favoring odontogenic differentiation and in vitro biomineralization without the addition of any inductive factors | Vagropoulou et al. (2021) [31] |
| Granular hydroxyapatite scaffold | SHED and DPSCs | gHA scaffold is an optimal scaffold as it induced osteogenesis in vitro. SHED had the highest osteogenic potential | Hagar et al. (2021) [32] |
| Polylactic acid and hydroxyapatite 3D-printed composite | DPSCs | Bone forming ability of composite in Winstar rats’ bone defects. Additionally, inflammatory reaction during biodegradation. | Gendviliene et al. (2021) [33] |
| Nanohydroxyapatite/collagen/poly(l-lactide) | SCAPs | These cells are alternative sources for alveolar bone engineering in regenerative medicine (mice). | Ling-Ling et al. (2021) [34] |
| Core/shell poly (methyl methacrylate) (PMMA)/silk fibroin (SF) fibers | DPSCs | Composite mats composed of core/shell PMMA/SF fibers could be considered a promising candidate for tissue engineering applications and drug delivery strategies | Atila et al. (2022) [35] |
| Chitosan and covalent tetra-armed poly (ethylene glycol) composite encapsulating acetylsalicylic acid (ASA) | PLSCs | The capacity of PDLSCs and ASA-laden CG to enhance new bone regeneration in situ using a mouse calvarial bone defect model. | Zhang et al. (2022) [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Ayala, F.J.; Aguilar-Pérez, F.J.; Nic-Can, G.I.; Rojas-Herrera, R.; Chuc-Gamboa, G.; Aguilar-Pérez, D.; Rodas-Junco, B.A. A Molecular View on Biomaterials and Dental Stem Cells Interactions: Literature Review. Appl. Sci. 2022, 12, 5815. https://doi.org/10.3390/app12125815
Aguilar-Ayala FJ, Aguilar-Pérez FJ, Nic-Can GI, Rojas-Herrera R, Chuc-Gamboa G, Aguilar-Pérez D, Rodas-Junco BA. A Molecular View on Biomaterials and Dental Stem Cells Interactions: Literature Review. Applied Sciences. 2022; 12(12):5815. https://doi.org/10.3390/app12125815
Chicago/Turabian StyleAguilar-Ayala, Fernando J., Fernando J. Aguilar-Pérez, Geovanny I. Nic-Can, Rafael Rojas-Herrera, Gabriela Chuc-Gamboa, David Aguilar-Pérez, and Beatriz A. Rodas-Junco. 2022. "A Molecular View on Biomaterials and Dental Stem Cells Interactions: Literature Review" Applied Sciences 12, no. 12: 5815. https://doi.org/10.3390/app12125815
APA StyleAguilar-Ayala, F. J., Aguilar-Pérez, F. J., Nic-Can, G. I., Rojas-Herrera, R., Chuc-Gamboa, G., Aguilar-Pérez, D., & Rodas-Junco, B. A. (2022). A Molecular View on Biomaterials and Dental Stem Cells Interactions: Literature Review. Applied Sciences, 12(12), 5815. https://doi.org/10.3390/app12125815







