Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions
Abstract
:1. Introduction
2. Sources of Daucosterol
| Plant Family | Country | Parts Used | References |
|---|---|---|---|
| Fallopia cillinerve Polygonaceae | China | Roots | [1] |
| Litsea cubeba Lauraceae | Vietnam | Not reported | [4] |
| Hechtia glomerata Zucc Bromeliaceae | Mexico | Leaves | [2] |
| Eleocharis dulcis Cyperaceae | China | Not reported | [33] |
| Ipomoea batatas Convolvulaceae | China | Not reported | [25] |
| Dendrobium huoshanense Orchidaceae | China | Stems | [5] |
| Dendrobium officinale Orchidaceae | |||
| Crataegus gracilior Rosaceae | Mexico | Flowers | [46] |
| Hyssopus cuspidatus Boriss. Lamiaceae | China | Aerial parts | [6] |
| Ficus deltoidea Moraceae | Indonesia | Leaves | [10] |
| Dioscorea batatas Dioscoreaceae | Korea | Not reported | [11] |
| Crateva adansonii Capparaceae | Cameroon | Stem bark | [24] |
| Leaves | [48] | ||
| Astragalus tanae Fabaceae | Italy | Aerial parts | [12] |
| Acanthopanax sessiliflorus (Rupr. and Maxim.) Seem. Araliaceae | China | Fruits | [49] |
| Centaurea resupinata subsp. dufourii Asteraceae | Algeria | Aerial parts | [7] |
| Ononis mitissima L. Fabaceae | Algeria | Aerial parts | [8] |
| Prangos ferulacea Apiaceae | Iran | Leaves and stems | [9] |
| Cassia italica Fabaceae | Saudi Arabia | Aerial parts | [3] |
| Dipsacus chinensis Caprifoliaceae | China | Roots | [50] |
| Dipsacus asperoides Caprifoliaceae | |||
| Dipsacus japonicas Caprifoliaceae | |||
| Dipsacus kangdigensis Caprifoliaceae | |||
| Dipsacus daliensis Caprifoliaceae | |||
| Rheum turkestanicum Polygonaceae | Iran | Whole plant Roots | [14,15] |
| Heracleum persicum Apiaceae | Iran | Whole plant | [15] |
| Landolphia owariensis Apocynaceae | Nigeria | Leaves | [13] |
| Streptocaulon griffithii Asclepiadaceae | China | Not reported | [51] |
| Streptocaulon griffithii Asclepiadaceae | China | Whole plant | [51] |
| Archidendron clypearia Fabaceae | Vietnam | Whole plant | [16] |
| Shibataea chinensis Nakai Gramineae | China | Leaves | [52] |
| Morus alba Moraceae | Korea | Leaves | [35] |
| Geranium collinum Geraniaceae | China | Roots | [53] |
| Lasianthus hartii Rubiaceae | China | Leaves | [54] |
| Salvia syriaca Lamiaceae | Iran | Roots | [55] |
| Sedum caeruleum Crassulaceae | Algeria | Aerial parts | [56] |
| Adenophora triphylla Campanulaceae | Korea | Not reported | [27] |
| Pulicaria inuloides Asteraceae | Yemen | Aerial parts | [57] |
| Salvia miltiorrhiza Lamiaceae | China | Roots | [28] |
| Salvia officinalis Lamiaceae | China | Roots | [28] |
| Rosa canina L. Rosaceae | Iran | Fruits | [58] |
| Dorema glabrum Fisch. and C.A. Mey. Apiaceae | Iran | Aerial parts | [60] |
| Clematis heracleifolia Ranunculaceae | Korea | Whole plant | [59] |
| Artemisia apiacea Asteraceae | Korea | Not reported | [61,99] |
| Pyrus spp. Rosaceae | China | Peels and pulps | [62] |
| Salvia sahendica Lamiaceae | Iran | Aerial parts | [36] |
| Punica granatum Lythraceae | Tunisia | Flowers | [64] |
| Helicteres isora L. Sterculiaceae | India | Fruits | [65] |
| Ceiba pentandra L. Bombacaceae | Seeds | ||
| Eria spicata Orchidaceae | China | Whole plant | [68] |
| Lysimachia clethroides Primulaceae | China | Aerial parts | [66] |
| Randia dumetorum Rubiaceae | Not reported | Bark | [67] |
| Litchi chinensis Sapindaceae | China | Seeds | [69] |
| Parasenecio pseudotaimingasa Asteraceae | Korea | Leaves | [17] |
| Grewia optiva Drummond ex Burret Tiliaceae | Pakistan | Stem bark | [18] |
| Urtica angustifolia Urticaceae | China | Leaves, roots, and stems | [74] |
| Salvia limbata Lamiaceae | Iran, Turkey, and Afghanistan | Aerial parts | [71] |
| Lindera glauca Lauraceae | Korea | Stems | [72] |
| Sphallerocarpus gracilis Apiaceae | China | Roots | [73] |
| Hypericum ascyron L. Hypericaceae | China | Whole plant | [74] |
| Paeonia lactiflora Paeoniaceae | Korea | Roots | [75] |
| Paeonia suffruticosa Paeoniaceae | Root bark | ||
| Alangium kurzi Alangiaceae | Indonesia | Stem bark | [76] |
| Boerhaavia diffusa Nyctaginaceae | Nigeria | Leaves | [77] |
| Cassia mimosoides var. nomame Makino Fabaceae | Korea | Seeds | [78] |
| Phyllenthus emblica L. Phyllanthaceae | China | Fruits | [20] |
| Mitragyna speciosa Rubiaceae | America | Leaves | [79] |
| Astragalus membranaceus Fabaceae | Korea | Roots | [80] |
| Bennettiodendron leprosipes Flacourtiaceae | China | Stem bark and twigs | [81] |
| Flacourtia ramontchi Flacourtiaceae | Bark and twigs | ||
| Alchornea cordifolia (Schumach. and Thonn.) Müll. Arg. Euphorbiaceae | Belgium | Leaves and root bark | [82] |
| Flueggea virosa Euphorbiaceae | China | Twigs and leaves | [83] |
| Eriobotrya fragrans Champ Rosaceae | China | Fruits and leaves | [22] |
| Portulaca oleracea L. Portulacaceae | Egypt | Not reported | [21] |
| Pteridium aquilinum Pteridaceae | China | Not reported | [84] |
| Brassica campestris ssp rapa Brassicaceae | Korea | Roots | [85] |
| Penthorum chinense Penthoraceae | China | Whole plant | [86] |
| Arctotis arctotoides Asteraceae | Not reported | Not reported | [23] |
| Selinum cryptotaenium Umbelliferae | China | Roots | [87] |
| Embelia ribes Myrsinaceae | China | Roots | [88] |
| Punica granatum Lythraceae | China | Flowers | [89] |
| Dioscorea opposita Dioscoreaceae | China | Aerial parts | [19] |
| Junellia aspera Verbenaceae | Spain | Aerial parts | [90] |
| Sitophilus oryzae Curculionidae | Not reported | ||
| Astragalus mongholicus Bunge Fabaceae | China | Roots | [91] |
| Hemiphragma heterophyllum Scrophulariaceae | China | Whole plant | [92] |
| Dendrobium moniliforme Orchidaceae | China | Stems | [95] |
| Punica granatum Lythraceae | China | Seeds | [93] |
| Nepeta cataria L. var. citriodora Lamiaceae | Poland | Seeds | [94] |
| Euphorbia altotibetic Euphorbiaceae | China | Whole plant | [96] |
| Rhodiola sachalinensis Crassulaceae | Korea | Roots | [97] |
| Gnetum montanum Gnetaceae | China | Not reported | [98] |
| Ajania fruticulosa Asteraceae | China | Aerial parts | [100] |
| Rhodiola fastigiata Crassulaceae | China | Rhizomes | [101] |
| Jatropha curcas Euphorbiaceae | China | Roots | [102] |
| Gentiana algida Gentianaceae | China | Whole plant | [103] |
| Gentiana siphonantha Gentianaceae | China | Rhizomes and roots | [104] |
| Gentiana scabra Bunge Gentianaceae | China | Roots | [105] |
| Rabdosia coetsa Lamiaceae | China | Leaves | [106] |
| Artemisia sieversiana Asteraceae | China | Aerial parts | [105] |
| Inula racemosa Asteraceae | Roots | ||
| Amanoa oblongifolia Euphorbiaceae | Peru | Stem bark | [107] |
| Rhodiola rosea L. Crassulaceae | Russia | Rhizomes | [108] |
| Acanthopanax sessiliflorum Araliaceae | Not reported | Roots | [109] |
| Leaves |
3. Extraction and Characterization
4. Evaluation of Biological Properties
4.1. Antioxidant Activity
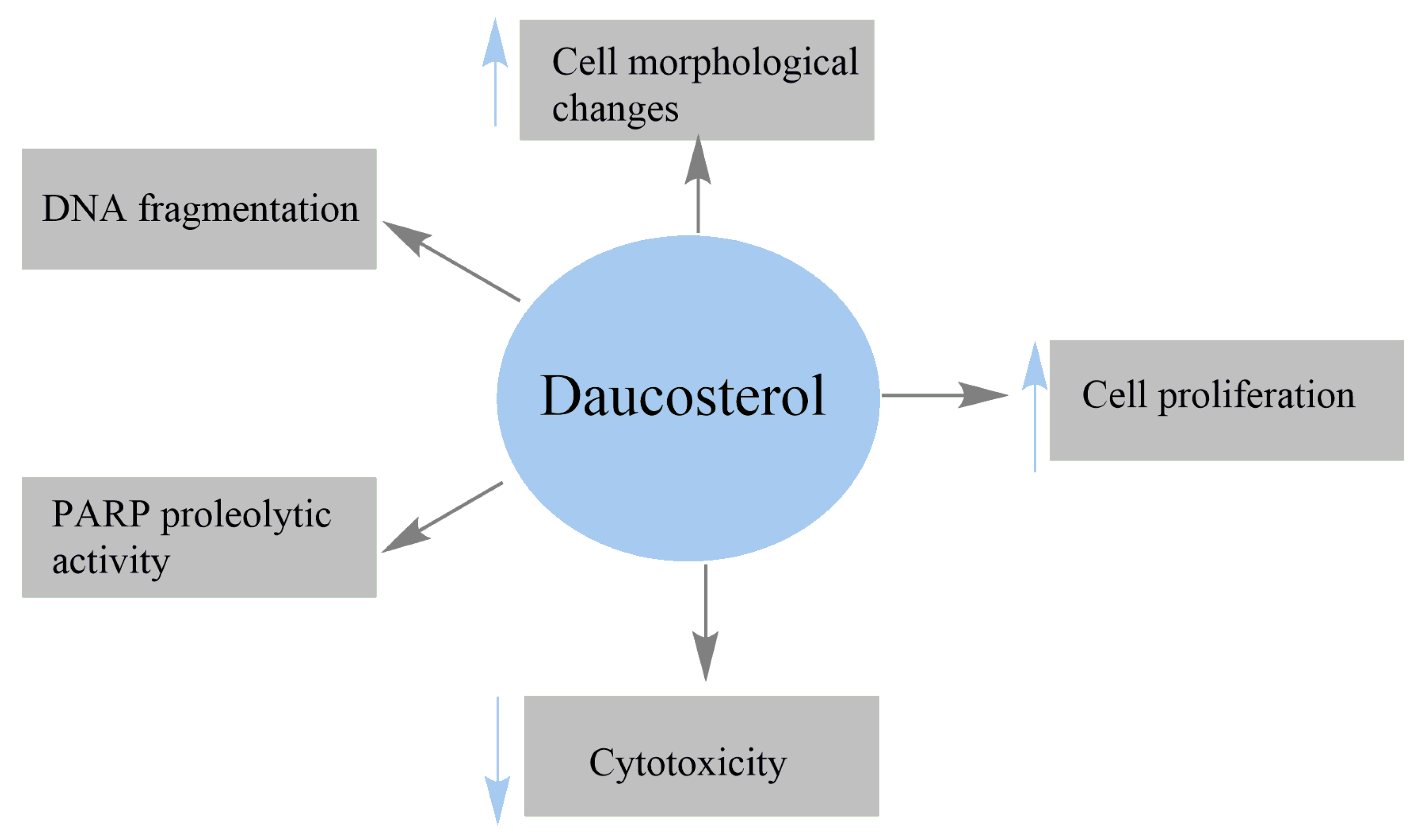
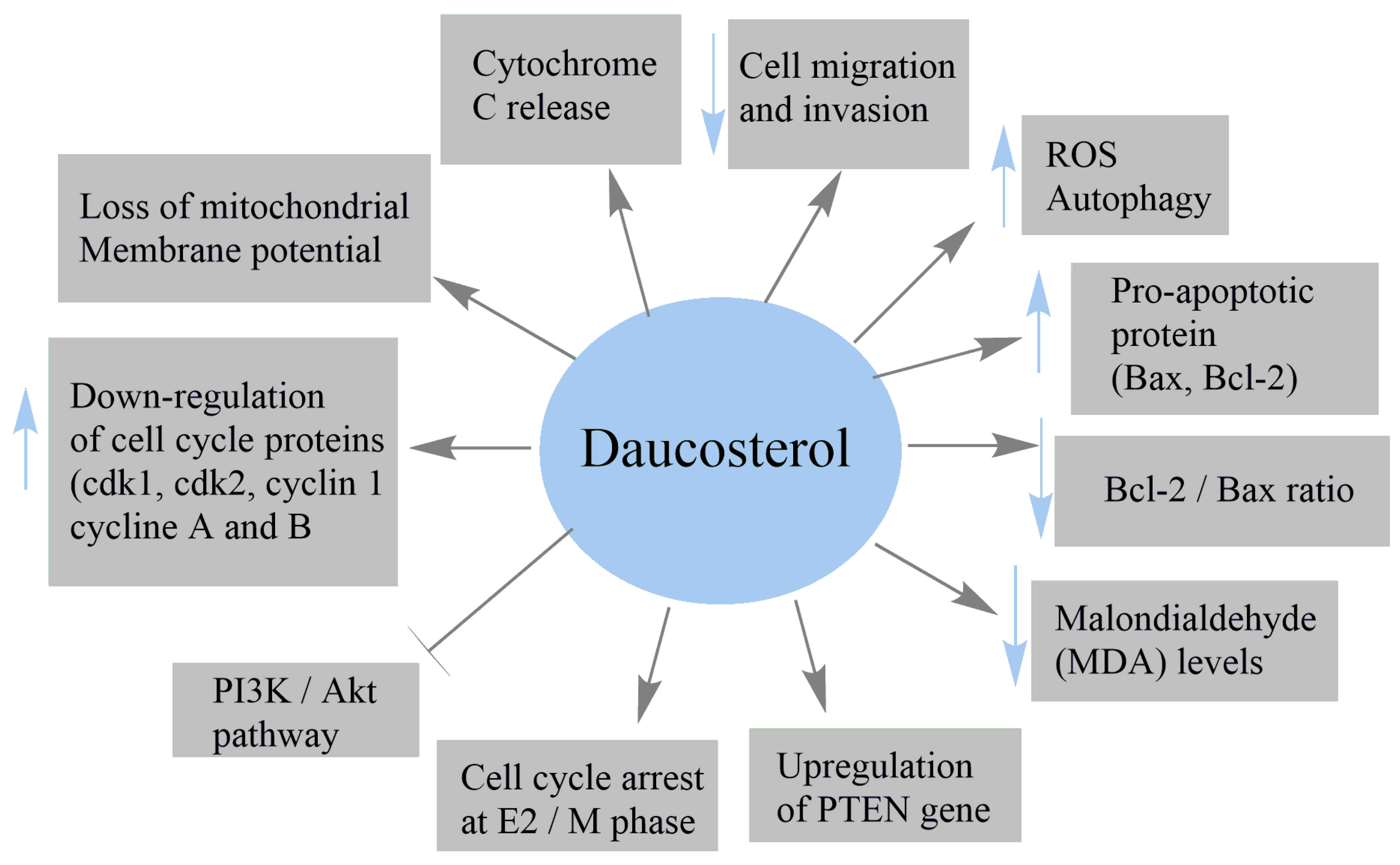
4.2. Anticancer Activity
4.3. Neuroprotective Activity
4.4. Anti-Inflammatory Activity
4.5. Immunomodulatory Effects
4.6. Antidiabetic Activity
4.7. Hypolipidemic Activity
5. Limitations and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.; Zhuang, H.; Jiang, H. Study on the Chemical Constituents of a Plant in the Soil of Taibai Mountain. Int. J. Sci. 2021, 8, 4. [Google Scholar]
- Stefani, T.; Morales-San Claudio, P.D.C.; Rios, M.Y.; Aguilar-Guadarrama, A.B.; González-Maya, L.; Sánchez-Carranza, J.N.; González-Ferrara, M.; Camacho-Corona, M. del R. UPLC–QTOF–MS Analysis of Cytotoxic and Antibacterial Extracts of Hechtia Glomerata Zucc. Nat. Prod. Res. 2022, 36, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Al-Haidari, R.A.; Al-Oqail, M.M. New Benzoic Acid Derivatives from Cassia Italica Growing in Saudi Arabia and Their Antioxidant Activity. Saudi Pharm. J. 2020, 28, 1112–1117. [Google Scholar] [CrossRef]
- Quach, N.T.; Nguyen, Q.H.; Vu, T.H.N.; Le, T.T.H.; Ta, T.T.T.; Nguyen, T.D.; Van Doan, T.; Van Nguyen, T.; Dang, T.T.; Nguyen, X.C.; et al. Plant-Derived Bioactive Compounds Produced by Streptomyces Variabilis LCP18 Associated with Litsea cubeba (Lour.) Pers as Potential Target to Combat Human Pathogenic Bacteria and Human Cancer Cell Lines. Braz. J. Microbiol. 2021, 52, 1215–1224. [Google Scholar] [CrossRef]
- Wan, J.; Gong, X.; Wen, C.; Wei, Y.; Kong, L.; Han, B.; Ouyang, Z. First Systematic Analysis for Chemical Composition by HPLC-ESI-MSn and Antioxidant Activity Comparison of Dendrobium Huoshanense and Dendrobium Officinale. Res. Sq. 2020; in review. [Google Scholar] [CrossRef]
- Shomirzoeva, O.; Li, J.; Numonov, S.; Atolikshoeva, S.; Aisa, H.A. Chemical Components of Hyssopus Cuspidatus Boriss.: Isolation and Identification, Characterization by HPLC-DAD-ESI-HRMS/MS, Antioxidant Activity and Antimicrobial Activity. Nat. Prod. Res. 2020, 34, 534–540. [Google Scholar] [CrossRef]
- Bouzghaia, B.; Moussa, M.T.B.; Goudjil, R.; Harkat, H.; Pale, P. Chemical Composition, in Vitro Antioxidant and Antibacterial Activities of Centaurea Resupinata Subsp. Dufourii (Dostál) Greuter. Nat. Prod. Res. 2021, 35, 4734–4739. [Google Scholar] [CrossRef]
- Besbas, S.; Mouffouk, S.; Haba, H.; Marcourt, L.; Wolfender, J.-L.; Benkhaled, M. Chemical Composition, Antioxidant, Antihemolytic and Anti-Inflammatory Activities of Ononis mitissima L. Phytochem. Lett. 2020, 37, 63–69. [Google Scholar] [CrossRef]
- Abdollahnezhad, H.; Bahadori, M.B.; Pourjafar, H.; Movahhedin, N. Purification, Characterization, and Antioxidant Activity of Daucosterol and Stigmasterol from Prangos Ferulacea. Lett. Appl. Biosci. 2021, 10, 2174–2180. [Google Scholar]
- Murni, A.; Yuliansih, P.; Mohamad, K.; Kita, M.; Hanif, N. A Glucosylated Steroid from the Active Fraction of Indonesian Ficus Deltoidea Leaves. AIP Conf. Proc. 2020, 2243, 030015. [Google Scholar] [CrossRef]
- Park, M.-K.; Cho, S.; Ahn, T.-K.; Kim, D.-H.; Kim, S.-Y.; Lee, J.-W.; Kim, J.-I.; Seo, E.-W.; Son, K.-H.; Lim, J.-H. Immunomodulatory Effects of β-sitosterol and Daucosterol Isolated from Dioscorea batatas on LPS-stimulated RAW 264.7 and TK-1 Cells. J. Life Sci. 2020, 30, 359–369. [Google Scholar] [CrossRef]
- Kavtaradze, N.; Alaniya, M.; Masullo, M.; Cerulli, A.; Piacente, S. New Flavone Glycosides from Astragalus Tanae Endemic to Georgia. Chem. Nat. Compd. 2020, 56, 70–74. [Google Scholar] [CrossRef]
- Ibekwe, N.N.; Akoje, V.O.; Igoli, J.O. Phytochemical Constituents of the Leaves of Landolphia Owariensis. Trop. J. Nat. Prod. Res. 2019, 3, 261–264. [Google Scholar] [CrossRef]
- Dehghan, H.; Salehi, P.; Amiri, M.S. Bioassay-Guided Purification of α-Amylase, α-Glucosidase Inhibitors and DPPH Radical Scavengers from Roots of Rheum Turkestanicum. Ind. Crops Prod. 2018, 117, 303–309. [Google Scholar] [CrossRef]
- Dehghan, H. Isolation of 17 Antidiabetic Compounds from Two Iranian Plants. Org. Agric. 2019. Available online: http://herb.bonyadhamayesh.ir/fa/ (accessed on 29 April 2022).
- Duong, N.T.; Vinh, P.D.; Thuong, P.T.; Hoai, N.T.; Thanh, L.N.; Bach, T.T.; Nam, N.H.; Anh, N.H. Xanthine Oxidase Inhibitors from Archidendron Clypearia (Jack.) I.C. Nielsen: Results from Systematic Screening of Vietnamese Medicinal Plants. Asian Pac. J. Trop. Med. 2017, 10, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Choi, J.Y.; Lee, K.H.; Lee, S. Isolation of Antibacterial Compounds from Parasenecio Pseudotaimingasa. Hortic. Environ. Biotechnol. 2012, 53, 561–564. [Google Scholar] [CrossRef]
- Uddin, G.; Siddiqui, B.S.; Alam, M.; Sadat, A.; Ahmad, A.; Uddin, A. Chemical Constituents and Phytotoxicity of Solvent Extracted Fractions of Stem Bark of Grewia Optiva Drummond Ex Burret. Middle-East. J. Sci. Res. 2011, 8, 85–91. [Google Scholar]
- Ma, C.; Wang, W.; Chen, Y.-Y.; Liu, R.-N.; Wang, R.-F.; Du, L.-J. Neuroprotective and Antioxidant Activity of Compounds from the Aerial Parts of Dioscorea Opposita. J. Nat. Prod. 2005, 68, 1259–1261. [Google Scholar] [CrossRef]
- Luo, W.; Zhao, M.; Yang, B.; Shen, G.; Rao, G. Identification of Bioactive Compounds in Phyllenthus emblica L. Fruit and Their Free Radical Scavenging Activities. Food Chem. 2009, 114, 499–504. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.M.; Aziz, M.A. Portulene, a New Diterpene from Portulaca oleracea L. J. Asian Nat. Prod. Res. 2008, 10, 1039–1043. [Google Scholar] [CrossRef]
- Hong, Y.; Qiao, Y.; Lin, S.; Jiang, Y.; Chen, F. Characterization of Antioxidant Compounds in Eriobotrya Fragrans Champ Leaf. Sci. Hortic. 2008, 118, 288–292. [Google Scholar] [CrossRef]
- Sultana, N.; Afolayan, A.J. A Novel Daucosterol Derivative and Antibacterial Activity of Compounds from Arctotis Arctotoides. Nat. Prod. Res. 2007, 21, 889–896. [Google Scholar] [CrossRef]
- Nguedia, M.Y.; Tueche, A.B.; Yaya, A.J.G.; Yadji, V.; Ndinteh, D.T.; Njamen, D.; Zingue, S. Daucosterol from Crateva Adansonii DC (Capparaceae) reduces 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Wistar rats. Environ. Toxicol. 2020, 35, 1125–1136. [Google Scholar] [CrossRef]
- Han, B.; Jiang, L.; Jiang, P.; Zhou, D.; Jia, X.; Li, X.; Ye, X. Daucosterol Linolenate from Sweet Potato Suppresses MCF7-Xenograft-Tumor Growth through Regulating PI3K/AKT Pathway. Planta Med. 2020, 86, 767–775. [Google Scholar] [CrossRef]
- Rajavel, T.; Banu Priya, G.; Suryanarayanan, V.; Singh, S.K.; Pandima Devi, K. Daucosterol Disturbs Redox Homeostasis and Elicits Oxidative-Stress Mediated Apoptosis in A549 Cells via Targeting Thioredoxin Reductase by a P53 Dependent Mechanism. Eur. J. Pharmacol. 2019, 855, 112–123. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, S.; Park, Y.I.; Kwon, K.H. Antioxidative and Neuroprotective Effects of Extract and Fractions from Adenophora triphylla. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1580–1588. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Rupasinghe, H. The Anticancer Properties of Phytochemical Extracts from Salvia Plants. Bot. Targets Ther. 2016, 6, 25–44. [Google Scholar] [CrossRef] [Green Version]
- Ji, Z.-H.; Xu, Z.-Q.; Zhao, H.; Yu, X.-Y. Neuroprotective Effect and Mechanism of Daucosterol Palmitate in Ameliorating Learning and Memory Impairment in a Rat Model of Alzheimer’s Disease. Steroids 2017, 119, 31–35. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Feng, C. Improvement of Cerebral Ischemia/Reperfusion Injury by Daucosterol Palmitate-Induced Neuronal Apoptosis Inhibition via PI3K/Akt/MTOR Signaling Pathway. Metab. Brain Dis. 2020, 35, 1035–1044. [Google Scholar] [CrossRef]
- Jang, J.; Kim, S.-M.; Yee, S.-M.; Kim, E.-M.; Lee, E.-H.; Choi, H.-R.; Lee, Y.-S.; Yang, W.-K.; Kim, H.-Y.; Kim, K.-H.; et al. Daucosterol Suppresses Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Int. Immuno Pharmacol. 2019, 72, 124–130. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, X.; Shang, C.; Phuong Thao, T.T.; Koyama, T. Inhibitory Properties of Saponin from Eleocharis Dulcis Peel against α-Glucosidase. RSC Adv. 2021, 11, 15400–15409. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, X.; Shang, C.; Thao, T.T.P.; Koyama, T. Inhibition and Interactions of Alpha-Amylase by Daucosterol from the Peel of Chinese Water Chestnut (Eleocharis dulcis). Food Funct. 2021, 12, 8411–8424. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.Y.; Park, J.H.; Jung, H.S.; Kim, J.S.; Kang, S.S.; Kim, Y.S.; Han, Y. Immunoregulatory Activity by Daucosterol, a β-Sitosterol Glycoside, Induces Protective Th1 Immune Response against Disseminated Candidiasis in Mice. Vaccine 2007, 25, 3834–3840. [Google Scholar] [CrossRef]
- Li, K.; Lee, M.L.; Que, L.; Li, M.; Kang, J.S.; Choi, Y.H.; Kim, K.M.; Jung, J.-C.; Hwang, D.Y.; Choi, Y.W. Lipolysis Effect of Daucosterol Isolated from Mulberry (Morus alba) Leaves. J. Life Sci. 2017, 27, 1500–1506. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Farimani, M.M. Inactivation of PI3K/Akt Pathway and Upregulation of PTEN Gene Are Involved in Daucosterol, Isolated from Salvia Sahendica, Induced Apoptosis in Human Breast Adenocarcinoma Cells. S. Afr. J. Bot. 2014, 93, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Jiang, P.; Liu, W.; Xu, H.; Li, Y.; Li, Z.; Ma, H.; Yu, Y.; Li, X.; Ye, X. Role of Daucosterol Linoleate on Breast Cancer: Studies on Apoptosis and Metastasis. J. Agric. Food Chem. 2018, 66, 6031–6041. [Google Scholar] [CrossRef]
- Wang, G.-Q.; Gu, J.-F.; Gao, Y.-C.; Dai, Y.-J. Daucosterol Inhibits Colon Cancer Growth by Inducing Apoptosis, Inhibiting Cell Migration and Invasion and Targeting Caspase Signalling Pathway. Bangladesh J Pharm. 2016, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Mir, S.A.; Dar, L.A.; Ali, T.; Kareem, O.; Rashid, R.; Khan, N.A.; Chashoo, I.A.; Bader, G.N. A Review on Its Phytochemistry and Pharmacology. Edible Plants Health Dis. 2022, 327–348. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, X.; Li, X.; Zheng, Y.; Liu, B.; Xiao, Y. Daucosterol Inhibits the Proliferation, Migration, and Invasion of Hepatocellular Carcinoma Cells via Wnt/β-Catenin Signaling. Molecules 2017, 22, 862. [Google Scholar] [CrossRef]
- Chung, M.J.; Lee, S.; Park, Y.I.; Lee, J.; Kwon, K.H. Neuroprotective Effects of Phytosterols and Flavonoids from Cirsium Setidens and Aster Scaber in Human Brain Neuroblastoma SK-N-SH Cells. Life Sci. 2016, 148, 173–182. [Google Scholar] [CrossRef]
- Sheng, Z.; Dai, H.; Pan, S.; Wang, H.; Hu, Y.; Ma, W. Isolation and Characterization of an α-Glucosidase Inhibitor from Musa spp. (Baxijiao) Flowers. Molecules 2014, 19, 10563–10573. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Wang, L.; Zhang, Y. Immunological Activities of Components from Leaves of Liriodendron Chinensis. Chin. Herb. Med. 2015, 7, 279–282. [Google Scholar] [CrossRef]
- Taheri, Y.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Ezzat, S.M.; Merghany, R.M.; Cho, W.C. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid.-Based Complem. Alter. Med. 2022, 2022, 4024331. [Google Scholar] [CrossRef]
- Jain, V.; Verma, S.K.; Katewa, S.S. Therapeutic Validation of Ipomoea digitata Tuber (Ksheervidari) for its Effect on Cardio-Vascular Risk Parameters. Ind. J. Trad. Knowl. 2011, 617–623. Available online: http://hdl.handle.net/123456789/12823 (accessed on 29 April 2022).
- Torres-Ortiz, D.A.; Eloy, R.; Moustapha, B.; César, I.-A.; Edmundo, M.-S.; Jesús Eduardo, C.-R.; Dulce María, R.-P. Vasorelaxing Effect and Possible Chemical Markers of the Flowers of the Mexican Crataegus Gracilior. Nat. Prod. Res. 2020, 34, 3522–3525. [Google Scholar] [CrossRef]
- Hernández-Pérez, A.; Bah, M.; Ibarra-Alvarado, C.; Rivero-Cruz, J.F.; Rojas-Molina, A.; Rojas-Molina, J.I.; Cabrera-Luna, J.A. Aortic relaxant activity of Crataegus gracilior Phipps and identification of some of its chemical constituents. Molecules 2014, 19, 20962–20974. [Google Scholar] [CrossRef]
- Zingue, S.; Gbaweng Yaya, A.J.; Michel, T.; Ndinteh, D.T.; Rutz, J.; Auberon, F.; Maxeiner, S.; Chun, F.K.-H.; Tchinda, A.T.; Njamen, D.; et al. Bioguided Identification of Daucosterol, a Compound That Contributes to the Cytotoxicity Effects of Crateva Adansonii DC (Capparaceae) to Prostate Cancer Cells. J. Ethnopharmacol. 2020, 247, 112251. [Google Scholar] [CrossRef]
- Chen, L.; Xin, X.; Feng, H.; Li, S.; Cao, Q.; Wang, X.; Vriesekoop, F. Isolation and Identification of Anthocyanin Component in the Fruits of Acanthopanax Sessiliflorus (Rupr. and Maxim.) Seem. by Means of High Speed Counter Current Chromatography and Evaluation of Its Antioxidant Activity. Molecules 2020, 25, 1781. [Google Scholar] [CrossRef] [Green Version]
- CHEN Da-xia1, 2 Compare and Cluster Analysis of Six Chemical Constituents in Dipsacus Medicinal Plants. Nat. Prod. Res. Dev. 2019, 30, 132. [CrossRef]
- Zhang, X.; Wang, K.-W.; Wang, H. Chemical Constituents of Streptocaulon Griffithii. Chem. Nat. Compd. 2018, 54, 803–805. [Google Scholar] [CrossRef]
- Kuang, Y.; Yang, S.X.; Sampietro, D.A.; Zhang, X.F.; Tan, J.; Gao, Q.X.; Liu, H.W.; Ni, Q.X.; Zhang, Y.Z. Phytotoxicity of Leaf Constituents from Bamboo (Shibataea chinensis Nakai) on Germination and Seedling Growth of Lettuce and Cucumber. Allelopath. J. 2017, 40, 133–142. [Google Scholar] [CrossRef]
- Numonov, S.; Edirs, S.; Bobakulov, K.; Qureshi, M.N.; Bozorov, K.; Sharopov, F.; Setzer, W.N.; Zhao, H.; Habasi, M.; Sharofova, M.; et al. Evaluation of the Antidiabetic Activity and Chemical Composition of Geranium Collinum Root Extracts—Computational and Experimental Investigations. Molecules 2017, 22, 983. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, C.; Liu, X.-X.; Zhang, Y.; Wang, K.; Cheng, Z.-Q. Chemical Constituents and Antioxidant Activity of Lasianthus Hartii. Chem. Nat. Compd. 2017, 53, 390–393. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Dinparast, L.; Valizadeh, H.; Farimani, M.M.; Ebrahimi, S.N. Bioactive Constituents from Roots of Salvia Syriaca L.: Acetylcholinesterase Inhibitory Activity and Molecular Docking Studies. S. Afr. J. Bot. 2016, 106, 1–4. [Google Scholar] [CrossRef]
- Bensouici, C.; Kabouche, A.; Karioti, A.; Öztürk, M.; Duru, M.E.; Bilia, A.R.; Kabouche, Z. Compounds from Sedum Caeruleum with Antioxidant, Anticholinesterase, and Antibacterial Activities. Pharm. Biol. 2016, 54, 174–179. [Google Scholar] [CrossRef]
- Galala, A.A.; Sallam, A.; Abdel-Halim, O.B.; Gedara, S.R. New Ent-Kaurane Diterpenoid Dimer from Pulicaria Inuloides. Nat. Prod. Res. 2016, 30, 2468–2475. [Google Scholar] [CrossRef]
- Asghari, B.; Salehi, P.; Farimani, M.M.; Ebrahimi, S.N. α-Glucosidase Inhibitors from Fruits of Rosa canina L. Rec. Nat. Prod. 2015, 9, 276–283. [Google Scholar]
- Kim, M.A.; Kim, M.J.; Chun, W.; Kwon, Y. Chemical Constituents and Their Acetylcholinesterase Inhibitory Activity of Underground Parts of Clematis heracleifolia. Korean J. Pharmacogn. 2015, 46, 6–11. [Google Scholar]
- Delnavazi, M.-R.; Hadjiakhoondi, A.; Delazar, A.; Ajani, Y.; Tavakoli, S.; Yassa, N. Phytochemical and Antioxidant Investigation of the Aerial Parts of Dorema Glabrum Fisch. and C.A. Mey. Iran. J. Pharm. Res. 2015, 14, 925–931. [Google Scholar]
- Lee, J.; Weon, J.B.; Yun, B.-R.; Eom, M.R.; Ma, C.J. Simultaneous Determination Three Phytosterol Compounds, Campesterol, Stigmasterol and Daucosterol in Artemisia Apiacea by High Performance Liquid Chromatography-Diode Array Ultraviolet/Visible Detector. Pharm. Mag. 2015, 11, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical Composition and Antioxidant and Anti-Inflammatory Potential of Peels and Flesh from 10 Different Pear Varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Gao, M.; Gao, P.; Gao, D.; Zhang, H.; Qiao, M. Radix Ranunculi ternati: Review of its chemical constituents, pharmacology, quality control and clinical applications. J. Pharm Pharmacol. 2022. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Vicendo, P.; Fterrich, A.; Bouajila, J. Chemical Composition and Antioxidant, Anti-Inflammatory, and Antiproliferation Activities of Pomegranate (Punica granatum) Flowers. J. Med. Food 2013, 16, 544–550. [Google Scholar] [CrossRef]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant Activity and Free Radical Scavenging Capacity of Phenolic Extracts from Helicteres Isora L. and Ceiba Pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zhang, Y.; Kang, W. Antioxidant and A-Glucosidase Inhibitory Compounds in Lysimachia Clethroides. AJPP 2012, 6, 3230–3234. [Google Scholar] [CrossRef] [Green Version]
- Singh, R. Chemical investigation and in vitro cytotoxic activity of randia dumetorum lamk bark. Int. J. Chem. Sci. 2012, 10, 1374–1382. [Google Scholar]
- Wang, L.; Wu, M.; Huang, J.; Wang, J.; Chen, Y.; Yin, B. Chemical Constituents of Eria Spicata. Chem. Nat. Compd. 2012, 48, 168–169. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Lin, L.; Xu, F. Five Chemical Constituents of the Ethyl Acetate Fraction from Ethanol Extract of Semen Litchi. JMPR 2012, 6, 168–170. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, X.; Jiang, Y.; Han, Y.; Zhou, Y. The Extraction, Identification and Quantification of Hypoglycemic Active Ingredients from Stinging Nettle (Urtica angustifolia). Afr. J. Biotechnol. 2011, 10, 9428–9437. [Google Scholar] [CrossRef]
- Saeidnia, S.; Gohari, A.R.; Malmir, M.; Moradi, A.F.; Ajani, Y. Tryptophan and Sterols from Salvia limbata. J. Med. Plants 2011, 10, 41–47. [Google Scholar]
- Huh, G.-W.; Park, J.-H.; Shrestha, S.; Lee, Y.-H.; Ahn, E.-M.; Kang, H.-C.; Baek, N.-I. Sterols from Lindera Glauca Blume Stem Wood. J. Appl. Biol. Chem. 2011, 54, 309–312. [Google Scholar] [CrossRef]
- Gao, C.; Lu, Y.; Tian, C.; Xu, J.; Guo, X.; Zhou, R.; Hao, G. Main Nutrients, Phenolics, Antioxidant Activity, DNA Damage Protective Effect and Microstructure of Sphallerocarpus Gracilis Root at Different Harvest Time. Food Chem. 2011, 127, 615–622. [Google Scholar] [CrossRef]
- Kang, W.-Y.; Song, Y.-L.; Zhang, L. α-Glucosidase Inhibitory and Antioxidant Properties and Antidiabetic Activity of Hypericum Ascyron L. Med. Chem. Res. 2011, 20, 809–816. [Google Scholar] [CrossRef]
- Koo, Y.K.; Kim, J.M.; Koo, J.Y.; Kang, S.S.; Bae, K.; Kim, Y.S.; Chung, J.-H.; Yun-Choi, H.S. Platelet Anti-Aggregatory and Blood Anti-Coagulant Effects of Compounds Isolated from Paeonia Lactiflora and Paeonia Suffruticosa. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 624–628. [Google Scholar] [CrossRef]
- Nur, S.M.; Soemitro, S.; Bahti, H.H.; Tarigan, P.; Shen, Y.M.; Rumampuk, R.J. Chemical Constituents from the Steam Bark of Alangium Kurzi, Craib. Indones. J. Chem. 2010, 1, 128–130. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Akinmoladun, A.C.; Ogunboye, A.A.; Akindahunsi, A.A. Antioxidant Activity and Hepatoprotective Property of Leaf Extracts of Boerhaavia Diffusa Linn against Acetaminophen-Induced Liver Damage in Rats. Food Chem. Toxicol. 2010, 48, 2200–2205. [Google Scholar] [CrossRef]
- Park, J.-H.; Kwon, S.-J. Isolation of Daucosterol and Naphthalene glucoside from Seeds of Cassia mimosoides var. nomame Makino. Korean J. Plant Resour. 2009, 22, 26–30. [Google Scholar]
- León, F.; Habib, E.; Adkins, J.E.; Furr, E.B.; McCurdy, C.R.; Cutler, S.J. Phytochemical Characterization of the Leaves of Mitragyna Speciosa Grown in USA. Nat. Prod. Commun. 2009, 4, 1934578X0900400705. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-S.; Lee, J.-H.; Lee, S.-H.; Kang, S.-S.; Jeong, C.-S. Suppressive Actions of Astragali Radix (AR) Ethanol Extract and Isolated Astragaloside I on HCl/Ethanol-Induced Gastric Lesions. Biomol. Ther. 2009, 17, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Chai, X.-Y.; Ren, H.-Y.; Xu, Z.-R.; Bai, C.-C.; Zhou, F.-R.; Ling, S.-K.; Pu, X.-P.; Li, F.-F.; Tu, P.-F. Investigation of Two Flacourtiaceae Plants: Bennettiodendron Leprosipes and Flacourtia Ramontchi. Planta Med. 2009, 75, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Mavar-Manga, H.; Haddad, M.; Pieters, L.; Baccelli, C.; Penge, A.; Quetin-Leclercq, J. Anti-Inflammatory Compounds from Leaves and Root Bark of Alchornea Cordifolia (Schumach. and Thonn.) Müll. Arg. J. Ethnopharmacol. 2008, 115, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-C. Chemical Constituents from Flueggea Virosa: Chemical Constituents from Flueggea Virosa. Chin. J. Nat. Med. 2008, 6, 251–253. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Hu, Y.; Wang, L.; Ding, Z.; Liu, Y.; Wang, J. Isolation of 5-Hydroxypyrrolidin-2-One and Other Constituents from the Young Fronds of Pteridium Aquilinum. J. Nat. Med. 2008, 62, 358–359. [Google Scholar] [CrossRef] [PubMed]
- Bang, M.-H.; Lee, D.-Y.; Oh, Y.-J.; Han, M.-W.; Yang, H.-J.; Chung, H.-G.; Jeong, T.-S.; Lee, K.-T.; Choi, M.-S.; Baek, N.-I. Development of Biologically Active Compounds from Edible Plant Sources XXII. Isolation of Indoles from the Roots of Brassica campestris ssp rapa and their hACAT Inhibitory Activity. Appl. Biol. Chem. 2008, 51, 65–69. [Google Scholar]
- Zhang, T.; Chen, Y.-M.; Zhang, G.-L. Novel Neolignan from Penthorum Chinense. J. Integr. Plant Biol. 2007, 49, 1611–1614. [Google Scholar] [CrossRef]
- Rao, G.-X.; Gao, Y.-L.; Lin, Y.-P.; Xiao, Y.-L.; Li, S.-H.; Sun, H.-D. Chemical Constituents of Selinum Cryptotaenium. J. Asian Nat. Prod. Res. 2006, 8, 273–275. [Google Scholar] [CrossRef]
- Lin, P.; Li, S.; Wang, S.; Yang, Y.; Shi, J. A Nitrogen-Containing 3-Alkyl-1,4-Benzoquinone and a Gomphilactone Derivative from Embelia Ribes. J. Nat. Prod. 2006, 69, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, W.; Wang, L.; Liu, R.; Ding, Y.; Du, L. Constituents of the Flowers of Punica granatum. Fitoterapia 2006, 77, 534–537. [Google Scholar] [CrossRef]
- Pungitore, C.R.; García, M.; Gianello, J.C.; Sosa, M.E.; Tonn, C.E. Insecticidal and Antifeedant Effects of Junellia Aspera (Verbenaceae) Triterpenes and Derivatives on Sitophilus Oryzae (Coleoptera: Curculionidae). J. Stored Prod. Res. 2005, 41, 433–443. [Google Scholar] [CrossRef]
- Yu, D.-H.; Bao, Y.-M.; Wei, C.-L.; An, L.-J. Studies of chemical constituents and their antioxidant activities from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 2005, 18, 297–301. [Google Scholar]
- Yang, M.-F.; Li, Y.-Y.; Li, B.-G.; Zhang, G.-L. A New Monoterpene Glycoside from Hemiphragma heterophyllum. J. Integr. Plant Biol. 2004, 46, 1454. [Google Scholar]
- Wang, R.-F.; Xie, W.-D.; Zhang, Z.; Xing, D.-M.; Ding, Y.; Wang, W.; Ma, C.; Du, L.-J. Bioactive Compounds from the Seeds of Punica Granatum (Pomegranate). J. Nat. Prod. 2004, 67, 2096–2098. [Google Scholar] [CrossRef]
- Klimek, B.; Modnicki, D. Terpenoids and sterols from Nepeta cataria L. var. citriodora (Lamiaceae). Acta Pol. Pharm. 2005, 62, 231–235. [Google Scholar] [PubMed]
- Bi, Z.-M.; Wang, Z.-T.; Xu, L.-S. Chemical Constituents of Dendrobium Moniliforme. Acta Bot. Sin. Engl. Ed. 2004, 46, 124–126. [Google Scholar]
- Li, P.; Feng, Z.X.; Ye, D.; Huan, W.; Da Gang, W.; Dong, L.X. Chemical Constituents from the Whole Plant of Euphorbia Altotibetic. Helv. Chim. Acta 2003, 86, 2525–2532. [Google Scholar] [CrossRef]
- Song, E.-K.; Kim, J.-H.; Kim, J.-S.; Cho, H.; Nan, J.-X.; Sohn, D.-H.; Ko, G.-I.; Oh, H.; Kim, Y.-C. Hepatoprotective Phenolic Constituents of Rhodiola Sachalinensis on Tacrine-Induced Cytotoxicity in Hep G2 Cells. Phytother. Res. 2003, 17, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Jiang, B.; Li, X.-M.; Zhang, H.-J.; Zhao, Q.-S.; Li, S.-H.; Sun, H.-D. Constituents of Gnetum Montanum. Fitoterapia 2002, 73, 40–42. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.S.; Jang, J.M.; Park, Y.; Kim, Y.B.; Kim, B.-K. Phytochemical Constituents from the Herba of Artemisia Apiacea. Arch. Pharm. Res. 2002, 25, 285–288. [Google Scholar] [CrossRef]
- Meng, J.C.; Hu, Y.F.; Chen, J.H.; Tan, R.X. Antifungal Highly Oxygenated Guaianolides and Other Constituents from Ajania Fruticulosa. Phytochemistry 2001, 58, 1141–1145. [Google Scholar] [CrossRef]
- Jin-rui, C.; Lin-gang, Q.; Min, L.; Si-ping, J.; Yu, K.; Zhong-wu, M.; Guan-fu, H. Studies on the Chemical Constituents of Rhodiola Fastigita S. H. Fu. J. Integr. Plant Biol. 1991, 33. Available online: https://www.jipb.net/EN/Y1991/V33/I1/ (accessed on 12 October 2021).
- Ling-yi, K.; Zhi-da, M.; Jian-xia, S.; Rui, F. Chemical Constituents from Roots of Jatropha Curcas. J. Integr. Plant Biol. 1996, 38. Available online: https://www.jipb.net/EN/Y1996/V38/I2/ (accessed on 12 October 2021).
- Tan, R.X.; Wolfender, J.-L.; Ma, W.G.; Zhang, L.X.; Hostettmann, K. Secoiridoids and Antifungal Aromatic Acids from Gentiana Algida. Phytochemistry 1996, 41, 111–116. [Google Scholar] [CrossRef]
- Tan, R.X.; Kong, L.D. Secoiridoids from Gentiana Siphonantha. Phytochemistry 1997, 46, 1035–1038. [Google Scholar] [PubMed]
- Tan, R.X.; Tang, H.Q.; Hu, J.; Shuai, B. Lignans and Sesquiterpene Lactones from Artemisia Sieversiana and Inula Racemosa. Phytochemistry 1998, 49, 157–161. [Google Scholar] [CrossRef]
- Wen-Wu, L.I.; Bo-Gang, L.I.; Li-Sheng, D.; Yao-Zu, C. The Chemical Constituents of Rabdosia Coetsa. J. Integr. Plant Biol. 1998, 40. Available online: https://www.jipb.net/EN/Y1998/V40/I5/ (accessed on 12 October 2021).
- Fang, X.; Nanayakkara, N.P.D.; Phoebe, C.H.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Plant Anticancer Agents XXXVII. Constituents of Amanoa Oblongifolia. Planta Med. 1985, 51, 346–347. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Zapesochnaya, G.G.; Shchavlinskii, A.N. Terpenoids of the Rhizomes OfRhodiola Rosea. Chem. Nat. Compd. 1985, 21, 593–597. [Google Scholar] [CrossRef]
- Elyakova, L.A.; Dzizenko, A.K.; Sova, V.V.; Elyakov, G.B. The Isolation of Daucosterol FromAcanthopanax Sessiliflorum. Chem. Nat. Compd. 1966, 2, 26–27. [Google Scholar] [CrossRef]
- Zhao, C.; She, T.; Wang, L.; Su, Y.; Qu, L.; Gao, Y.; Xu, S.; Cai, S.; Shou, C. Daucosterol Inhibits Cancer Cell Proliferation by Inducing Autophagy through Reactive Oxygen Species-Dependent Manner. Life Sci. 2015, 137, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.P.; Wu, H.; Wang, Y.; Weng, X.C. Isolation of Some Compounds from Nutmeg and Their Antioxidant Activities. Czech J. Food Sci. 2012, 30, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Rajavel, T.; Mohankumar, R.; Archunan, G.; Ruckmani, K.; Devi, K.P. Beta Sitosterol and Daucosterol (Phytosterols Identified in Grewia Tiliaefolia) Perturbs Cell Cycle and Induces Apoptotic Cell Death in A549 Cells. Sci. Rep. 2017, 7, 3418. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.; Huang, X.; Liao, T.; Li, G.; Yu, X.; You, Y.; Huang, Y. Daucosterol Induces Autophagic-Dependent Apoptosis in Prostate Cancer via JNK Activation. BioSci. Trends Adv. Publ. 2019, 13, 160–167. [Google Scholar] [CrossRef] [Green Version]
- El Omari, N.; Bakrim, S.; Bakha, M.; Lorenzo, J.M.; Rebezov, M.; Shariati, M.A.; Bouyahya, A. Natural bioactive compounds targeting epigenetic pathways in cancer: A review on alkaloids, terpenoids, quinones, and isothiocyanates. Nutrients 2021, 13, 3714. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Oumeslakht, L.; Zeouk, I.; Aboulaghras, S.; Balahbib, A.; El Omari, N. The role of epigenetic modifications in human cancers and the use of natural compounds as epidrugs: Mechanistic pathways and pharmacodynamic actions. Biomolecules 2022, 12, 367. [Google Scholar] [CrossRef]
- El Omari, N.; Bakha, M.; Imtara, H.; Guaouguaoua, F.E.; Balahbib, A.; Zengin, G.; Bouyahya, A. Anticancer mechanisms of phytochemical compounds: Focusing on epigenetic targets. Environ. Sci. Poll. Res. 2021, 28, 47869–47903. [Google Scholar] [CrossRef]
- Jia, M.; Pang, S.; Liu, X.; Mao, Y.; Wu, C.; Zhang, H. Effect of Dietary Phytochemicals on the Progression of Breast Cancer Metastasis Based on the in Vivo Detection of Circulating Tumor Cells. J. Funct. Foods 2020, 65, 103752. [Google Scholar] [CrossRef]
- Scholl, S.; Augustin, A.; Loewenstein, A.; Rizzo, S.; Kuppermann, B.D. General pathophysiology of macular edema. Eur. J. Ophthalmol. 2011, 21 (Suppl. S6), 10–19. [Google Scholar] [CrossRef]
- Huang, L.-J.; Gao, W.-Y.; Li, X.; Zhao, W.-S.; Huang, L.-Q.; Liu, C.-X. Evaluation of the in Vivo Anti-Inflammatory Effects of Extracts from Pyrus Bretschneideri Rehd. J. Agric. Food Chem. 2010, 58, 8983–8987. [Google Scholar] [CrossRef]
- Kim, M.J.; Wang, H.S.; Lee, M.W. Anti-Inflammatory Effects of Fermented Bark of Acanthopanax Sessiliflorus and Its Isolated Compounds on Lipopolysaccharide-Treated RAW 264.7 Macrophage Cells. Evid. Based Complement. Altern. Med. 2020, 2020, e6749425. [Google Scholar] [CrossRef]
- Bui Thanh, T.; Vu Duc, L.; Nguyen Thanh, H.; Nguyen Tien, V. In Vitro Antioxidant and Anti-Inflammatory Activities of Isolated Compounds of Ethanol Extract from Sanchezia Speciosa Leonard’s Leaves. J. Basic Clin. Physiol. Pharm. 2017, 28, 79–84. [Google Scholar] [CrossRef]
- de Carvalho, A.T.; Paes, M.M.; Cunha, M.S.; Brandão, G.C.; Mapeli, A.M.; Rescia, V.C.; Oesterreich, S.A.; Villas-Boas, G.R. Ethnopharmacology of Fruit Plants: A Literature Review on the Toxicological, Phytochemical, Cultural Aspects, and a Mechanistic Approach to the Pharmacological Effects of Four Widely Used Species. Molecules 2020, 25, 3879. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A Review on Chemical-Induced Inflammatory Bowel Disease Models in Rodents. Korean J. Physiol. Pharm. 2014, 18, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Lee, J.-H. Berberine Synergy with Amphotericin B against Disseminated Candidiasis in Mice. Biol. Pharm. Bull. 2005, 28, 541–544. [Google Scholar] [CrossRef] [Green Version]
- Herzyk, D.J.; Gore, E.R.; Polsky, R.; Nadwodny, K.L.; Maier, C.C.; Liu, S.; Hart, T.K.; Harmsen, A.G.; Bugelski, P.J. Immunomodulatory Effects of Anti-CD4 Antibody in Host Resistance against Infections and Tumors in Human CD4 Transgenic Mice. Infect. Immun. 2001, 69, 1032–1043. [Google Scholar] [CrossRef] [Green Version]
- Saeidnia, S.; Ara, L.; Hajimehdipoor, H.; Read, R.W.; Arshadi, S.; Nikan, M. Chemical Constituents of Swertia Longifolia Boiss. with α-Amylase Inhibitory Activity. Res. Pharm. Sci. 2016, 11, 23–32. [Google Scholar]
- Ivorra, M.; D’ocon, M.P.; Payá, M.; Villar, Á. Antihyperglycemic and Insulin-Releasing Effects of Beta-Sitosterol 3-Beta-D-Glucoside and Its Aglycone, Beta-Sitosterol. Arch. Int. De Pharmacodyn. Et De Ther. 1988, 296, 224–231. [Google Scholar]
- Benny, M.; Antony, B.; Aravind, A.; Gupta, K.; Joseph, B.; Benny, I. Purification and characterization of anti-hyperglycemic bioactive molecule from costus pictus d. don. Int. J. Pharm. Sci. Res. 2020, 11, 2075–2081. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Singh, S.P.; Srivastava, A.; Puri, A. Main Extracts and Hypolipidemic Effects of the Bauhinia Racemosa Lam. Leaf Extract in HFD-Fed Hamsters. Nat. Prod. Res. 2013, 27, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharm. 2019, 10, 1449. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Guo, R.; Guo, W.; Hong, J.; Li, L.; Ni, L.; Sun, J.; Liu, B.; Rao, P.; Lv, X. Monascus Yellow, Red and Orange Pigments from Red Yeast Rice Ameliorate Lipid Metabolic Disorders and Gut Microbiota Dysbiosis in Wistar Rats Fed on a High-Fat Diet. Food Funct. 2019, 10, 1073–1084. [Google Scholar] [CrossRef]
- Mironova, V.N.; Kalashnikova, L.A. [Hypolipidemic action of beta-D-glycoside beta-sitosterol in the rat]. Farm. Toksikol. 1982, 45, 45–47. [Google Scholar]
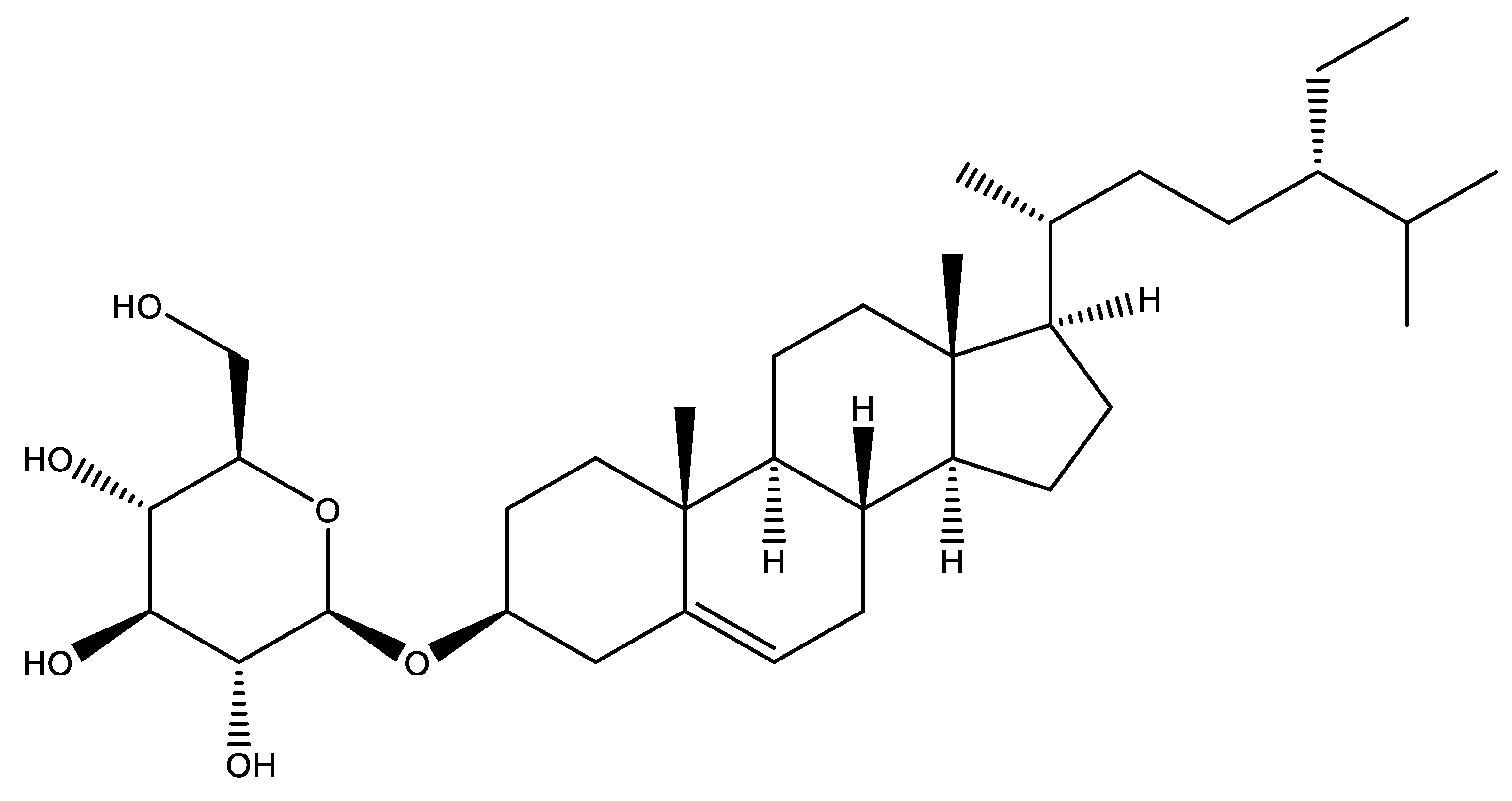

| Plant Family | Parts Used | Extraction Method | Extraction Parameters | Purification Method | Yields | References |
|---|---|---|---|---|---|---|
| Prangos ferulacea Apiaceae | Leaves and stems | Maceration | 750 g extracted by 3 L of n-hexane, dichloromethan, ethyl acetate, and methanol | NMR and FT-IR | - | [9] |
| Cassia italica Fabaceae | Aerial parts | Not reported | Not reported | NMR and HMBC | - | [3] |
| Ononis mitissima L. Fabaceae | Aerial parts | Not reported | Not reported | 1D and 2D NMR, mass spectrometry | - | [8] |
| Centaurea resupinata subsp. dufourii Asteraceae | Aerial parts | Not reported | Not reported | NMR techniques (1H NMR, 13C NMR, COSY, HSQC, HMBC) and mass spectroscopy (ESI-MS) | - | [7] |
| Acanthopanax sessiliflorus (Rupr. and Maxim.) Seem. Araliaceae | Fruits | Not reported | Not reported | Electro-spray ionization/mass spectrometry (ESI/MS), 1H- and 13C-NMR | - | [49] |
| Hyssopus cuspidatus Boriss. Lamiaceae | Aerial parts | Not reported | 17 kg were extracted in triplicate with EtOH (75%) at room temperature (50 L each time). The crude EtOH extract was concentrated under reduced pressure, followed by suspension in water and successive extraction with petroleum ether, ethyl acetate, and n-butanol | Silica gel column chromatography (SGCC) and further purified with MeOH | 15 mg | [6] |
| Rheum turkestanicum Polygonaceae | Roots | Maceration | 3.8 kg extracted with 8 L of n-hexane (24 h × 2) | 1H-, 13C-, 2D NMR, EI-MS, and single-crystal X-ray diffraction | - | [14] |
| Morus alba Moraceae | Leaves | Ultrasound | 1933.76 g extracted in triplicate and successively with n-hexane, EtOAc, and MeOH in sonicator at room temperature (1 h) | NMR, using 1H, 13C, DEPT, COSY, HSQC, and HMBC NMR | - | [35] |
| Pulicaria inuloides Asteraceae | Aerial parts | Not reported | - | 1H-NMR, 13C-NMR, and HMQC | - | [57] |
| Salvia syriaca Lamiaceae | Roots | Maceration | 2.2 kg of powdered material were extracted with acetone (3 × 10 L) by maceration at room temperature | Preparative thin layer chromatography (TLC) (CHCl3–MeOH (85:15)) | 45 mg | [55] |
| Adenophora triphylla Campanulaceae | Not reported | Not reported | Not reported | 1H-NMR, 13C-NMR, and EI mass spectra | - | [27] |
| Sedum caeruleum Crassulaceae | Aerial parts | Not reported | 1500 g of powder was extracted with 80% MeOH. After evaporating the methanol under vacuum, the residue was dissolved in water and extracted with petroleum ether, chloroform, ethyl acetate, and butanol | UV, 1D, 2D NMR, and MS | 7.2 mg | [56] |
| Rosa canina L. Rosaceae | Fruits | Maceration | 1.6 kg of powder was extracted with 4 L of n-hexane, ethyl acetate, acetone, and methanol | 1H- and 13C-NMR | - | [58] |
| Dorema glabrum Fisch. and C.A. Mey. Apiaceae | Aerial parts | Maceration | 0.8 kg was macerated with methanol (4 L × 5) at room temperature | UV and 1H, 13C-NMR | - | [60] |
| Salvia sahendica Lamiaceae | Aerial parts | maceration | 3 kg extracted with Me2CO (7 × 5 L) | 1H and 13C NMR | - | [36] |
| Pyrus spp. Rosaceae | Peels and pulps | Not reported | 10 g extracted with methanol: water (6:4), acid (solvent A) and 1% (v/v) formic acid in acetonitrile (solvent B) | - | - | [62] |
| Punica granatum Lythraceae | Flowers | Not reported | 5 g of powder was extracted with 80% ethanol | - | - | [64] |
| Helicteres isora L. Sterculiaceae | Fruits | Not reported | Powdered material was extracted by stirring with 50 mL of 50% methanol at 25 °C for 24 h and centrifuged at 7000 rpm for 10 min. The pellet was re-extracted with an additional 50 mL of 50% methanol | - | - | [65] |
| Ceiba pentandra L. Bombacaceae | Seeds | Not reported | Powdered material was extracted by stirring with 50 mL of 50% methanol at 25 °C for 24 h and centrifuged at 7000 rpm for 10 min. The pellet was re-extracted with an additional 50 mL of 50% methanol | - | - | [65] |
| Litchi chinensis Sapindaceae | Seeds | Not reported | 10 kg exhaustively extracted three times with 95% ethanol (50 L) at room temperature | - | - | [69] |
| Randia dumetorum Rubiaceae | Bark | Not reported | - | 13C-NMR spectra using 2D NMR (HSQC, HMBC, and DQF-COSY) | [67] | |
| Lysimachia clethroides Primulaceae | Aerial parts | Not reported | 6.75 kg extracted three times with 75% alcohol for 7 days at room temperature. The concentrated extract (480 g), after evaporation of the solvent in a vacuum pump, was suspended in water and extracted with petroleum ether, EtOAC, and n-BuOH | 1H and 13C NMR | - | [66] |
| Urtica angustifolia Urticaceae | Leaves, roots, and stems | Decoction | 1 kg was extracted with water (90 °C, 20 BV) 3 times (15 min/time) | TLC, IR, and ESI-MS spectral | - | [70] |
| Sphallerocarpus gracilis Apiaceae | Roots | Not reported | - | - | - | [73] |
| Hypericum ascyron L. Hypericaceae | Whole plant | Not reported | 450 g was refluxed three times with petroleum ether, EtOAC, and MeOH for 2 h | 13C-NMR | 20.9 mg | [74] |
| Grewia optiva Drummond ex Burret. Tiliaceae | Stem bark | Not reported | 7 kg was extracted three times with ethanol at room temperature. The combined ethanolic extracts were partitioned between EtOAc and water. The EtOAc layer was washed with H2O, dried (anhydrous Na2SO4), and evaporated under reduced pressure to give a gummy residue that was further fractionated into petroleum ether soluble and insoluble fractions | 1D and 2D NMR (HMQC, HMBC, COSY, NOESY, and J-resolved) and EI and HRMS | 6 mg | [18] |
| Boerhaavia diffusa Nyctaginaceae | Leaves | Maceration | 150 g extracted with 1 L of distilled water for 24 h | [77] | ||
| Astragalus membranaceus Fabaceae | Roots | Not reported | 17.8 kg was chopped into small pieces and refluxed with 70% ethanol for 3 h at 70–80 °C | - | - | [80] |
| Phyllenthus emblica L. Phyllanthaceae | Fruits | Not reported | 1100 g extracted with 95% ethanol at room temperature for 7 days | SGCC and TLC | - | [20] |
| Portulaca oleracea L. Portulacaceae | Not reported | Not reported | 1450 g extracted with CHCl3 | 1H and 13C aided with HMQC | - | [21] |
| Eriobotrya fragrans Champ Rosaceae | Fruits and leaves | Not reported | 4.5 kg extracted with 95% EtOH three times for 3 days with 2 shakings per day | 13C-NMR | 20.8 mg | [22] |
| Alchornea cordifolia (Schumach. and Thonn.) Müll. Arg. Euphorbiaceae | Leaves and root bark | Not reported | - | 1D and 2D NMR spectra were recorded in CDCl3 | - | [82] |
| Arctotis arctotoides Asteraceae | Not reported | NMR (COSY, NOESY, HMQC, and HMBC) and mass spectra | [23] | |||
| Dioscorea opposita Dioscoreaceae | Aerial parts | Not reported | 4 kg extracted with 95% ethanol under reflux. The ethanol crude extract (115 g) was suspended in water and partitioned successively with petroleum ether, EtOAc, and n-butanol | Silica gel column chromatography using CHCl3-MeOH mixtures (95:5 f 3:1) | 300 mg | [19] |
| Astragalus mongholicus Bunge Fabaceae | Roots | Maceration | 10 kg extracted with 90% ethanol at room temperature. The alcoholic solution was concentrated under vacuum The concentrated extract was diluted with water. The water solution was successively extracted with petroleum ether, ethyl acetate, and, finally, n-butanol | 13C-NMR | - | [91] |
| Punica granatum Punicaceae | Seeds | Not reported | 4 kg extracted with 95% ethanol under reflux. The ethanol crude extract (489 g) was suspended in water and partitioned successively with petroleum ether, EtOAc, and n-butanol | 1H and 13C NMR | 205 mg | [93] |
| Artemisia sieversiana Asteraceae | Aerial parts | Not reported | 14 kg extracted twice for 37 h at room temperature with MeOH | Combination of spectral methods (IR, EIMS, H and 2CNMR, DEPT, COSY, NOESY, and HETCOR) | 64 mg | [105] |
| Methods Used | Key Results | References |
|---|---|---|
| DPPH | IC50 = 490 ± 2.9 µg/Ml | [9] |
| DPPH | RSA% = 19.7% at 100 mg/mL | [3] |
| DPPH | IC50 = 27.3 ± 0.015 µg/mL | [8] |
| H2O2 | I% = 31.18 ± 0.5% | |
| FTC | I% = 37.12 ± 0.44% | |
| FRAP | EC50 = 122.23 ± 0.014 µg EAA/mg ex | |
| PPM | EC50 = 16.44 ± 0.0012 µg EAA/mg ex | |
| DPPH | IC50 = 36.263 ± 0.005 µg/mL | [7] |
| DPPH | No effect | [6] |
| DPPH | IC50 = 155.0 ± 0.5 μM | [14] |
| DPPH | IC50 = 224.1 ± 8.2 µg/mL | [60] |
| DPPH | IC50 = 6.0 ± 0.1 mg/L | [64] |
| ABTS | IC50 = 4.8 ± 0.04 mg/mL | [111] |
| ABTS | I% = 27% at 0.04 mg/mL | |
| FRAP | EC50 = 1.09 ± 0.12 mg/mL | |
| DPPH | EC50 > 250 µg/mL | [69] |
| ABTS | EC50 = 143.4 µg/mL | |
| DPPH | IC50 = 11.42 ± 0.07 µg/mL | [66] |
| ABTS | IC50 = 9.02 ± 0.11 µg/mL | |
| DPPH | IC50 = 108.14 ± 9.54 µg GAE/mL | [73] |
| FRAP | IC50 = 4.91 ± 0.39 µmol Fe(II)/g of DW | |
| ABTS | IC50 = 1.66 ± 0.15 µmol TE/g of DW | |
| DPPH | IC50 = 38.86 µg/mL | [20] |
| ABTS | IC50 = 0.71 ± 0.01 µg/mL |
| Cell Lines | Key Results | References |
|---|---|---|
| Human breast adenocarcinoma MCF-7 | Suppressed the proliferation of MCF-7 cells Induced the cytotoxicity of MCF-7 cells Modulated Bax, Bcl2, and PARP Reduced the mitochondrial membrane potential Increased the levels of cytochrome c (Cyt c) released Upregulated the PTEN gene and inhibited the PI3K/Akt pathway Decreased the intracellular GSH content | [36] |
| Human breast cancer MCF-7 | IC50 = 16.95 μM Inhibited colony formation of MCF-7 cells Induced autophagy Induced the conversion and aggregation of LC3-II Increased the expression of Beclin-1 | [110] |
| Gastric cancer MGC803 | IC50 = 19.96 μM Induced autophagy Induced the conversion and aggregation of LC3-II Increased the expression of Beclin-1 | |
| Gastric cancer BGC823 | IC50 = 3.13 μM Inhibited colony formation of BGC823 cells Increased ROS production Induced autophagy Induced the conversion and aggregation of LC3-II Increased the expression of Beclin-1 | |
| Gastric cancer AGS | IC50 = 24.19 μM Induced autophagy Induced the conversion and aggregation of LC3-II Increased the expression of Beclin-1 | |
| Human colon cancer HCT-116 | IC50 = 26.6 μM at 24 h IC50 = 47.3 μM at 48 h Decreased the percentage of migrated cells by 14.4% at 100 μM Increased the percentage of apoptotic cells by 74.2% at 100 μM Induced cell-cycle arrest at sub-G1 phase | [38] |
| Human lung cancer A549 | Inhibited the proliferation of A549 cells IC50 = 17.46 μg/mL at 48 h Perturbed cell cycle Induced apoptotic cell death | [112] |
| Human HCC HepG2; Human HCC SMMC-7721 | Reduced the proliferation of HepG2 and SMMC-7721 cells Decreased cell migration and invasion abilities of both cells Reduced the levels of β-catenin and p-β-catenin Suppressed the expression of Wnt/ β-catenin signaling proteins | [40] |
| Breast cancer MCF-7; MCF-7 xenografts in nude mice | IC50 = 53.27 μg/mL Inhibited cell viability in ER-positive MCF-7 cells Induced apoptosis in MCF-7 cells Diminished the expression of Bcl-xl, Bcl-2, and XIAP Increased Bax, Bad, and activated caspase Inactivated the upstream PI3K/Akt/NF-κB pathway | [37] |
| Breast cancer MDA-MB-231 | IC50 > 1000 μg/mL | |
| Breast cancer 4T1 | IC50 > 1000 μg/mL Blocked metastasis progression Decreased the number of visible metastasis foci Inhibited metastasis size distribution in lung tissue | |
| Nontumorigenic breast epithelial MCF-10A | No cytotoxicity | |
| Human prostate cancer (PC3 and LNCap) | Inhibited cell proliferation Induced cell-cycle arrest Promoted apoptosis and autophagy Increased phosphorylation of c-Jun N-terminal kinase (JNK) | [113] |
| Human lung cancer A549 | IC50 = 20.9 Μm Inhibited the growth of A549 cells Increased reactive oxygen species (ROS) level Promoted intrinsic apoptotic cell death Increased the expression of caspase-3, caspase-9, Bax, PARP inactivation, and Cyt c release Diminished the expression of Bcl-2 protein Inhibited the thioredoxin (TrxR) redox system | [26] |
| Breast cancer MCF-7; MCF-7 xenografts in nude mice | Induced cytotoxicity Decreased the proliferation rates Increased the number of apoptotic cells Activated the expressions of caspase 3 and PARP1 Reduced the tumor volume Decreased the levels of CEA, CA125, and CA153 Increased the expression of cleaved caspase 3 Decreased the BCL-2 and VEGF Downregulated the expression of PI3K/Akt Repressed insulin-induced PI3K/Akt activation | [25] |
| MDA-MB-231 | Exhibited a weak effect on cell proliferation | |
| 4T1 nontumorigenic | Exhibited a weak effect on cell proliferation | |
| Breast epithelial MCF-10A | Exhibited a weak effect on cell proliferation | |
| 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Wistar rats | Reduced tumor volume Decreased the levels of protein and malondialdehyde (MDA) Reduced cancer antigen (CA) 15-3 level Decreased MDA levels Increased catalase activity Reduced proliferation of mammary duct cells | [24] |
| Prostate carcinoma LNCaP | Inhibited cell growth and proliferation | [48] |
| Prostate carcinoma DU145 | Inhibited cell growth and proliferation Increased the number of late apoptotic cells Increased the number of cells in S phase Decreased the number of G0/G1 cells Downregulated the cell-cycle proteins (cdk1, pcdk1, cyclin A and B) | |
| Prostate carcinoma PC3 | Inhibited cell growth and proliferation Increased the number of late apoptotic cells Downregulated the cell-cycle proteins (cdk1, pcdk1, cyclin A and B) Downregulated cdk2 Downregulated Akt, pAKT, and Bcl-2 proteins Upregulated the pro-apoptotic protein Bax | |
| Breast tumor BALB/c mouse model | Suppressed primary tumor growth Reduced lung metastases Increased the number of captured CTCs in the blood circulation | [117] |
| Murine H22 hepatoma allograft model in ICR mice | Inhibited murine hepatoma H22 cell growth Induced intracellular ROS generation Induced autophagy | [110] |
| Experimental Approaches | Key Results | References |
|---|---|---|
| Oxygen–glucose deprivation/reperfusion-mediated injury in OGD/R model | Reduced neuronal loss and apoptotic rate Suppressed caspase-3 activity Upregulated the expression of IGF1 protein Activated the AKT signal pathway Diminished the downregulation of the Mcl-1 and Bcl-2 Decreased the expression level of protein Bax | [40] |
| H2O2-induced cell death of human brain neuroblastoma SK-N-SH cells | Inhibited cell death and LDH activity Reduced intracellular ROS levels by 37.7% Reduced H2O2-induced apoptotic cell death Reduced H2O2-mediated fragmented DNA Increased CAT and SOD2 mRNA levels Attenuated H2O2-induced phosphorylation of p38 and JNK | [41] |
| Cerebral ischemia/reperfusion (I/R) rat model | Decreased apoptotic cell death Suppressed iNOS expression in ischemic zone Upregulated the PI3K/Akt/mTOR signaling pathway | [30] |
| Experimental Approaches | Key Results | References |
|---|---|---|
| α-amylase inhibitors | Inhibition = 57.5 ± 3.1% in a 10 mg/mL | [126] |
| α-glucosidase inhibitors | IC50 = 247.35 mg/L Inhibition constant = 2.34 mg/L | [42] |
| Normal and hyperglycemic rats | Increased fasting plasma insulin levels Enhanced oral glucose tolerance Improved glucose-induced insulin release | [127] |
| Molecular docking | Inhibited human α-glucosidase | [53] |
| α-glucosidase inhibitors | IC50 = 13.3 ± 1.9 µM | [58] |
| Glucose tolerance test | Significant hypoglycemic activity | [128] |
| α-glucosidase inhibitors | IC50 = 5.67 mg/L | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. https://doi.org/10.3390/app12125779
El Omari N, Jaouadi I, Lahyaoui M, Benali T, Taha D, Bakrim S, El Menyiy N, El Kamari F, Zengin G, Bangar SP, et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Applied Sciences. 2022; 12(12):5779. https://doi.org/10.3390/app12125779
Chicago/Turabian StyleEl Omari, Nasreddine, Imane Jaouadi, Manal Lahyaoui, Taoufiq Benali, Douae Taha, Saad Bakrim, Naoual El Menyiy, Fatima El Kamari, Gökhan Zengin, Sneh Punia Bangar, and et al. 2022. "Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions" Applied Sciences 12, no. 12: 5779. https://doi.org/10.3390/app12125779
APA StyleEl Omari, N., Jaouadi, I., Lahyaoui, M., Benali, T., Taha, D., Bakrim, S., El Menyiy, N., El Kamari, F., Zengin, G., Bangar, S. P., Lorenzo, J. M., Gallo, M., Montesano, D., & Bouyahya, A. (2022). Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Applied Sciences, 12(12), 5779. https://doi.org/10.3390/app12125779












