Machine-Learning Applications in Oral Cancer: A Systematic Review
Abstract
:1. Introduction
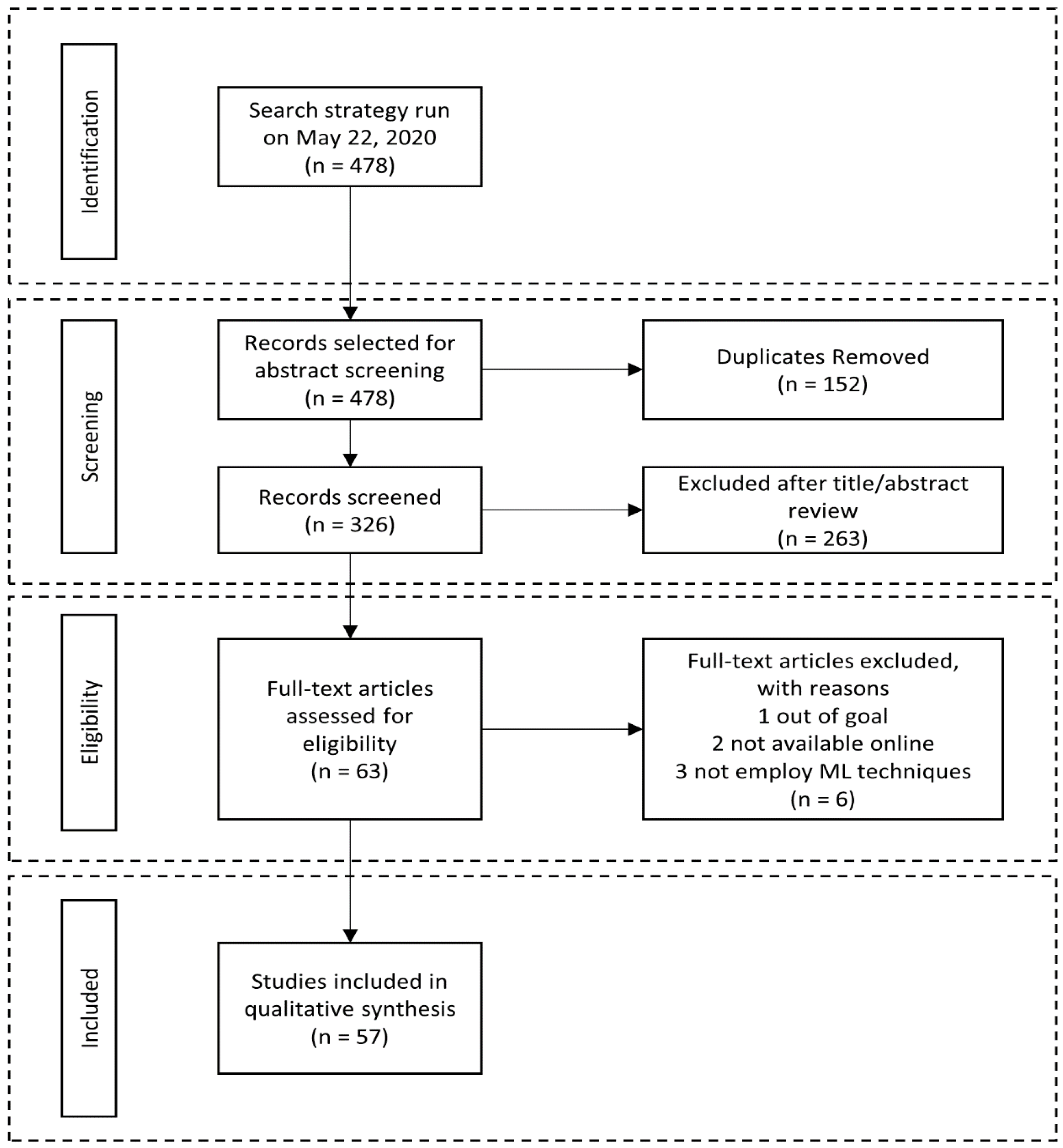
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria and Study Selection Process
- -
- Articles reporting data related to the machine-learning applications in oral cancer disease.
- -
- Only original articles in English language were considered.
- -
- Case reports, lectures, data in brief, reviews, in vitro studies (on animals and on human cell lines) and non-original data were excluded from this study.
- -
- Articles that did not involve a concrete machine-learning application in oral cancer disease were excluded.
2.3. Data Collection and Extraction
3. Results
3.1. Risk of Bias
3.2. Predictive Model Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, Z.; Hu, C.; He, H.; Li, Y.; Lyu, J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the global burden of disease study. Cancer Commun. 2020, 40, 81–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lip, Oral Cavity Fact Sheet. 2020. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/1-Lip-oral-cavity-fact-sheet.pdf (accessed on 1 March 2022).
- Kowalski, L.P.; de Oliveira, M.M.; Lopez, R.V.M.; e Silva, D.R.M.; Ikeda, M.K.; Curado, M.P. Survival trends of patients with oral and oropharyngeal cancer treated at a cancer center in São Paulo, Brazil. Clinics 2020, 75, e1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roi, A.; Roi, C.I.; Andreescu, N.I.; Riviş, M.; Badea, I.D.; Meszaros, N.; Rusu, L.C.; Iurciuc, S. Oral cancer histopathological subtypes in association with risk factors: A 5-year retrospective study. Rom. J. Morphol. Embryol. 2020, 61, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.F.; Silva, K.D.D.; Horta, M.C.R.; de Aguiar, M.C.F. Can morphological features evaluated in oral cancer biopsies influence in decision-making? A preliminary study. Pathol. Res. Pract. 2020, 216, 153138. [Google Scholar] [CrossRef]
- Yap, T.; Celentano, A.; Seers, C.; McCullough, M.J.; Farah, C.S. Molecular diagnostics in oral cancer and oral potentially malignant disorders—A clinician’s guide. J. Oral Pathol. Med. 2020, 49, 1–8. [Google Scholar] [CrossRef]
- Tapia-Castillo, A.; Carvajal, C.A.; López-Cortés, X.; Vecchiola, A.; Fardella, C.E. Novel metabolomic profile of subjects with non-classic apparent mineralocorticoid excess. Sci. Rep. 2021, 11, 17156. [Google Scholar] [CrossRef]
- Kulkarni, S.; Seneviratne, N.; Baig, M.S.; Khan, A.H.A. Artificial intelligence in medicine: Where are we now? Acad. Radiol. 2020, 27, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, A.F.; Eisenberg, M.C.; Meza, R. Age effects and temporal trends in HPV-related and HPV-unrelated oral cancer in the United States: A multistage carcinogenesis modeling analysis. PLoS ONE 2016, 11, e0151098. [Google Scholar] [CrossRef]
- Sorrell, I.; Shipley, R.J.; Hearnden, V.; Colley, H.E.; Thornhill, M.H.; Murdoch, C.; Webb, S.D. Combined mathematical modelling and experimentation to predict polymersome uptake by oral cancer cells. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Qi, M.; Yuan, Y.; Duan, S.; Tao, X. Machine learning–based MRI texture analysis to predict the histologic grade of oral squamous cell carcinoma. Am. J. Roentgenol. 2020, 215, 1184–1190. [Google Scholar] [CrossRef]
- Chu, C.S.; Lee, N.P.; Adeoye, J.; Thomson, P.; Choi, S. Machine learning and treatment outcome prediction for oral cancer. J. Oral Pathol. Med. 2020, 49, 977–985. [Google Scholar] [CrossRef]
- Shan, J.; Jiang, R.; Chen, X.; Zhong, Y.; Zhang, W.; Xie, L.; Cheng, J.; Jiang, H. Machine learning predicts lymph node metastasis in early-stage oral tongue squamous cell carcinoma. J. Oral Maxillofac. Surg. 2020, 78, 2208–2218. [Google Scholar] [CrossRef]
- Hadzic, S.; Gojkov-Vukelic, M.; Pasic, E.; Dervisevic, A. Importance of early detection of potentially malignant lesions in the prevention of oral cancer. Mater. Socio-Med. 2017, 29, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Wolff, R.F.; Moons, K.G.M.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A tool to assess the risk of bias and applicability of prediction model studies. Ann. Intern. Med. 2019, 170, 51. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Wu, Q.; Li, Q.; Yao, M.; Zhou, H. A 3-mRNA-based prognostic signature of survival in oral squamous cell carcinoma. PeerJ 2019, 7, e7360. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.S.; Tan, J.W.; Chang, S.-W.; Yap, H.J.; Abdul Kareem, S.; Zain, R.B. A genetic programming approach to oral cancer prognosis. PeerJ 2016, 4, e2482. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.-H.; Chen, W.-M.; Hsieh, Y.-S.; Fan, Y.-C.; Yang, P.E.; Kang, S.-T.; Liao, C.-T. A novel multi-gene detection platform for the analysis of miRNA expression. Sci. Rep. 2018, 8, 10684. [Google Scholar] [CrossRef] [Green Version]
- Paul, R.R. A novel wavelet neural network based pathological stage detection technique for an oral precancerous condition. J. Clin. Pathol. 2005, 58, 932–938. [Google Scholar] [CrossRef] [Green Version]
- Sunny, S.; Baby, A.; James, B.L.; Balaji, D.; Aparna, N.V.; Rana, M.H.; Gurpur, P.; Skandarajah, A.; D’Ambrosio, M.; Ramanjinappa, R.D.; et al. A smart tele-cytology point-of-care platform for oral cancer screening. PLoS ONE 2019, 14, e0224885. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, V.; Kumar Singh, A.; Acharya, V. A systematic approach to prioritize drug targets using machine learning, a molecular descriptor-based classification model, and high-throughput screening of plant derived molecules: A case study in oral cancer. Mol. Biosyst. 2015, 11, 3362–3377. [Google Scholar] [CrossRef]
- Cheng, C.S.; Shueng, P.W.; Chang, C.C.; Kuo, C.W. Adapting an evidence-based diagnostic model for predicting recurrence risk factors of oral cancer. J. Univ. Comput. Sci. 2018, 24, 742–752. [Google Scholar]
- Kan, C.; Lee, A.Y.; Nieman, L.T.; Sokolov, K.; Markey, M.K. Adaptive spectral window sizes for extraction of diagnostic features from optical spectra. J. Biomed. Opt. 2010, 15, 047012. [Google Scholar] [CrossRef]
- Downer, M.C.; Jullien, J.A.; Speight, P.M. An interim determination of health gain from oral cancer and precancer screening: 3. Preselecting high risk individuals. Community Dent. Health 1998, 15, 72–76. [Google Scholar] [PubMed]
- Lu, C.; Lewis, J.S.; Dupont, W.D.; Plummer, W.D.; Janowczyk, A.; Madabhushi, A. An oral cavity squamous cell carcinoma quantitative histomorphometric-based image classifier of nuclear morphology can risk stratify patients for disease-specific survival. Mod. Pathol. 2017, 30, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, M.; ten Bosch, L.; Kuik, D.J.; Langendijk, J.A.; Leemans, C.R.; Leeuw, I.V. Artificial neural network analysis to assess hypernasality in patients treated for oral or oropharyngeal cancer. Logop. Phoniatr. Vocol. 2011, 36, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Muthu Rama Krishnan, M.; Pal, M.; Bomminayuni, S.K.; Chakraborty, C.; Paul, R.R.; Chatterjee, J.; Ray, A.K. Automated classification of cells in sub-epithelial connective tissue of oral sub-mucous fibrosis—An SVM based approach. Comput. Biol. Med. 2009, 39, 1096–1104. [Google Scholar] [CrossRef]
- Das, N.; Hussain, E.; Mahanta, L.B. Automated classification of cells into multiple classes in epithelial tissue of oral squamous cell carcinoma using transfer learning and convolutional neural network. Neural Netw. 2020, 128, 47–60. [Google Scholar] [CrossRef]
- Krishnan, M.M.R.; Acharya, U.R.; Chakraborty, C.; Ray, A.K. Automated diagnosis of oral cancer using higher order spectra features and local binary pattern: A comparative study. Technol. Cancer Res. Treat. 2011, 10, 443–455. [Google Scholar] [CrossRef]
- Rahman, T.Y.; Mahanta, L.B.; Das, A.K.; Sarma, J.D. Automated oral squamous cell carcinoma identification using shape, texture and color features of whole image strips. Tissue Cell 2020, 63, 101322. [Google Scholar] [CrossRef]
- Aubreville, M.; Knipfer, C.; Oetter, N.; Jaremenko, C.; Rodner, E.; Denzler, J.; Bohr, C.; Neumann, H.; Stelzle, F.; Maier, A. Automatic classification of cancerous tissue in laserendomicroscopy images of the oral cavity using deep learning. Sci. Rep. 2017, 7, 11979. [Google Scholar] [CrossRef] [Green Version]
- Das, D.K.; Bose, S.; Maiti, A.K.; Mitra, B.; Mukherjee, G.; Dutta, P.K. Automatic identification of clinically relevant regions from oral tissue histological images for oral squamous cell carcinoma diagnosis. Tissue Cell 2018, 53, 111–119. [Google Scholar] [CrossRef]
- Van Staveren, H.J.; Van Veen, R.L.P.; Speelman, O.C.; Witjes, M.J.H.; Star, W.M.; Roodenburg, J.L.N. Classification of clinical autofluorescence spectra of oral leukoplakia using an artificial neural network: A pilot study. Oral Oncol. 2000, 36, 286–293. [Google Scholar] [CrossRef]
- Ge, S.; Lu, H.; Li, Q.; Logan, H.L.; Dodd, V.J.; Bian, J.; Shenkman, E.A.; Guo, Y. Classification tree analysis of factors associated with oral cancer exam. Am. J. Health Behav. 2019, 43, 635–647. [Google Scholar] [CrossRef]
- de Veld, D.C.G.; Skurichina, M.; Witjes, M.J.H.; Duin, R.P.W.; Sterenborg, H.J.C.M.; Roodenburg, J.L.N. Clinical study for classification of benign, dysplastic, and malignant oral lesions using autofluorescence spectroscopy. J. Biomed. Opt. 2004, 9, 940–950. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, G.; Nagata, T.; Mattern, D.; Schmelzeisen, R.; Schumacher, M. Comparison of fuzzy inference, logistic regression, and classification trees (CART): Prediction of cervical lymph node metastasis in carcinoma of the tongue. Methods Inf. Med. 2003, 42, 572–577. [Google Scholar] [CrossRef]
- Das, D.K.; Mitra, P.; Chakraborty, C.; Chatterjee, S.; Maiti, A.K.; Bose, S. Computational approach for mitotic cell detection and its application in oral squamous cell carcinoma. Multidimens. Syst. Signal Process. 2017, 28, 1031–1050. [Google Scholar] [CrossRef]
- Jeyaraj, P.R.; Samuel Nadar, E.R. Computer-assisted medical image classification for early diagnosis of oral cancer employing deep learning algorithm. J. Cancer Res. Clin. Oncol. 2019, 145, 829–837. [Google Scholar] [CrossRef]
- Ariji, Y.; Fukuda, M.; Kise, Y.; Nozawa, M.; Yanashita, Y.; Fujita, H.; Katsumata, A.; Ariji, E. Contrast-enhanced computed tomography image assessment of cervical lymph node metastasis in patients with oral cancer by using a deep learning system of artificial intelligence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 458–463. [Google Scholar] [CrossRef]
- Yu, M.; Yan, H.; Xia, J.; Zhu, L.; Zhang, T.; Zhu, Z.; Lou, X.; Sun, G.; Dong, M. Deep convolutional neural networks for tongue squamous cell carcinoma classification using Raman spectroscopy. Photodiagnosis Photodyn. Ther. 2019, 26, 430–435. [Google Scholar] [CrossRef]
- Dong, F.; Tao, C.; Wu, J.; Su, Y.; Wang, Y.; Wang, Y.; Guo, C.; Lyu, P. Detection of cervical lymph node metastasis from oral cavity cancer using a non-radiating, noninvasive digital infrared thermal imaging system. Sci. Rep. 2018, 8, 7219. [Google Scholar] [CrossRef]
- Karadaghy, O.A.; Shew, M.; New, J.; Bur, A.M. Development and assessment of a machine learning model to help predict survival among patients with oral squamous cell carcinoma. JAMA Otolaryngol. Neck Surg. 2019, 145, 1115–1120. [Google Scholar] [CrossRef]
- Spyridonos, P.; Gaitanis, G.; Bassukas, I.D.; Tzaphlidou, M. Evaluation of vermillion border descriptors and relevance vector machines discrimination model for making probabilistic predictions of solar cheilosis on digital lip photographs. Comput. Biol. Med. 2015, 63, 11–18. [Google Scholar] [CrossRef]
- Banerjee, S.; Pal, M.; Chakrabarty, J.; Petibois, C.; Paul, R.R.; Giri, A.; Chatterjee, J. Fourier-transform-infrared-spectroscopy based spectral-biomarker selection towards optimum diagnostic differentiation of oral leukoplakia and cancer. Anal. Bioanal. Chem. 2015, 407, 7935–7943. [Google Scholar] [CrossRef]
- Zlotogorski-Hurvitz, A.; Dekel, B.Z.; Malonek, D.; Yahalom, R.; Vered, M. FTIR-based spectrum of salivary exosomes coupled with computational-aided discriminating analysis in the diagnosis of oral cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liang, X.; Fu, Y.; Liu, Y.; Zheng, L.; Liu, F.; Li, T.; Yin, X.; Qiao, X.; Xu, X. Identification of AUNIP as a candidate diagnostic and prognostic biomarker for oral squamous cell carcinoma. EBioMedicine 2019, 47, 44–57. [Google Scholar] [CrossRef] [Green Version]
- Winck, F.V.; Prado Ribeiro, A.C.; Ramos Domingues, R.; Ling, L.Y.; Riaño-Pachón, D.M.; Rivera, C.; Brandão, T.B.; Gouvea, A.F.; Santos-Silva, A.R.; Coletta, R.D.; et al. Insights into immune responses in oral cancer through proteomic analysis of saliva and salivary extracellular vesicles. Sci. Rep. 2015, 5, 16305. [Google Scholar] [CrossRef]
- Hsu, C.-W.; Chen, Y.-T.; Hsieh, Y.-J.; Chang, K.-P.; Hsueh, P.-C.; Chen, T.-W.; Yu, J.-S.; Chang, Y.-S.; Li, L.; Wu, C.-C. Integrated analyses utilizing metabolomics and transcriptomics reveal perturbation of the polyamine pathway in oral cavity squamous cell carcinoma. Anal. Chim. Acta 2019, 1050, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, V.; Acharya, V. Integrated network analysis and logistic regression modeling identify stage-specific genes in oral squamous cell carcinoma. BMC Med. Genom. 2015, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bur, A.M.; Holcomb, A.; Goodwin, S.; Woodroof, J.; Karadaghy, O.; Shnayder, Y.; Kakarala, K.; Brant, J.; Shew, M. Machine learning to predict occult nodal metastasis in early oral squamous cell carcinoma. Oral Oncol. 2019, 92, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Srivastava, S.; Roy, R.; Anand, A.; Gaurav, K.; Husain, N.; Jain, S.; Sonkar, A.A. Malignancy prediction among tissues from oral SCC patients including neck invasions: A 1H HRMAS NMR based metabolomic study. Metab. Off. J. Metab. Soc. 2020, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Vittal, S.; Karthikeyan, G. Modeling association detection in order to discover compounds to inhibit oral cancer. J. Biomed. Inform. 2018, 84, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Abdul-Kareem, S.; Merican, A.F.; Zain, R.B. Oral cancer prognosis based on clinicopathologic and genomic markers using a hybrid of feature selection and machine learning methods. BMC Bioinform. 2013, 14, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, A.; Paul, R.R.; Chaudhuri, K.; Chatterjee, J.; Pal, M.; Banerjee, P.; Mukherjee, K.; Banerjee, S.; Dutta, P.K. Performance analysis of different wavelet feature vectors in quantification of oral precancerous condition. Oral Oncol. 2006, 42, 914–928. [Google Scholar] [CrossRef]
- Brickley, M.R.; Cowpe, J.G.; Shepherd, J.P. Performance of a computer simulated neural network trained to categorise normal, premalignant and malignant oral smears. J. Oral Pathol. Med. 1996, 25, 424–428. [Google Scholar] [CrossRef]
- Campisi, G.; Calvino, F.; Carinci, F.; Matranga, D.; Carella, M.; Mazzotta, M.; Rubini, C.; Panzarella, V.; Santarelli, A.; Fedele, S.; et al. Peri-tumoral inflammatory cell infiltration in OSCC: A reliable marker of local recurrence and prognosis? An investigation using artificial neural networks. Int. J. Immunopathol. Pharmacol. 2011, 24, 113–120. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Tsai, T.; Chen, H.-M.; Chen, C.-T.; Chiang, C.-P. PLS-ANN based classification model for oral submucous fibrosis and oral carcinogenesis. Lasers Surg. Med. 2003, 32, 318–326. [Google Scholar] [CrossRef]
- McRae, M.P.; Modak, S.S.; Simmons, G.W.; Trochesset, D.A.; Kerr, A.R.; Thornhill, M.H.; Redding, S.W.; Vigneswaran, N.; Kang, S.K.; Christodoulides, N.J.; et al. Point-of-care oral cytology tool for the screening and assessment of potentially malignant oral lesions. Cancer Cytopathol. 2020, 128, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Uthoff, R.D.; Song, B.; Sunny, S.; Patrick, S.; Suresh, A.; Kolur, T.; Keerthi, G.; Spires, O.; Anbarani, A.; Wilder-Smith, P.; et al. Point-of-care, smartphone-based, dual-modality, dual-view, oral cancer screening device with neural network classification for low-resource communities. PLoS ONE 2018, 13, e0207493. [Google Scholar] [CrossRef]
- Dey, S.; Sarkar, R.; Chatterjee, K.; Datta, P.; Barui, A.; Maity, S.P. Pre-cancer risk assessment in habitual smokers from DIC images of oral exfoliative cells using active contour and SVM analysis. Tissue Cell 2017, 49, 296–306. [Google Scholar] [CrossRef]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of tumor grade and nodal status in oropharyngeal and oral cavity squamous-cell carcinoma using a radiomic approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Nayak, G.S.; Kamath, S.; Pai, K.M.; Sarkar, A.; Ray, S.; Kurien, J.; D’Almeida, L.; Krishnanand, B.R.; Santhosh, C.; Kartha, V.B.; et al. Principal component analysis and artificial neural network analysis of oral tissue fluorescence spectra: Classification of normal premalignant and malignant pathological conditions. Biopolymers 2006, 82, 152–166. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Fu, Y.; Liu, T.; Liu, X.; Zhang, X.; Fu, J.; Guan, X.; Chen, T.; Chen, X.; et al. Quantitative prediction of oral cancer risk in patients with oral leukoplakia. Oncotarget 2017, 8, 46057–46064. [Google Scholar] [CrossRef] [Green Version]
- Majumder, S.K.; Ghosh, N.; Gupta, P.K. Relevance vector machine for optical diagnosis of cancer. Lasers Surg. Med. 2005, 36, 323–333. [Google Scholar] [CrossRef]
- Muthu Rama Krishnan, M.; Shah, P.; Chakraborty, C.; Ray, A.K. Statistical analysis of textural features for improved classification of oral histopathological images. J. Med. Syst. 2012, 36, 865–881. [Google Scholar] [CrossRef]
- Nawandhar, A.; Kumar, N.; Veena, R.; Yamujala, L. Stratified squamous epithelial biopsy image classifier using machine learning and neighborhood feature selection. Biomed. Signal Process. Control 2020, 55, 101671. [Google Scholar] [CrossRef]
- Tseng, W.-T.; Chiang, W.-F.; Liu, S.-Y.; Roan, J.; Lin, C.-N. The application of data mining techniques to oral cancer prognosis. J. Med. Syst. 2015, 39, 59. [Google Scholar] [CrossRef]
- Brouwer de Koning, S.G.; Baltussen, E.J.M.; Karakullukcu, M.B.; Dashtbozorg, B.; Smit, L.A.; Dirven, R.; Hendriks, B.H.W.; Sterenborg, H.J.C.M.; Ruers, T.J.M. Toward complete oral cavity cancer resection using a handheld diffuse reflectance spectroscopy probe. J. Biomed. Opt. 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Sharma, N.; Om, H. Usage of probabilistic and general regression neural network for early detection and prevention of oral cancer. Sci. World J. 2015, 2015, 234191. [Google Scholar] [CrossRef] [Green Version]
- Campisi, G.; Di Fede, O.; Giovannelli, L.; Capra, G.; Greco, I.; Calvino, F.; Maria Florena, A.; Lo Muzio, L. Use of fuzzy neural networks in modeling relationships of HPV infection with apoptotic and proliferation markers in potentially malignant oral lesions. Oral Oncol. 2005, 41, 994–1004. [Google Scholar] [CrossRef]
- Carnielli, C.M.; Macedo, C.C.S.; De Rossi, T.; Granato, D.C.; Rivera, C.; Domingues, R.R.; Pauletti, B.A.; Yokoo, S.; Heberle, H.; Busso-Lopes, A.F.; et al. Combining discovery and targeted proteomics reveals a prognostic signature in oral cancer. Nat. Commun. 2018, 9, 3598. [Google Scholar] [CrossRef]
- Muzio, L.L.; D’Angelo, M.; Procaccini, M.; Bambini, F.; Calvino, F.; Florena, A.M.; Franco, V.; Giovannelli, L.; Ammatuna, P.; Campisi, G. Expression of cell cycle markers and human papillomavirus infection in oral squamous cell carcinoma: Use of fuzzy neural networks. Int. J. Cancer 2005, 115, 717–723. [Google Scholar] [CrossRef]
- Steinkraus, D.; Buck, I.; Simard, P.Y. Using GPUs for machine learning algorithms. In Proceedings of the Eighth International Conference on Document Analysis and Recognition (ICDAR’05), Seoul, Korea, 31 August–1 September 2005; Volume 2, pp. 1115–1120. [Google Scholar]
- Jafari, A.; Najafi, S.; Moradi, F.; Kharazifard, M.; Khami, M. Delay in the diagnosis and treatment of oral cancer. J. Dent. 2013, 14, 146–150. [Google Scholar]
- Ford, P.J.; Farah, C.S. Early detection and diagnosis of oral cancer: Strategies for improvement. J. Cancer Policy 2013, 1, e2–e7. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Gallegos, R.; Figueroa, C. Biomarkers of progression to oral cancer in patients with dysplasia: A systematic review. Mol. Clin. Oncol. 2020, 13, 42. [Google Scholar] [CrossRef]
- Rivera, C. The challenge of the state of susceptibility to oral cancer. J. Oral Res. 2015, 4, 8–9. [Google Scholar] [CrossRef]
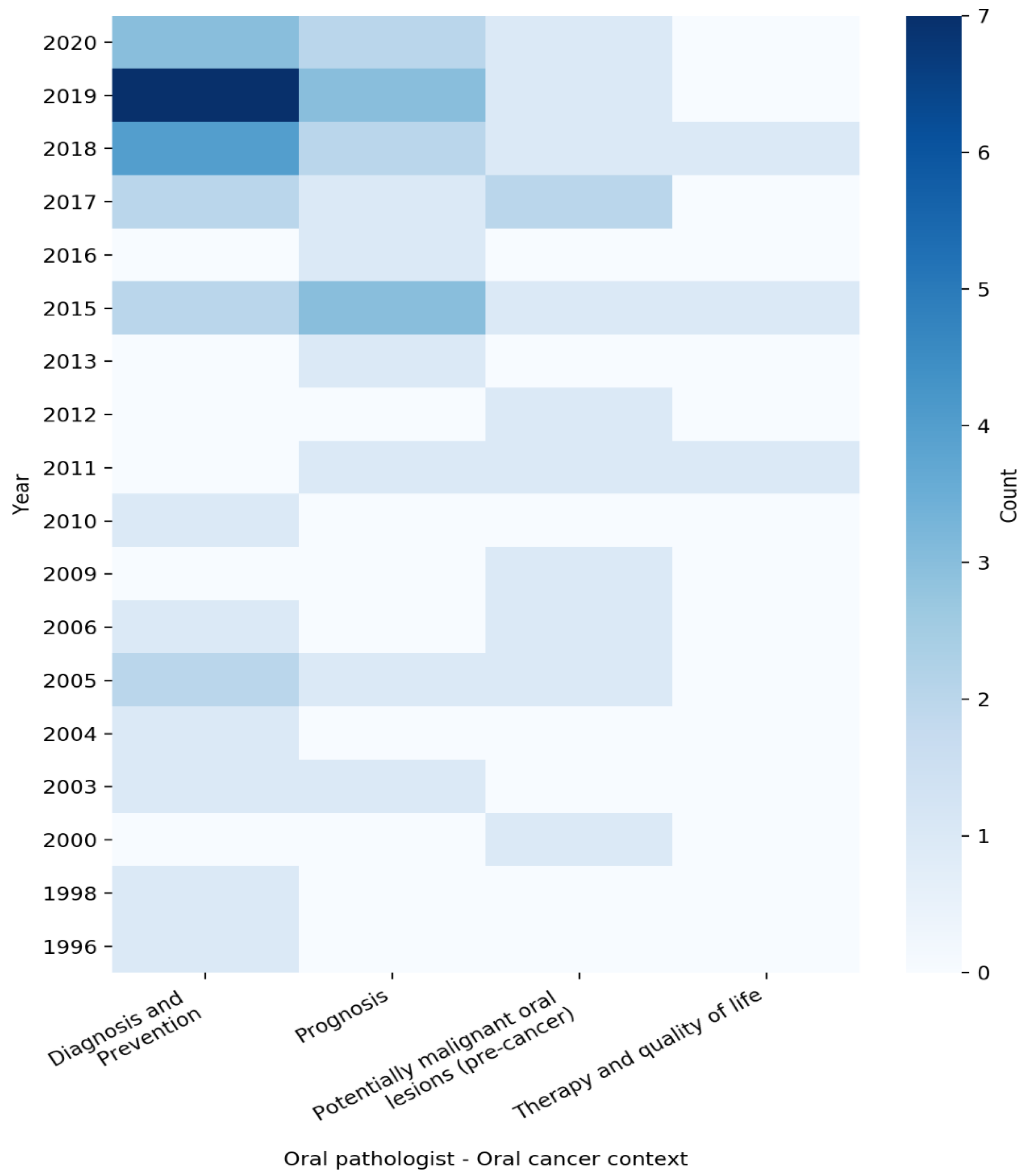
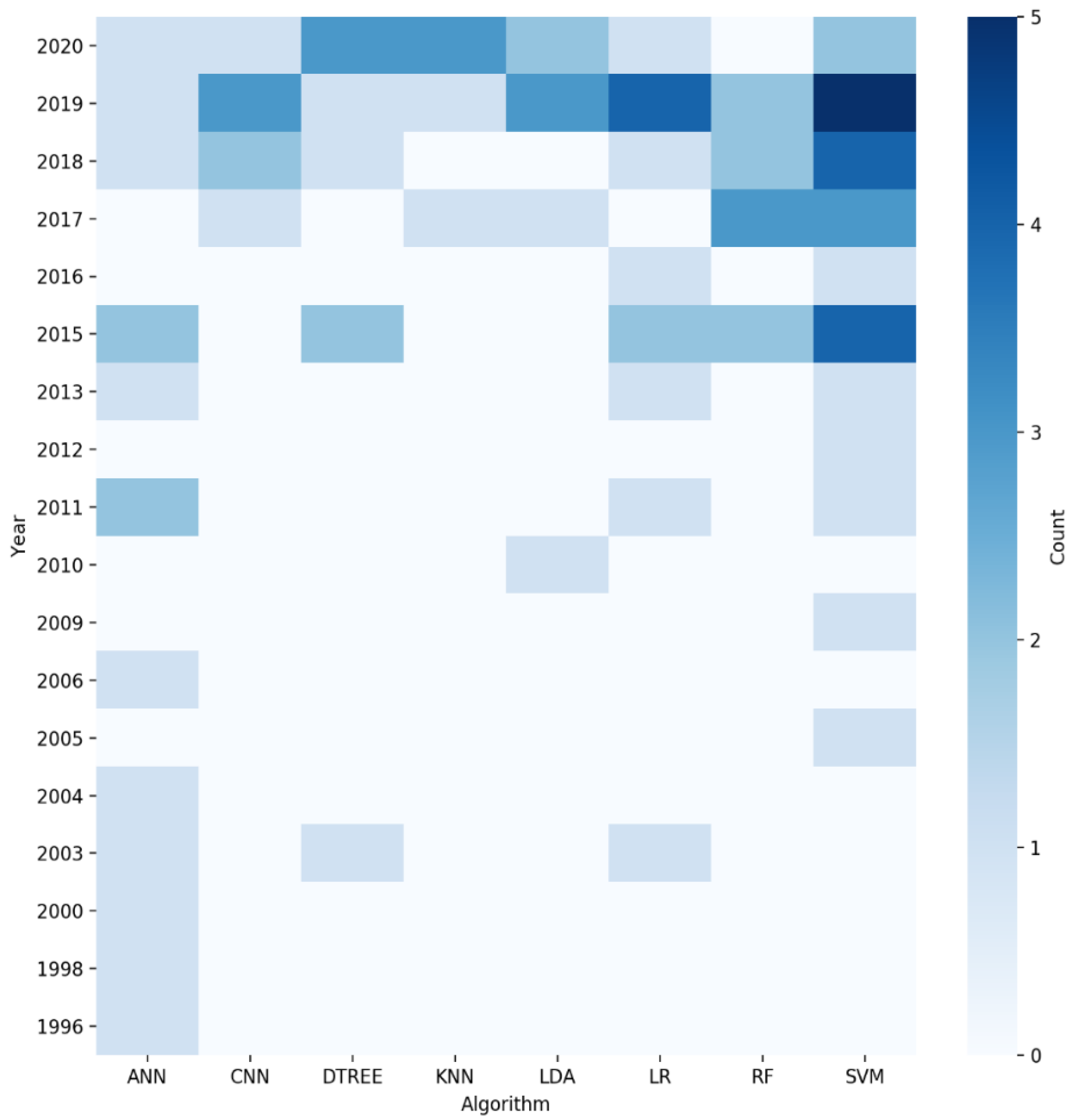
| Ref. | Year | Clinical Context | Applied Algorithm | Input Features | n Samples | ROB | Concluding Remarks |
|---|---|---|---|---|---|---|---|
| [17] | 2019 | Prognosis | XGBOOST | Expression profiles and clinical data | 291 | + | A three-mRNA signature (CLEC3B, C6, and CLCN1) successfully predicted the survival of OSCC patients |
| [18] | 2016 | Prognosis | GP, SVM, LR | Personal details, medical history, p53, p63 | 31 | + | Genetic programing (GP) an ideal prediction model for cancer clinical and genomic data |
| [19] | 2018 | Diagnosis and Prevention | SVM | miRNA expression | 122 | + | Using the platform with an ML algorithm, it discovers miRNA expression patterns capable of separating healthy subjects from OSCC patients |
| [20] | 2005 | Potentially malignant oral lesions (pre-cancer) | WNN | TEM images of collagen fibers from oral subepithelial region | 145 | + | The trained network was able to classify normal and oral pre-cancer stages |
| [21] | 2019 | Potentially malignant oral lesions (pre-cancer) | SVM, RF, LR, LDA, KNN | Cytology images | 60 | + | Applicability of tele-cytology for accurate, remote diagnosis and use of automated ANN-based analysis in improving its efficacy |
| [22] | 2015 | Therapy and quality of life | CTREE, RF, BA, SVM | Gene expression data | 486 | + | Analyzed the dysregulated gene pairs between control and tumor samples and then implemented an ensemble-based feature selection approach to prioritize targets in oral squamous cell carcinoma (OSCC) for therapeutic exploration |
| [23] | 2018 | Prognosis | KSTAR, IBK, RFC, RT | Personal details, medical history, smoking, betel nut chewing, and drinking | 1428 | + | Evidence-based diagnostic model using machine-learning techniques for the prediction of risk factors of recurrent oral cancer |
| [24] | 2010 | Diagnosis and Prevention | LDA | Spectral data | 57 | + | Presents an approach to adaptively adjust spectral window sizes for feature extraction from optical spectra |
| [25] | 1998 | Diagnosis and Prevention | ANN | Personal details, dental attendance, and smoking and drinking habits | 2027 | + | Sensitivity analysis using a decision model to simulate opportunistic screening for oral cancer and pre-cancer |
| [26] | 2017 | Prognosis | LDA, QDA, RF, SVM | Images of H&E-stained tissue sections | 115 | + | Investigates computer-extracted image features of nuclear shape and texture on digitized images of H&E-stained tissue sections for risk stratification of oral cavity squamous cell carcinoma patients compared with standard clinical and pathologic parameters |
| [27] | 2011 | Therapy and quality of life | ANN | Speech recording | 51 | - | Applicability of neural network feature analysis of nasalance in speech to assess hypernasality in speech of patients treated for oral or oropharyngeal cancer |
| [28] | 2009 | Potentially malignant oral lesions (pre-cancer) | SVM | Images of SECT (sub-epithelial connective tissue) of NOM and OS | 20 | + | Automated classification method using SVM for understanding the deviation of normal structural profile of oral mucosa during precancerous changes |
| [29] | 2020 | Diagnosis and Prevention | CNN | Histopathological images | 8321 | + | CNN-based multi-class grading method of OSCC could be used for diagnosis of patients with OSCC |
| [30] | 2011 | Potentially malignant oral lesions (pre-cancer) | SVM | Images of surface epithelium from oral mucosa | 158 | + | Classification based on textural features for the development of a computer-assisted screening of oral sub-mucous fibrosis (OSF) |
| [31] | 2020 | Diagnosis and Prevention | DTREE, SVM, KNN, LDA, LR | Histopathological images | 720 | + | SVM and linear discriminant classifier gave the best result for texture and color features, respectively, from the histopathological images |
| [32] | 2017 | Diagnosis and Prevention | CNN | Images of confocal laser endomicroscopy (CLE) | 7894 | + | Novel automatic approach for OSCC diagnosis using deep-learning technologies on CLE images |
| [33] | 2018 | Diagnosis and Prevention | CNN, RF | Microscopic images of the oral mucosa | 100 | + | CNN approach is proposed for segmentation of different constituent layers from oral mucosa histology images |
| [34] | 2000 | Potentially malignant oral lesions (pre-cancer) | ANN | Spectral data | 28 | - | Neural networks provide a very good discrimination between autofluorescence spectra of leukoplakia and normal tissue |
| [35] | 2019 | Diagnosis and Prevention | CTA | Medical/dental experience, psychosocial factors, demographics | 2401 | + | Classification tree analysis (CTA) to identify population subgroups that are less likely to have an oral cancer examination (OCE) |
| [36] | 2004 | Diagnosis and Prevention | ANN, KLLC, PCA | Spectral data | 134 | + | Classification and detection of invisible tissue alterations through autofluorescence spectroscopy applying PCA and ANN methods |
| [37] | 2003 | Prognosis | LR, DTREE | Tumor size, mode of invasion, and keratinization | 118 | + | Three statistical methods for the prediction of lymph node metastasis in carcinoma of the tongue are compared |
| [38] | 2017 | Diagnosis and Prevention | RF | Histopathological images of OSCC | 150 | + | Automated technique for accomplishing the task of mitotic cell count from related histopathological images |
| [39] | 2019 | Diagnosis and Prevention | CNN | Hyperspectral images | 100 | + | Proposed regression-based partitioned CNN learning algorithm for a complex medical image of oral cancer diagnosis |
| [40] | 2019 | Diagnosis and Prevention | CNN | Computed tomography scan images | 441 | + | Deep-learning image classification system for the diagnosis of lymph node metastasis on CT images |
| [41] | 2019 | Diagnosis and Prevention | CNN, SVM, LDA | Spectral data | 1440 | + | Classification method that discriminates tongue squamous cell carcinoma (TSCC) from non-tumorous tissue |
| [42] | 2018 | Diagnosis and Prevention | SVM | Infrared (IR) thermal imaging | 90 | + | Automatic analysis by an entropy gradient support vector machine (EGSVM) using a digital infrared thermal imaging system |
| [43] | 2019 | Prognosis | RF, DJU, LR, ANN | Personal details, tumor, and treatment characteristics | 33,065 | + | Describes a model using machine learning to help predict 5-year overall survival among patients with oral squamous cell carcinoma (OSCC) |
| [44] | 2015 | Potentially malignant oral lesions (pre-cancer) | RVM, SVM, MLC | Images of lip border | 150 | + | Using robust macro-morphological descriptors of the vermillion border from non-standardized digital photographs with a probabilistic model (RVM) for solar cheilosis recognition |
| [45] | 2015 | Diagnosis and Prevention | SVM | Spectral data | 47 | + | Classification of two oral lesions, namely oral leukoplakia (OLK) and oral squamous cell carcinoma (OSCC), was performed with SVM using different combinations of spectral features |
| [46] | 2019 | Diagnosis and Prevention | LDA, SVM | Spectral data | 34 | ? | Showed the specific IR spectral signature for OC salivary exosomes, which was accurately differentiated from HI exosomes based on detecting subtle changes in the conformations of proteins, lipids, and nucleic acids using optimized ANN |
| [47] | 2019 | Diagnosis and Prevention | LR | Tissue microarray chips | 105 | + | The effectiveness of Aurora kinase A and Ninein interacting protein (AUNIP) in diagnosing OSCC was evaluated by machine learning |
| [48] | 2015 | Prognosis | SVM | Protein intensity | 30 | + | Proteomeof whole saliva and salivary extracellular vesicles (EVs) from patients with OSCC and healthy individuals were analyzed. The proteomics data could classify OSCC with 90% accuracy |
| [49] | 2019 | Diagnosis and Prevention | SVM | Putrescine, glycyl-leucine, and phenylalanine | 31 | + | With three-marker panel, consisting of putrescine, glycyl-leucine, and phenylalanine using a support vector machine (SVM) model that can discriminate paired cancerous (T) from adjacent noncancerous (AN) tissues |
| [50] | 2015 | Prognosis | LR | Gene expression profiles | 486 | + | The proposed network-driven integrative analytical approach can identify multiple genes significantly related to an OSCC stage |
| [51] | 2019 | Prognosis | SVM, GB, LR, DTREE | Clinicopathologic data | 782 | + | Machine learning improves prediction of pathologic nodal metastasis in patients with clinical T1-2N0 OCSCC compared to methods based on DOI |
| [52] | 2020 | Prognosis | PLS-DA, OPLS-DA | Spectral data | 180 | + | Spectral data on 180 tissues comprising tumor, margin, and bed from 43 OSCC patients were used to perform machine-learning models to identify malignancy status |
| [53] | 2018 | Therapy and quality of life | DFG | Family, gene, compound, bile mutation, GWAS phenotype, OMIM phenotype, kidney mutation, and oral mutation | 400 | + | Algorithm Medusa in parallel with binary classification was used in order to find potential compounds to inhibit oral cancer |
| [54] | 2013 | Prognosis | ANFIS, ANN, SVM, LR | Clinicopathologic and genomic data | 31 | + | The results revealed that the prognosis is superior with the presence of both clinicopathologic and genomic markers |
| [55] | 2006 | Potentially malignant oral lesions (pre-cancer) | WNN | TEM images of collagen fibers from oral subepithelial region | 145 | + | The trained network could classify normal fibers from less advanced and advanced stages of OSF successfully |
| [56] | 1996 | Diagnosis and Prevention | ANN | Intra-oral smears | 348 | + | A neural network differentiated between normal/non-dysplastic mucosa and dysplastic/malignant mucosa |
| [57] | 2011 | Prognosis | LR, ANN | Medical history | 211 | ? | Suggests the importance of routinely investigating PTI in OSCCs as useful marker of tumoral behavior and prognosis |
| [58] | 2003 | Diagnosis and Prevention | PLS, ANN | Spectral data | 97 | + | The PLS-ANN classification algorithm based on autofluorescence spectroscopy at 330 nm excitation is useful for in vivo diagnosis of OSF, as well as oral pre-malignant and malignant lesions |
| [59] | 2020 | Potentially malignant oral lesions (pre-cancer) | KNN | Demographics, lesion characteristics, and cell phenotypes | 999 | + | Cytopathology tools represent a practical solution for rapid PMOL assessment, with the potential to facilitate screening and longitudinal monitoring in primary, secondary, and tertiary clinical care settings |
| [60] | 2018 | Potentially malignant oral lesions (pre-cancer) | CNN | Images oral cavity | 170 | + | CNN was implemented in the cloud and used for automatic image analysis and classification of pairs of images into “suspicious” and “non-suspect” |
| [61] | 2017 | Potentially malignant oral lesions (pre-cancer) | SVM | DIC images of oral exfoliative cells | 119 | + | The selected morphological and textural features of epithelial cells are compared with the non-smoker (-ve control group) group and clinically diagnosed pre-cancer patients (+ve control group) using SVM classifier |
| [62] | 2020 | Prognosis | DTREE, ANN, NB, KNN | Contrast-enhanced CT images | 40 | + | A radiomic ML approach applied to PTLs is able to predict TG and NS in patients with OC and OP SCC |
| [63] | 2006 | Diagnosis and Prevention | ANN, PCA | Spectral data | 143 | + | Spectral analyses were used to classify and discriminate among normal, pre-malignant, and malignant conditions on oral tissue. Sensitivity and specificity gave results of > 92% in PCA and ANN |
| [64] | 2017 | Potentially malignant oral lesions (pre-cancer) | RF, SVM, KNN | Exfoliative cytology, histopathology, and clinical follow-up data | 364 | ? | Developed an exfoliative cytology-based method for quantitative prediction of cancer risk in patients with oral leukoplakia |
| [65] | 2005 | Diagnosis and Prevention | SVM, RVM | Spectral data | 325 | + | The Bayesian framework of RVM formulation makes it possible to predict the posterior probability of class membership in discriminating early SCC from the normal squamous tissue sites of the oral cavity in contrast to dichotomous classification provided by the non-Bayesian SVM |
| [66] | 2012 | Potentially malignant oral lesions (pre-cancer) | BC, SVM | Images of normal and OSF oral mucosa | 119 | + | Bayesian classification and support vector machines (SVM) to classify normal and OSF |
| [67] | 2020 | Diagnosis and Prevention | SVM, DTREE, NCA, LDA | H&E-stained microscopic images of squamous epithelial layer | 676 | + | ML-based automatic OSCC classifier named as stratified squamous epithelial biopsy image classifier (SSE-BIC) to categorize H&E-stained microscopic images of squamous epithelial layer in four different classes: normal, well-differentiated, moderately differentiated, and poorly differentiated |
| [68] | 2015 | Prognosis | DTREE, LR, ANN | Medical history | 673 | - | Determines the differences between the symptoms shown in past cases where patients died or survived oral cancer |
| [69] | 2018 | Diagnosis and Prevention | SVM | Spectral data | 186 | + | Diffuse reflectance spectra were used to discriminate tumor from healthy tissue, and SVM models were used to classify them |
| [70] | 2015 | Diagnosis and Prevention | LIR, DTREE, RF, TREEB, ANN, CCNN, PNN/GRNN | Medical history | 1025 | - | Data-mining model using probabilistic neural network and general regression neural network (PNN/GRNN) for early detection and prevention of oral malignancy |
| [71] | 2005 | Diagnosis and Prevention | FNN | Age, gender, smoking, alcohol, bcl-2, PCNA, surviving | 21 | ? | FNN were effectively used to analyze the relationship between oral leukoplakia and HPV infection |
| [72] | 2018 | Prognosis | RF, DTREE, NB, LR, SVM, ANN | Peptides and proteins | 40 | + | Proteomics analysis of proteins in saliva in combination with machine-learning methods were applied to study prognosis classification |
| [73] | 2005 | Prognosis | FNN | Age, gender, smoking, alcohol, bcl-2, PCNA, surviving | 21 | ? | FNNs were used to build up a predictive model to study the relationship between HPV infection and different variables in the OSCC |
| Performance Metricas Mean (SD; n) | ||||
|---|---|---|---|---|
| Accuracy % | Sensitivity % | Specificity % | AUC | |
| SVM | 85.83 (10.01; 19) | 86.45 (8.06; 17) | 88.20 (10.72; 15) | 0.83 (0.15; 9) |
| ANN | 75.72 (13.08; 8) | 76.90 (13.65; 9) | 84.59 (13.44; 8) | 0.69 (0.14; 5) |
| LR | 75.47 (12.67; 9) | 76.53 (13.68; 6) | 77.51 (10.78; 4) | 0.7 (0.14; 4) |
| ANOVA | p-value = 0.037 | p-value = 0.058 | p-value = 0.270 | p-value = 0.213 |
| Diagnosis and Prevention | ||||
| SVM | 90.22 (5.79; 8) | 87.19 (4.69; 7) | 89.99 (7.80;7) | 0.95 (0.04; 3) |
| ANN | 84.03 (20.18; 2) | 84.51 (7.97; 5) | 83.28 (15.48; 5) | 0.6 (0.18; 2) |
| LR | 93.50 (9.19; 2) | 87.00 (0; 1) | ---- | --- |
| ANOVA | p-value = 0.570 | p-value = 0.761 | p-value = 0.343 | p-value = 0.039 |
| Prognosis | ||||
| SVM | 74.72 (13.19; 5) | 78.90 (11.95; 4) | 74.00 (26.87; 2) | 0.75 (0.16; 5) |
| ANN | 71.55 (11.69; 5) | 61.17 (7.80; 3) | 80.15 (4.44; 2) | 0.76 (0.09; 3) |
| LR | 68.21 (5.97; 6) | 73.05 (15.98; 4) | 74.35 (10.69; 3) | 0.7 (0.14; 4) |
| ANOVA | p-value = 0.600 | p-value = 0.249 | p-value = 0.903 | p-value = 0.861 |
| Potentially malignant oral lesions (pre-cancer) | ||||
| SVM | 89.69 (2.65; 5) | 90.61 (5.38; 6) | 90.86 (3.34; 6) | --- |
| ANN | --- | 86.00 (0; 1) | 100 (0; 1) | --- |
| LR | 83.00 (0; 1) | 80.00 (0; 1) | 87.00 (0; 1) | --- |
| ANOVA | p-value = 0.082 | p-value = 0.255 | p-value = 0.083 | --- |
| Therapy and quality of life | ||||
| SVM | 87.00 (0; 1) | --- | --- | 0.89 (0; 1) |
| ANN | 80.00 (0; 1) | --- | --- | --- |
| LR | --- | --- | --- | --- |
| ANOVA | --- | --- | --- | --- |
| Method | Count |
|---|---|
| Hold out | 18 |
| 5-fold CV | 11 |
| 10-fold CV | 10 |
| LOOCV | 7 |
| 3-fold CV | 3 |
| 7-fold CV | 2 |
| 4-fold CV | 2 |
| 9-fold CV | 1 |
| Without validation (not mentioned) | 3 |
| Total | 57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cortés, X.A.; Matamala, F.; Venegas, B.; Rivera, C. Machine-Learning Applications in Oral Cancer: A Systematic Review. Appl. Sci. 2022, 12, 5715. https://doi.org/10.3390/app12115715
López-Cortés XA, Matamala F, Venegas B, Rivera C. Machine-Learning Applications in Oral Cancer: A Systematic Review. Applied Sciences. 2022; 12(11):5715. https://doi.org/10.3390/app12115715
Chicago/Turabian StyleLópez-Cortés, Xaviera A., Felipe Matamala, Bernardo Venegas, and César Rivera. 2022. "Machine-Learning Applications in Oral Cancer: A Systematic Review" Applied Sciences 12, no. 11: 5715. https://doi.org/10.3390/app12115715
APA StyleLópez-Cortés, X. A., Matamala, F., Venegas, B., & Rivera, C. (2022). Machine-Learning Applications in Oral Cancer: A Systematic Review. Applied Sciences, 12(11), 5715. https://doi.org/10.3390/app12115715






